Abstract
Renal cell carcinoma (RCC) accounts for 3% all of solid organ tumours and is slightly more common in men in the age range of 60 to 70 years. Skin metastases occur in 3% to 6% of RCCs. There are only approximately 30 cases of scalp metastases secondary to RCC in the literature. They usually occur late in metastatic disease and are a bad prognostic marker. A 67-year-old Caucasian male presented with a metastatic scalp lesion, 10 years post-radical treatment for RCC. His initial diagnosis was a T3bN0M0 RCC. He presented with a raised erythematous lesion on his parietal scalp, the histology of which demonstrated late metastatic recurrence. Shortly after this, he developed diffuse metastatic disease. Metastatic RCC can occur many years after initial diagnosis and present in many forms. Cutaneous metastatic lesions of RCC can mimic many other dermatologic conditions and carries an ominous prognosis. It is therefore important not only for the urologist, but also general practitioners and patients to be vigilant of any new skin lesion as a portent of impending metastatic disease.
Introduction
Renal cell cancer (RCC) accounts for 3% of all solid tumours1 with an increasing incidence worldwide.2 There is expected to be a total of 57 760 cases of RCC diagnosed in the United States in 2009 with 12 980 estimated deaths.3 Despite developments in imaging and treatment of metastatic RCC with immunotherapy (interferon) and vascular endothelial growth factor targeting agents (e.g., sunitinib), the prognosis remains poor with 20% to 50% of patients with localized disease relapsing post-nephrectomy.4 Up to 30% of patients present with metastases and the median survival for metastatic disease is 10 to 12 months.5 The most common sites of metastatic RCC recurrence are the lung, lymph nodes, liver and bone.6 Cutaneous metastases are rare (3% to 6% of cases) and scalp metastases are extremely uncommon. There are approximately 30 cases in the literature.
Case presentation
A 67-year-old man presented to the general surgical service at our institution with an occipital scalp lesion which was increasing in size. This scalp lesion had been present for 3 months. It was a raised purple nodule, 1.1 × 1 cm in diameter. It had the appearance of a small hemangioma. There were no other abnormalities on examination. The lesion was excised in an outpatient setting under local anesthetic by a consultant general surgeon and closed primarily.
The histology revealed a dermal tumour composed of a nest of pleomorhic cells with abundant clear cytoplasm and prominent nucleoli with negative margins. This was confirmed as metastatic RCC.
The patient’s history was remarkable for having undergone a right radical nephrectomy 10 years previously. The histology of the primary tumour was a maximum size of 3.5 cm, a conventional clear cell carcinoma with oncocytic change. The tumour was confined within the capsule, but there was renal vein involvement within the kidney. The margins were negative and there were no lymph nodes identified. Overall, it was staged as T3bN0M0, Fuhrman grade 3. He was on routine follow-up since his radical nephrectomy with annual chest and abdominal imaging.
Two months after the excision of the scalp lesion, the patient presented to the emergency room with lethargy, weakness, nausea and anuria. He was diagnosed with acute renal failure with a creatinine of 384 μmol/L and urea of 18.3 mmol/L. Subsequent imaging with computed tomography (CT) revealed a new metastatic lesion in his left ureteric wall. An emergency percutaneous nephrostomy was sited. An antegrade nephrostogram confirmed an obstructing upper ureteric lesion and endoscopic biopsies were taken with a biopsy probe and basket. These biopsies were highly vascular and stained strongly positive for CD10 (marker for RCC); overall they were highly suspicious for metastatic RCC. His bone scan was negative for malignant disease. The CT also demonstrated diffuse metastatic disease (right rectus abdominus muscle, pancreas and brain), which were new since his last surveillance CT.
His case was discussed at a multidisciplinary meeting and it was decided to treat his single brain metastasis (<3 cm right thalamus) with steroids, whole brain and then stereotactic radiotherapy. His renal failure resolved and he was started on sunitinib malate; however, his metastatic disease progressed and he died 9 months later.
Discussion
Renal cell carcinoma recurs in 20% to 40% of patients with clinically localized disease post-nephrectomy7 and most recurrences occur in the first 3 years. Frequent sites include lung (75%), local lymph nodes (65%), bone (40%), liver (40%), brain (5%) and contra-lateral kidney.4 Approximately 2% to 10% of patients develop adrenal metastases.8
The most common tumours to metastasize to the skin are breast, lung and colon cancer and overall the scalp is affected in 6.9% of cases.9 The chest and abdomen are the most common sites for all cutaneous metastases, whereas the face and scalp are the least common.9 Scalp metastases are thought to result from hematogenous spread or direct extension of nodal disease and develop there due to the high degree of vascularity and immobility. Cancers of the genito-urinary tract account for 10% of cutaneous metastatic lesions.10 Renal cell carcinoma is the most common genito-urinary cancer to metastasise to the skin11 and accounts for 6.8% of cutaneous metastases. They are more commonly found in men.12 Although it is usually seen at the later stages of the disease, RCC may present initially with a synchronous cutaneous metastasis.13
Renal metastatic cutaneous lesions are described as well-circumscribed, cutaneous nodules that are flesh-coloured, violaceous, blue or appear as a cutaneous horn. They are frequently very vascular14 and pulsatile. They may mimic other conditions, such as osteomyelitis, septic arthritis, gout, pyogenic granuloma or osteoarthritic disease.15 Histologically, they may have a similar appearance to the primary lesion; however, they are frequently poorly differentiated. The cells tend to be clear, pale-staining filled with intracytoplasmic lipid and glycogen embedded in a fibrous and highly vascular stroma.16 Immunochemistry techniques show positivity for epithelial markers, keratin, epithelial membrane antigen, carcinoembryonic antigen and vimentin.17 The RCC marker antigen, a monoclonal antibody directed against a normal proximal renal tubule antigen, is a relatively specific marker for cutaneous metastases of RCC.18
As in this case, patients may present with a long time interval from their curative radical nephrectomy with meta-static disease. Late recurrences are defined as those occurring 10 or more years after the initial diagnosis. The overall late recurrence rate is about 11%.19 There is a reported case of a patient surviving 45 years before recurrence.20 Once they present with cutaneous metastases, patients’ survival is poor; in one study the mean survival was only 7 months. In the same group, 90% of patients had a second metastasis in at least one other site.21 The mean 5-year survival rate of patients with a cutaneous metastasis is 13% to 50% if there is one lesion present and 0% to 8% in patients with multiple lesions.22
Cutaneous metastases can be diagnosed on excisional biopsy or by fine needle biopsy.23 Treatment is usually excision and can be curative if it is solitary or palliative in disseminated metastatic disease. There is one reported case of scalp metastasis responding completely to hypo-fractionated radiotherapy (13 fractions of 375 cGy) in combination with sorafenib24 in a patient with pulmonary and scalp metastases. Radiotherapy can also be used as a palliative treatment in cutaneous lesions in combination with excision if necessary11 and, interestingly, there is a report of complete spontaneous regression of a scalp metastases.25
Conclusion
As cutaneous metastases are underestimated and underdiagnosed, all patients’ skin should be examined routinely by physicians and patients to rule out evidence of cutaneous lesions. Unfortunately, cutaneous metastases can present many years after apparent curative surgery for RCC and portent an ominous prognosis.
Fig. 1.
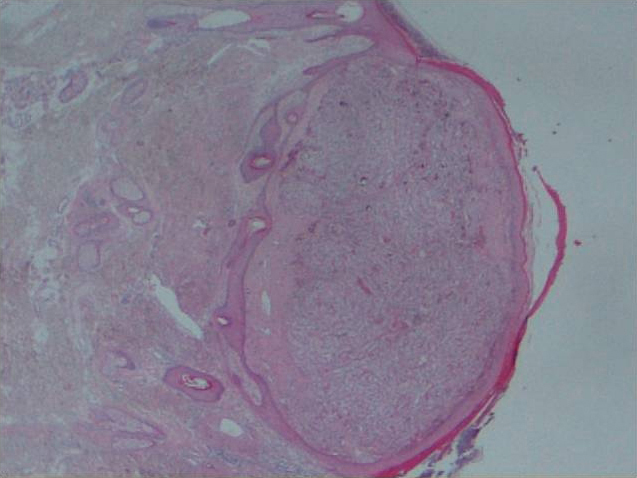
Well-circumscribed tumour in the dermis, not involving the epidermis.
Fig. 2.

Tumour with optically clear cytoplasm, blue pleomorphic nuclei, metastatic renal cell carcinoma was confirmed on immunohistochemistry.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 9th ed. Philadelpha, PA: Elsevier; 2006. [Google Scholar]
- 2.Mathew A, Devesa SS, Fraumeni JF, Jr, et al. Global incidences in kidney cancer incidence, 1973–1992. Eur J Cancer Prev. 2002;11:171–8. doi: 10.1097/00008469-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Flanigan R. Metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003;4:385–90. doi: 10.1007/s11864-003-0039-2. [DOI] [PubMed] [Google Scholar]
- 5.Motzer R. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–40. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 6.Pan D, Niall O, Sharma H, et al. Isolated scalp nodule in patient with undiagnosed RCC. ScientificWorldJournal. 2006;6:2430–2. doi: 10.1100/tsw.2007.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chin A, Lam JS, Figlin RA, et al. Surveillance strategies for renal cell carcinoma patietns following nephrectomy. Rev Urol. 2006;8:1–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Antonelli A, Cozzoli A, Simeone C, et al. Surgical treatment of adrenal metastases from renal cell carcinoma: a single centre experience of 45 patients. BJU Int. 2006;97:505–8. doi: 10.1111/j.1464-410X.2006.05934.x. [DOI] [PubMed] [Google Scholar]
- 9.Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of the data. South Med J. 2003;96:164–7. doi: 10.1097/01.SMJ.0000053676.73249.E5. [DOI] [PubMed] [Google Scholar]
- 10.Saeed S, Keehn C, Morgan M. Cutaneous metastases: a clinical, pathological and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419–30. doi: 10.1111/j.0303-6987.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 11.Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021–6. doi: 10.1016/j.urology.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Páez Borda A, Nacarino Corbacho L, Diego García A, et al. Cutaneous and gynecologic metastases discloing renal cell carcinoma: the diagnostic and therapeutic implications [in Spanish] Arch Esp Urol. 1992;45:341–5. [PubMed] [Google Scholar]
- 13.Porter N, Anderson H, Al-Dujaily S. Renal cell carcinoma presenting as a solitary cutaneous facial metastasis: case report and rewiew of the literature. Int Semin Surg Oncol. 2006;12:27. doi: 10.1186/1477-7800-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanson R, Kneafsey B, Royston D. Cutaneous lesions, not just skin deep! Irish J Med Sci. 2006;175:66–8. doi: 10.1007/BF03169005. [DOI] [PubMed] [Google Scholar]
- 15.Perdonà S, Autorino R, Gallo L, et al. Renal cell carcinoma with solitary toe metastasis. Int J Urol. 2005;12:401–4. doi: 10.1111/j.1442-2042.2005.01060.x. [DOI] [PubMed] [Google Scholar]
- 16.Peris K, Fargnoli MC, Lunghi F, et al. Unusually large cutaneous metasteases of renal cell carcinoma. Acta Derm Venereol. 2001;81:77–8. doi: 10.1080/000155501750208407. [DOI] [PubMed] [Google Scholar]
- 17.García Torrelles M, Beltrán Armada JR, Verges Prosper A, et al. Skin metastases from renal cell carcinoma [in Spanish] Actas Urol Esp. 2007;31:556–8. doi: 10.1016/s0210-4806(07)73682-3. [DOI] [PubMed] [Google Scholar]
- 18.Perna AG, Ostler DA, Ivan D, et al. Renal cell carcinoma marker (RCC-Ma) is specific for cutaneous metastasis of renal cell carcinoma. J Cutan Pathol. 2007;34:381–5. doi: 10.1111/j.1600-0560.2006.00623.x. [DOI] [PubMed] [Google Scholar]
- 19.McNichols D, Segura J, DeWeerd J. Renal ell carcinoma: long-term survival and late recurrence. J Urol. 1981;126:17–23. doi: 10.1016/s0022-5347(17)54359-1. [DOI] [PubMed] [Google Scholar]
- 20.Tapper H, Klein H, Rubenstein W, et al. Recurrent renal cell carcinoma after 45 years. Clin Imaging. 1997;21:237–5. doi: 10.1016/s0899-7071(96)00042-3. [DOI] [PubMed] [Google Scholar]
- 21.Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164–7. doi: 10.1159/000030440. [DOI] [PubMed] [Google Scholar]
- 22.Menter A, Boyd A, McCaffrre D. Recurrent renal cell carcinoma presenting as skin nodules: two case reports and review of the literature. Cutis. 1989;44:305–8. [PubMed] [Google Scholar]
- 23.Spitz DJ, Reddy V, Selvaggi SM, et al. Fine-needle biopsy of scalp lesions. Diagn Cytopathol. 2000;23:35–8. doi: 10.1002/1097-0339(200007)23:1<35::aid-dc8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Gay HA, Cavalieri R, Allison RR, et al. Complete response in a cutaneous facial metastatic nodule from renal cell carcinoma after hypofractionated radiotherapy. Dermatol Online J. 2007;13:6. [PubMed] [Google Scholar]
- 25.Chang K, Chan K, Lam C. Spontaneous regression of a renal cell metastases. Hong Kong Med J. 1999;5:72–5. [PubMed] [Google Scholar]


