Abstract
Background and Purpose: There are a limited number of studies comparing the Aperio mechanical thrombectomy device to other stent-based devices. In this paper, we compared the Aperio thrombectomy device to the Solitaire AB, FR and Revive devices in a model of the middle cerebral artery (MCA) within a modified pulsatile flow system. Methods: Thrombi made of lamb’s blood were placed into a pulsatile flow system perfused with Hartmann’s solution at 80 bpm with a mean pressure of 90 mm Hg. 30 experiments were run with each device. Results: Recanalization rates were similar for all three devices (90% with the Solitaire AB, FR, 80% with the Revive, and 90% with the Aperio). The mean number of attempts to retrieve the thrombus was also similar for all three devices (1.7 with the Solitaire AB, FR, 2.1 with the Revive, 1.6 with the Aperio). Clot fragmentation and embolization rates revealed no statistical significance but there was a trend towards lower embolization rates with the Aperio (23% compared to 40% with the Solitaire AB, FR and 47% with the Revive). The Aperio was the fastest to recanalize the MCA (mean of 66 seconds compared to 186 seconds for the Solitaire AB, FR and 169 seconds for the Revive). Conclusions: In this in vitro setting, the Aperio device seems to be an efficacious and safe device when compared to other similar clinically used mechanical thrombectomy devices. Larger clinical trials are warranted.
Keywords: Stroke, mechanical thrombectomy, flow model, Aperio, recanalization, Solitaire, Revive
Introduction
Stroke is the third most common cause of death in the UK. It is estimated that one in four men and one in five women who are aged 45 years are expected to experience a stroke after their 85th year [1].
The acute management of an ischemic stroke is to restore blood flow to the affected area as quickly as possible to reduce long-term disability and therefore improve patient outcomes [2]. Intravenous thrombolysis with alteplase within 4.5 hours of onset has been shown to be the most effective intervention to restore perfusion to cerebral tissue [3]. However, this form of intervention is limited to a small number of stroke patients mainly due to a narrow therapeutic window and other contraindications such as an increased tendency to bleed [4,5]. According to the National Sentinel Stroke Clinical Audit, only 5% of stroke patients received intravenous thrombolysis in the UK in 2010 [6].
Mechanical thrombectomy is emerging as an alternative approach to recanalization of an occluded cerebral artery. It involves the introduction of a mechanical thrombectomy device via a guiding catheter through the femoral artery and leading up to the occlusion in the cerebral artery to retrieve the clot. Thrombectomy is approved for longer therapeutic windows of 6-8 hours and achieves higher recanalization rates, when compared to the recanalization rate for intravenous thrombolysis (66%) of the middle cerebral artery [7]. Therefore, thrombectomy can theoretically be offered to a larger number of patients with stroke. The indications for mechanical thrombectomy include failure to recanalize after intravenous thrombolysis or with onset of symptoms out of the therapeutic window [2,8]. Mechanical thrombectomy is not without its disadvantages. The devices used may cause the clot to fragment and embolize distally potentially causing further ischemia and therefore damage. There is also a risk of device-induced vasospasm and arterial wall injury which can compromise ischemia and lead to hemorrhage [9].
There are a limited number of studies comparing the Aperio (Acandis, Pforzheim, Germany) device to other clinically used mechanical thrombectomy devices. Wenger et. al [10] compared the Aperio system to the Solitaire AB, FR (Covedien) and Merci (Stryker) devices in vitro whereas a recent study by Roth et. al [11] compared the Aperio to the Solitaire AB, FR devices in a swine model. More studies to test differences in recanalization between the Aperio and other distal stent-based devices are warranted. In this study, we compared the Aperio to its stent-based counterparts: the Revive (Micrus, Codman and Shurtleff, Raynham, USA) and Solitaire AB, FR devices in a model of the middle cerebral artery within a modified pulsatile flow system.
Materials and methods
Mechanical thrombectomy devices
The Solitaire AB, FR, the Revive and the Aperio devices are stent-based mechanical thrombectomy devices with retrievable stents (Figure 1) that are deployed distally and over the clot.
Figure 1.
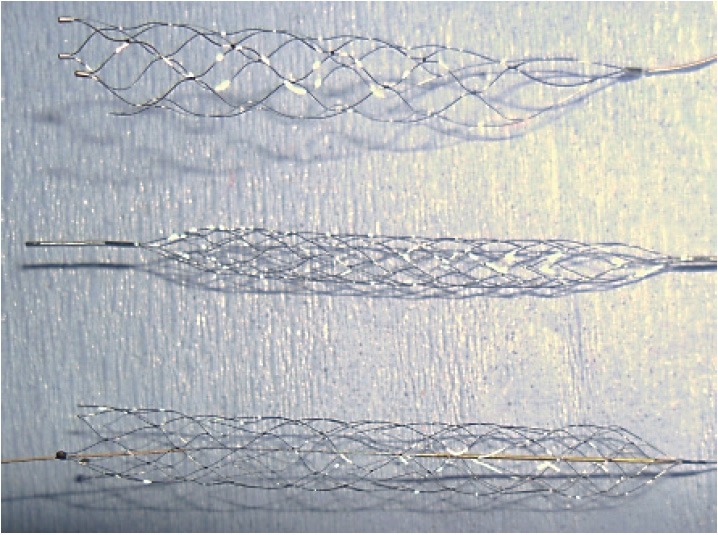
The three stent-based mechanical thrombectomy devices. Pictured from top to bottom: the Solitaire AB, FR, the Revive, and the Aperio. Devices are not to scale.
The Solitaire AB, FR devices are self-expanding nitinol stents with closed-cells and open-ended designs. The Solitaire FR is exactly the same as the AB device, but it is certified for use clinically. For the purposes of the experiments that were run in this study, the AB and FR were grouped when referring to the Solitaire device. The stent deploys in a recommended vessel diameter of 2-4 mm for the 4 x 15/4 x 20 mm version or a vessel diameter of 3-5.5 mm for the 6 x 20/6 x 30 mm version. The device uses a 0.021 or 0.027 inch microcatheter (Rebar 18, Covedien) for delivery of the device and has a push wire length of 1, 800 mm [12]. In this study, the 4 x 20 mm version with a microcatheter diameter of 0.021 inches was used.
The Revive device is a self-expanding stent with various cell shapes that spiral around the longitudinal axis of the stent. One major difference to the Solitaire AB, FR and Aperio devices is that the Revive has a closed-ended basket. The stent is 4.5 x 22 mm and the device uses a 0.021 inch microcatheter for delivery. High radial force and a decrease in cell size from the proximal to the distal retrieval zone are special characteristics of the device.
The Aperio device is also a self-expanding stent, containing both closed and partially opened cells with an open-ended design. It is 4.5 x 40 mm and is recommended for usage in vessel diameters ranging from 2-4 mm. The device is provided with its own Acandis microcatheter, which is 0.027 inches in diameter. The push wire length is 1, 550 mm and the recommended guide wire diameter ranges from 0.010-0.014 inches. Three radiopaque markers enhance the visibility of the device expansion while performing thrombectomy [13].
Thrombus formation and introduction into the system
Freshly obtained lamb’s blood (AJ Greens Abattoir, Stoke, UK) was left to clot naturally for 24 hours. Thrombi were cut from the main thrombus cake into approximately 5 x 30 mm smaller clots to ensure they would occlude and remain in the model cerebral artery. The clots were then washed in Hartmann’s solution and left to dry. This method has been previously used to generate thrombi [14]. Only clots that weighed in the range of 0.4-0.6 g were used.
To occlude the target area in the middle cerebral artery, the generated thrombus was put in the beaker of Hartmann’s solution while the peristaltic pump was off. With the pump turned on in the reverse flow direction, the thrombus travelled in the collateral vessel past the middle cerebral artery due to its lower resistance. The peristaltic pump was then turned off and the collateral vessel clamped. With the correct physiological flow, the pump was turned on at 80 bpm resulting in the thrombus being introduced into the middle cerebral artery occluding the target area. The collateral vessel was then unclamped to allow free flow through the system. Only thrombi that resulted in the occlusion of the target area (Figure 2) were used.
Figure 2.
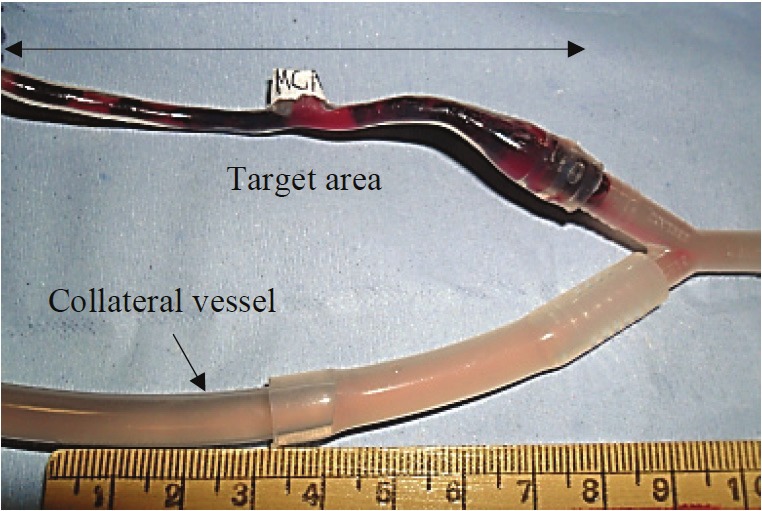
The target area occluded within the model middle cerebral artery.
The pulsatile flow system
The modified pulsatile flow system (Figure 3) has not been used before but is based on previously used and established models [10,14]. A beaker filled with Hartmann’s solution was placed in a water bath (Grant Instruments, Cambridge, UK) at 37°C and 1, 220 mm above the silicone model (ELSTRAT Geneva, Switzerland) of the middle cerebral artery to create a mean arterial pressure of 90 mm Hg (1, 220 mm H2O/1.36 mm Hg). The model of the middle cerebral artery had a luminal diameter of 3 mm, meeting the recommendations for vessel diameter for each of the three devices. The beaker was made to contain 400 ml of Hartmann’s solution, which brought the solution level to the correct height. A second collateral vessel was set up alongside the model of the middle cerebral artery. A peristaltic pump (Watson Marlow, Falmouth, Cornwall, UK) was used to perfuse the system at 80 bpm. A 30 ml syringe was attached to a three-way valve and added to manually aspirate while retrieving the thrombus with the devices. To assess embolization, a filter was introduced into the system.
Figure 3.
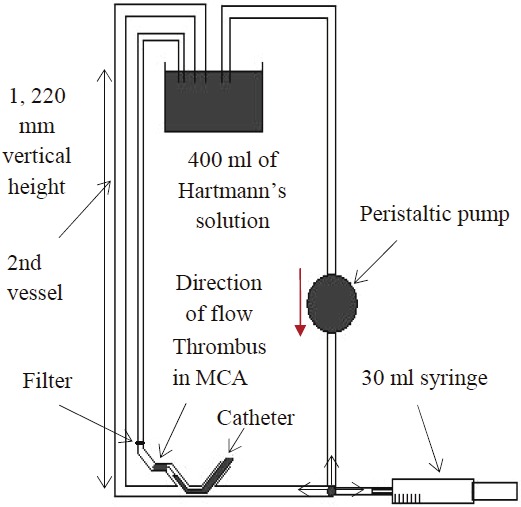
Schematic diagram of the flow system.
Device techniques used in the system
All three of the devices were used according to standardized techniques. Additionally, an interventional neuroradiologist (N.S.) ensured correct usage of the devices.
A 7F catheter, attached to a hemostatic valve, was used as the guiding catheter for all three devices. The guiding catheter was integrated into the system and securely fastened to standardize the finish time (retrieval of the thrombus up to the end of the guiding catheter) for each experiment. A 0.014 inch guide wire (Boston Scientific, FA, USA) used for all three devices was passed through the guiding catheter, into the middle cerebral artery, and positioned 5-10 mm distal to the thrombus occluding the target area. The recommended microcatheter size for each device (0.021 inches for the Solitaire AB, FR and Revive devices; 0.027 inches for the Aperio device) was fed down the guiding catheter, through the guide wire, and positioned distal to the thrombus. The guide wire was then removed and the device advanced through the microcatheter past the thrombus. To deploy each device, the microcatheter was slowly retracted which allowed full entrapment of the thrombus within the device. Constant suction was applied via the 30 ml syringe while retrieving the thrombus.
Assessments
Recanalization was defined as removal of the thrombus from the target area with complete and free flow of the Hartmann’s solution through the previously occluded middle cerebral artery. This was assessed visually and was scored as 0 (failed or incomplete removal of the thrombus) or 1 (thrombus successfully removed in its entirety). A maximum of five attempts was permitted to retrieve the thrombus completely before recording it as a failed attempt.
The time until successful recanalization was defined as starting the timer from the removal of the guide wire from the microcatheter when it was distal to the clot and in position, and stopping the timer at the complete retrieval of the thrombus from the middle cerebral artery and up to the guiding catheter. The timer was still running up until the end of the fifth attempt of thrombus retrieval.
Thrombus fragmentation was assessed visually by inspecting the retrieved thrombus within the struts of each device after each experiment ended. It was scored as 0 (thrombus retrieved in one whole piece without fragmentation) or 1 (thrombus retrieved in more than one piece).
Thrombus embolus was assessed at the end of each experiment by visual inspection of the filter that was placed distal to the middle cerebral artery (Figure 4). It was scored as 0 (no thrombus embolus or emboli caught in the distal filter) or 1 (thrombus embolus or emboli caught in the distal filter).
Figure 4.
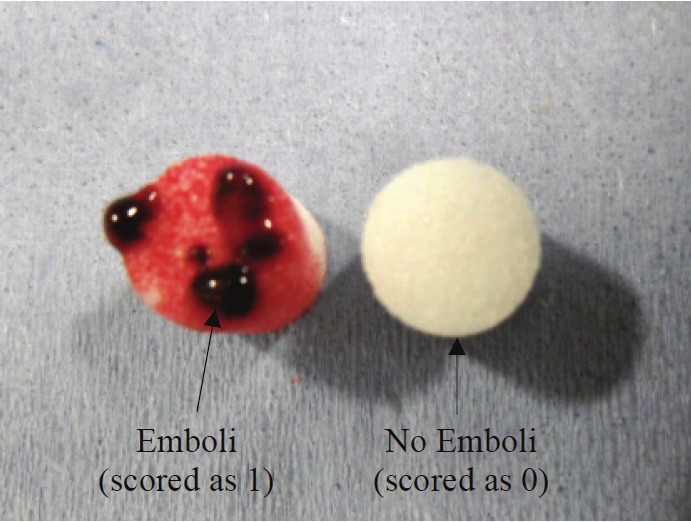
On the left, is a recovered filter with emboli shown after use of the Aperio. On the right, is a clean filter for comparison. Each filter was 5 mm in diameter.
All experiments were run and assessed by the same researcher.
Results
The general results are summarized in Table 1.
Table 1.
Results for each of the three devices
| Device | Solitaire AB, FR | Revive | Aperio |
|---|---|---|---|
| Number of experiments (n) | 30 | 30 | 30 |
| Number of attempts (mean, (SD)) | 1.7 (1.3) | 2.1 (1.6) | 1.6 (1.3) |
| Recanalization rates (n, (%)) | 27 (90%) | 24 (80%) | 27 (90%) |
| Fragmentation rates (n, (%)) | 8 (27%) | 15 (50%) | 14 (47%) |
| Embolization rates (n, (%)) | 12 (40%) | 14 (47%) | 7 (23%) |
| Time to successful recanalization (mean, (SD)) (s) | 186 (21) | 169 (37) | 66 (11) |
n: number, SD: standard deviation, s: seconds.
Number of attempts taken
The mean number of attempts taken for each of the devices was 1.7 (SD 1.3, CI 95% [1.2 - 2.2]), 2.1 (SD 1.6, CI 95% [1.5 - 2.7]), and 1.6 (SD 1.3, CI 95% [1.1 - 2.1]) for the Solitaire AB, FR, Revive, and Aperio devices, respectively. There were no significant differences between the devices in the number of attempts taken to retrieve the thrombus (p = 0.50, Kruskal-Wallis test). There were also no significant differences while individually comparing the Solitaire AB, FR versus the Revive (p = 0.59, Mann-Whitney test), the Solitaire AB, FR versus the Aperio (p = 0.50, Mann-Whitney test), and the Revive versus the Aperio (p = 0.25, Mann-Whitney test).
Thrombus fragmentation
Thrombus fragmentation rates for each of the devices was 8/30 (27%), 15/30 (50%), and 14/30 (47%) for the Solitaire AB, FR, Revive, and Aperio devices, respectively. There were no significant differences in rates of thrombus fragmentation between the three devices (p = 0.14, 2 x 3 2-tailed Chi-squared test). There were also no significant differences while individually comparing the Solitaire AB, FR versus the Revive (p = 0.11, 2-tailed Fisher’s exact test), the Solitaire AB, FR versus the Aperio (p = 0.18, 2-tailed Fisher’s exact test), and the Revive versus the Aperio (p = 1.00, 2-tailed Fisher’s exact test).
Thrombus emboli
Thrombus embolization rates for each of the devices was 12/30 (40%), 14/30 (47%), and 7/30 (23%) for the Solitaire AB, FR, Revive, and Aperio devices, respectively. There were no significant differences in rates of thrombus embolization between the three devices (p = 0.15, 2 x 3 2-tailed Chi-squared test). There were also no significant differences while individually comparing the Solitaire AB, FR versus the Revive (p = 0.79, 2-tailed Fisher’s exact test), the Solitaire AB, FR versus the Aperio (p = 0.27, 2-tailed Fisher’s exact test), and the Revive versus the Aperio (p = 0.10, 2-tailed Fisher’s exact test).
Time taken for successful recanalization
The mean time it took for successful recanalization with each of the three devices was 186 s (SD 21, CI 95% [178 – 195]), 169 s (SD 37, CI 95% [153 – 185]), and 66 s (SD 11, CI 95% [62 – 71]) for the Solitaire AB, FR, Revive, and Aperio devices, respectively. There was a significant difference in the mean time it took for successful recanalization between the three devices (p = < 0.0001, Kruskal-Wallis test). There were also significant differences with the Solitaire AB, FR versus the Aperio (p = < 0.0001, Mann-Whitney test), the Revive versus the Aperio (p = < 0.0001, Mann-Whitney test), and the Solitaire AB, FR versus the Revive (p = 0.01, Mann-Whitney test) when compared individually.
Recanalization rates
Recanalization rates for each of the devices was 27/30 (90%), 24/30 (80%), and 27/30 (90%) for the Solitaire AB, FR, Revive, and Aperio devices, respectively. There were no significant differences in rates of recanalization between the three devices (p = 0.42, 2 x 3 2-tailed Chi-squared test). There were also no significant differences while individually comparing the Solitaire AB, FR versus the Revive (p = 0.47, 2-tailed Fisher’s exact test), the Solitaire AB, FR versus the Aperio (p = 1.00, 2-tailed Fisher’s exact test), and the Revive versus the Aperio (p = 0.47, 2-tailed Fisher’s exact test).
Device-thrombus interaction
Device-thrombus interaction was assessed subjectively by the researcher undergoing the experiments. All three of the devices were easy to use and navigated well within the flow system. However, the Aperio device was harder to manoeuvre within the system when compared to the other two devices. The guide wire and the microcatheter passed between the thrombus and the vessel wall in all of the experiments that were run. The Solitaire AB, FR device seemed to enmesh into the clot immediately through the centre, grasping the clot well within its stent. It seemed that thrombi that occluded a larger portion of the target area in the middle cerebral artery were easier to grasp by the Aperio device and were less likely to fragment or embolize. All three of the devices were retrieved slowly with their corresponding microcatheters in order to avoid losing the thrombus.
Complications
There were no complications in using any of the three devices within the model and in retrieving the thrombi.
Discussion
All three of the distal stent-based devices removed the majority of thrombi that occluded the target area within the model of the middle cerebral artery.
Recanalization rates were similar when comparing all three devices, showing no significant differences. The Aperio device was able to recanalize the occluded vessel as many times as the Solitaire AB, FR device, with all three devices achieving rates of at least 80%. These recanalization rates are comparable to other studies performed in vitro and in vivo of stent-based mechanical thrombectomy devices, achieving recanalization rates between 90-100% [11,15,16].
Furthermore, recanalization rates have been shown to be inversely related to the number of attempts taken to retrieve a clot [17]. If recanalization can be achieved in the first attempt, this usually relates to improved patient outcome. The number of attempts with the Aperio did not differ significantly compared to the other devices (mean of 1.6 passes to remove the thrombus). One of the few studies currently available shows a restoration of flow obtained in 1-3 passes with the Aperio [10].
Thrombus fragmentation rates showed no significance between the three devices. Sometimes, the clot would fragment within the flow system or within the middle cerebral artery which made it difficult to determine whether the devices caused it to do so on retrieval or not. In our study, the Aperio showed similar fragmentation rates to the other two devices, but a previous study found that the Solitaire AB, FR device was more likely to fragment a clot than the Aperio [10]. This is clinically relevant as fragmentation of the thrombus can lead to distal embolization therefore worsening the patients’ prognosis [18].
Thrombus embolization rates were similar for all three devices, with no significant differences. The Aperio caused a clot embolus in 23% of the experiments whereas the Solitaire AB, FR and Revive devices caused an embolus closer to 50% of experiments. This may have been due to the longer length and area of capture of the Aperio compared to the other two devices, which enabled it to catch clots that broke off proximally. Statistical significance would most likely be identified by running a much larger number of experiments than were run in this study. A device that causes less clot emboli will be favored over another, as clot embolization to distal vessels could result in further damage depending on the characteristics of the embolus [19].
Furthermore, the Aperio was significantly faster at restoring blood flow to the blocked area than the other two devices. This is most likely due to the thicker feeding wire of the device and larger microcatheter diameter [13], which allowed a better grip and faster feeding of the device into its microcatheter. A faster restoration of blood flow to the ischemic area of the brain prevents permanent brain damage and high mortality rates [20]. However, the times reflected in this study are much quicker than if it were performed in vivo as the distance from the catheter entry point to the occlusion was much shorter. Other factors such as visibility through the tubing and the tortuous anatomy of the circle of Willis also play an important role.
Homogeneous clots are more likely to fragment than clots formed in humans [21]. Devicethrombus interaction between the three devices was similar in that all three deployed successfully into the thrombus. Clinically, the low trend in embolization rates seen in the Aperio device has to be balanced against its relatively more difficult use within the tortuous anatomy of the intracranial vessels. This is possibly due to its longer length. Compression of the thrombus on retrieval was seen in all three devices. However, this is typical of distal thrombectomy devices [9]. Since the thrombi generated in this study represented homogeneous clots, high fragmentation and embolization rates when the device engaged the clot was seen in all three.
Limitations
As these experiments were conducted in vitro, several factors such as vessel wall interaction with the thrombus and device as well as the physiological reactions to device usage such as vasospasm could not be replicated. Devices may cause dissection of the vessel or cause additional thrombi to form from emboli in vivo [22]. Even though Hartmann’s solution was used in part due to the ease of direct visibility it provided, it does not simulate the characteristics of human blood. Fragmentation and embolization rates may have been exaggerated due to the use of homogeneous clots, as discussed above. The model middle cerebral artery was straight and did not reflect its tortuous anatomy intracranially, which could have eased the use of the devices in terms of navigation and quickened times to successful recanalization. It was also difficult to compare techniques between the devices as the Solitaire AB, FR device requires a balloon guide catheter to maximize results [23], which was not used in these experiments. Another limitation of this study is that devices were used more times than they are indicated for. This could potentially affect devices that were pushed through a smaller microcatheter as this would have put more strain on the stent and could negatively affect the stent radial force.
Conclusions
Three stent-based mechanical thrombectomy devices were compared in a modified model of a pulsatile flow system. The Aperio device achieved similar recanalization rates, fragmentation rates and embolization rates to its counterparts. It seems to be a safe addition to the neurointerventionalists’ range of options in treating patients via mechanical thrombectomy. Larger clinical trials are warranted.
Disclosures statement
None. The authors are not affiliated with the device companies at the time the experiments were carried out.
References
- 1.Wolfe CD. The impact of stroke. Br Med Bull. 2000;56:275–286. doi: 10.1258/0007142001903120. [DOI] [PubMed] [Google Scholar]
- 2.Thomassen L, Bakke SJ. Endovascular reperfusion therapy in acute ischaemic stroke. Acta Neurol Scand. 2007;187:22–29. doi: 10.1111/j.1600-0404.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 3.Tekle WG, Chaudhry SA, Fatima Z, Ahmed M, Khalil S, Hassan AE, Rodriguez GJ, Suri FK, Qureshi AI. Intravenous Thrombolysis in Expanded Time Window (3-4.5 hours) in General Practice with Concurrent Availability of Endovascular Treatment. J Vasc Interv Neurol. 2012;5:22–26. [PMC free article] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Murray V, Berge E, Del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2009;4:CD000213. doi: 10.1002/14651858.CD000213.pub2. [DOI] [PubMed] [Google Scholar]
- 5.IST-collaborative group. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3] ): a randomised controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Sentinel Stroke Clinical Audit Round. Royal College of Physicians. http://www.rcplondon.ac.uk/sites/default/files/national-sentinel-stroke-audit-2010-public-report-and-appendices_0.pdf. 2011. Accessed July 8, 2012. [Google Scholar]
- 7.Costalat V, Machi P, Lobotesis K, Maldonado I, Vendrell JF, Riquelme C, Mourand I, Milhaud D, Héroum C, Perrigault PF, Arquizan C, Bonafé A. Rescue, combined, and stand-alone thrombectomy in the management of large vessel occlusion stroke using the solitaire device: a prospective 50-patient single-center study: timing, safety, and efficacy. Stroke. 2011;42:1929–1935. doi: 10.1161/STROKEAHA.110.608976. [DOI] [PubMed] [Google Scholar]
- 8.Benmira S, Banda ZK, Bhattacharya V. The start of a new era for stroke treatment: mechanical thrombectomy devices. Curr Neurovasc Res. 2011;8:75–85. doi: 10.2174/156720211794520242. [DOI] [PubMed] [Google Scholar]
- 9.Gralla J, Schroth G, Remonda L, Nedeltchev K, Slotboom J, Brekenfeld C. Mechanical thrombectomy for acute ischemic stroke: thrombus-device interaction, efficiency, and complications in vivo. Stroke. 2006;37:3019–3024. doi: 10.1161/01.STR.0000248457.55493.85. [DOI] [PubMed] [Google Scholar]
- 10.Wenger K, Nagl F, Wagner M, Berkefeld J. Improvement of stent retriever design and efficacy of mechanical thrombectomy in a flow model. [published online ahead of print June 15, 2012] Cardiovasc Intervent Radiol. 2012 doi: 10.1007/s00270-012-0420-2. http://www.springerlink.com/content/hp71p623441x61m1/. Accessed July 8, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Roth C, Junk D, Papanagiotou P, Keuler A, Körner H, Schumacher M, Reith W. A comparison of 2 stroke devices: the new aperio clot-removal device and the solitaire AB/FR. [published online ahead of print February 2, 2012.] Am J Neuroradiol. 2012 doi: 10.3174/ajnr.A2962. http://www.ajnr.org/content/early/2012/02/02/ajnr.A2962.abstract?rss=1. Accessed July 8, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miteff F, Faulder KC, Goh AC, Steinfort BS, Sue C, Harrington TJ. Mechanical thrombectomy with a self-expanding retrievable intracranial stent (Solitaire AB): experience in 26 patients with acute cerebral artery occlusion. Am J Neuroradiol. 2011;32:1078–1081. doi: 10.3174/ajnr.A2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohde S, Bösel J, Hacke W, Bendszus M. Stent retriever technology: concept, application and initial results. [published online ahead of print November 23, 2011] J Neurointerv Surg. 2011 doi: 10.1136/neurintsurg-2011-010160. http://jnis.bmj.com/content/early/by/section. Accessed July 8, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Tennuci C, Pearce G, Wong J, Nayak S, Jones T, Lally F, Roffe C. Comparison of the effectiveness of three methods of recanalization in a model of the middle cerebral artery: thrombus aspiration via a 4F catheter, thrombus aspiration via the GP thromboaspiration device, and mechanical thrombectomy using the Solitaire thrombectomy device. Stroke Res Treat. 2011;2011:Article 186424. doi: 10.4061/2011/186424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bösel J, Hacke W, Bendszus M, Rohde S. Treatment of acute ischemic stroke with clot retrieval devices. Curr Treat Options Cardiovasc Med. 2012;14:260–272. doi: 10.1007/s11936-012-0172-y. [DOI] [PubMed] [Google Scholar]
- 16.Rohde S, Haehnel S, Herweh C, Pham M, Stampfl S, Ringleb PA, Bendszus M. Mechanical thrombectomy in acute embolic stroke: preliminary results with the Revive device. Stroke. 2011;42:2954–2956. doi: 10.1161/STROKEAHA.111.616763. [DOI] [PubMed] [Google Scholar]
- 17.Loh Y, Jahan R, McArthur DL, Shi ZS, Gonzalez NR, Duckwiler GR, Vespa PM, Starkman S, Saver JL, Tateshima S, Liebeskind DS, Viñuela F. Recanalization rates decrease with increasing thrombectomy attempts. Am J Neuroradiol. 2010;31:935–939. doi: 10.3174/ajnr.A1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latchaw RE. Mechanical thrombectomy devices for treating acute ischemic stroke. Endovascular Today. Acute Stroke Management. 2007 [Google Scholar]
- 19.Martinez H, Zoarski GH, Obuchowski AM, Stallmayer MJ, Papangelou A, Airan-Javia S. Mechanical thrombectomy of the internal carotid artery and middle cerebral arteries for acute stroke by using the retriever device. Am J Neuroradiol. 2004;33:1812–1815. [PMC free article] [PubMed] [Google Scholar]
- 20.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA, IMS I, II Investigators. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–1072. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kan I, Yuki I, Murayama Y, Viñuela FA, Kim RH, Vinters HV, Viñuela F. A novel method of thrombus preparation for use in a swine model for evaluation of thrombectomy devices. Am J Neuroradiol. 2010;31:1741–1743. doi: 10.3174/ajnr.A1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liebig T, Reinartz J, Hannes R, Miloslavski E, Henkes H. Comparative in vitro study of five mechanical embolectomy systems: effectiveness of clot removal and risk of distal embolization. Neuroradiology. 2008;50:43–52. doi: 10.1007/s00234-007-0297-y. [DOI] [PubMed] [Google Scholar]
- 23.Machi P, Costalat V, Lobotesis K, Maldonado IL, Vendrell JF, Riquelme C, Bonafé A. Solitaire FR thrombectomy system: immediate results in 56 consecutive acute ischemic stroke patients. J Neurointerv Surg. 2011;4:62–66. doi: 10.1136/jnis.2010.004051. [DOI] [PMC free article] [PubMed] [Google Scholar]


