Abstract
Context
Good sleep is an important recovery method for prevention and treatment of overtraining in sport practice. Whether sleep is regulated by melatonin after red-light irradiation in athletes is unknown.
Objective
To determine the effect of red light on sleep quality and endurance performance of Chinese female basketball players.
Design
Cohort study.
Setting
Athletic training facility of the Chinese People's Liberation Army and research laboratory of the China Institute of Sport Science.
Patients or Other Participants
Twenty athletes of the Chinese People's Liberation Army team (age = 18.60 ± 3.60 years) took part in the study. Participants were divided into red-light treatment (n = 10) and placebo (n = 10) groups.
Intervention(s)
The red-light treatment participants received 30 minutes of irradiation from a red-light therapy instrument every night for 14 days. The placebo group did not receive light illumination.
Main Outcome Measure(s)
The Pittsburgh Sleep Quality Index (PSQI) questionnaire was completed, serum melatonin was assessed, and 12-minute run was performed at preintervention (baseline) and postintervention (14 days).
Results
The 14-day whole-body irradiation with red-light treatment improved the sleep, serum melatonin level, and endurance performance of the elite female basketball players (P < .05). We found a correlation between changes in global Pittsburgh Sleep Quality Index and serum melatonin levels (r = −0.695, P = .006).
Conclusions
Our study confirmed the effectiveness of body irradiation with red light in improving the quality of sleep of elite female basketball players and offered a nonpharmacologic and noninvasive therapy to prevent sleep disorders after training.
Key Words: Pittsburgh Sleep Quality Index, melatonin, 12-minute run
Key Points
Red-light illumination positively affected sleep quality and endurance performance variables in Chinese female basketball players.
Red-light illumination is a positive nonpharmacologic and noninvasive therapy to prevent sleep disorders after training.
Good sleep is a prerequisite for optimal performance.1 Given that people spend about one-third of their lives asleep, sleep has substantial functions for development, daily functioning, and health.2 Perhaps no daytime behavior has been associated more closely with improved sleep than exercise.3 Researchers have shown that exercise serves as a positive function for sleep. Regular exercise consistently has been associated with better sleep.4 Moreover, the American Academy of Sleep Medicine considers physical exercise to be a modality of nonpharmacologic treatment for sleep disorders.4 When studying the influence of exercise on sleep, most investigators have compared acute exercise and sedentary control treatments.5 In their study of chronic moderate-intensity endurance exercise, Driver and Taylor6 also provided compelling evidence that exercise promotes sleep.
However, exercise can negatively affect sleep quality. Exercising immediately before going to sleep is detrimental to sleep quality.7 Athletes train very hard to improve their on-field performances, but excessive training may lead to a decrease in performance, which is known as overtraining syndrome. Researchers8 have shown that symptoms of overtraining indicate poor-quality sleep. Good sleep is an important recovery method for prevention and treatment of overtraining in sport practice.9
Evidence is compelling that chronic exposure to bright light (3000 lux) can enhance sleep.10 Guilleminault et al11 suggested that the effects of exposure to light may be more powerful than those associated with exercise. In a recent study in which red-light therapy (wavelength = 670 nm, light dose = 4 J/cm2) was used, Yeager et al12 indicated that red light could restore glutathione redox balance upon toxicologic insult and enhance both cytochrome c oxidase and energy production, all of which may be affected by melatonin. Melatonin is a neurohormone that is produced by the pineal gland and regulates sleep and circadian functions.13 No one knows whether sleep is regulated by melatonin after red-light irradiation in athletes. Researchers14,15 have demonstrated that phototherapy improves muscle regeneration after exercise. Red light could protect human erythrocytes in preserved diluted whole blood from the damage caused by experimental artificial heart-lung machines.16 However, the effect of red-light illumination on endurance performance is a new topic in sport science.
Sleep quality can be defined subjectively by self-report17 or by more objective measures, such as polysomnography or actigraphy.18 Subjective sleep quality has been assessed most widely with the Pittsburgh Sleep Quality Index (PSQI).17 The PSQI is a comprehensive 18-item self-report questionnaire assessing sleep disturbances in the previous month. It derives ordinal scores for 7 clinically relevant domains of sleep: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances (eg, awakenings from sleep due to discomfort, bad dreams), use of sleeping medication, and daytime dysfunction (feeling sleepy during the day due to a poor night's sleep). Scores from these separate components are combined to derive a global measure of sleep quality.19
As demonstrated in these studies, acute or chronic exercise may lead to good- or bad-quality sleep. However, the effects of red light on sleep quality and endurance performance have not been investigated sufficiently. Therefore, the purpose of our study was to determine the effect of red light on the sleep quality and endurance performance of Chinese female basketball players.
METHODS
Participants
Twenty female athletes of the Chinese People's Liberation Army team (age = 18.60 ± 3.60 years) participated in the study. Participant characteristics are described in Table 1. All participants were healthy and were not using medications regularly or temporarily during the measurements. Athletes were excluded if they had participated in less than 80% of the scheduled team physical training and basketball sessions for the last 3 months or used any kind of nutritional supplements or pharmacologic agents. All participants provided written informed consent, and the study was approved by the Ethical Committee of the China Institute of Sport Science.
Table 1.
Baseline Characteristics of the Participants (Mean ± SD)
| Variable |
Treatment Group |
|
| Red Light |
Placebo |
|
| Age, y | 19.30 ± 4.30 | 17.90 ± 2.81 |
| Maximal oxygen consumption, mL·kg−1·min−1 | 48.88 ± 3.86 | 47.84 ± 2.51 |
| Height, m | 1.83 ± 6.09 | 1.83 ± 5.68 |
| Mass, kg | 71.24 ± 8.26 | 70.75 ± 6.71 |
| Body mass index | 21.08 ± 1.84 | 21.37 ± 2.33 |
Design
We used a randomized parallel pretest-posttest design. Participants were assigned randomly to either a red-light therapy intervention group (n = 10) or non–red-light therapy intervention group (placebo group, n = 10). Measurements were collected at preintervention (baseline) and postintervention (14 days). The exercise training schedule of the 2 groups was unchanged during the 14 days; the red-light treatment group used a red-light therapy instrument every night for total body irradiation for 30 minutes. The training routine of the athletes during the 14 intervention days included 12 exercise sessions with the following specifications: 2 hours of morning training, 2 hours of afternoon training, and no training on Sunday.
The red-light treatment participants lay in the supine position, and continuous illumination was performed using noncoherent red light from a whole-body red-light treatment machine (Shanghai Dayou PDT Technology Co, Ltd, Shanghai, China) with an average wavelength of 658 nm and light dose of 30 J/cm2. The whole body received the phototherapy treatment (Figure 1). In general studies, investigators have used 14 days20,21 or 7 days22 as 1 session period, so we chose 14 days as a trial time. The placebo participants also lay in the supine position under the red-light device but did not receive any light illumination. All participants wore swimsuits to enhance irradiation from the device and were blind to the treatment.
Figure 1.
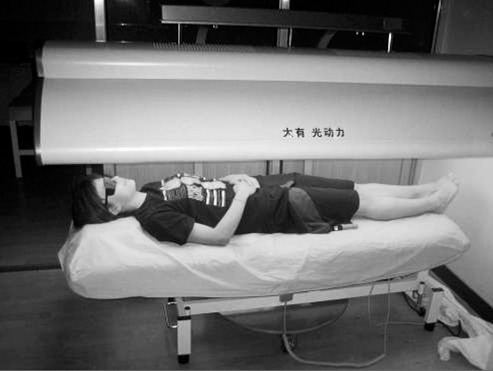
Participant receiving the red-light treatment.
Measurement
Sleep Quality
Sleep quality was measured by the Chinese version of the PSQI.17 The 19-item measure assesses sleep quality and disturbances over a half-month time interval. The total PSQI score ranges from 0 to 21, and higher scores reflect poorer-quality sleep.17 The 7 items of this instrument measure several aspects of insomnia: difficulties with onset and maintenance of sleep, satisfaction with the current sleep pattern, interference with daily functioning, noticeable impairment attributed to sleep problems, degree of distress, and concern caused by any sleeping problems.
Cooper 12-Minute Run
Participants were instructed to complete as many laps as possible on a 400-m outdoor track during the 12-minute test period. Emphasis was placed on pacing oneself throughout the test. The test administrators (J.Z., D.L., and J.X.) counted the laps completed during the 12-minute test period while calling out the time elapsed at 3, 6, and 9 minutes and orally encouraging the participants. At the end of the 12-minute period, the test administrator instructed the participants to stop and used a measuring wheel to determine the fraction of the last lap completed by each participant. This distance was added to the distance determined by the number of laps completed to give the total distance covered during the test.
Serum Melatonin
In humans, the serum level of melatonin, which is derived mainly from the pineal gland, demonstrates a clear increase at night and a decrease during the day.23,24 Given that the masking effects of activities (eg, exercise, sleep, and food intake25,26) have little effect on the daily pattern of the circulating melatonin level, melatonin secretion appears to directly reflect the function of the biological clock as a specific marker of circadian rhythm.27 We drew blood samples in the morning (8:00 am) of preintervention and postintervention. Melatonin in the serum was measured in pictograms per milliliter using an enzyme-linked immunosorbent assay kit (Melatonin ELISA; IBL, Hamburg, Germany).
Statistical Analysis
Data were analyzed using descriptive statistics, 2-way analyses of variance (ANOVAs), and t tests for independent means. Isolated comparisons between groups (experimental, control) and times (preintervention, postintervention) were performed only in cases where time × group interactions were found. We used Pearson product moment correlation coefficients to determine the relationships among sleep quality, serum melatonin, and endurance performance. The α level was set at .05. We used SPSS (version 16.0; IBM Corporation, Armonk, NY) for data analysis.
RESULTS
Participants
We found no differences in any of the baseline characteristics between the groups.
Sleep Quality
We found an effect for group (F1,18 = 5.62, P = .03) and a time × group interaction for global PSQI scores (F1,18 = 5.66, P = .03; Figure 2). At preintervention, we found no difference between the groups (t18 = −0.53, P = .60). At postintervention, participants in the red-light treatment group demonstrated greater improvement in global PSQI scores than the placebo group (t18 = −4.55, P < .001). Descriptive statistics and statistical values for the PSQI subscores are listed in Table 2. Among the subscores, we found a time × group interaction for subjective sleep quality (F1,18 = 6.70, P = .02) and effects of group for sleep duration (F1,18 = 5.36, P = .03) and sleep latency (F1,18 = 5.65, P = .03). We noted an effect of time for daytime dysfunction (F1,18 = 6.40, P = .02). We did not observe an effect of group or a time × group interaction for habitual sleep efficiency (F1,18 = 2.49, P = .13 and F1,18 = 2.84, P = .11, respectively) or sleep disturbance (F1,18 = 0.21, P = .65 and F1,18 = 3.32, P = .09, respectively) variables.
Figure 2.
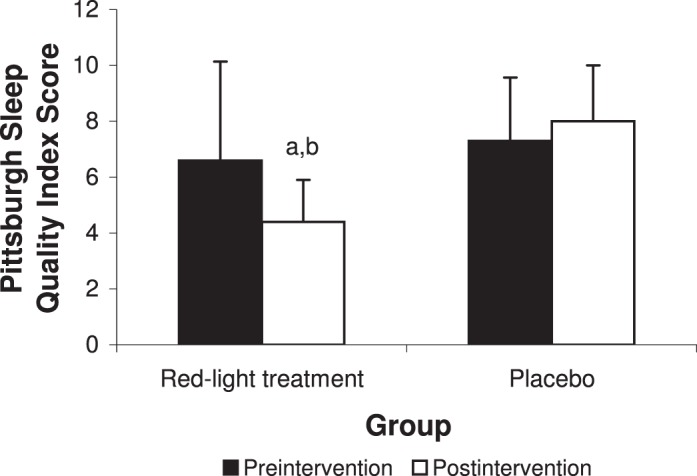
Subjective sleep quality (Pittsburgh Sleep Quality Index) for the red-light treatment and placebo groups (mean ± SD). a Indicates different from preintervention (P < .05). b Indicates difference between groups at postintervention (P < .01).
Table 2.
Subjective Sleep Quality (Mean ± SD)
| Pittsburgh Sleep Quality Index Subscoresa |
Treatment Group |
Time |
Time × Group |
Group |
||||||
| Red Light |
Placebo |
F1,18 |
P |
F1,18 |
P |
F1,18 |
P |
|||
| Preintervention |
Postintervention |
Preintervention |
Postintervention |
|||||||
| Sleep quality | 1.80 ± 0.92 | 1.30 ± 0.48 | 1.90 ± 0.74 | 2.20 ± 0.63 | 0.42 | .53 | 6.70 | .02 | 3.24 | .09 |
| Sleep latency | 1.80 ± 1.03 | 0.30 ± 0.48 | 0.70 ± 0.48 | 0.80 ± 0.42 | 0.31 | .58 | 0.31 | .58 | 5.65 | .03 |
| Sleep duration | 0.30 ± 0.48 | 0.30 ± 0.48 | 0.70 ± 0.48 | 0.90 ± 0.57 | 2.25 | .15 | 2.25 | .15 | 5.36 | .03 |
| Habitual sleep efficiency | 0.90 ± 0.57 | 0.40 ± 0.52 | 0.90 ± 0.57 | 1.00 ± 0.67 | 1.26 | .28 | 2.84 | .11 | 2.49 | .13 |
| Sleep disturbance | 1.50 ± 0.97 | 0.90 ± 0.74 | 1.30 ± 0.95 | 1.40 ± 0.70 | 1.69 | .21 | 3.32 | .09 | 0.21 | .65 |
| Use of sleeping medications | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | NA | NA | NA | NA | NA | NA |
| Daytime dysfunction | 6.60 ± 3.53 | 1.20 ± 0.63 | 2.00 ± 0.82 | 1.90 ± 0.74 | 6.40 | .02 | 3.60 | .07 | 2.15 | .16 |
Abbreviation: NA, not applicable.a Higher scores reflect worse sleep quality.
Serum Melatonin
We found an effect for group (F1,18 = 18.84, P < .001) and a time × group interaction for serum melatonin level (F1,18 = 14.08, P = .001). At preintervention, we demonstrated no difference between the red-light treatment (22.2 ± 7.2 pg/mL) and placebo (21.7 ± 6.8 pg/mL) groups (t18 = 0.17, P = .87). At postintervention, participants in the red-light treatment group (38.8 ± 6.7 pg/mL) demonstrated greater improvement in serum melatonin level than the placebo group (23.8 ± 7.3 pg/mL; t18 = 4.96, P < .001; Figure 3).
Figure 3.
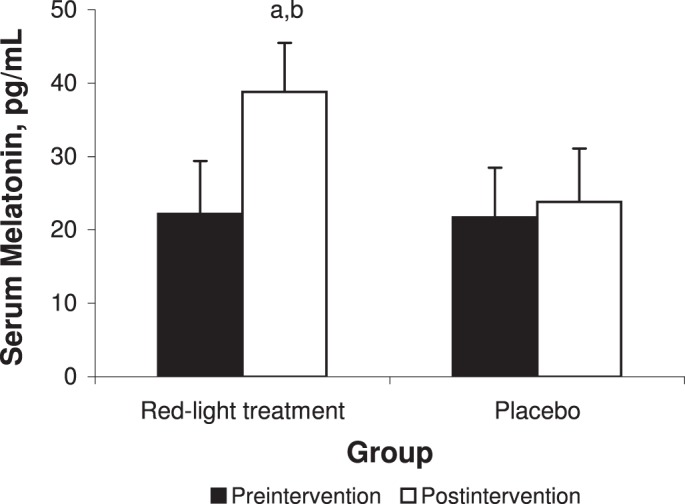
>Serum levels of melatonin for the red-light treatment and placebo groups. a Indicates different from preintervention (P < .01). b Indicates difference between groups at postintervention (P < .01).
Endurance Performance
We noted an effect of time for distance (F1,18 = 12.76, P = .004). We observed a trend toward improvement but no time × group interaction (F1,18 = 1.72, P = .22). A difference was found between preintervention and postintervention of the red-light treatment group (t18 = 3.54, P = .005; Figure 4).
Figure 4.
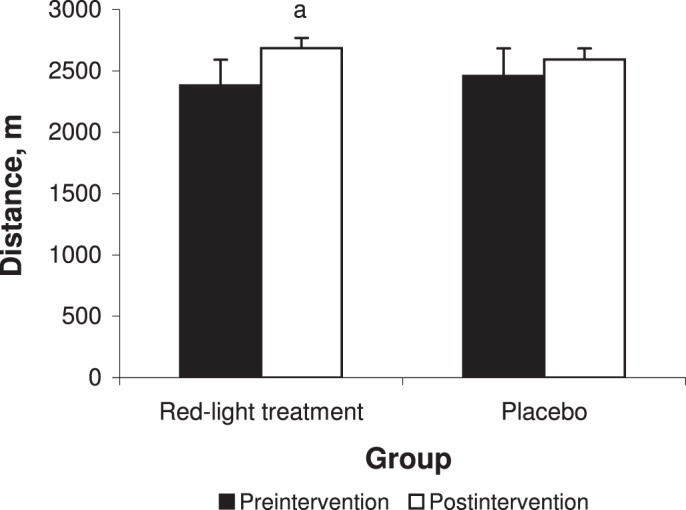
Cooper 12-minute run performances in the red-light treatment and placebo groups. a Indicates different from preintervention (P < .05).
Relationship Among Improvements in Sleep Quality, Serum Melatonin Level, and Endurance Performance
We demonstrated a correlation between changes in global PSQI and serum melatonin levels (r = −0.695, P = .006; Table 3). We also saw a trend toward a negative relationship between change in global PSQI and distance of the 12-minute run test at preintervention and postintervention for all participants (r = −0.353, P = .07; Table 3). In addition, when the results of the postintervention were analyzed alone, we found a negative correlation between change in the distance of the 12-minute run test and global PSQI (r = −0.579, P = .02).
Table 3.
Relationships Among Improvements in Global Pittsburgh Sleep Quality Index, Serum Melatonin, and Endurance Performance
| Measurement |
r |
P |
| Serum melatonin, pg/mL | −0.695a | .006 |
| 12-min run test, m | −0.353 | .07 |
Indicates difference (P < .01).
DISCUSSION
Our results indicated that a 14-day program of red-light treatment improved sleep and serum melatonin levels. Although the statistical analysis did not reveal differences between groups for running distance in the aerobic exercise test, the percentage increase in the red-light treatment group (12.8%) was higher than the percentage increase in the control group (5.5%; P < .05).
The PSQI revealed that the improvements in global PSQI scores and sleep quality were greater at postintervention in the red-light treatment group than in the placebo group. In addition, we found an effect of time for daytime dysfunction (F1,8 = 6.40, P = .02). Sack et al28 suggested a role of melatonin in facilitating sleepiness during the night by inhibiting a central nervous system wakefulness-generating system. The positive effect seen in our study may be due to relatively higher melatonin levels after the red-light illumination. Our results are in accordance with those reported in previous studies, showing that melatonin might be a principal component of red-light therapy.12 In their analysis of the effects of light on melatonin levels and rhythms in humans, Wright and Lack29 showed that, whereas shorter wavelengths of blue (430 nm) and green (540 nm) light suppress salivary melatonin and shift the melatonin rhythm, red light (610 nm and 660 nm) has no effect on melatonin suppression and slightly shortens the time before dim-light onset of melatonin secretion. Recently, Figueiro and Rea22 demonstrated that blue light reduced nocturnal levels of melatonin, whereas red light increased them. However, our observations contradicted those reported in studies of adults with insomnia in which researchers30 reported negative relationships between the red-light condition and improved sleep variables and daytime symptoms. Conflicting results in the literature may stem from studying different participants. In our study, the participants were female basketball players who did not have severe insomnia.
We observed an effect of time on distance (F1,8 = 12.76, P = .004), such that 12-minute run distance was longer after 14 days of red-light illumination in basketball players. For the 12-minute run distance, an effect was noted for time but not for group; no time × group interaction was seen. Therefore, we could not draw a clear conclusion that red-light illumination induced positive changes in endurance performance among basketball players. With regard to the association of red light and exercise, the data are quite scarce. As far as we know, only 1 study31 of red light and exercise in human participants has been published. The study was carried out in healthy, physically active male volunteers, and treatment with light-emitting diodes produced a smaller decrease in maximal isometric torque after high-intensity concentric isokinetic exercise. Ihsan32 demonstrated that laser promoted arteriolar vasodilation and improved the peripheral microcirculation. In addition, phototherapy could improve the muscle regeneration after exercise.33 Therefore, observations by us and by Baroni et al31 may be mainly related to increased arteriolar vasodilation and peripheral microcirculation after red-light illumination.
We found a correlation between changes in global PSQI and serum melatonin levels (r = −0.695, P = .006). A negative relationship existed between decreases in sleep quality and improvements in endurance performance as determined by 12-minute run distance, but this was only a trend (r = −0.353, P = .07). We also demonstrated a correlation between sleep quality and distance of the 12-minute run test during the postintervention period. This in part may be due to sleep providing recovery from autonomic reactivity, psychoemotional tension, and hormonal responses.34 These results suggest that endurance performance was mediated by mechanisms other than sleep quality alone.
CONCLUSIONS
We have demonstrated that red-light illumination positively affected sleep quality and endurance performance variables in Chinese female basketball players. Based on previous studies,6,12,14,15,33 we can infer that red-light treatment contributes to increased melatonin secretion in the pineal gland and muscle regeneration. To our knowledge, we are the first to demonstrate the positive effects of red-light treatment on sleep and aerobic performance, which is an interesting link with practical application to sports training. Although more studies involving phototherapy, sleep, and exercise performance need to be performed, red-light treatment is a possible nonpharmacologic and noninvasive therapy to prevent sleep disorders after training.
ACKNOWLEDGMENTS
This research project was supported by National Key Technologies R&D Program Fund of China (2006BAK37B06).
We thank Professor Craig G. Crandall for editing and Dr James Pearson for proofreading the manuscript. We also thank Bin Fan and Qingde Shi for analyzing and entering the data; Baoxin Feng, Peifang Zong, Wenyuan Shang, Weiying Zhang, and Pengfei Li for their technical assistance; and our volunteers for their willingness to participate in this project.
REFERENCES
- 1.Skein M, Duffield R, Edge J, Short MJ, Mundel T. Intermittent-sprint performance and muscle glycogen after 30 h of sleep deprivation. Med Sci Sports Exerc. 2011;43(7):1301–1311. doi: 10.1249/MSS.0b013e31820abc5a. [DOI] [PubMed] [Google Scholar]
- 2.Gerber M, Brand S, Holsboer-Trachsler E, Puhse U. Fitness and exercise as correlates of sleep complaints: is it all in our minds? Med Sci Sports Exerc. 2010;42(5):893–901. doi: 10.1249/MSS.0b013e3181c0ea8c. [DOI] [PubMed] [Google Scholar]
- 3.Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24(2):355–365. doi: 10.1016/j.csm.2004.12.003. xi. [DOI] [PubMed] [Google Scholar]
- 4.Myllymaki T, Kyrolainen H, Savolainen K et al. Effects of vigorous late-night exercise on sleep quality and cardiac autonomic activity. J Sleep Res. 2011;20(1 pt 2):146–153. doi: 10.1111/j.1365-2869.2010.00874.x. [DOI] [PubMed] [Google Scholar]
- 5.Youngstedt SD, Kripke DF, Elliott JA. Is sleep disturbed by vigorous late-night exercise? Med Sci Sports Exerc. 1999;31(6):864–869. doi: 10.1097/00005768-199906000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Driver HS, Taylor SR. Exercise and sleep. Sleep Med Rev. 2000;4(4):387–402. doi: 10.1053/smrv.2000.0110. [DOI] [PubMed] [Google Scholar]
- 7.van Straten A, Cuijpers P. Self-help therapy for insomnia: a meta-analysis. Sleep Med Rev. 2009;13(1):61–71. doi: 10.1016/j.smrv.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Tanskanen M, Atalay M, Uusitalo A. Altered oxidative stress in overtrained athletes. J Sports Sci. 2010;28(3):309–317. doi: 10.1080/02640410903473844. [DOI] [PubMed] [Google Scholar]
- 9.Roose J, de Vries WR, Schmikli SL, Backx FJ, van Doornen LJ. Evaluation and opportunities in overtraining approaches. Res Q Exerc Sport. 2009;80(4):756–764. doi: 10.1080/02701367.2009.10599617. [DOI] [PubMed] [Google Scholar]
- 10.Campbell SS, Dawson D, Anderson MW. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J Am Geriatr Soc. 1993;41(8):829–836. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 11.Guilleminault C, Clerk A, Black J, Labanowski M, Pelayo R, Claman D. Nondrug treatment trials in psychophysiologic insomnia. Arch Intern Med. 1995;155(8):838–844. [PubMed] [Google Scholar]
- 12.Yeager RL, Oleske DA, Sanders RA, Watkins JB, III, Eells JT, Henshel DS. Melatonin as a principal component of red light therapy. Med Hypotheses. 2007;69(2):372–376. doi: 10.1016/j.mehy.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 13.Radogna F, Diederich M, Ghibelli L. Melatonin: a pleiotropic molecule regulating inflammation. Biochem Pharmacol. 2010;80(12):1844–1852. doi: 10.1016/j.bcp.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 14.Leal Junior EC, Lopes-Martins RA, Rossi RP et al. Effect of cluster multi-diode light emitting diode therapy (LEDT) on exercise-induced skeletal muscle fatigue and skeletal muscle recovery in humans. Lasers Surg Med. 2009;41(8):572–577. doi: 10.1002/lsm.20810. [DOI] [PubMed] [Google Scholar]
- 15.Leal Junior EC, Lopes-Martins RA, Baroni BM et al. Comparison between single-diode low-level laser therapy (LLLT) and LED multi-diode (cluster) therapy (LEDT) applications before high-intensity exercise. Photomed Laser Surg. 2009;27(4):617–623. doi: 10.1089/pho.2008.2350. [DOI] [PubMed] [Google Scholar]
- 16.Itoh T, Murakami H, Orihashi K et al. Low power laser protects human erythrocytes in an in vitro model of artificial heart-lung machines. Artif Organs. 2000;24(11):870–873. doi: 10.1046/j.1525-1594.2000.06624.x. [DOI] [PubMed] [Google Scholar]
- 17.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 18.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 19.Barclay NL, Eley TC, Buysse DJ, Rijsdijk FV, Gregory AM. Genetic and environmental influences on different components of the Pittsburgh Sleep Quality Index and their overlap. Sleep. 2010;33(5):659–668. doi: 10.1093/sleep/33.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desmet KD, Paz DA, Corry JJ et al. Clinical and experimental applications of NIR-LED photobiomodulation. Photomed Laser Surg. 2006;24(2):121–128. doi: 10.1089/pho.2006.24.121. [DOI] [PubMed] [Google Scholar]
- 21.Whelan HT, Connelly JF, Hodgson BD et al. NASA light-emitting diodes for the prevention of oral mucositis in pediatric bone marrow transplant patients. J Clin Laser Med Surg. 2002;20(6):319–324. doi: 10.1089/104454702320901107. [DOI] [PubMed] [Google Scholar]
- 22.Figueiro MG, Rea MS. The effects of red and blue lights on circadian variations in cortisol, alpha amylase, and melatonin. Int J Endocrinol. 2010;2010:829351. doi: 10.1155/2010/829351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynch HJ, Wurtman RJ, Moskowitz MA, Archer MC, Ho MH. Daily rhythm in human urinary melatonin. Science. 1975;187(4172):169–171. doi: 10.1126/science.1167425. [DOI] [PubMed] [Google Scholar]
- 24.Miles A, Philbrick DR. Melatonin and psychiatry. Biol Psychiatry. 1988;23(4):405–425. doi: 10.1016/0006-3223(88)90291-0. [DOI] [PubMed] [Google Scholar]
- 25.Lynch HJ, Jimerson DC, Ozaki Y, Post RM, Bunney WE, Jr, Wurtman RJ. Entrainment of rhythmic melatonin secretion in man to a 12-hour phase shift in the light/dark cycle. Life Sci. 1978;23(15):1557–1563. doi: 10.1016/0024-3205(78)90583-0. [DOI] [PubMed] [Google Scholar]
- 26.Vaughan GM, Allen JP, Tullis W, Siler-Khodr TM, de la Pena A, Sackman JW. Overnight plasma profiles of melatonin and certain adenohypophyseal hormones in men. J Clin Endocrinol Metab. 1978;47(3):566–571. doi: 10.1210/jcem-47-3-566. [DOI] [PubMed] [Google Scholar]
- 27.Gastel JA, Roseboom PH, Rinaldi PA, Weller JL, Klein DC. Melatonin production: proteasomal proteolysis in serotonin N-acetyltransferase regulation. Science. 1998;279(5355):1358–1360. doi: 10.1126/science.279.5355.1358. [DOI] [PubMed] [Google Scholar]
- 28.Sack RL, Hughes RJ, Edgar DM, Lewy AJ. Sleep-promoting effects of melatonin: at what dose, in whom, under what conditions, and by what mechanisms? Sleep. 1997;20(10):908–915. doi: 10.1093/sleep/20.10.908. [DOI] [PubMed] [Google Scholar]
- 29.Wright HR, Lack LC. Effect of light wavelength on suppression and phase delay of the melatonin rhythm. Chronobiol Int. 2001;18(5):801–808. doi: 10.1081/cbi-100107515. [DOI] [PubMed] [Google Scholar]
- 30.Lack L, Wright H, Kemp K, Gibbon S. The treatment of early-morning awakening insomnia with 2 evenings of bright light. Sleep. 2005;28(5):616–623. doi: 10.1093/sleep/28.5.616. [DOI] [PubMed] [Google Scholar]
- 31.Baroni BM, Leal Junior EC, Geremia JM, Diefenthaeler F, Vaz MA. Effect of light-emitting diodes therapy (LEDT) on knee extensor muscle fatigue. Photomed Laser Surg. 2010;28(5):653–658. doi: 10.1089/pho.2009.2688. [DOI] [PubMed] [Google Scholar]
- 32.Ihsan FR. Low-level laser therapy accelerates collateral circulation and enhances microcirculation. Photomed Laser Surg. 2005;23(3):289–294. doi: 10.1089/pho.2005.23.289. [DOI] [PubMed] [Google Scholar]
- 33.Baroni BM, Leal Junior EC, De Marchi T, Lopes AL, Salvador M, Vaz MA. Low level laser therapy before eccentric exercise reduces muscle damage markers in humans. Eur J Appl Physiol. 2010;110(4):789–796. doi: 10.1007/s00421-010-1562-z. [DOI] [PubMed] [Google Scholar]
- 34.Hynynen E, Uusitalo A, Konttinen N, Rusko H. Heart rate variability during night sleep and after awakening in overtrained athletes. Med Sci Sports Exerc. 2006;38(2):313–317. doi: 10.1249/01.mss.0000184631.27641.b5. [DOI] [PubMed] [Google Scholar]


