Abstract
Context
Tournament season can provoke overreaching syndrome in professional tennis players, which may lead to deteriorated performance. Thus, appropriate recovery methods are crucial for athletes in order to sustain high-level performance and avoid injuries. We hypothesized that whole-body cryostimulation could be applied to support the recovery process.
Objective
To assess the effects of 5 days of whole-body cryostimulation combined with moderate-intensity training on immunologic, hormonal, and hematologic responses; resting metabolic rate; and tennis performance in a posttournament season.
Design
Controlled laboratory study.
Setting
National Olympic Sport Centre.
Patients or Other Participants
Twelve high-ranking professional tennis players.
Intervention(s)
Participants followed a moderate-intensity training program. A subgroup was treated with the 5-day whole-body cryostimulation (−120°C) applied twice a day. The control subgroup participated in the training only.
Main Outcome Measure(s)
Pretreatment and posttreatment blood samples were collected and analyzed for tumor necrosis factor α, interleukin 6, testosterone, cortisol, and creatine kinase. Resting metabolic rate and performance of a tennis drill were also assessed.
Results
Proinflammatory cytokine (tumor necrosis factor α) decreased and pleiotropic cytokine (interleukin 6) and cortisol increased in the group exposed to cryostimulation. In the same group, greater stroke effectiveness during the tennis drill and faster recovery were observed. Neither the training program nor cryostimulation affected resting metabolic rate.
Conclusions
Professional tennis players experienced an intensified inflammatory response after the completed tournament season, which may lead to overreaching. Applying whole-body cryostimulation in conjunction with moderate-intensity training was more effective for the recovery process than the training itself. The 5-day exposure to cryostimulation twice a day ameliorated the cytokine profile, resulting in a decrease in tumor necrosis factor α and an increase in interleukin 6.
Key Words: overreaching, cytokine, cortisol, recovery, resting metabolic rate
Key Points
Professional tennis players who underwent moderate-intensity training and whole-body cryostimulation after the tournament season displayed a synergistic anti-inflammatory effect.
Concentrations of tumor necrosis factor α decreased and interleukin-6 increased.
Cryostimulation may be a useful adjunct to a postseason training program.
Cryostimulation is a general term used to describe localized cold therapy, water immersion, and ice-pack therapy as well as whole-body cryostimulation.1,2 It is a popular rehabilitation method because it limits secondary tissue damage and functions as a support for training programs. Whole-body cryostimulation relies on the exposure of a whole organism to an extremely low temperature (below −100°C) in a special chamber for 2 to 3 minutes. This form of cryostimulation was first introduced and modeled for therapeutic purposes in the 1970s by Yamauchi et al.3
Due to its limited availability, whole-body cryostimulation is used rarely.4,5 However, it may accelerate the recovery process more effectively than localized cold therapy,6 depending on both the area treated and the frequency of application.7,8 High-intensity training and competition may result in overreaching and overtraining, which are characterized by performance deterioration.9,10 Therefore, the appropriate use of cold exposure may be particularly important for professional athletes in supporting and enhancing recovery after such activity.
Strenuous exercise results in excessive synthesis and release of proinflammatory cytokines. Overreaching athletes experience a large inflammatory response to exercise,11 and elevated proinflammatory cytokines are associated with increased catabolic processes.12
Exposure to extremely low temperatures may initiate theromogenesis, with or without shivering, to increase metabolic heat production to 2- to 5-fold resting levels.13 Consequently, such cold exposure may accelerate the resting metabolic rate (RMR).
Our experiment was conducted in November and December, after the end of the competitive tennis season. This short break is considered both a regenerative period after the previous season and a preseason for the next year. Only a few authors have investigated this midseason period, and they mostly focused on the detraining effect.14,15 Therefore, the purpose of our study was to examine the effects of whole-body cryostimulation in conjunction with a moderate-intensity training program on professional tennis players during the midseason break. We assumed that whole-body cryostimulation would influence the immunologic, hormonal, and hematologic responses; resting metabolic rate; and tennis performance and thus contribute to a more effective muscle-recovery process.
METHODS
Participants
Twelve high-ranking professional, national-level, male tennis players (ATP singles ranking = 150–900, ATP doubles ranking = 11–15) took part in our experiment during a 2-week tennis camp at the National Olympic Sport Centre in Cetniewo, Poland, during the November-to-December break in the season. All participants lived in the same accommodations and followed the same training schedule and diet. The daily energy content of food offered in the menu did not exceed 4200 kcal. The recommended protein dose varied from 1.3 to 1.5 g/kg of body mass.
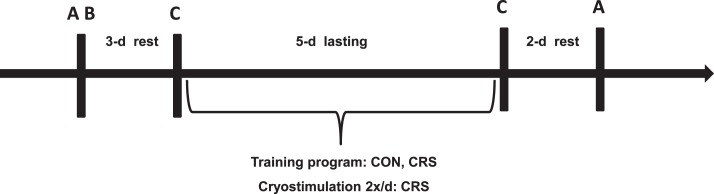
We designed the experimentation schedule and collaborated on all details of the training program with the coaches (Figure, Table 1). The main portion of the study consisted of 5 days of moderate-intensity training and cryostimulation applied twice a day. Before this stage, we assessed body composition, resting energy expenditure, maximal aerobic capacity, and cytokine and hormone levels (Figure: time A,B). The assessment was followed by 3 days of rest; the tennis drill was performed on the fourth day (time C). After the training and cryostimulation program was completed, the tennis drill was repeated on the following day. The participants rested for the 2 following days, after which their cytokine levels and resting energy expenditure were assessed again (time A).
Figure.
Schematic presentation of the experimental design. A, 8:00 am: Assessment of body composition and resting energy expenditure; blood collection for hematologic measures, tumor necrosis factor α, interleukin-6, creatine kinase, cortisol, and testosterone. B, 10:00 am: Assessment of aerobic capacity with exercise at incrementally increases. C: Tennis drills, 2 × 6 min at work-to-rest ratio of 1:1; blood collection for lactic acid measurement. Abbreviations: CON, control group; CRS, cryostimulation group.
Table 1.
Training Programa
| Day |
Before Lunch |
Training Intensity |
After Lunch |
Training Intensity |
| Monday | CRYO (9:30 am) | CRYO (5:30 pm) | ||
| Training A (10:15–11:00 am) | Low | Training D (7:00–8:00 pm) | Moderate | |
| Training B (12:00 pm–1:30 pm) | 60% of 1 RM | |||
| Training C (1:45–2:30 pm) | Moderate | |||
| Tuesday | CRYO (9:30 am) | CRYO (5:30 pm) | ||
| Training A (10:15–11:00 am) | Low | Training F (7:00–8:00 pm) | Moderate | |
| Training E (12:00 am–2:00 pm) | Moderate | |||
| Wednesday | CRYO (9:30 am) | CRYO (5:30 pm) | ||
| Training A (10:15–11:00 am) | Low | |||
| Training B (12:00 pm–1:30 pm) | 60% of 1 RM | |||
| Training C (1:45–2:30 pm) | Moderate | |||
| Thursday | CRYO (9:30 am) | CRYO (5:30 pm) | ||
| Training A (10:15–11:00 am) | Low | Training A (6:30–7:30 pm) | Low | |
| Training G (12:00 pm–1:00 pm) | Moderate | |||
| Training E (1:15–2:00 pm) | High | |||
| Friday | CRYO (9:30 am) | CRYO (5:30 pm) | ||
| Training A (10:15–11:00 am) | Low | |||
| Training B (12:00 pm–1:30 pm) | 60% of 1 RM | |||
| Training C (1:45–2:30 pm) | Moderate | |||
| Saturday and Sunday | Rest |
Abbreviations: CRYO, whole-body cryostimulation; RM, repetition maximum.
Training A: Stretching exercise, hold-relax technique.
Training B: Strength training for local strength endurance (8 basic tennis exercises, each at 60% of 1 RM, involving arms and shoulders as follows: bench press, dumbbell pullovers, T-bar rows, reverse curls; legs as follows: squats, lunges; trunk as follows: crunches, dumbbell side bends).
Training C: Agility games with tennis balls on small court (main stress on coordination, agility, accuracy).
Training D: Conditioning exercise, team sports; volleyball: short games with short periods (few seconds) with high intensity, average heart rate at 80% to 90% maximum. Most vital elements during games were appropriate mechanical performance of exercises and scoring maximum number of points.
Training E: Conditioning exercise, team sports; soccer: short games with short periods (few seconds) with high intensity, average heart rate at 80% to 90% maximum. Most vital elements during games were appropriate mechanical performance of exercises and scoring maximum number of points.
Training F: Ice skating with main focus on balance and free style; average heart rate 60% to 70% of maximum.
Training G: Endurance, continuous distance running for 60 minutes, average heart rate 70% to 80% of maximum.
The course of the main experiment differed for the cryostimulation (CRS; n = 6) and control (CON; n = 6) groups, to which participants were randomly assigned. However, in 2 cases, we took the participants' known hypersensitivity to low temperatures into consideration and assigned them to CON. Both groups followed the same training schedule during the main stage of the experiment. All the details of the training program reported by the coaches are shown in Table 1. The schedule was aimed at maintaining the players' physical conditioning via moderate-intensity exercise. Most of the drills and exercises used in the experiment were familiar to the athletes from their regular national tennis association examination camps in previous years.
Anthropometric Measurements
Body mass and composition were estimated using a bioelectrical impedance floor scale (model TBF-300 Body Fat Monitor/Scale Analyzer; Tanita, Tokyo, Japan; accuracy = ±0.1 kg). These measurements were taken 1 hour before breakfast and after the participants had voided their bladder and bowels. During the measurement, they wore only briefs and remained barefoot. Duplicate measures were taken with the participant standing, in accordance with the manufacturer's guidelines; the average value was used for the final analysis. The device was calibrated before each test session. Measurement accuracy was 98%.16
Resting Metabolic Rate
The RMR and percentage of energy derived from carbohydrate, fat, and protein was estimated using 3 sets of telemetric analyzers (model MetaMax 3B; Cortex Biophysik, Leipzig, Germany) and an indirect calorimetric program based on the basic primary variables: V˚o2, V˚co2, rate of nitrogen excretion, and respiratory quotient. Assessment lasted from 6:30 to 8:00 am and took place after a restful night's sleep (7 hours), 12 hours of fasting, and 48 hours without vigorous physical activity. Simultaneously, measurements were obtained in a hotel for each pair of roommate players. All players were instructed to remain calm in a relaxed and supine position before and throughout the entire RMR session. The room was darkened, and noise was kept to a minimum. Ambient room temperature varied between 23° and 24°C. Measured values of V˚o2 and V˚co2 during the last 20 minutes of the 30-minute measurement period were averaged and used to calculate RMR. Urine was collected 24 hours before the measurement to determine the concentration of urinary nitrogen, using a kit (model B7550; Pointe Scientific, Inc, Canton, MI) and a spectrophotometer (model PU8730; Koninklije Philips Electronics NV, Surrey, United Kingdom). The same protocol for measuring RMR was repeated after 5 days of whole-body cryostimulation and 2 days of recovery.17
Aerobic Power Measurement
Maximal aerobic capacity was determined during an incremental tennis exercise and expressed as maximal oxygen consumption (V˚o2max). The examination was aimed at establishing physical capacity and intensity of the anaerobic threshold. Breath-by-breath pulmonary gas exchange was measured (model MetaMax 3B; Cortex Biophysik) throughout the exercise. Participants performed a continuously graded multistage field tennis test according to the protocol of Smekal et al18: 3-minute exercise stages separated by 1-minute breaks for machine adjustments, based on typical tennis movements when reaching for a ball thrown from the ball machine (model Hot Shot DXSR-1594; Prince Sports, Inc, Bordentown, NJ). The landing point for the balls was set about 2 m in front of the baseline. Participants were instructed to hit the balls by alternating between backhand and forehand. They were allowed a 3-minute warm-up period before the test. Immediately after the warm-up, V˚o2max testing began. The test was ended at either the player's request or at the investigator's command, when the athlete was no longer able to fulfill the test criteria (ie, no longer able to perform strokes with acceptable technique and precision). During the break for machine adjustments between exercise stages, the participant was in a seated position. During the initial phase of the exercise, the number of balls shot from the machine was 12 per minute, whereas in the final phase it varied from 28 to 32 per minute, according to individual endurance abilities. The number of balls shot from the ball machine was adjusted manually. During each stage of the test, stroke effectiveness ratings were recorded as percentage of shots hitting the target zones (data not shown). The stroke ratings in the study by Smekal et al18 were good predictors of tournament performance (r = 0.94).
Before each athlete's test, the O2 and CO2 analyzers were recalibrated using standard gases of known concentrations in accordance with the manufacturer's guidelines. Heart rates were monitored continuously by telemetry monitor (model T31; Polar Electro-Oy, Kempele, Finland) during each test session and the first 5-minute passive recovery after the test.
Tennis Drill
The movement used in the tennis drill was exactly the same as in the V˚o2max test described earlier except that intensity remained constant at the level of the individual's anaerobic threshold. The frequency of the ball machine was 16 to 18 balls per minute. The average oxygen uptake at the anaerobic threshold level corresponded to the average intensity of a tennis match.19 The activity consisted of two 6-minute exercises separated by a 6-minute break; therefore, the work-to-rest ratio equaled 1:1. Breath-by-breath pulmonary gas measurement was also used to control exercise intensity. During the exercise, the stroke effectiveness was recorded. Additionally, in order to determine the physiologic cost of the exercise, blood lactate was assessed. Stroke effectiveness was expressed as the percentage of shots hitting the target zones, which consisted of 2 areas in the corners of the court, bordered by the boundary line, singles lines, and marking lines and 1 meter from the service and center lines.
Whole-Body Cryostimulation
The CRS group was exposed to cold twice a day (9:30 am and 5:30 pm) in a cryogenic chamber at the National Olympic Sport Centre in Cetniewo, Poland. Before the experiment, each participant was examined by a physician for any contraindications to such treatment. Most participants had used whole-body cryostimulation previously. Each participant gave his written consent before the study, and the Bioethical Committee of the Regional Medical Society in Gdańsk approved the study according to the Declaration of Helsinki. Each cryostimulation session lasted 3 minutes at a temperature of −120°C. Entry to the cryochamber was preceded by a 20- to 30-second adaptation period in the vestibule at a temperature of −60°C. The participants were dressed in shorts, socks, gloves, and a hat covering their auricles. They did not participate in any other treatment after the whole-body cryostimulation to avoid obscuring the cryogenic effect.
Blood Analysis
Blood samples were collected for 2 main purposes. First, cytokines (interleukin 6 [IL-6] and tumor necrosis factor α [TNFα]), hormone levels (testosterone and cortisol), hematologic values, and creatine kinase (CK) were assessed. Measurements were taken at point A of the Figure at the initial and final stages of the experiment. Second, the lactate level (LA) at point C, before and after the test, was established. Measurements were taken before, immediately after, and after 5 minutes of rest following the tennis drill. The samples for determining the cytokine and hormone concentrations were always collected between 8:00 and 8:30 am from the antecubital vein. After collection, the samples were immediately placed in ice at 4°C. Within 10 minutes, they were centrifuged at 2500g and 4°C for 10 minutes. Aliquots of plasma were stored at −70°C.
Hematologic measurements were determined by conventional methods using an LH 750 Hematology Analyzer (Beckman-Coulter Inc, Brea, CA). Plasma TNFα and IL-6 levels were determined by enzyme immunoassay methods using commercial kits (TNF: kit HSTA00D; IL-6: kit HS600B; R&D Systems, Minneapolis, MN). Detection limits for TNFα and IL-6 were 0.038 pg·ml−1 and 0.04 pg·ml−1, respectively. The intra-assay coefficients of variation were <2.5% for TNFα and <8.0% for IL-6.
Cortisol concentration was determined with electrochemiluminescence immunoassay (model Elecsys 2010; Roche, Nutley, NJ) and testosterone with the chemiluminescence immunoassay (COBAS 6000/E601, Roche).
Plasma creatine kinase activity was used as a marker of muscle damage and was evaluated by CK kit (Emapol, Gdańsk, Poland). The CK detection limit for the applied kit was 6 U·L−1, whereas its intra-assay coefficient of variation was 1.85%. Lactate concentration in the capillary blood was assessed before and 5 minutes after training (model LKM 140; Dr Lange, Dusseldorf, Germany), with an intra-assay coefficient of variation of 2.5%.
Statistical Calculations
Statistical analysis was performed using Statistica for Windows (version 8.0; StatSoft, Tulsa, OK). A 2 (group) × 2 (time) repeated-measures analysis of variance was calculated to determine the significance of the differences between groups and between precryostimulation and postcryostimulation sessions. The level of significance was set at .05 for all analyses. Additionally, in order to elaborate on the differential significance between groups before and after the 5 days of whole-body cryostimulation, the method of multiple comparisons (post hoc honestly significant Tukey test) was applied. Furthermore, due to the small number of participants, we also calculated the effect size (partial η2), which ranges between 0 and 1. Using the Cohen rule of thumb as well as the conversion table for η2, the interpretations of the partial η2 value are unequivocal. However, the most restrictive interpretation method assigns values to the effect size as follows: 0.1 constitutes a small effect; 0.3, a medium effect; and 0.5, a large effect.
RESULTS
All participants completed the study, and no adverse events were reported. The combination of 5 days of whole-body cryostimulation and training had no effect on body weight or composition (Table 2).
Table 2.
Participants' Demographic and Anthropometric Characteristics (Mean ± SD)a
| Characteristic |
Group |
|||
| Cryostimulation |
Control |
|||
| Before Study |
After Study |
Before Study |
After Study |
|
| Age, y | 23 ± 3.0 | 23 ± 3.0 | 20 ± 2.0 | 20 ± 2.0 |
| Height, cm | 185.1 ± 2.5 | 185.1 ± 2.5 | 182.5 ± 5.7 | 182.5 ± 5.7 |
| Weight, kg | 79.7 ± 6.2 | 79.3 ± 5.9 | 81.4 ± 7.1 | 81.2 ± 6.9 |
| Fat-free mass, kg | 72.6 ± 4.6 | 72.5 ± 4.5 | 73.6 ± 5.7 | 73.5 ± 5.6 |
| Fat, kg | 7.0 ± 3.2 | 6.7 ± 3.1 | 7.7 ± 3.0 | 7.6 ± 2.9 |
| Fat, % | 8.5 ± 3.2 | 8.3 ± 2.8 | 9.3 ± 3.0 | 9.2 ± 2.9 |
| Body mass index, kg·m−2 | 23.2 ± 1.8 | 23.1 ± 1.7 | 24.4 ± 1.9 | 24.3 ± 1.9 |
In most participants, the hematologic values remained unchanged except for the white blood cell and basophil levels (Table 3). An increase in basophil percentage was observed in the CRS group, whereas the value decreased in the CON group. The effect size for this change (expressed as partial η2) was 0.53. The levels of cytokines and hormones were noticeably different for the 2 groups after the main part of the experiment. Multiple-comparisons testing (post hoc honestly significant difference Tukey test) indicated that, in the CRS group, the cytokine TNFα concentration decreased by 60%, whereas in the CON group, it fell by 35%. At the same time, the concentration of IL-6 in the CRS group increased by 23%; in the CON group, a slight drop of 7.7% was recorded. These differences were statistically significant. Also, the effect sizes for these measurements demonstrated that the changes of 63% and 60% for TNFα and IL-6, respectively, were attributed to cryostimulation (Table 3).
Table 3.
Effects of Whole-Body Cryostimulation on Hormonal Responses and Cytokine Concentrations in Tennis Players (Mean ± SD)
| Variable |
Group |
Differences |
P Value |
F1,10 |
Effect Size, Partial η2 |
Test Power (α = .05) |
|||
| Cryostimulation |
Control |
||||||||
| Before Study |
After Study |
Before Study |
After Study |
||||||
| Tumor necrosis factor α, pg·mL−1 | 2.5 ± 0.3 | 1.0 ± 0.1 | 2.6 ± 0.6 | 1.7 ± 0.1 | Group × time | .002 | 17.0 | 0.63 | 0.959 |
| Interleukin-6, pg·mL−1 | 1.3 ± 0.2 | 1.6 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 | Group × time | .003 | 14.76 | 0.60 | 0.932 |
| Testosterone, nmol·L−1 | 18.9 ± 1.6 | 21.4 ± 2.5 | 22.5 ± 3.8 | 19.4 ± 3.6 | Group × time | .01 | 8.07 | 0.45 | 0.727 |
| Cortisol, nmol·L−1 | 412.0 ± 43.2a | 463.0 ± 77.5 | 513.7 ± 63.1 | 417.6 ± 35.6 | Group × time | .008 | 10.7 | 0.52 | 0.838 |
| White blood cells, 103/μL | 5.6 ± 0.9a | 6.1 ± 1.0 | 6.7 ± 2.1 | 7.0 ± 1.5 | Group × time | .002 | 16.25 | 0.62 | 0.951 |
| Lymphoctes, % | 35.0 ± 4.1 | 34.0 ± 3.2 | 31.5 ± 8.9 | 31.9 ± 5.4 | None | ||||
| Basophils, % | 10.1 ± 0.8 | 11.1 ± 0.5 | 10.4 ± 2.1 | 9.8 ± 2.1 | Group × time | .007 | 11.31 | 0.53 | 0.857 |
| Neutrophils, % | 53.7 ± 3.5 | 53.9 ± 4.0 | 57.9 ± 13.0 | 58.2 ± 10.0 | None | ||||
| Red blood cells, 103/μL | 5.1 ± 0.1 | 5.2 ± 0.3 | 5.1 ± 0.5 | 5.4 ± 0.7 | None | ||||
| Platelets, 103/μL | 202.3 ± 24.4 | 220.5 ± 30.4 | 246.3 ± 33.0 | 238.2 ± 23.0 | None | ||||
| Hemoglobin, g·dL−1 | 15.0 ± 0.3 | 15.3 ± 0.4 | 15.3 ± 1.0 | 15.2 ± 0.8 | None | ||||
| Creatine kinase, U·L−1 | 305.0 ± 81.9 | 241.4 ± 48.9 | 286.7 ± 11.0 | 295.5 ± 65.5 | Group × time | .01 | 9.63 | 0.49 | 0.798 |
Differences between groups at baseline (P = .004).
In addition, we investigated the level of CK as a marker of damaged tissue. In both groups, this level was slightly elevated before the main part of the experiment, which could have resulted from insufficient recovery after the tournament season. After the 5-day training program, CK in the CRS group decreased from 305.0 to 241.4 U·L−1, whereas in the CON group, it remained almost the same (286.7 U·L−1 before the main part of the investigation and 295.5 U·L−1 after). The partial η2 for the observed changes was 0.49.
Although the cortisol and testosterone values corresponded to reference physiologic values, they were altered in very different ways. The baseline cortisol level was different for the groups (P = .004). However, in the CRS group, the cortisol concentration increased from 412.0 to 463.0 nmol·L−1, whereas in the CON group, it dropped from 513.7 to 417.6 nmol·L−1 (Table 3). The average values of cortisol for the groups after the end of the investigation were not different. Yet significant changes were noted within the relative levels of testosterone. In the CRS group at the end of the experiment, testosterone had increased (from 18.9 to 21.4 nmol·L−1); in the CON group, it decreased slightly (22.5 to 19.4 nmol·L−1). The partial η2 for testosterone was 0.45 and for cortisol was 0.52, suggesting a considerable effect size (Table 3).
Analysis of the RMR using indirect calorimetry showed no statistically significant differences occurred, either between groups or with time. The percentage contributions of carbohydrate and fat in energy production remained constant, but the protein contribution increased in the CRS group by 2% and decreased slightly in the CON group (0.5%). Nonetheless, the relative values of the oxidized proteins did not differ in the statistical evaluation (Table 4).
Table 4.
Resting Metabolic Rate and Percentage of Substrate Before and After Whole-Body Cryostimulationa (Mean ± SD)
| Variable |
Group |
|||
| Cryostimulation |
Control | |||
|
Before Study |
After Study |
Before Study |
After Study |
|
| Resting metabolic rate, kcal | 1920 ± 171 | 1916 ± 183 | 2017 ± 79 | 2010 ± 95 |
| Carbohydrate, % | 57.5 ± 7.2 | 57.3 ± 3.2 | 62.6 ± 6.2 | 61.8 ± 2.8 |
| Fat, % | 29.6 ± 6.4 | 30.6 ± 5.3 | 25.1 ± 9.4 | 26.5 ± 4.3 |
| Protein, % | 12.8 ± 2.3 | 14.8 ± 2.2 | 12.1 ± 4.0 | 11.6 ± 2.2 |
| Carbohydrate, g·kg−1 | 3.5 ± 0.4 | 3.4 ± 0.2 | 3.9 ± 0.4 | 3.8 ± 0.3 |
| Fat, g·kg−1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.2 | 0.7 ± 0.1 |
| Protein, g·kg−1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
No differences were seen for group or over time.
To check the influence of the specific tennis drill on the physiologic cost, we assessed oxygen uptake during the exercise and heart rate and lactate level before and after the main experiment. We took into consideration the oxygen uptake in minutes 2 and 6 of each stage of the tennis drill. We also calculated the differences between those values. The larger the difference in oxygen uptake, the greater the physiologic cost of the exercise. In the CRS group after the main experiment, the lower value for the difference in the oxygen uptake was recorded in both stages of the drill. It dropped from 18.2 to 12.5 and 18.3 to 16.6 mL·kg−1·min−1 for the first and second parts of the tennis drill, respectively, whereas in the CON group, it remained at the same level throughout both parts of the experiment. The differences were statistically significant, and the effect size confirmed the influence of the cold exposure in the CRS group (Table 5). With repeated performance of the tennis drill after the 5-day program, stroke effectiveness improved in the CRS and CON groups by 6% and 5%, respectively. This high stroke effectiveness was maintained in the second phase of the exercise only by the CRS group; in the CON group, growing fatigue led to its decrease. Moreover, heart rate during the tennis drill and recovery periods was lower in the CRS group but remained at the same level in the CON group for both stages of the tennis drills. The effect sizes for heart rate during the recovery after the first and second tennis drills were 0.76 and 0.79, respectively. Immediately after completing the exercise, the blood lactate concentration decreased in both groups. In the fifth minute of rest after the end of the exercise, the lactate level was lower in the CRS group (2.8 mmol·L−1) than in the CON group (3.3 mmol·L−1).
Table 5.
Effect of Whole-Body Cryostimulation on Energetic Cost of Specific Tennis Drills in National Tennis Players (Mean ± SD)
| Tennis Drill |
Variable |
Group |
Differences |
P Value |
F1,10 |
Effect Size, Partial η2 |
Test Power (α = .05) |
|||
| Cryostimulation |
Control |
|||||||||
| Before Study |
After Study |
Before Study |
After Study |
|||||||
| I | V˚O2max, mL·kg−1·min−1 | 61.0 ± 3.0 | 61.0 ± 3.0 | 60.6 ± 5.8 | 61.0 ± 3.0 | None | ||||
| V˚O2 at 2 min, mL·kg−1·min−1 | 31.2 ± 3.4 | 31.8 ± 3.6 | 32.0 ± 7.4 | 32.8 ± 7.7 | None | |||||
| V˚O2 at 6 min, mL·kg−1·min−1 | 49.3 ± 3.5 | 44.3 ± 2.5 | 48.5 ± 2.6 | 48.0 ± 2.4 | Time | .003 | 14.41 | 0.59 | 0.927 | |
| Group × time | .001 | 18.82 | 0.65 | 0.973 | ||||||
| ΔV˚O2,a mL·kg−1·min−1 | 18.2 ± 5.7 | 12.5 ± 4.9 | 16.5 ± 7.6 | 16.0 ± 7.6 | Time | .0009 | 21.59 | 0.68 | 0.986 | |
| Group × time | .002 | 15.25 | 0.60 | 0.938 | ||||||
| Heart rate, beats/min | 170 ± 4 | 168 ± 2 | 177 ± 6 | 173 ± 7 | Time | .009 | 10.30 | 0.51 | 0.824 | |
| Stroke effectiveness, % | 82.0 ± 6.0 | 88.0 ± 5.0 | 77.0 ± 4.0 | 79.0 ± 3.0 | Time | .0003 | 28.59 | 0.74 | 0.997 | |
| Group × time | .02 | 7.78 | 0.44 | 0.711 | ||||||
| Heart rate after 5-min rest, beats/min | 113 ± 5 | 103 ± 7 | 119 ± 6 | 111 ± 5 | Time | .0002 | 31.46 | 0.76 | 0.998 | |
| II | V˚O2 at 2 min, mL·kg−1·min−1 | 33.6 ± 3.7 | 33.5 ± 3.3 | 34.2 ± 6.7 | 34.1 ± 4.9 | None | ||||
| V˚O2 at 6 min, mL·kg−1·min−1 | 52.5 ± 3.2 | 50.1 ± 3.7 | 51.0 ± 3.5 | 50.6 ± 2.3 | Time | .04 | 5.24 | 0.34 | 0.543 | |
| ΔV˚O2,a mL·kg−1·min−1 | 18.3 ± 5.4 | 16.6 ± 4.8 | 16.8 ± 5.6 | 16.5 ± 5.7 | Time | .04 | 5.18 | 0.34 | 0.538 | |
| Heart rate, beats/min | 176 ± 3 | 168 ± 2.0 | 179 ± 4 | 173.0 ± 7 | Time | .001 | 19.17 | 0.66 | 0.975 | |
| Stroke effectiveness, % | 84.0 ± 5.0 | 90.0 ± 6.0 | 78.0 ± 7.0 | 79.0 ± 7.0 | Time | .001 | 19.28 | 0.66 | 0.976 | |
| Group × time | .02 | 6.94 | 0.41 | 0.662 | ||||||
| Heart rate after 5-min rest, beats/min | 115 ± 6 | 103 ± 7 | 119.0 ± 5 | 111.0 ± 6 | Time | .0009 | 38.93 | 0.79 | 0.999 | |
| Lactate at end of drill, mmol·L−1 | 5.8 ± 1.1 | 5.2 ± 0.6 | 6.0 ± 1.4 | 5.5 ± 0.9 | None | |||||
| Lactate after 5-min rest, mmol·L−1 | 4.2 ± 0.7 | 2.8 ± 0.1 | 4.1 ± 0.9 | 3.3 ± 0.7 | Time | .002 | 15.2 | 0.60 | 0.938 | |
DISCUSSION
A growing body of evidence suggests that the systemic inflammation response contributes significantly to the overtraining syndrome.9,11,20 Excessive, high-volume, or high-intensity professional training with insufficient rest may cause repetitive trauma, resulting in an acute-phase response, and may stimulate circulating monocytes to produce pro-inflammatory cytokines (eg, TNFα, IL-1β) resulting in the systemic inflammation.11,20 In our study, we observed that most of the athletes had an elevated baseline concentration of the pro-inflammatory cytokine TNFα (Table 3) after the recently completed tournament season.
Our main finding is that 5 days of whole-body cryostimulation in conjunction with moderate-intensity training considerably improved both the blood cytokine profile and physical performance. Moreover, this is the first study performed on professional athletes in which the pro-inflammatory cytokine concentration TNFα decreased after such a short period of whole-body cryostimulation. Previous authors21,22 exposed participants to extremely low temperatures once a day for 10 days. However, Hirvonen et al 6observed that whole-body cryostimulation applied 3 times a day for 7 days was very effective in reducing the inflammatory process in patients with rheumatoid arthritis. Based on this observation, we designed our 5-day twice-daily protocol for whole-body cryostimulation. Our experiment was conducted during a posttournament season camp aimed at providing sufficient recovery while avoiding a decrease in physical performance. The proposed whole-body cryostimulation protocol may be useful for tennis players during short breaks between tournaments for more effective recovery in the future.
At the beginning of our tennis camp, the participants were characterized by elevated levels of TNFα and CK, reflecting the sustained overreaching that had occurred after the intense competitive season. Similarly, an elevated concentration of TNFα was observed in basketball players during a playoff round and in judo athletes after a high-intensity training session.23,24 In our experiment, we noted the decreasing concentration of TNFα in all tennis players after 5 days of moderate-intensity training. However, the decrease was more pronounced when the training was accompanied by the cryostimulation protocol. The concentration of TNFα decreased 2.5-fold in the CRS group and only 1.5-fold in the CON group. These data are consistent with the previous observation of an anti-inflammatory effect of low-intensity exercise.25 Furthermore, our results indicate that whole-body cryostimulation can potentiate the effect of training. Nemet et al4 also observed a decrease in another pro-inflammatory cytokine mediator, IL-1β, under the influence of ice packs applied locally during recovery from sprint-interval training. Still, this localized cryostimulation had no effect on the concentration of IL-6,4 possibly due to its limited range of influence and insufficiently cold temperature.
The 5-day program of moderate-intensity training and cryostimulation increased the cytokine IL-6 by 23% over the baseline value, yet the level of IL-6 remained unchanged in the CON group. An increase in IL-6 was also recorded within a group of untrained, healthy men who underwent whole-body cryostimulation once a day for 10 days.21
Moreover, IL-6 concentration increased exponentially during physical effort in relation to the intensity and duration of an exercise, mass of the working muscles, and the individual's endurance capacity.25,26 Recent studies27,28 highlight the fact that muscle-derived IL-6 is an important factor in regulating metabolism and stimulating the regenerative and proliferative processes of the satellite cells. Its role as an energy sensor was summarized in the latest published study,28 in which skeletal muscle contraction was emphasized as a major source of IL-6 production. Thus, exposure to an extremely low temperature might have triggered muscle shivering, resulting in an increase in IL-6. Nevertheless, these are only assumptions because we did not assess the source of IL-6, which requires advanced methods such as muscle biopsies and real-time polymerase chain reaction.29
However, the elevated release of IL-6 may have also been provoked by a change in the number of leukocytes in the CRS group after the cryostimulation protocol, as noted in previous work.21,30 Still, in the CON group, we observed the elevated number of leukocytes simultaneously with an insignificant change in the IL-6 concentration. The cytokine IL-6 has been classified as a pleiotropic cytokine that is involved in a number of metabolic pathways, which makes determining and interpreting its source and role very challenging. Also, other tissues could be sources of IL-6. Diestel et al31 found that in vitro endothelial cells increased the expression of IL-6 in response to cold. Our data clearly showed that whole-body cryostimulation led to an increased concentration of IL-6, which was associated with a drop in the TNFα blood level. Previous authors12 had already reported that IL-6 might inhibit TNF production, but further studies are needed to confirm this mechanism in humans during whole-body cryostimulation.
The functions of IL-6 produced by skeletal muscle include mobilization of energetic substrates and release of cortisol.32 In fact, we observed rises in both IL-6 and cortisol in the CRS group but not in the CON group. Still, the effects of cold exposure on plasma cortisol concentrations are equivocal.13 Cortisol level was not affected by localized ice-pack application4 or a single cryochamber treatment,22 yet it increased in kayakers exposed to cryostimulation combined with an extensive training program for 10 days.22 In our research, the level of cortisol was assessed at rest and always at the same time of the day in order to avoid the influence of diurnal circadian rhythm. The cortisol levels were considerably different between the groups from the beginning of our experiment, and we continued to observe opposite tendencies. In the CRS group, the cortisol level increased during the main part of the experiment, whereas in the CON group, it decreased (Table 3). It should be noted, however, that cortisol values at both the beginning and end of the experiment were within normal limits in both groups. Testosterone and cortisol levels are frequently measured to determine the anabolic and catabolic effects of training and recovery.9 Even though our participants' cortisol and testosterone values did not exceed the reference ranges, changes resulting from the shifts in their levels ultimately caused the opposite results within the groups.
Previous research33demonstrated that cortisol may enhance lipolysis, suggesting that it contributes to increased lipid oxidation. Banfi et al2 summarized the effect of whole-body cryostimulation in athletes but did not comment on RMR. To examine the influence of cold exposure on RMR, we measured it before and after our main experiment. Contrary to our initial expectations, 5 days of whole-body cryostimulation did not increase RMR or change the percentage of fat used as a metabolic substrate (Table 4), regardless of the increased cortisol level (Table 3). Due to exposure to an extremely low temperature, the rate of blood flow through vessels changed, yet the effect of cryostimulation on prolonged thermogenesis was rather limited. We recorded a slight increase in the percentage of protein in the energy yield in the CRS group, which may suggest enhanced protein turnover. The observed changes were rather insignificant, though, and do not constitute a basis for any confident conclusions.
In the CRS group, we also observed changes in the hematologic values, including increases in the white blood cell count and percentage of basophils, yet the ranges of those shifts corresponded to the reference values. Published data on morphologic values after a cryotherapy treatment are not consistent. The change in white blood cell count could have resulted from the enhanced concentration of IL-6 and cortisol, which may have infiltrated to the tissues.29 Our results correspond with observations published by Lubkowska et al.21
To learn if changes in the cytokine profile were accompanied by changes in physical performance, the athletes completed tennis drills. Intensity, as determined by oxygen consumption, was adjusted to the average oxygen uptake during a tennis match.19 We had assessed the oxygen consumption in the second and sixth minutes of each stage of each tennis drill and calculated the differences between them, which indicated that fatigue developed during the exercise. In the CRS group, these differences decreased after the main part of the experiment in both stages of the exercise, suggesting the slower onset of fatigue. We did not observe any statistical differences in the CON group (Table 5).
Interestingly, we noted considerably increased stroke effectiveness of both groups, as expressed in the percentage of balls hitting the target zones. However, improved stroke effectiveness was seen in the CRS group during both the first and second performances of the drill, whereas in the CON group, it was seen only during the first (Table 5). Based on these observations, we assume that moderate-intensity training in conjunction with 5-day whole-body cryostimulation may delay mental deterioration, which is often observed in tennis players.14,15 Furthermore, in the CRS group, a considerably more effective recovery rate was observed during and after the drill. Heart rate (measured 5 minutes after each tennis drill) was lower in the CRS group, indicating more effective cardiorespiratory system function. The reduced level of TNFα and less disturbance of homeostasis could have resulted in less neural-muscle coordination disruption and faster recovery in the CRS group, which may have improved stroke effectiveness. Such results were not obtained in the CON group. Enhancing the efficiency of the recovery processes and preventing the worsening of physical performance during detraining constitute 2 of the main targets for coaches, athletic trainers, and physiotherapists. They are particularly difficult goals to achieve in tennis.14 For the best ranking possible, tennis players take part in many tournaments to score points in the general classifications. Planning and implementing an appropriate training program in tennis are, therefore, demanding and difficult tasks. Additionally, the unpredictable occurrences and effects of many factors—such as number of matches played, intensity, and duration—on a player must be taken into consideration. Furthermore, Kovacs et al15 observed that during the unsupervised period of detraining, physical capacity decreases significantly, which means that breaks between successive tournament seasons are especially vital. Training intensity should sustain (or develop) the physical performance recorded at the beginning of a break, yet at the same time, it ought to provide an effective recovery method. Because of the upcoming tournament season, we did not examine how long the improved performance was maintained, which constitutes a limitation of our study. Our short training program, supported by whole-body cryostimulation, was expected to prevent overreaching as well as to sustain physical performance without triggering an acute immune response.
To the best of our knowledge, this is the first study showing the synergistic anti-inflammatory effect of moderate-intensity training and whole-body cryostimulation in professional athletes. Our results suggest that such cryostimulation may function as appropriate support for a training program to ensure an effective midseason break. Finally, given the limited number of participants in our experiments, chosen from among high-ranking athletes, we encourage future authors to undertake larger studies on the effects of whole-body cryostimulation.
ACKNOWLEDGMENTS
Funding for this project was provided by the Gdańsk University of Physical Education and Sport, Poland. We thank Radosław Szymanik, Polish National Team coach, for help in conducting the research and the players for their engagement in the performance of the imposed training program. We also thank Janusz Springer for language assistance.
REFERENCES
- 1.McDermott BP, Casa DJ, Ganio MS et al. Acute whole-body cooling for exercise-induced hyperthermia: a systematic review. J Athl Train. 2009;44(1):84–93. doi: 10.4085/1062-6050-44.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banfi G, Lombardi G, Colombini A, Melegati G. Whole-body cryotherapy in athletes. Sports Med. 2010;40(6):509–517. doi: 10.2165/11531940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi T, Kim S, Nogami S, Kawano AD. Extreme cold treatment (−150°C) on the whole body in rheumatoid arthritis [abstract] Rev Rhum. 1981;48(suppl):P1054. [Google Scholar]
- 4.Nemet D, Meckel Y, Bar-Sela S et al. Effect of local cold-pack application on systemic anabolic and inflammatory response to sprint-interval training: a prospective comparative trial. Eur J Appl Physiol. 2009;107(4):411–417. doi: 10.1007/s00421-009-1138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffield R, Steinbacher G, Fairchild TJ. The use of mixed-method, part-body pre-cooling procedures for team-sport athletes training in the heat. J Strength Cond Res. 2009;23(9):2524–2532. doi: 10.1519/JSC.0b013e3181bf7a4f. [DOI] [PubMed] [Google Scholar]
- 6.Hirvonen HE, Mikkelsson MK, Kautiainen H, Pohjolainen TH, Leirisalo-Repo M. Effectiveness of different cryotherapies on pain and disease activity in active rheumatoid arthritis: a randomised single blinded controlled trial. Clin Exp Rheumatol. 2006;24(3):295–301. [PubMed] [Google Scholar]
- 7.Hubbard TJ, Aronson SL, Denegar CR. Does cryotherapy hasten return to participation? A systematic review. J Athl Train. 2004;39(1):88–94. [PMC free article] [PubMed] [Google Scholar]
- 8.Costello JT, Donnelly AE. Cryotherapy and joint position sense in healthy participants: a systematic review. J Athl Train. 2010;45(3):306–316. doi: 10.4085/1062-6050-45.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehmann M, Foster C, Netzer N, et al. Physiological responses to short- and long-term overtraining in endurance athletes. In: Kreider RB, Fry AC, O'Toole ML, editors. Overtraining in Sport. Champaign, IL: Human Kinetics; 1998. pp. 19–46. In. eds. [Google Scholar]
- 10.Gleeson M. Biochemical and immunological markers of overtraining. J Sports Sci Med. 2002;1(2):31–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Smith LL. Cytokine hypothesis of overtraining: a physiological adaptation to excessive stress? Med Sci Sports Exerc. 2000;32(2):317–331. doi: 10.1097/00005768-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K, Nakaji S, Yamada M et al. Systemic inflammatory response to exhaustive exercise: cytokine kinetics. Exerc Immunol Rev. 2002;8:6–48. [PubMed] [Google Scholar]
- 13.Castellani JW. M Brenner IK, Rhind SG. Cold exposure: human immune responses and intracellular cytokine expression. Med Sci Sports Exerc. 2002;34(12):2013–2020. doi: 10.1097/00005768-200212000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Hornery DJ, Farrow D, Mujika I, Young W. Fatigue in tennis: mechanisms of fatigue and effect on performance. Sports Med. 2007;37(3):199–212. doi: 10.2165/00007256-200737030-00002. [DOI] [PubMed] [Google Scholar]
- 15.Kovacs MS, Pritchett R, Wickwire PJ, Green JM, Bishop P. Physical performance changes after unsupervised training during the autumn/spring semester break in competitive tennis players. Br J Sports Med. 2007;41(11):705–710. doi: 10.1136/bjsm.2007.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziemann E, Grzywacz T, Luszczyk M et al. Aerobic and anaerobic changes with high-intensity interval training in active college-aged men. J Strength Cond Res. 2011;25(4):1104–1112. doi: 10.1519/JSC.0b013e3181d09ec9. [DOI] [PubMed] [Google Scholar]
- 17.Schutz Y. The basis of direct and indirect calorimetry and their potentials. Diabetes Metab Rev. 1995;11(4):383–408. doi: 10.1002/dmr.5610110406. [DOI] [PubMed] [Google Scholar]
- 18.Smekal G, Pokan R, von Duvillard SP et al. Comparison of laboratory and “on-court” endurance testing in tennis. Int J Sports Med. 2000;21(4):242–249. doi: 10.1055/s-2000-310. [DOI] [PubMed] [Google Scholar]
- 19.Smekal G, von Duvillard SP, Rihacek C et al. A physiological profile of tennis match play. Med Sci Sports Exerc. 2001;33(6):999–1005. doi: 10.1097/00005768-200106000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Main LC, Dawson B, Grove JR, Landers GJ, Goodman C. Impact of training on changes in perceived stress and cytokine production. Res Sports Med. 2009;17(2):121–132. doi: 10.1080/15438620802689757. [DOI] [PubMed] [Google Scholar]
- 21.Lubkowska A, Szygula Z, Klimek AJ, Torii M. Do sessions of cryostimulation have influence on white blood cell count, level of IL6 and total oxidative and antioxidative status in healthy men? Eur J Appl Physiol. 2010;109(1):67–72. doi: 10.1007/s00421-009-1207-2. [DOI] [PubMed] [Google Scholar]
- 22.Wozniak A, Wozniak B, Drewa G, Mila-Kierzenkowska C, Rakowski A. The effect of whole-body cryostimulation on lysosomal enzyme activity in kayakers during training. Eur J Appl Physiol. 2007;100(2):137–142. doi: 10.1007/s00421-007-0404-0. [DOI] [PubMed] [Google Scholar]
- 23.Zembron-Lacny A, Slowinska-Lisowska M, Ziemba A. Integration of the thiol redox status with cytokine response to physical training in professional basketball players. Physiol Res. 2010;59(2):239–245. doi: 10.33549/physiolres.931774. [DOI] [PubMed] [Google Scholar]
- 24.Laskowski R, Ziemann E, Olek RA, Zembron-Lacny A. The effect of three days of judo training sessions on the inflammatory response and oxidative stress markers. J Hum Kinet. 2011;30:65–73. doi: 10.2478/v10078-011-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 26.Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33(3):114–119. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen BK. Muscles and their myokines. J Exp Biol. 2011;214(pt 2):337–346. doi: 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen BK. Muscular interleukin-6 and its role as an energy sensor. Med Sci Sports Exerc. 2012;44(3):392–396. doi: 10.1249/MSS.0b013e31822f94ac. [DOI] [PubMed] [Google Scholar]
- 29.Keller C, Steensberg A, Pilegaard H et al. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J. 2001;15(14):2748–2750. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- 30.Brenner IK, Castellani JW, Gabaree C et al. Immune changes in humans during cold exposure: effects of prior heating and exercise. J Appl Physiol. 1999;87(2):699–710. doi: 10.1152/jappl.1999.87.2.699. [DOI] [PubMed] [Google Scholar]
- 31.Diestel A, Roessler J, Berger F, Schmitt KR. Hypothermia downregulates inflammation but enhances IL-6 secretion by stimulated endothelial cells. Cryobiology. 2008;57(3):216–222. doi: 10.1016/j.cryobiol.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10. and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285(2):E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 33.Fischer CP. Interleukin-6 in acute exercise and training: what is biological relevance? Exerc Immunol Rev. 2006;12:6–33. [PubMed] [Google Scholar]