Abstract
In the search for opioid ligands with mixed functional activity, a series of 5′-(4-chlorophenyl)-4,5α-epoxypyridomorphinans possessing alkoxy or acyloxy groups at C-14 was synthesized and evaluated. In this series, the affinity and functional activity of the ligands were found to be influenced by the nature of the substituent at C-14 as well as by the substituent at N-17. Whereas the incorporation of a 3-phenylpropoxy group at C-14 on N-methylpyridomorhinan gave a dual MOR agonist/DOR agonist 17h its incorporation on N-cyclopropylmethylpyridomorphinan gave a MOR agonist/DOR antagonist 17d. Interestingly, 17d, in contrast to 17h, did not produce tolerance or dependence effects on prolonged treatment in cells expressing MOR and DOR. Moreover, 17d displayed greatly diminished analgesic tolerance as compared to morphine on repeated administration, thus supporting the hypothesis that ligands with MOR agonist/DOR antagonist functional activity could emerge as novel analgesics devoid of tolerance, dependence and related side effects.
INTRODUCTION
Opioids analgesics are the most effective and widely used drugs for the treatment of moderate-to-severe pain. The clinical usefulness of opioid analgesics, however, is limited by side effects such as respiratory depression, constipation, analgesic tolerance, physical dependence and addiction liabilities. The development of analgesic tolerance significantly diminishes the analgesic effectiveness on repeated administration, and physical dependence necessitates continued opioid administration to avoid withdrawal symptoms. The development of tolerance and concerns over the risk of developing physical dependence and addiction significantly hamper the optimal use of opioids in the treatment of chronic pain conditions.1–4 Analgesic effects of opioids are mediated by three major types of opioid receptors, the μ-, δ-, and κ-opioid receptors (MORs, DORs and KORs, respectively).5–8 The analgesic activity as well as the side effects such as euphoria, respiratory depression, tolerance, physical dependence, and opioid-induced bowel dysfunction produced by morphine and related clinically effective opioids are primarily mediated by MORs.9–11 Whereas the mechanisms underlying the development of tolerance and other side effects are complex, the interactions between MOR and DOR in particular have been implicated as playing a key role in modulating analgesic activity as well as the side effects associated with opioids.12–15
On the basis of the findings that (i) DOR null mice do not develop analgesic tolerance to morphine,16 (ii) administration of DOR antisense oligonucleotides attenuates morphine dependence,17,18 and (iii) DOR antagonists such as naltrindole19 and TIPP[ψ]20 are able to prevent the development of morphine tolerance and dependence, we and others have been pursuing the concept of developing ligands possessing mixed MOR agonist/DOR antagonist properties as analgesic agents potentially devoid of tolerance, dependence and related side effects.21–23 In our initial effort,24 we found that annulation of an arylpyridine such as 3-(4-chlorophenyl)pyridine onto the C-ring of the opioid antagonist naltrexone (1, Chart 1) gave a ligand 6 with potent antagonist activity at DOR and agonist activity at MOR in ex vivo assays using guinea pig ileum and mouse vas deferens tissues. This compound, however, did not display MOR agonist activity in functional assays using cells expressing MOR.25 The strategy of annulation of chlorophenylpyridine ring to the morphinan C-ring when applied to naloxone (2), 14-deoxynaltrexone (3), oxymorphone (4) and hydromorphone (5) gave ligands 7–10. Of these pyridomorphinans, the hydromorphone-derived compound 10 displayed the desired MOR agonist/DOR antagonist profile of activity. Although the compound displayed only modest agonist activity at MOR, its propensity to induce antinociceptive tolerance was found to be lower than that of morphine.26
Chart 1a.
aCPM = cyclopropylmethyl
Morphinan-6-one ligands possessing a methyl group on the morphinan nitrogen in general are known to display opioid agonist activity whereas those possessing a cyclopropylmethyl (CPM) group display opioid antagonist activity.1,27,28 Among structural variations at the 14-position of the morphinan-6-ones, of particular interest is the incorporation of alkoxy and arylalkoxy ether functions explored extensively by Schmidhammer and coworkers.29–35 Their studies have shown that incorporation of groups such as benzyloxy or 1-naphthylmethoxy on agonist templates led to ligands such as 11 and 12 that displayed considerably higher antinociceptive potencies.34 Interestingly, their studies also revealed that incorporation of an alkoxy group such as 3-phenylpropoxy not only increased the binding affinities at all three opioid receptor types but also converted antagonists such as 1 and 2 into agonist ligands (13 and 14) possessing potent analgesic activity and no measurable antagonist activity in vivo.33,34
In addition to morphinan-6-ones possessing an ether function at the 14-position, ligands possessing an ester function such as 15 and 16 have also been investigated. Recently, Zhang and coworkers reported on the synthesis and activity of a series of aromatic and heteroaromatic esters of naltrexone.36 Most of these ligands were found to be MOR antagonists in the [35S]GTP-γ-S binding assay. Husbands and coworkers studied cinnamoyl ester derivatives such as 16, derived from the antagonist 1, to compare the activity profile with the more extensively studied 14-cinnamoyl amides.37 They found that 16 displayed primarily MOR antagonist activity in in vitro isolated tissue assays. Whereas the compound showed no agonist activity in the warm-water tail-withdrawal assay, it produced substantial inhibition of acetic acid-induced writhing. Results from agonist selectivity experiments showed that the antinociceptive effects of 16 are primarily mediated through its agonist activity at MOR and DOR.37
In view of these observations indicating that an alkoxy or acyloxy substituent at the 14-position of the morphinan framework can have a substantial influence on the binding and functional activity at opioid receptor subtypes it was of interest to explore the effect of incorporating such groups in the pyridomorphinan scaffold in our search for new ligands possessing MOR agonist/DOR antagonist activity. The target compounds that we explored, generically represented by 17 and 18, included N-methyl or N-CPM group at the morphinan nitrogen. Presented herein are the results of the investigation that led to the identification of potent dual MOR/DOR agonists and mixed MOR agonist/DOR antagonist ligands along with their analgesic effects and propensity to induce tolerance and dependence.
RESULTS AND DISCUSSION
Synthesis
For the synthesis of the desired target compounds, the previously reported 17-cyclopropylmethyl- and 17-methyl-3,14-dihydroxypyridomorphinans 624 and 926 served as suitable starting materials. For the synthesis of 14-alkoxy target compounds we found it convenient to perform dialkylation at the phenolic hydroxyl at the 3-position and the tertiary alcohol at the 14-position followed by selective dealkylation of the phenolic ether function. Thus, dimethylation of 6 with dimethyl sulfate or dialkylation of 6 or 9 with appropriate alkyl bromides using sodium hydride as the base yielded the corresponding dialkyl derivatives 19a–d and 19f. Treatment of these with boron tribromide led to selective removal of the alkyl group from the ether function at C-3 yielding the target compounds 17a–d and 17f. For the preparation of 17e, 17g and 17h, the oxycodone-derived pyridomorphinan 2026 was used as the starting material. Alkylation of 20 with the appropriate alkylating agent followed by 3-O-demethylation of the resulting diethers 19e, 19g and 19h delivered the desired target compounds (Scheme 1).
Scheme 1.
Synthesis of 14-Alkoxypyridomorphinans 17a–ha
aReagents and conditions: (a) NaH, DMF, Me2SO4 or R′Br, 0 °C to rt; (b) BBr3, CH2Cl2, −78 °C to rt.
The starting materials 6 and 9 were treated with an excess of the appropriate acid chloride and the resulting intermediates were treated with aqueous base to remove the acyl group from the phenolic hydroxyl group to obtain the desired 14-O-acylated target compounds 18a–f. The yields of the final products in these acylation reactions were only modest possibly due to elimination reactions setting in as side reactions. For example, isolation of the products from the reaction of 6 with benzoyl chloride after a 5 h reaction time gave the dibenzoate 21 and the elimination product 22 in 60:40 ratio. When the reaction was allowed to proceed for a longer period of time (16 h) the elimination product became the main product with a distribution ratio of 26:74 for 21 and 22. These benzoates 21 and 22 could be converted to the free phenolic compounds 18a and 23, respectively, by treatment with K2CO3 in aqueous methanol (Scheme 2).
Scheme 2.
Synthesis of 14-Acyloxypyridomorphinans 18a–f and 21–23a
aReagents and conditions: (a) R′COCl, Et3N, DMF or PhMe; (b) K2CO3, MeOH-H2O, rt; (c) PhCOCl, Et3N, DMF.
Ligand Binding at the Opioid Receptors
All target compounds were evaluated for binding affinities at DOR, MOR and KOR using a radioligand displacement assay with membranes prepared from CHO cells stably expressing these receptors. The radioligands [3H]DADLE, [3H]DAMGO and [3H]U69,593 were used for labeling the DOR, MOR and KOR sites, respectively. These evaluations were performed as previously described.38–40 The affinity and selectivity data for the target compounds are given in Table 1. With the exception of 18a and 18d, all of the ligands displayed high affinity binding at DOR with Ki µ5 nM. In general, all of the ligands displayed relatively non-selective binding profiles at all three opioid receptor subtypes. The 14-methoxy compound 17a arising from 14-O-methylation displayed a binding profile somewhat similar to that of the parent compound 6. In contrast, methylation of 9 produced the ligand 17e that displayed markedly improved binding affinity at MOR and KOR. Among the N-CPM compounds, installation of the arylalkyl groups such as benzyl, cinnamyl and 3-phenylpropyl on the oxygen at C-14 (compounds 17b, 17c, and 17d) consistently increased the binding affinity at MOR. A similar trend of increasing MOR affinity is seen among the N-Me compounds 17f, 17g, and 17h. Placement of a benzoyloxy group at C-14 is generally not well tolerated. The two benzoyloxy compounds 18a and 18d are 45- and 10-fold weaker in binding to DOR compared to their 14-benzyloxy counterparts, 17b and 17f, respectively. These benzoyloxy ligands also displayed a comparable decrease in affinity at MOR and KOR indicating a general unfavorable interaction trend among all receptor subtypes. In contrast, installation of phenylacetyl and phenylpropionyl groups (18b, 18c, 18e and 18f) gave ligands with moderate to high affinity at all three receptors. In fact the phenylpropoxy and phenylpropionyl ligands possessing three atom separation between 14-O and the pendant phenyl group (17d vs 18c and 17h vs 18f) displayed somewhat comparable affinity profiles. The relatively high affinity of these ligands at all three receptors may be attributable to the conformational flexibility afforded by the longer chain to position the pendant phenyl group to occupy a suitable binding pocket for favorable hydrophobic or aryl-π interactions at the ligand binding pocket. The binding profile of compound 23 lacking an ether function with unsaturation between C-8 and C-14 resembled that of the saturated (6) or the methoxy (17a) analogues exhibiting lower affinity at MOR compared to affinities at DOR and KOR.
Table 1.
Binding Affinities of the Pyridomorphinans at DOR, MOR and KOR
 | |||||||
|---|---|---|---|---|---|---|---|
| Ki ± SEM (nM) | selectivity ratio | ||||||
| Compd | R | R' | DORa | MORb | KORc | MOR/DOR | KOR/DOR |
| 17a | CPM | Me | 1.95 ± 0.14 | 41.9 ± 2.8 | 5.37 ± 0.48 | 22 | 2.8 |
| 17b | CPM | C6H5CH2 | 1.91 ± 0.08 | 8.42 ± 0.29 | 5.33 ± 0.27 | 4.4 | 2.8 |
| 17c | CPM | C6H5CH=CHCH2 | 3.74 ± 0.32 | 3.03 ± 0.38 | 3.31 ± 0.30 | 0.81 | 0.89 |
| 17d | CPM | C6H5CH2CH2CH2 | 1.20 ± 0.12 | 0.66 ± 0.06 | 1.82 ± 0.11 | 0.55 | 1.5 |
| 17e | Me | Me | 1.63 ± 0.08 | 7.89 ± 0.33 | 27.99 ± 1.59 | 4.8 | 17 |
| 17f | Me | C6H5CH2 | 1.78 ± 0.15 | 4.69 ± 0.17 | 49.0 ± 6.0 | 2.6 | 28 |
| 17g | Me | C6H5CH=CHCH2 | 1.14 ± 0.11 | 0.41 ± 0.04 | 1.55 ± 0.09 | 0.36 | 1.4 |
| 17h | Me | C6H5CH2CH2CH2 | 1.04 ± 0.08 | 0.54 ± 0.04 | 1.53 ± 0.10 | 0.52 | 1.5 |
| 18a | CPM | C6H5CO | 87 ± 8 | 192 ± 21 | 117 ± 6 | 2.2 | 1.3 |
| 18b | CPM | C6H5CH2CO | 1.33 ± 0.10 | 1.04 ± 0.25 | 3.10 ± 0.19 | 0.78 | 2.3 |
| 18c | CPM | C6H5CH2CH2CO | 4.13 ± 0.29 | 2.58 ± 0.12 | 12.0 ± 13 | 0.62 | 2.9 |
| 18d | Me | C6H5CO | 17.0 ± 1.0 | 84.0 ± 7.0 | 112 ± 4 | 4.9 | 6.6 |
| 18e | Me | C6H5CH2CO | 0.97 ± 0.04 | 1.43 ± 0.08 | 17.0 ± 1.0 | 1.5 | 18 |
| 18f | Me | C6H5CH2CH2CO | 0.96 ± 0.05 | 0.94 ± 0.05 | 4.41 ± 0.23 | 0.98 | 4.6 |
| 23 | CPM | — | 2.71 ± 0.11 | 13.0 ± 0.4 | 5.97 ± 0.19 | 4.8 | 2.2 |
| 6d | CPM | H | 2.20 ± 0.20 | 51.0 ± 8.0 | 20.0 ± 1.0 | 23 | 9 |
| 9d | Me | H | 3.90 ± 0.20 | 230 ± 10 | 468 ± 17 | 59 | 120 |
In vitro Functional Activity at the Opioid Receptors
Compound selections and in vitro functional activity determinations were performed with the primary aim of identifying ligands possessing the desired MOR agonist/DOR antagonist activity. The agonist efficacy (Emax) and potency (EC50) values were determined using previously described [35S]GTP-γ-S binding assays with cells expressing MOR, DOR or KOR. The agonist Emax values were normalized to the stimulation produced by the standard agonists DAMGO, DADLE and U69,593 at MOR, DOR and KOR, respectively. The antagonist potency of the ligands was determined using a [35S]GTP-γ-S binding assay by measuring the shift in EC50 value of standard agonists. The functional activity data thus obtained are presented in Table 2. As might be expected, with the exception of 18d, all of the compounds 17e–h, 18e and 18f possessing the classical MOR agonist N-Me structural feature displayed full agonist efficacy at MOR with Emax values >100. The weak MOR agonist potency and partial efficacy of 18d is in conformity with its poor binding affinity at MOR. In terms of agonist potency, whereas the methyl and benzyl ethers were weak (17e, EC50 = 379 nM; 17f, EC50 = 301 nM), the cinnamyl (17g) and the 3-phenylpropyl (17h) ethers were nearly 100-fold more potent with EC50 values of 4.27 nM and 2.15 nM, respectively. Compared to these ethers, the esters displayed diminished MOR agonist potencies (18e EC50 = 87 nM; 18f EC50 = 48 nM). All of the esters (18a–c) as well as the unsaturated compound 23 possessing the classical antagonist N-CPM structural feature did indeed turn out to be antagonists at MOR. Similarly, the methyl, benzyl and cinnamyl ethers 17a–c possessing the N-CPM group also displayed a non-agonist profile at MOR. Most interestingly, however, the phenylpropyl ether 17d possessing the N-CPM group displayed an agonist profile at MOR (Emax = 72%, EC50 = 1.74 nM). This transformative influence on the functional activity at MOR brought about by installation of a 3-phenylpropoxy group at the 14-position of the 17-cyclopropylmethyl-4,5-epoxypyridomorphinan is similar to the effect of such a group on 17-cyclopropylmethyl-4,5-epoxy-6-oxomorphinans. 33
Table 2.
Functional Activity of the Pyridomorphinans at DOR, MOR and KOR
| antagonist activity | antagonist activity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| compd | DOR Ke (nM) |
MOR Ke (nM) |
KOR Ke (nM) |
DOR EC50 (nM) |
DOR E max (%) |
MOR EC50 (nM) |
MOR E max (%) |
KOR EC50 (nM) |
KOR E max (%) |
| 17a | a | a | a | no stimb | nac | no stim | na | a | a |
| 17b | 0.64 ± 0.11 | 11 ± 1 | 13 ± 3 | no stim | na | no stim | na | 27 ± 9 | 15 ± 1 |
| 17c | a | a | a | no stim | na | no stim | na | a | a |
| 17d | 0.091 ± 0.01 | d | 1.35 ± 0.28 | no stim | na | 1.74 ± 0.20 | 72 ± 2 | no stim | na |
| 17e | 1.60 ± 0.35 | d | 17 ± 0.13 | no stim | na | 379 ± 50 | 118 ± 4 | 633 ± 108 | 36 ± 2 |
| 17f | 4.54 ± 0.81 | d | 83 ± 14 | 11 ± 1 | 52 ± 1 | 301 ± 28 | 119 ± 3 | 215 ± 54 | 32 ± 2 |
| 17g | e | e | e | 0.59 ± 0.05 | 84 ± 2.0 | 4.27 ± 0.57 | 130 ± 3 | e | e |
| 17h | e | e | 6.9 ± 0.02 | 0.27 ± 0.03 | 85 ± 2.0 | 2.15 ± 0.21 | 125 ± 2 | no stim | na |
| 18a | 218 ± 2 | 261 ± 36 | 126 ± 13 | no stim | na | no stim | na | no stim | na |
| 18b | 0.37 ± 0.04 | 0.48 ± 0.06 | 7 ± 2 | no stim | na | no stim | na | 26 ± 9 | 34 ± 2 |
| 18c | 1.86 ± 0.21 | 13 ± 1.7 | 14 ± 2 | no stim | na | no stim | na | 13 ± 4 | 25 ± 1 |
| 18d | 45 ± 6 | 1273 ± 166 | 426 ± 47 | no stim | na | 4623 ± 1439 | 55 ± 8 | no stim | na |
| 18e | 3.38 ± 0.34 | d | 198 ± 20 | 19 ± 6 | 27 ± 3 | 87 ± 10 | 122 ± 3 | 109 ± 24 | 36 ± 2 |
| 18f | 1.64 ± 0.30 | d | 18 ± 0.2 | 5.52 ± 0.53 | 59 ± 2 | 48 ± 5 | 105 ± 2 | 136 ± 60 | 16 ± 2 |
| 23 | 0.58 ± 0.10 | 8.0 ± 0.89 | 19 ± 3.0 | no stim | na | no stim | na | no stim | na |
Not tested due to lack of agonist activity at MOR.
No stimulation of [35S]GTP-γ-S binding at 10 µM.
Not applicable.
Not tested due to full agonist activity at MOR.
Not tested due to agonist activity at both MOR and DOR.
We had earlier demonstrated that pyridomorphinans in general, and those possessing an aryl group such as the 4-chlorophenylgroup at the 5′-position on the pyridine ring in particular, showed a non-agonist functional profile at DOR, irrespective of whether the ligands possessed a MOR-agonist methyl group (9 and 10, Chart 1) or a MOR-antagonist CPM group (6 and 8, Chart 1) on the morphinan nitrogen. The functional activity profile of the current series of compounds at DOR, however, is influenced by the nature of the substituent at C-14. In the current series of compounds, all of the ligands possessing an N-CPM group were antagonists at DOR including 17d. However, ligands possessing N-methyl group (17f, 17g, 17h, 18e and 18f) displayed weak to potent partial agonist activity at DOR. Among the N-methyl compounds, the 14-methoxy compound 17e is the only exception retaining antagonist activity at DOR. Interestingly, the N-CPM containing MOR agonist ligand 17d also turned out to be the most potent DOR antagonist with a Ke of 0.091 nM.
At KOR, most of the tested ligands displayed weak partial agonist activity (Emax <36%) with varying potencies as antagonists. The phenylpropoxy compound 17d was devoid of agonist activity at KOR. Thus, the incorporation of the phenylpropoxy group on a pyridomorphinan possessing the N-CPM group did not induce agonist activity at DOR or KOR as it did at MOR. This is in contrast to the results from incorporation of a phenylpropoxy group on 6-oxomorphinans reported by Schmidhammer and coworkers, who found that introduction of a phenylpropoxy group at the C14-position of 1 yielded the agonist 13, devoid of any antagonist activity.33 The findings of the present study, together with the structure-activity relationships observed in earlier studies,24,26,41,42 indicate that the substituted pyridine moiety fused to the C-ring of morphinans and the CPM substituent at the morphinan nitrogen serve as pharmacophoric elements that stabilize the inactive conformation of DOR and KOR regardless of the nature of the substituent at C-14. In contrast, installation of a group such as phenylpropoxy at C-14, even on frameworks possessing the N-CPM and pyridomorphinan groups, is capable of imparting agonist interaction at MOR. Among the compounds of the present study, 17d emerged as a compound of particular interest as it displayed a balanced profile of potent agonist activity at MOR coupled with potent antagonist activity at DOR and KOR.
In Vitro Studies on Tolerance and Dependence
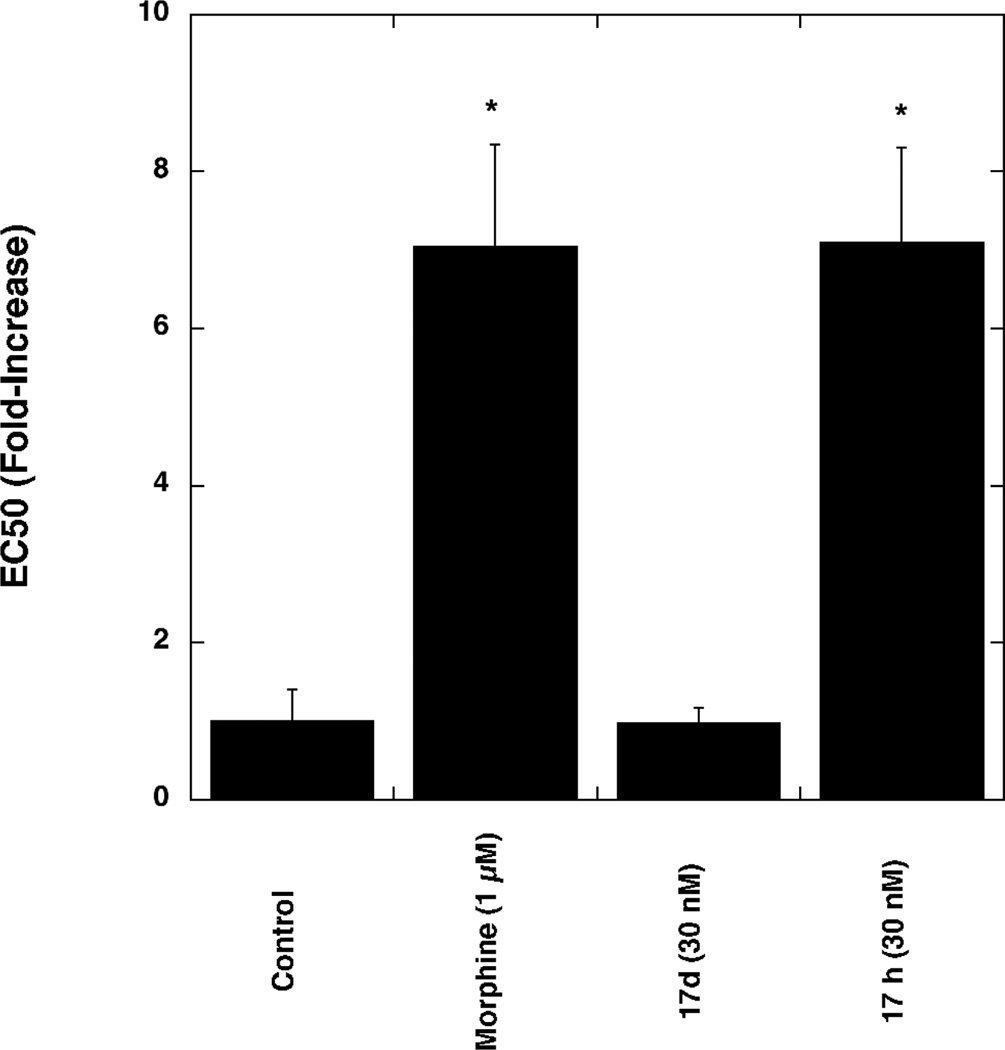
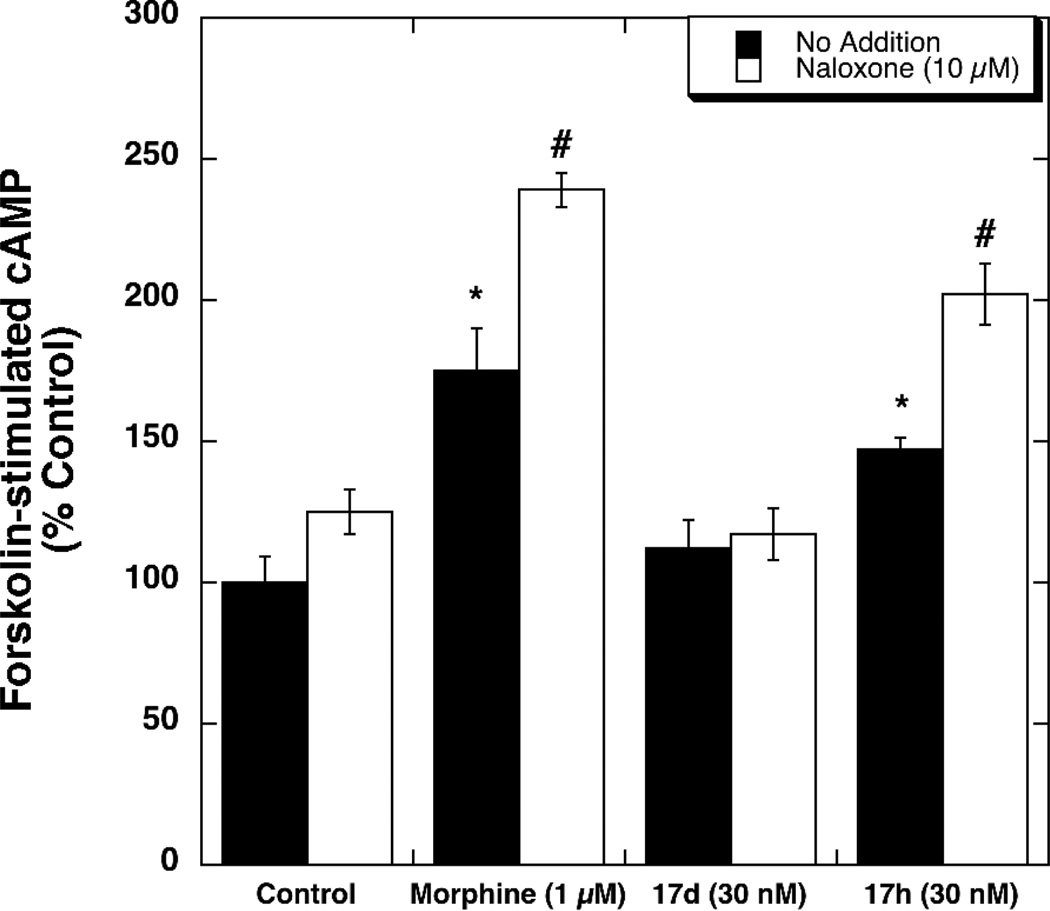
Having identified 17d as a MOR agonist/DOR antagonist we conducted two experiments using CHO cells co-expressing MOR and DOR (dimer cells) to determine the effect of 17d on the development of tolerance and dependence, respectively. As a control, we included the structurally similar MOR/DOR dual agonist 17h in these experiments. Dimer cells were treated for 20 h with medium (control), morphine (1 µM), 17d (30 nM) or 17h (30 nM). These concentrations were chosen to be approximately 25-fold greater than the corresponding EC50 values for stimulation of [35S]GTP-γ-S binding to membranes prepared from dimer cells (EC50 for 17d = 1.2 ± 0.1 nM, EC50 for 17h = 0.34 ± 0.02 nM). As reported in Figure 1, chronic morphine and chronic 17h resulted in an ~ 7-fold increase in the EC50 value for DAMGO-mediated inhibition of forskolin-stimulated cAMP accumulation. Unlike morphine, 17d and 17h decreased the Emax value for DAMGO-mediated inhibition of forskolin-stimulated cAMP by 40% and 20%, respectively. The mechanism(s) underlying the decreased efficacy observed with chronic 17d and 17h treatment is currently under investigation.
Figure 1.
Comparison of the effects of chronic drug treatment on DAMGO-mediated inhibition of forskolin-stimulated cAMP accumulation in MOR/DOR dimer cells: MOR/DOR dimer cells were treated for 20 h with morphine (1 µM), or 17d (30 nM), or 17h (30 nM), respectively. DAMGO-mediated inhibition of forskolin-stimulated cAMP accumulation was performed as described in Methods section. Data are presented as the EC50 ratio (fold over control). Each value is the mean ± SEM (n=3). *p<0.05 when compared with the control cells (two-tailed Student’s t-test).
In the “dependence” experiments (Figure 2), dimer cells were treated chronically as described above. After 20 h treatment, the cells were washed to remove drugs, and the degree of cAMP accumulation produced by forskolin/IBMX (100 µM/500 µM) was determined in the absence and presence of 10 µM naloxone. Chronic morphine produced a significant increase in forskolin-stimulated cAMP accumulation, a phenomenon called “cAMP superactivation.”6 The combination of forskolin plus naloxone produced a further increase in cAMP accumulation, a phenomenon called “naloxone-induced cAMP overshoot.” The MOR agonist/DOR agonist compound (17h) produced effects similar to that of morphine, whereas the MOR agonist/DOR antagonist compound (17d) did not.
Figure 2.
Comparison of the effects of chronic drug treatment on spontaneous and naloxone (10 µM)-induced cAMP overshoot in MOR/DOR dimer cells: MOR/DOR dimer cells were treated for 20 h with morphine (1 µM), or 17d (30 nM), or 17h (30 nM), respectively. The forskolin-stimulated cAMP accumulation was assessed as described in Methods. Each value is the mean ± SEM (n=3). *p<0.05 when compared with no addition condition of the control cells. #p<0.05 when compared with no addition condition of the morphine-treated or 17h-treated cells (two-tailed Student’s t-test).
Chronic treatment of cells that express MOR with MOR agonists produce a variety of cellular adaptations that together produce tolerance and dependence.6 In this study we assessed three such changes: 1) tolerance, as determined by shifts in the DAMGO-dose response curve for inhibition of forskolin-stimulated cAMP accumulation, 2) cAMP superactivation and 3) the naloxone-induced cAMP overshoot. These latter two measures are generally considered as cellular signs related to dependence. The cAMP overshoot arises from chronic-morphine induced formation of constitutively active receptors, which are receptors that activate G proteins in the absence of an agonist. The constitutively active MORs inhibit forskolin-stimulated cAMP. Since naloxone is an inverse agonist, it decreases the activity of the constitutively active MORs. This relieves the inhibitory effect of the constitutively active receptors on forskolin-stimulated cAMP, resulting in a further increase in forskolin-stimulated cAMP accumulation.40,43
The results observed here with chronic morphine treatment of dimer cells are similar to what we observed previously using CHO cells that stably express the cloned human MOR.40 In contrast, chronic treatment of dimer cells with the MOR agonist/DOR antagonist 17d did not produce either tolerance, as defined as an increase in the EC50 value for DAMGO-mediated inhibition of forskolin-stimulated cAMP, or dependence, as defined by the presence of cAMP superactivation or a naloxone-induced cAMP overshoot. The MOR agonist/DOR agonist 17h had similar effects to that of morphine.
Numerous studies have replicated the original observations in whole animals that DOR antagonists reduce or block the development of morphine tolerance and dependence.19,44 This is the first study that we are aware of that demonstrates this phenomenon at the cellular level, and this cell-based system should be useful for investigating the involvement of delta receptors in the development of morphine tolerance and dependence. Moreover, these data support the hypothesis that the mu/delta heterodimer may be a crucial mediator of tolerance and dependence.45
Analgesic Activity and Tolerance Studies in Mice
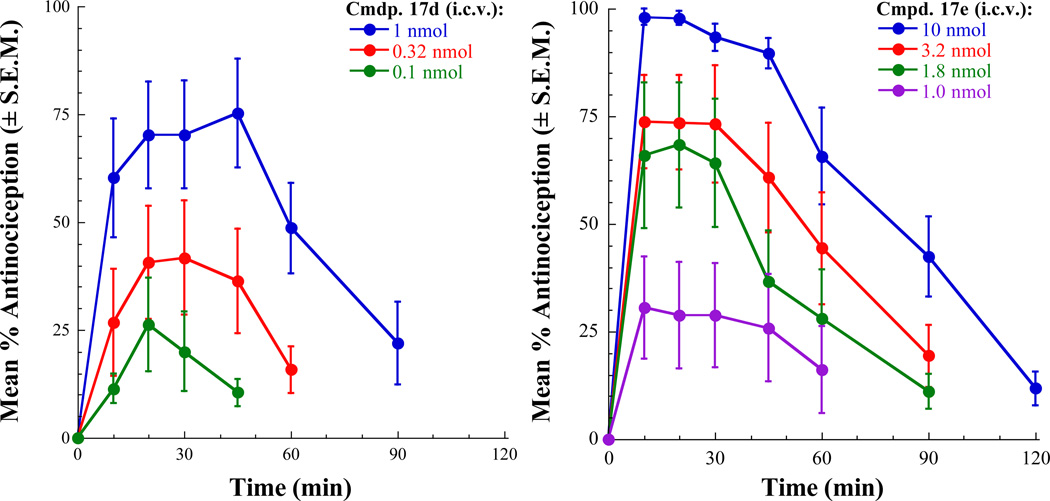
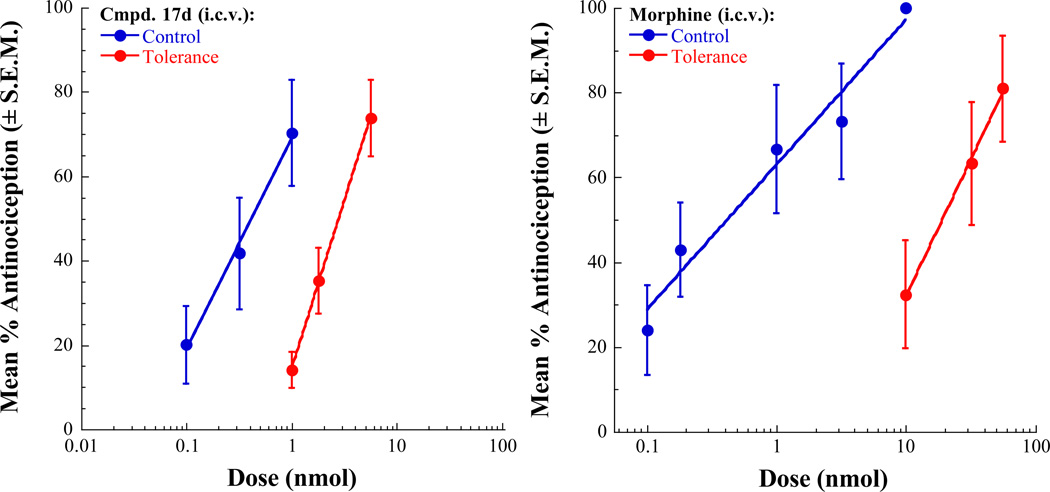
The analgesic activity of selected ligands were tested in mice using the 55 °C warm-water tail-withdrawal test as described previously.46 Compounds that were evaluated in this study were 17d, 17e, 17g, and 17h. These compounds were administered by the intracerebroventricular (i.c.v.) route. All of the tested compounds produced dose-related antinociception in the 55°C tail-flick assay. Morphine and 17e, 17g and 17h produced maximal or near-maximal agonist effects at the highest doses tested. Antinociceptive activity of 17d maxed out at approximately 75% (a 10 nmol dose did not produce an additional effect). The antinociceptive effects of these compounds were blocked by naloxone pretreatment confirming that the analgesic activity of these compounds is mediated through opioid receptors. The antinociceptive dose-response curves for 17d and 17e are shown in Figure 3. The calculated antinociceptive A50 values of all the tested compounds and the morphine control are listed in Table 3. In this assay, compounds 17d, 17g, and 17h displayed potency equivalent to or better than that of morphine. This is attributable to potent agonist activity of these ligands at MOR (17d) or at both MOR and DOR (17g and 17h) as determined in the [35S]GTP-γ-S assays. Despite the very weak MOR agonist potency displayed by 17e in the [35S]GTP-γ-S assay (MOR agonist EC50 = 379 nM), this compound produced significant analgesic effect and was only 6-fold weaker than other tested ligands. As evaluated in the in vitro tolerance assays, it was of interest to determine the propensity of the two compounds, 17d, a MOR agonist/DOR antagonist and 17h, a MOR-DOR dual agonist, to induce analgesic tolerance. The studies were carried out using the tolerance development assay involving repeated injection of the test compound (twice daily for 3 days). The degree of tolerance development is indicated by the fold-shift in the antinociceptive A50 values when tested in naive control mice and in the repeated injection paradigm. In this repeated administration paradigm, morphine produces a significant development of tolerance inducing a 44-fold shift in A50 value (control A50 = 0.43 nmol, tolerance A50 = 19.04 nmol). In contrast, 17d displayed only a 7.9-fold shift in antinociceptive potency (control A50 = 0.35 nmol, tolerance A50 = 2.78 nmol) on repeated administration, thus confirming that the MOR agonist/DOR antagonist ligand indeed produces significantly less tolerance than morphine (Figure 4). Based on the results from the in vitro tolerance study, the MOR-DOR dual agonist ligand 17h might be expected to display robust tolerance development in the repeated injection paradigm. Unfortunately, undue toxicity displayed by 17h as well as the alternative MOR-DOR dual agonist 17g on repeated administration precluded the determination of induction of analgesic tolerance by these dual agonists. In the [35S]GTP-γ-S binding assay, the two compounds 17d and 17h displayed functional antagonism at KOR. While KOR mediated effects may have contributed to the responses observed with these compounds in vivo, it is more likely that the MOR-DOR interactions are primarily responsible for tolerance and dependence related activities of these compounds based on the results from the in vitro tolerance and dependence experiments using MOR-DOR dimer cells devoid of KOR.
Figure 3.
Antinociceptive dose– and time–response curves for 17d and 17e in the 55 °C warm-water tail-withdrawal assay.
Table 3.
Analgesic Activity of Selected Ligands in the Mouse Warm-Water Tail-Withdrawal Assaya
| compd | maximal antinociception |
dose for maximal antinociception |
antinociceptive A50 values |
95% confidence limits |
|---|---|---|---|---|
| 17d | 75% | 1 nmol | 0.35 nmol | 0.18–0.69 nmol |
| 17e | 98% | 10 nmol | 1.44 nmol | 1.02–2.03 nmol |
| 17g | 91% | 1 nmol | 0.23 nmol | 0.16–0.33 nmol |
| 17h | 92% | 1 nmol | 0.23 nmol | 0.18–0.30 nmol |
| Morphine | 100% | 10 nmol | 0.43 nmol | 0.38–0.51 nmol |
Compounds were administered i.c.v. and the A50 values calculated at time of peak effect.
Figure 4.
Antinociceptive dose–response curves for naive control mice and mice injected repeatedly with A90 doses of 17d (left panel) or morphine (right panel) i.c.v. twice daily for 3 days.
SUMMARY AND CONCLUSIONS
Several lines of evidence suggest physical or functional interactions between MOR and DOR and that activation of MOR while simultaneously inhibiting DOR can produce analgesic effects with diminished propensity for developing tolerance. With the goal of identifying novel small molecule ligands with mixed MOR agonist/DOR antagonist profiles, the current investigation focused on 4,5α-epoxypyridomorphinans possessing an ether or ester function at C-14. The observed structure-activity relationships clearly indicate that the intrinsic activity of such ligands is influenced by the nature of the substituent on the morphinan nitrogen (Me or CPM) as well as the group at C-14. Remarkably, the installation of a 3-phenylpropoxy group at C-14 on the framework containing a CPM group on the morphinan nitrogen led to a selective transformation of functional activity to that of an agonist at MOR with retention of antagonist activity at DOR and KOR, thus producing a ligand with the desired MOR agonist/DOR antagonist profile. Although the structural basis for this selective transformation of functional activity remains to be elucidated, it is likely that the placement of a group such as phenylpropoxy group on suitable morphinan antagonist frameworks could transform them to mixed function ligands possessing agonist activity at MOR and antagonist activity at DOR and KOR.
Pharmacological evaluations with the MOR agonist/DOR antagonist ligand 17d demonstrated that the mixed function ligand indeed produces diminished tolerance and dependence effects in a cellular model system as compared to the MOR/DOR dual agonist ligand 17h. Moreover, the MOR agonist/DOR antagonist 17d, when tested using the repeated administration procedure in mice, produced greatly diminished tolerance development as compared to morphine. These results suggest that developing small molecules with mixed MOR agonist/DOR antagonist profile could be a fruitful approach in the search for analgesics devoid of some of the side effects associated with currently available opioid drugs.
Experimental Section
General Methods
Melting points were determined in open capillary tubes with a Mel-Temp melting point apparatus and are uncorrected. 1H NMR spectra were recorded on a Nicolet 300NB spectrometer operating at 300.635 MHz. Chemical shifts are expressed in parts per million downfield from tetramethylsilane. Spectral assignments were supported by proton decoupling. Mass spectra were recorded on a Varian MAT 311A double-focusing mass spectrometer in the fast atom bombardment (FAB) mode or on a Bruker BIOTOF II in electrospray ionization (ESI) mode. Elemental analyses were performed by Atlantic Microlab, Inc. (Atlanta, GA) or by the Spectroscopic and Analytical Laboratory of Southern Research Institute. Analytical results indicated by elemental symbols were within ± 0.4% of the theoretical values. Thin layer chromatography (TLC) was performed on Analtech silica gel GF 0.25 mm plates. Flash column chromatography was performed with E. Merck silica gel 60 (230–400 mesh). Yields are of purified compounds and were not optimized. On the basis of NMR and combustion analysis data, all final compounds reported in the manuscript are >95% pure.
5′-(4-Chlorophenyl)-17-(cyclopropylmethyl)-6,7-didehydro-4,5α-epoxy-3-hydroxy-14-methoxypyrido[2′,3′:6,7]morphinan (17a)
Step 1
Sodium hydride (0.096 g, 4.0 mmol, 60% dispersion in mineral oil, washed with hexanes) was added to a stirred solution of 6 (0.487 g, 1.0 mmol) in DMF (7 mL) at 0–5 °C. The mixture was stirred at 0 °C for 10 min, treated dropwise with dimethyl sulfate (0.277 g, 2.2 mmol) and then allowed to warm to room temperature. The mixture was stirred at room temperature overnight and then was quenched by addition of small pieces of ice. The mixture was diluted with water (20 mL) and extracted with CHCl3 (2 × 25 mL). The combined extracts were washed with water and brine, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to yield 0.3 g (58%) of 5′-(4-chlorophenyl)-17-(cyclopropylmethyl)-6,7-didehydro-3,14-dimethoxy-4,5α-epoxypyrido[2′,3′:6,7]morphinan (19a). ESI MS m/z 515 (MH)+. The crude product thus obtained was used in the next step without further purification.
Step 2
A solution of 19a (0.26 g, 0.5 mmol) in anhydrous CH2Cl2 (7 mL) was cooled to −78 °C and treated dropwise with boron tribromide (0.75 g, 3.0 mmol). The mixture was stirred at −78 °C for 1 h and then allowed to come to room temperature. After quenching the reaction by addition of drops of ice-cold water, the mixture was extracted with CHCl3 (2 × 50 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The crude product was purified over silica gel column using CHCl3–MeOH–NH4OH (98:1.5:0.5) to yield 0.18 g (72 %) of desired product 17a: mp 170–173 °C; TLC Rf 0.39 (CHCl3–MeOH, 95:5); 1H NMR (DMSO-d6) δ 0.12–0.54 (2 m, 4H, cyclopropyl CH2CH2), 0.84–0.88 (m, 1H, cyclopropyl CH), 1.43–1.47 (m, 1H, C-15 H), 2.16–2.68 (m, 7H, C-15 H, C-8 H, C-10 H, C-16 H2, NCH2), 3.01 (d, 1H, J = 17.2 Hz, C-8 H), 3.11 (m, 1H, C-10 H), 3.17 (s, 3H, OCH3), 3.6 (m, 1H, C-9 H), 5.32 (s, 1H, C-5 H), 6.52 (s, 2H, C-2 H, C-1 H), 7.53–7.56 (m, 2H, C-3″ H, C-5″ H), 7.71–7.77 (m, 3H, C-4′ H, C-2″ H, C-6″ H), 8.76 (s, 1H, C-6′ H), 9.04 (s, 1H, C-3 OH). ESI MS m/z 501 (MH)+. Anal. (C30H29ClN2O3·H2O) C, H, N.
14-(Benzyloxy)-5′-(4-chlorophenyl)-17-(cyclopropylmethyl)-6,7-didehydro-4,5α-epoxy-3-hydroxypyrido[2′,3′:6,7]morphinan (17b)
Step 1
A solution of 6 (0.487 g, 1.0 mmol) in DMF (5.0 mL) was reacted with sodium hydride (0.12 g, 3.0 mmol, 60% dispersion in mineral oil, washed with hexane) and benzyl bromide (0.35 g, 2.2 mmol) as described in Step 1 for the preparation of 17a. Purification of the crude product by chromatography over a column of silica using CHCl3–MeOH, 99:1 yielded 0.46 g (69%) of 3,14-bis(benzyloxy)-5′-(4-chlorophenyl)-17-(cyclopropylmethyl)-6,7-didehydro-4,5α-epoxypyrido[2′,3′:6,7]morphinan (19b). ESI MS m/z 667 (MH)+.
Step 2
A solution of 19b (0.46 g, 0.7 mmol) in anhydrous CH2Cl2 (5.0 mL) was cooled to −78 °C and treated dropwise with boron tribromide (3.0 mL of 1 M solution in CH2Cl2, 3.0 mmol). The mixture was stirred at −78 °C for 1 h and then allowed to attain to room temperature. The reaction was quenched by the addition of water (10 mL). The mixture was extracted with CHCl3 (2 × 50 mL), dried with anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure. The residue was purified over the column of silica gel using EtOAc–hexane (1:1) as the eluent. The product obtained was crystallized from EtOAc to afford 0.098 g (25%) of 17b: mp 130–132 °C; TLC Rf 0.42 (CHCl3–MeOH, 95:5); 1H NMR (DMSO-d6) δ 0.08–0.19 and 0.44–0.54 (m, 4H, cyclopropyl CH2CH2), 0.84–0.96 (m, 1H, cyclopropyl CH), 1.50 (d, 1H, J = 10.6 Hz, C-15 H), 2.20–2.28 (m, 1H, C-15 H), 2.34–2.82 (m, 7H, C-16 H2, C-10 H, C-8 H2, NCH2-cyclopropyl), 3.10–3.23 (m, 1H, C-10 H), 3.82 (d, 1H, J = 5.7 Hz, C-9 H), 4.35 (dd, 2H, J = 11.1 and 11.4Hz, OCH2), 5.38 (s, 1H, C-5 H), 6.52–6.57 (m, 2H, C-1 H, C-2 H), 7.10–7.45 (m, 5H, C6H5), 7.53–7.56 (m, 2H, C-3″ H, C-5″ H), 7.70–7.74 (m, 3H, C-4′ H, C-2″ H, C-6″ H), 8.79 (d, 1H, J = 2.2 Hz, C-6′ H), 8.96 (s, 1H, C-3 OH). ESI MS m/z 577 (MH)+. Anal. (C36H33ClN2O3·H2O) C, H, N.
5′-(4-Chlorophenyl)-14-cinnamyloxy-17-(cyclopropylmethyl)-6,7-didehydro-4,5α-epoxy-3-hydroxypyrido[2′,3′:6,7]morphinan (17c)
Step 1
To a stirred solution of 6 (0.974 g, 2.0 mmol) in DMF (15 mL) was added sodium hydride (60% dispersion in mineral oil, 0.288 g, 6.0 mmol) at 0–5 °C. After stirring at 0 °C for 10 minutes, cinnamyl bromide (0.871 g, 4.4 mmol) was added dropwise. The mixture was stirred at room temperature overnight, and the reaction was quenched by careful addition of small pieces of ice. The mixture was diluted with water and extracted with CHCl3 (2 × 50 mL). The combined organic extracts were washed with water, brine and dried over anhydrous sodium sulfate. The crude product obtained after removal of the solvent under reduced pressure was purified over a column of silica using EtOAc–hexane 20:80 to yield 0.524 g (36%) of 5′-(4-chlorophenyl)-17-(cyclopropylmethyl)-3,14-(dicinnamyloxy)-6,7-didehydro-4,5α-epoxypyrido[2′,3′:6,7]morphinan (19c). ESI MS m/z 719 (MH)+. The product thus obtained was used in the next step without further purification.
Step 2
A solution of 19c (0.524 g, 0.73 mmol) in anhydrous CH2Cl2 (7 mL) was cooled to −78 °C. Boron tribromide (1.50 g, 6.0 mmol) was added dopwise. After stirring for 1 h, the reaction mixture was allowed to attain room temperature. The reaction mixture was quenched by addition of drops of ice-cold water. After dilution with water, the crude product was extracted with CHCl3 (2 × 100 mL), dried over anhydrous sodium sulfate, filtered and evaporated under reduced pressure. The crude product was purified by column chromatography over silica using EtOAc–hexane 25:75 as the eluent to obtain 0.268 g (61%) of 17c: mp 138–140 °C; TLC Rf 0.35 (CHCl3–MeOH, 95:5); 1H NMR (DMSO-d6) δ 0.06–0.23 (m, 2H, cyclopropyl CH2), 0.43–0.57 (2m, 2H, cyclopropyl CH2), 0.83–0.99 (m, 1H, cyclopropyl CH), 1.43–1.53 (m, 1H, C-15 H), 2.12–2.76 (m, 7H, C-16 H2, C-8 H, C-10 H, NCH2-cyclopropyl, C-15 H), 2.99 (m, 2H, C-8 H, C-10 H), 3.70 (d, 1H, J = 5.61 Hz, C-9 H), 4.05–4.39 (m, 2H, OCH2), 5.41 (s, 1H, C-5 H), 6.05–6.35 (m, 2H, CH=CH), 6.50 (s, 2H, C-2 H, C-1 H), 6.88–7.18 (m, 5H, C6H5), 7.45–7.78 (m, 5H, C-5″ H, C-3″ H, C-4′ H, C-2″ H, C-6″ H), 8.8 (d, 1H, J = 2.2 Hz, C-6′ H), 9.06 (s, 1H, C-3 OH). ESI MS m/z 603 (MH)+. Anal. (C38H35ClN2O3·0.5H2O) C, H, N.
5′-(4-Chlorophenyl)-17-(cyclopropylmethyl)-6,7-didehydro-4,5α-epoxy-3-hydroxy-14-(3-phenylpropoxy)pyrido[2′,3′:6,7]morphinan (17d)
Step 1
To a stirred solution of 6 (1.948 g, 4.0 mmol) in DMF (40 mL) was added sodium hydride (0.96 g, 24 mmol, 60% dispersion in mineral oil, washed with hexanes) at 0–5 °C. After allowing the mixture to stir for 10 minutes, 3-phenylpropyl bromide (1.752 g, 8.8 mmol) was added dropwise. The reaction mixture was allowed to come to room temperature and stirred for 2 days. Excess of sodium hydride was decomposed with drops of ice-cold water, the mixture was then diluted with water and extracted with CHCl3 (2 × 100 mL). The organic extracts were washed with water and brine, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The residue was purified by chromatography over a column of silica gel using EtOAc–hexane 20:80 as the eluent to obtain 5′-(4-Chlorophenyl)-17-(cyclopropylmethyl)-6,7-didehydro-4,5α-epoxy-3,14-bis(3-phenylpropoxy)pyrido[2′,3′:6,7]morphinan (19d). Yield 0.96 g (34%). ESI MS m/z 723 (MH)+.
Step 2
A solution of 19d (0.92 g, 1.27 mmol) in anhydrous CH2Cl2 (25 mL) was cooled to −78 °C. Boron tribromide (3.18 g, 12.7 mmol) was added dropwise and the mixture was stirred for 1 h. The mixture was then allowed to come to room temperature and the reaction was quenched by addition of drops of ice-cold water. The mixture was diluted with water and extracted with CHCl3. The organic layer was dried over anhydrous sodium sulfate, concentrated under reduced pressure and the residue was purified by chromatography over a column of silica using EtOAc–hexane 1:1 to obtain 0.23 g (28%) of the desired product (17d): mp 118–120 °C; TLC Rf 0.36 (CHCl3–MeOH, 95:5); 1H NMR (DMSO-d6) δ 0.06–0.23 (m, 2H, cyclopropyl CH2), 0.43–0.57 (2m, 2H, cyclopropyl CH2), 0.83–0.99 (m, 1H, cyclopropyl CH), 1.43–1.53 (m, 1H, C-15 H), 2.12–2.76 (m, 7H, C-16 H2, C-8 H, C-10 H, NCH2, C-15 H), 2.99 (m, 2H, C-8 H, C-10 H), 3.70 (d, 1H, J = 5.61 Hz, C-9 H), 4.05–4.39 (m, 2H, OCH2), 5.41 (s, 1H, C-5 H), 6.05–6.35 (m, 4H, CH2CH2), 6.50 (s, 2H, C-2 H, C-1 H), 6.88–7.18 (m, 5H, C6H5) 7.45–7.78 (m, 5H, C-5″ H, C-3″ H, C-4′ H, C-2″ H, C-6″ H), 8.80 (d, 1H, J = 2.2 Hz, C-6′ H), 9.06 (s, 1H, C-3 OH). ESI MS m/z 605 (MH)+. Anal. (C38H37ClN2O3) C, H, N.
5′-(4-Chlorophenyl)-6,7-didehydro-4,5α-epoxy-3-hydroxy-14-methoxy-17-methylpyrido[2′,3′:6,7]morphinan (17e)
Step 1
A stirred solution of 20 (0.69 g, 1.5 mmol) in DMF (15 mL) was cooled to 0–5 °C and sodium hydride (0.21 g, 45.25 mmol, 60% dispersion in mineral oil, washed with hexanes) was added. The mixture was stirred at 0 °C for 10 minutes and then treated dropwise with dimethyl sulfate (0.277 g, 1.8 mmol). The mixture was stirred at room temperature overnight and excess sodium hydride was destroyed by addition of ice-cold water. The mixture was diluted with water and the product was extracted with CHCl3 (2 × 25 mL). The organic extracts were washed with water and brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was purified over a column of silica using CHCl3–MeOH–NH4OH 97:2.5:0.5 as the eluent to obtain 0.29 g (41%) of the 3,14-dimethoxy compound 19e: mp 290–294 °C (dec); TLC Rf 0.35 (CHCl3–MeOH, 95:5); 1H NMR (DMSO-d6) δ 1.42–1.46 (m, 1H, C-15 H), 2.16–2.63 (m, 5H, C-8 H2, C-10 H, C-15 H, C-16 H), 2.33 (s, 3H, NCH3), 3.01 (d, 1H, J = 17.12 Hz, C-16 H), 3.11 (s, 3H, C-14 OCH3), 3.23–3.42 (m, 2H, C-10 H, C-9 H), 3.67 (s, 3H, C-3 OCH3), 5.36 (s, 1H, C-5 H), 6.65 (d, 1H, J = 8.2 Hz, C-2 H), 6.70 (d, 1H, J = 8.2 Hz, C-1 H), 7.52–7.57 (m, 2H, C-2′ H, C-6″ H), 7.71–7.74 (m, 2H, C-3′ H, C-5″ H), 7.77 (d, 1H, J = 2.1 Hz, C-4′ H), 8.78 (d, 1H, J = 2.1 Hz, C-6′ H). ESI MS m/z 475 (MH)+.
Step 2
The dimethoxy compound 19e (0.360 g, 0.76 mmol) was dissolved in anhydrous CH2Cl2 (15 mL), cooled to −78 °C, and treated dropwise with boron tribromide (1M solution in CH2Cl2, 1.5 mL, 1.5 mmol). After maintaining the mixture at −78 °C for 1 h, it was allowed to warm to room temperature. The reaction was quenched by addition of ice cold water. The crude product was extracted with CHCl3, dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure and the crude product was purified by column chromatography over silica using CHCl3–MeOH–NH4OH 97.5:2:0.5 to obtain 0.12 g (35%) of 17e: mp 296–299 °C (dec.); TLC Rf 0.23 (CHCl3–MeOH, 95:5); 1H NMR (DMSO-d6) δ 1.42–1.46 (m, 1H, C-15 H), 1.70–2.63 (m, 5H, C-8 H2, C-10 H, C-15 H, C-16 H), 2.35 (s, 3H, NCH3), 3.00 (d, 1H, J = 17.0 Hz, C-16 H), 3.10 (s, 3H, C-14 OCH3), 3.14–3.50 (m, 2H, C-10 H, C-9 H), 5.32 (s, 1H, C-5 H), 6.49–6.56 (m, 2H, C-2 H, C-1 H), 7.52–7.57 (m, 2H, C-2″ H, C-6″ H), 7.71–7.74 (m, 2H, C-3″ H, C-5″ H), 7.77 (m, 1H, C-4′ H), 8.78 (d, 1H, J = 1.98 Hz, C-6′ H), 9.05 (s, 1H, C-3 OH). ESI MS m/z 461 (MH)+. Anal. (C27H25ClN2O3·0.25H2O) C, H, N.
14-Benzyloxy-5′-(4-chlorophenyl)-6,7-didehydro-4,5α-epoxy-3-hydroxy-17-methylpyrido[2′,3′:6,7]morphinan (17f)
Step 1
To a stirred solution of 9 in DMF (10 mL) at 0 °C was added sodium hydride (0.288 g, 6.0 mmol, 60% dispersion in mineral oil, washed with hexane). After stirring at 0 °C for 20 min, benzyl bromide (0.425 g 6.0 mmol) was added and the mixture was stirred at room temperature overnight. The reaction mixture was cautiously treated with ice-cold water, diluted with water, and extracted with CHCl3. The organic extracts were dried over anhydrous sodium sulfate, concentrated, and the residue obtained after removal of the solvent was purified over a column of silica using CHCl3–MeOH 99:1 to obtain 0.57 g (46%) of 3,14-Bis(benzyloxy)-5′-(4-chlorophenyl)-6,7-didehydro-4,5α-epoxy-17-methylpyrido[2′,3′:6,7]morphinan (19f).
Step 2
A solution of 19f (0.54 g, 1.0 mmol) in anhydrous CH2Cl2 (10 mL) was cooled to −78 °C and treated dropwise with boron tribromide (3.0 mL, 1 M solution in CH2Cl2, 3.0 mmol). After allowing the mixture to stir at −78 °C for 1 h, it was allowed to warm to room temperature. The mixture was quenched by addition of water (10 mL), extracted with CHCl3 (2 × 50 mL), dried and concentrated. The crude product thus obtained was purified by chromatography over a column of silica using CHCl3–MeOH 98:2 as the eluent. The product was crystallized from ethyl acetate to yield 0.12 g (23%) of the desired product (17f): mp 228–232 °C; TLC Rf 0.5 (CHCl3–MeOH, 92.5:7.5); 1H NMR (CDCl3), δ 1.49–1.52 (m, 1H, C-15 H), 2.21–2.28 (m, 1H, C-15 H), 2.37 (s, 3H, NCH3), 2.45–2.61 (m, 4H, C-16 H2, C-10 H, C-8 H), 3.12 (d, 1H, J = 17.1 Hz, C-10 H), 3.25 (s, 1H, C8 H), 3.52 (d, 1H, J = 5.5 Hz, C-9 H), 4.37 (dd, 2H, J = 11.5 and 11.4 Hz OCH2), 5.32 (s, 1H, C-5 H), 6.52–6.57 (m, 2H, C-1 H, C-2 H), 7.07–7.18 (m, 5H, C6H5), 7.53–7.59 (m, 2H, C-3″ H, 5″ H), 7.69–7.73 (m, 3H, C-4′ H, C-2″ H, 6″ H), 8.79 (d, 1H, J = 1.95 Hz, C-6′ H), 9.05 (s, 1H, C-3 OH). ESI MS m/z 537 (MH)+. Anal. (C33H29ClN2O3·0.25·H2O) C, H, N.
5′-(4-Chlorophenyl)-14-cinnamyloxy-6,7-didehydro-4,5α-epoxy-3-hydroxy-17-methylpyrido[2′,3′:6,7]morphinan (17g)
Step 1
Compound 20 (0.460 g, 1.0 mmol) was reacted with sodium hydride (0.144 g, 6.0 mmol, 60% dispersion in mineral oil) and cinnamyl bromide (0.202 g, 1.1 mmol) in DMF (15 mL) as described in step 1 for 17e to obtain 0.18 g (31%) of 5′-(4-Chlorophenyl)-14-cinnamyloxy-6,7-didehydro-4,5α-epoxy-3-methoxy-17-methylpyrido[2′,3′:6,7]morphinan (19g): mp 210–212°C; TLC Rf 0.46 (CHCl3–MeOH, 95:5); 1H NMR (DMSO-d6) δ 1.46–1.51 (m, 1H, C-15 H), 2.15–2.63 (2m, 5H, C-15 H, C-8 H, C-10 H, C-16 H2), 2.36 (s, 3H, NCH3), 3.05 (d, 1H, J = 17.1 Hz, C-8 H), 3.25–3.28 (m, 1H, C-10 H), 3.45–3.49 (m, 1H, C-9 H), 3.68 (s, 3H, C-3 OCH3), 4.07–4.25 (m, 2H, CH2CH=), 5.45 (s, 1H, C-5 H), 6.08–6.30 (m, 2H, -CH=CH-), 6.67 (d, 1H, J = 8.5 Hz, C-2 H), 6.71 (d, 1H, J = 8.2 Hz, C-1 H), 7.12–7.25 (m, 5H, phenyl), 7.50 (dd, 2H, J = 6.7 and 6.7 Hz, C-3″ H, C-5″ H), 7.60 (m, 2H, C-2″ H, C-6″ H), 7.71 (d, 1H, J = 2.0 Hz, C- 4′ H), 8.75 (s, 1H, C-6′ H). ESI MS m/z 577 (MH)+.
Step 2
Compound 19g (0.288 g, 0.5 mmol) was O-demethylated using boron tribromide and the product obtained after column chromatography using EtOAc–hexane (75:25) was crystallized from EtOAc to yield 0.102 g (57%) of the desired product 17g: mp 148–150 °C; TLC Rf 0.33 (CHCl3–MeOH, 90:10); 1H NMR (DMSO-d6) δ 1.46–1.51 (m, 1H, C-15 H), 2.20–2.66 (2m, 5H, C-15 H, C-8 H, C-10 H, C-16 H2), 2.36 (s, 3H, NCH3), 3.05 (d, 1H, J = 17.1 Hz, C-8 H), 3.22–3.28 (m, 1H, C-10 H), 3.43–3.49 (m, 1H, C-9 H) 4.07–4.25 (m, 2H, CH2CH=), 5.41 (s, 1H, C-5 H), 6.08–6.29 (m, 2H, CH=CH), 6.50–6.57 (m, 2H, C-2 H, C-1 H), 7.10–7.25 (m, 5H, C6H5), 7.46–7.52 (m, 2H, C-3″ H, C-5″ H), 7.62–7.68 (m, 2H, C-2″ H, C-6″ H), 7.71 (d, 1H, J = 2.0 Hz, C-4′ H), 8.78 (s, 1H, C-6′ H), 9.07 (s, 1H, C-3 OH). ESI MS m/z 563 (MH)+. Anal. (C35H31ClN2O3·0.75H2O) C, H, N.
5′-(4-Chlorophenyl)-6,7-didehydro-4,5α-epoxy-3-hydroxy-17-methyl-14-(3-phenylpropoxy)pyrido[2′,3′:6,7]morphinan (17h)
Step 1
Compound 20 (0.96 g, 2.0 mmol) was reacted with sodium hydride (0.320 g, 4.0 mmol, 60% dispersion in mineral oil) and 3-phenylpropyl bromide (0.46 g, 2.6 mmol) in DMF (20 mL) as described in step 1 for 17e to obtain 0.28 g (24%) of 5′-(4-Chlorophenyl)-14-cinnamyloxy-6,7-didehydro-4,5α-epoxy-3-methoxy-17-methyl-14-(3-phenylpropoxy)pyrido[2′,3′:6,7]morphinan (19h). ESI MS m/z 579 (MH)+.
Step 2
Compound 19h (0.15 g, 0.26 mmol) was O-demethylated using boron tribromide to obtain 0.09 g (62%) of the desired product (17h): mp 128–130 °C; TLC Rf 0.37 (CHCl3–MeOH, 95:5); 1H NMR (DMSO-d6) δ 1.39–1.53 (m, 1H, C-15 H), 1.52–1.66 (m, 2H, CH2CH2Ph), 2.09–2.66 (m, 7H, CH2Ph, C-16 H2, C-8 H, C-10 H, C-15 H), 2.49 (s, 3H, NCH3), 2.95 (d, 1H, J = 16.81 Hz, C-8 H), 3.15–3.72 (m, 4H, OCH2, C-10 H, C-9 H), 5.38 (s, 1H, C-5 H), 6.50 (s, 2H, C-2 H, C-1 H), 6.88–7.18 (m, 5H, C6H5), 7.48–7.58 (m, 2H, C-5″ H, C-3″ H), 7.65–7.75 (m, 3H, C-4′ H, C-2″ H, C-6″ H), 8.8 (d, 1H, J = 2.09 Hz, C-6′ H), 9.04 (s, 1H, C-3 OH). ESI MS m/z 565 (MH)+. Anal. (C35H33ClN2O3·0.25H2O) C, H, N.
14-Benzoyloxy-5′-(4-chlorophenyl)-17-(cyclopropylmethyl)-6,7-didehydro-4,5α-epoxy-3-hydroxypyrido[2′,3′:6,7]morphinan (18a)
To a solution of 6 (0.486 g, 1.0 mmol) in anhydrous DMF (10 mL), was added benzoyl chloride (0.421 g, 3.0 mmol) and triethylamine (0.42 mL). The reaction mixture was heated at 100 °C for 5 h under argon. The mixture was concentrated under reduced pressure, diluted with water, and extracted with CHCl3. The organic extracts were dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness. The residue obtained was dissolved in methanol (24 mL) and treated with saturated aqueous K2CO3 to adjust the pH of the mixture to 9–10. The basic solution was stirred at room temperature for 3.5 hours. The mixture was then concentrated under reduced pressure, diluted with water, and extracted with CHCl3. Workup of the extract and purification of the crude product on a column of silica using CHCl3–MeOH 98:2 yielded 0.192 g (32%) of the desired product 18a: mp 158–162 °C; TLC Rf 0.56 (CHCl3–MeOH, 95:5); 1H NMR (DMSO-d6) δ 0.07–0.17 and 0.32–0.35 (m, 4H, cyclopropyl CH2CH2), 0.58–0.62 (m, 1H, cyclopropyl CH), 1.67–1.71 (m, 1H, C-15 H), 2.17–2.45 (m, 3H, NCH2-cyclopropyl, C-15 H), 2.63–2.82 (m, 4H, C-16 H2, C-10 H, C-8 H), 3.15 (d, 1H, J = 18.5 Hz, C-10 H), 3.54 (d, 1H, J = 17.9 Hz, C-8 H), 4.68 (d, 1H, J = 6.0 Hz, C-9 H), 5.71 (s, 1H, C-5 H), 6.58 (s, 2H, C-1 H, C-2 H), 7.43–7.89 (m, 10H, C6H5, C-3″ H, C-5″ H, C-4′ H, C-2″ H, C-6″ H), 8.82 (d, 1H, J = 2.1 Hz, C-6′ H), 9.15 (s, 1H, C-3 OH). ESI MS m/z 591 (MH)+. Anal. (C36H31ClN2O4·0.5H2O) C, H, N.
5′-(4-Chlorophenyl)-17-(cyclopropylmethyl)-6,7-didehydro-4,5α-epoxy-3-hydroxy-14-(phenylacetoxy)pyrido[2′,3′:6,7]morphinan (18b)
This compound was prepared using the method described above for the preparation of 18a using toluene as the solvent instead of DMF. The reaction of 6 (0.486 g, 1.0 mmol), phenylacetyl chloride (0.50 g, 3.0 mmol) and triethylamine (0.6 mL) in toluene (10 mL) followed by basic workup of the reaction mixture and purification over a column of silica using EtOAc–hexane 60:40 yielded 0.101 g (17%) of the desired product 18b: mp 118–120 °C; TLC Rf 0.46 (CHCl3–MeOH, 92.5:7.5); 1H NMR (DMSO-d6) δ 0.33–0.47 (m, 4H, cyclopropyl CH2CH2), 0.64–88 (m, 1H, cyclopropyl CH), 1.51 (d, 1H, J = 10.3 Hz, C-15 H), 2.08–2.72 (m, 7H, C-16 H2, C-15 H, C-10 H, C-8 H, NCH2), 3.05 (d, 1H, J = 18.8 Hz, C-10 H), 3.55–3.70 (m, 3H, CH2CO, C-8 H), 4.51 (d, 1H, J = 6.0 Hz, C-9 H), 5.34 (s, 1H, C-5 H), 6.53 (s, 2H, C-1 H, C-2 H), 6.92–7.06 (m, 5H, C6H5), 7.53 (m, 2H, C-3″ H, C-5″ H), 7.62 (d, 1H, J = 2.1 Hz, C-4′ H), 7.62–7.72 (m, 2H, C-2″ H, C-6″ H), 8.81 (d, 1H, J = 2.0 Hz, C-6′ H), 9.14 (s, C-3 OH). ESI MS m/z 605 (MH)+. Anal. (C37H33ClN2O4·H2O) C, H, N.
5′-(4-Chlorophenyl)-17-(cyclopropylmethyl)-6,7-didehydro-4,5α-epoxy-3-hydroxy-14-(3-phenylpropionyloxy)pyrido[2′,3′:6,7]morphinan (18c)
This compound was prepared by using the method similar to that employed for the preparation of 18a. Yield 19%. mp 96–98 °C; TLC Rf 0.47 (CHCl3–MeOH, 95:5); 1H NMR (DMSO-d6) δ 0.08–0.11 and 0.39–0.48 (m, 4H, cyclopropyl CH2CH2), 0.67–71 (m, 1H, cyclopropyl CH), 1.57 (d, 1H, J = 10.7 Hz, C-15 H), 2.15–2.25 (m, 2H, CH2CH2Ph), 2.37–2.80 (m, 9H, C-16 H2, C-15 H, C-10 H, C-8 H, NCH2, CH2CH2Ph), 3.08 (d, 1H, J = 18.9 Hz, C-10 H), 3.54 (d, 1H, J = 17.6 Hz, C-8 H), 4.48 (d, 1H, J = 5.9 Hz, C-9 H), 5.46 (s, 1H, C-5 H), 6.55 (s, 2H, C-1 H, C-2 H), 6.98–7.16 (m, 5H, C6H5), 7.53–7.57 (m, 2H, C-3″ H, 5″ H), 7.71–7.75 (m, 3H, C-4′ H, C-2″ H, C-6″ H), 8.86 (d, 1H, J = 2.0 Hz, C-6′ H), 9.11 (s, 1H, C-3 OH). ESI MS m/z 618 (MH)+. Anal. (C38H35ClN2O4·0.5H2O) C, H, N.
14-Benzoyloxy-5′-(4-chlorophenyl)-6,7-didehydro-4,5α-epoxy-3-hydroxy-17-methylpyrido[2′,3′:6,7]morphinan (18d)
This compound was prepared by reacting 9 (0.446 g, 1.0 mmol) with benzoyl chloride (0.425 g, 3.0 mmol) in the presence of triethylamine (0.62 mL, 5.0 mmol) in toluene (7 mL). Basic workup and purification of the reaction mixture yielded 0.068 g (12%) of the desired product: mp 280–284 °C; TLC Rf 0.39 (CHCl3–MeOH, 95:5); 1H NMR (CDCl3) δ 1.68 (d, 1H, J = 10.3 Hz, C-15 H), 2.25 (s, 3H, NCH3), 2.90 (d, 1H, J = 3.3 Hz, C-15 H), 2.60–2.83 (m, 4H, C-16 H2, C-10 H, C-8 H), 3.26 (s, 1H, C-10 H), 3.71 (d, 1H, J = 17.4 Hz, C-8 H), 4.36 (d, 1H, J = 6.1 Hz, C-9 H), 5.72 (s, 1H, C-5 H), 6.59–6.64 (m, 2H, C-1 H, C-2 H), 7.40–7.52 (m, 4H, 3″ H, 5″ H, m-protons of Bz), 7.54–7.62 (m, 1H, p-proton of Bz), 7.56–7.75 (m, 2H, C-2″ H, C-6″ H), 7.79 (d, 1H, J = 1.94 Hz, C-4′ H), 7.84–7.89 (m, 2H, o-protons of Bz), 8.81 (d, 1H, J = 2.1 Hz, C-6′ H), 9.16 (s, 1H, C-3 OH). ESI MS m/z 551 (MH)+. Anal. (C33H27ClN2O4) C, H, N.
5′-(4-Chlorophenyl)-6,7-didehydro-4,5α-epoxy-3-hydroxy-17-methyl-14-(phenylacetoxy)pyrido[2′,3′:6,7]morphinan (18e)
Prepared as described above for 18d using phenylacetyl chloride as the reagent. Yield 16%. mp 194–196 °C; TLC Rf 0.68 (CHCl3–MeOH, 92.5:7.5); 1H NMR (CDCl3), δ 1.58 (d, 1H, J = 10.3 Hz, C-15 H), 2.25 (s, 3H, NCH3) 1.20–2.83 (m, 5H, C-16 H2, C-15 H, C-10 H, C-8 H), 3.26 (s, 1H, C-10 H), 3.17 (d, 1H, J = 18.5 Hz, C8 H), 3.42–3.71 (m, 3H, CH2Ph, C-8 H), 4.15 (d, 1H, J = 6.0 Hz, C-9 H), 5.32 (s, 1H, C-5 H), 6.53 (s, 2H, C-1 H, C-2 H), 6.93–7.06 (m, 5H, C6H5), 7.53 (d, 2H, J = 2.0 Hz, C-3″ H, C-5″ H), 7.61 (d, 1H, J = 1.94 Hz, C-4′ H), 7.68 (d, 2H, J = 6.6 Hz, C-2″ H, C-6″ H), 8.81 (d, 1H, J = 2.0 Hz, C-6′ H). ESI MS m/z 565 (MH)+. Anal. (C34H29ClN2O4·0.25H2O) C, H, N.
5′-(4-Chlorophenyl)-6,7-didehydro-4,5α-epoxy-3-hydroxy-17-methyl-14-(3-phenylpropionyloxy)pyrido[2′,3′:6,7]morphinan (18f)
Prepared as described above for 18d using phenylpropionyl chloride as the reagent. Yield (22%): mp 242–244 °C; TLC Rf 0.63 (CHCl3–MeOH, 92.5:7.5); 1H NMR (CDCl3), δ 1.55–1.59 (m, 1H, C-15 H), 2.15–2.22 (m, 1H, C-15 H), 2.25 (s, 3H, NCH3), 2.38–2.76 (m, 8H, C-16 H2, C-15 H, C-8 H, H2CH2Ph), 3.18 (d, 1H, J = 19.8 Hz, C-10 H), 3.50 (d, 1H, J = 18.5 Hz, C-8 H), 4.17 (d, 1H, J = 6.0 Hz, C-9 H), 5.45 (s, 1H, C-5 H), 6.53 (s, 2H, C-1 H, C-2 H), 6.98–7.10 (m, 5H, C6H5), 7.54–7.57 (m, 2H, C-3″ H, C-5″ H), 7.71–7.76 (m, 3H, C-4′ H, C-2″ H, C-6″ H), 8.85 (d, 1H, J = 2.1 Hz, C-6′ H), 9.11 (s, 1H, C-3 OH). ESI MS m/z 579 (MH)+. Anal. (C35H31ClN2O4) C, H, N.
3,14-Dibenzoyloxy-5′-(4-chlorophenyl)-17-(cyclopropylmethyl)-6,7-didehydro-4,5α-epoxy-3-hydroxypyrido[2′,3′:6,7]morphinan (21) and 3-Benzoyloxy-5′-(4-chlorophenyl)-17-(cyclopropylmethyl)-6,7,8,14-tetradehydro-4,5α-epoxypyrido[2′,3′:6,7]morphinan (22)
To a solution of 6 (0.972 g, 2.0 mmol) in anhydrous DMF (15 mL), and benzoyl chloride (1.2 g, 6.0 mmol) was added triethylamine (1.67 mL, 12.0 mmol). The reaction mixture was heated at 100 °C for 5 h under argon. The mixture was cooled, diluted with H2O (150 mL) and the product was extracted with CHCl3. The organic extracts were washed with water and brine and dried over anhydrous sodium sulfate. The residue obtained after the removal of the solvent under reduced pressure was chromatographed over a column of silica using EtOAc–hexane 60:40 as the eluent. Collection of fractions containing the faster moving component and workup gave 21: Yield 0.67 g (48%). TLC Rf 0.74 (CHCl3–MeOH, 97.5:2.5); 1H NMR (DMSO-d6) δ 0.02–0.08 and 0.34–0.40 (m, 4H, cyclopropyl CH2CH2), 0.55–0.67 (m, 1H, cyclopropyl CH), 1.74–1.85 (m, 1H, C-15 H), 2.21–2.48 (m, 1H, C-15 H), 2.22–2.48 (m, 2H, N-CH2-cyclopropylmethyl), 2.72–2.96 (m, 4H, C-16 H2, C-10 H, C-8 H), 3.28–3.40 (s, 1H, C-8 H), 3.84 (d, 1H, J = 17.6 Hz, C-10 H), 4.78 (d, 1H, J = 6.0 Hz, C-9 H), 5.89 (s, 1H, C-5 H), 6.87 (d, 1H, J = 8.2 Hz, C-1 H), 7.02 (d, 1H, J = 6.2 Hz, C-2 H), 7.42–7.57 (m, 4H, m-protons of 3-Bz, C-3″ H, C-5″ H), 7.52–7.66 (m, 3H, m- and p-protons of 14-Bz), 7.72–7.82 (m, 3H, p-protons of 14-Bz, C-2″ H, C-6″ H), 7.86 (d, 1H, J = 1.7 Hz C-4′ H), 7.90–7.94 (m, 2H, o-protons of 14-Bz), 8.09–8.20 (m, 2H, o-protons of C-3 Bz), 8.81 (m, 1H, C-6′ H). ESI MS m/z 695 (MH)+ Anal. (C43H35ClN2O5·0.25H2O) C, H, N. Elution and workup of the slower moving component gave 22: Yield 0.48 g (35%). mp 132–134 °C; TLC Rf 0.58 (CHCl3–MeOH, 95:5); 1H NMR (DMSO-d6) δ 0.15–0.19 and 0.50–0.54 (m, 4H, cyclopropyl CH2CH2), 0.85–0.91 (m, 1H, cyclopropyl CH), δ 1.77 (d, J = 11.7 Hz, 1H, C-15 H), 2.30–2.37 (m, 1H, C-15 H), 2.50–2.58 (m, 2H, NCH2-cyclopropyl), 2.76–2.94 (m, 3H, C-16 H2, C-10 H), 3.46 (s, 1H, C-10 H), 4.41 (d, 1H, J = 7.0 Hz, C-9 H), 5.88 (s, 1H, C-5 H), 6.31 (s, 1H, C-8 H), 6.73 (d, 1H, J = 8.3 Hz, C-2 H), 6.95 (d, 1H, J = 8.2 Hz, C-1 H), 7.55–7.78 (m, 7H, m- and p-protons of C-3 Bz and C-3″ H, C-5″ H, C-2″ H, C-6″ H), 7.85 (d, 1H, J = 2.2 Hz, C-4′ H), 8.10–8.11 (m, 2H, o-protons of C3-Bz), 8.72 (d, 1H, J = 2.1 Hz, C-6′ H). ESI MS m/z 573 (MH)+. Anal. (C36H29ClN2O3·0.75H2O) C, H, N.
5′-(4-Chlorophenyl)-17-(cyclopropylmethyl)-3-hydroxy-6,7,8,14-tetradehydro-4,5α-epoxypyrido[2′,3′:6,7]morphinan (23)
A solution of 22 (0.42 g, 0.73 mmol) was dissolved in MeOH (14 mL) and saturated aqueous K2CO3 was added dropwise to adjust the pH of the solution to 9–10. The mixture was stirred at room temperature for 3.5 h, diluted with H2O, and extracted with CHCl3. The organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residue obtained was purified by chromatography over a column of silica using CHCl3–MeOH 98:2 as the eluent to obtain 0.25 g (74 %) of 23: mp 164–166 °C; TLC Rf 0.38 (CHCl3–MeOH, 95:5); 1H NMR (DMSO-d6) δ 0.01–0.17 and 0.47–0.54 (m, 4H, cyclopropyl CH2CH2), 0.82–0.89 (m, 1H, cyclopropyl CH), 1.70 (d, 1H, J = 12.1 Hz, C-15 H), 2.23–2.25 (m, 1H, C-15 H), 2.45–2.47 (m, 2H, C-8 H2), 2.70–2.89 (m, 3H, C-16 H2, C-10 H), 3.16–3.21 (d, 1H, J = 18.0 Hz, C-10 H), 4.04–4.10 (m, 1H, C-9 H), 5.74 (s, 1H, C-5 H), 6.20 (s, 1H, C-8 H), 6.45–6.52 (m, 2H, C-1 H, C-2 H), 7.55–7.57 (m, 2H, C-3″ H, C-5″ H), 7.71–7.75 (m, 3H, C-4′ H, C-2″ H, C-6″ H), 8.76 (d, 1H, J = 2.2 Hz, C-6′ H), 9.13 (s, 1H, C-3 OH). ESI MS m/z 469 (MH)+. Anal. (C29H25ClN2O2·0.75H2O) C, H, N.
Opioid Binding Assays
As described earlier,38 the recombinant CHO cells (hMOR-CHO, hDOR-CHO and hKOR-CHO) were produced by stable transfection with the respective human opioid receptor cDNA, and provided by Dr. Larry Toll (SRI International, CA). The cells were grown on plastic flasks in DMEM (90%) (hDOR-CHO and hKOR-CHO) or DMEM/ F-12 (45%/ 45%) medium (hMOR-CHO) containing 10% FetalClone II (HyClone) and Geneticin (G-418: 0.10–0.2 mg/ml) (Invitrogen) under 95% air/5% CO2 at 37 °C. Cell monolayers were harvested and frozen at -80 °C. The hKOR-CHO, hMOR-CHO and hDOR-CHO cells were used for opioid binding experiments. For the [35S]GTP-γ-S binding experiments, hKOR-CHO and hMOR-CHO cells were used for assaying KOR and MOR receptor function. Excellent signal-to-noise ratio were obtained using the NG108-15 neuroblastoma×glioma cell for the DOR [35S]GTP-γ-S binding assay. Thus, hDOR-CHO cells were used for DOR binding assays, and the NG108-15 cells were used for the DOR [35S]GTP-γ-S binding assay.
Radioligands [3H][d-Ala2-MePhe4,Gly-ol5]enkephalin ([3H]DAMGO, SA = 44–48 Ci/mmol), [3H][d-Ala2, d-Leu5]enkephalin ([3H]DADLE, SA = 40–50 Ci/mmol) and and [3H](-)-U69,593 (SA = 50 Ci/mmol) were used to label MOR, DOR and KOR binding sites, respectively. On the day of the assay, cell pellets were thawed on ice for 15 minutes then homogenized with a polytron in 10 mL/pellet of ice-cold 10 mM Tris-HCl, pH 7.4. Membranes were then centrifuged at 30,000 × g for 10 minutes, resuspended in 10 mL/pellet ice-cold 10mM Tris-HCl, pH 7.4 and again centrifuged 30,000 × g for 10 min. Membranes were then resuspended in 25 °C 50 mM Tris-HCl, pH 7.4 (~100 mL/pellet hMOR-CHO, 50 mL/pellet hDOR-CHO and 120 mL/pellet hKOR-CHO). All assays took place in 50 mM Tris-HCl, pH 7.4, with a protease inhibitor cocktail [bacitracin (100 µg/mL), bestatin (10 µg/mL), leupeptin (4 µg/mL) and chymostatin (2 µg/mL)], in a final assay volume of 1.0 mL. All drug dilution curves were made up with buffer containing 1 mg/mL BSA. Nonspecific binding was determined using 20 µM levallorphan ([3H]DAMGO and [3H]DADLE) and 1 µM (-)-U69,593 (for [3H]U69,593 binding). [3H]Radioligands were used at ~ 2 nM concentrations. Triplicate samples were filtered with Brandel Cell Harvesters (Biomedical Research & Development Inc., Gaithersburg, MD), over Whatman GF/B filters, after a 2 hr incubation at 25 °C. The filters were punched into 24-well plates to which was added 0.6 mL of LSC-cocktail (Cytoscint). Samples were counted, after an overnight extraction, in a Trilux liquid scintillation counter at 44% efficiency. Opioid binding assays had ~30 µg protein per assay tube. Inhibition curves were generated by displacing a single concentration of radioligand by 10 concentrations of drug.
[35S]GTP-γ-S Binding Assays
The [35S]GTP-γ-S assays were conducted as described elsewhere.38 In this description, buffer “A” is 50 mM Tris-HCl, pH 7.4, containing 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA and buffer “B” is buffer A plus 1.67 mM DTT and 0.15% BSA. On the day of the assay, cells were thawed on ice for 15 min and homogenized using a polytron in 50 mM Tris-HCl, pH 7.4, containing 4 µg/mL leupeptin, 2 µg/mL chymostatin, 10 µg/mL bestatin and 100 µg/mL bacitracin. The homogenate was centrifuged at 30,000 × g for 10 min at 4 °C, and the supernatant discarded. The membrane pellets were resuspended in buffer B and used for [35S]GTP-γ-S binding assays. [35S]GTP-γ-S binding was determined as described previously. Briefly, test tubes received the following additions: 50 µL buffer A plus 0.1% BSA, 50 µL GDP in buffer A/0.1% BSA (final concentration = 40 µM), 50 µL drug in buffer A/0.1% BSA, 50 µL [35S]GTP-γ-S in buffer A/0.1% BSA (final concentration = 50 pM), and 300 µL of cell membranes (50 µg of protein) in buffer B. The final concentrations of reagents in the [35S]GTP-γ-S binding assays were: 50 mM Tris-HCl, pH 7.4, containing 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA, 1 mM DTT, 40 µM GDP and 0.1% BSA. Incubations proceeded for 3 h at 25 °C. Nonspecific binding was determined using GTP-γ-S (40 µM). Bound and free [35S]GTP-γ-S were separated by vacuum filtration (Brandel) through GF/B filters. The filters were punched into 24-well plates to which was added 0.6 mL LSC-cocktail (Cytoscint). Samples were counted, after an overnight extraction, in a Trilux liquid scintillation counter at 27% efficiency.
Data Analysis and Statistics
These methods are described elsewhere.39,40 For opioid binding experiments, the pooled data of three experiments (typically 30 data points) are fit to the two-parameter logistic equation for the best-fit estimates of the IC50 and N values: Y=100/(1+([INHIBITOR]/IC50)N), where “Y” is the percent of control value. Ki values for test drugs are calculated according to the standard equation: Ki = IC50/(1+[radioligand]/Kd]). For the [3H]radioligands, the following Kd values (nM±SD, n=3) were used in the Ki calculation: [3H]DAMGO (0.93±0.04), [3H]DADLE (1.9±0.3) and [3H](-)-U69,593 (11±0.6). The corresponding Bmax values were (fmol/mg protein±SD, n=3): [3H]DAMGO (1912±68), [3H]DADLE (3655±391) and [3H](-)-U69,593 (3320±364).
For the [35S]GTP-γ-S binding experiments, the percent stimulation of [35S]GTP-γ-S binding was calculated according to the following formula: (S – B)/B × 100, where B is the basal level of [35S]GTP-γ-S binding and S is the stimulated level of [35S]GTP-γ-S binding. Agonist dose-response curves (ten points/curve) were generated, and the data of several experiments, 3 or more, were pooled. The EC50 values (the concentration that produces fifty percent maximal stimulation of [35S]GTP-γ-S binding) and Emax were determined using either the program MLAB-PC (Civilized Software, Bethesda, MD), KaleidaGraph (Version 3.6.4, Synergy Software, Reading, PA) or Prism 4.0 (GraphPad Software, Inc, San Diego, CA). In most cases, the percent stimulation of the test compound is reported as a percent of the maximal stimulation of 1000 nM DAMGO, 500 nM SNC80 or 500 nM (-)-U50,488 in the appropriate cell type. For determination of Ke values using the “shift” experimental design, agonist (DAMGO, (-)-U50,488 or SNC80) dose-response curves were generated, using the appropriate cell type, in the absence and presence (ten points/curve) of a test compound. The data of several experiments, 3 or more, were pooled, and the Ke values were calculated according to the equation: [Test Drug]/(EC50–2/EC50–2 – 1), where EC50–2 is the EC50 value in the presence of the test drug and EC50–1 is the value in the absence of the test drug.
Cell culture and cAMP assay
CHO cells co-expressing cloned MOR and DOR (cMyc-mδ-HµCHO cells) were produced by stable transfection with the mouse DOR N-cMyc tag and human MOR cDNA, and were originally provided by Dr. J.B. Wang (University of Maryland Baltimore Campus, Baltimore, MD). Cells were grown on plastic flasks in F-12 Nutrient Mixture (HAM, GIBCO) containing 10% fetal bovine serum, 100 units/mL penicillin, 100 µg/mL streptomycin, 400 µg/mL hygromycin B (for DOR selection), and 400 µg/mL geneticin (for MOR selection) under 95% air/5% CO2 at 37 °C. After 80% confluence, cell monolayers were plated in 24-well plates and grown in F-12 Nutrient Mixture (HAM, GIBCO) containing 10% fetal bovine serum, 100 units/mL penicillin, 100 µg/mL streptomycin, 400 µg/mL hygromycin B and 400 µg/mL geneticin under 95% air/5% CO2 at 37 °C. On the day of the experiment, cells (control or drug-treated) were washed three times with serum free medium, and incubated with serum free medium containing IBMX (500 µM). After a 20-min incubation at 37 °C, medium was removed and then cells incubated with fresh serum free medium containing IBMX (500 µM) and forskolin (100 µM) and appropriate agonist or antagonist for 15 min (assay for opioid inhibition of cAMP accumulation) or 10 min (assay for naloxone-induced cAMP overshoot) at 37 °C. The reaction was terminated by aspiration of the medium and the addition of 0.5 mL of 0.1 N HCl. After chilling plates at 4 °C for at least 1 h, 0.4 mL was removed, neutralized, vortexed and centrifuged at 13,000 rpm for 5 min, supernatants were used for cAMP assay. These assay monitored inhibition of [3H]cAMP binding to cAMP-dependent protein kinase. Assays took place in 50 mM Tris-HCl, pH 7.4, containing 100 mM NaCl and 5 mM EDTA. After a 2 h incubation at 4 °C (protected from light), bound and free [3H]cAMP were separated by vacuum filtration through Whatman GF/B filters with two 4 mL washes with ice-cold 10 mM Tris-HCl, pH 7.4. Filters were punched into wells of plate to which was added 0.6 mL LSC-cocktail (CytoScint) and counted in a liquid scintillation counter at 44% efficiency.
Data Analysis and Statistics
The amount of cAMP in the samples was quantitated from a cAMP standard curve ranging from 0.25 to 256 pmol of cAMP/assay. Forskolin (100 µM) stimulated cAMP formation in the absence of agonist was defined as 100%. The EC50 (the concentration of agonist that produces fifty percent inhibition of forskolin stimulated cAMP formation) and Emax (% of maximal inhibition of forskolin stimulated cAMP) were calculated using program Prism version 4 (GraphPad Software, San Diego, CA). Data from three experiments were analyzed using Prism. Results are presented as the mean ± S.E.M.
Sources. [3H]cAMP (adenosine 3′5′-cyclic phosphate, [2,8-3H] (SA = 43 Ci/mmol)) was purchased from PerkinElmer Life and Analytical Sciences, Inc. (Waltham, MA). Forskolin, 3′5′-cyclic AMP (cAMP), 3-isobutyl-1-methylxanthine (IBMX) and protein kinase, 3′5′-cyclic-AMP binding protein were obtained from Sigma Chemical Co. (St. Louis, MO). Other reagents were obtained from sources reported eariler.47
Antinociceptive Studies
Male ICR mice (Harlan) were used for all evaluations. Mice were housed in a temperature and humidity controlled vivarium on a 12:12 h light:dark cycle with unlimited access to food and water prior to the formal procedures. Graded doses of morphine or the test compounds were injected intracerebroventricularly (i.c.v.) under light ether anesthesia.46 Morphine sulfate was dissolved in distilled water and injected in a volume of 5 µL. All test compounds were evaluated as free bases. The test compounds were dissolved in 100% DMSO and injected in a volume of 5 µL. Antinociceptive assays were performed at various times after injection.
Warm-Water Tail-Withdrawal Assay
Naive mice were baselined in the 55 °C warm-water tail-withdrawal test as previously described.46,48 Doses of morphine or the test compound were injected i.c.v., and antinociception was assessed at 10, 20, 30, 45, 60, 80, 120 and 180 min postinjection. Percent antinociception was calculated using the formula: %MPE (maximal possible effect) = 100 × (test - control)/(cutoff - control) where control is the pre-drug observation, test is the post-drug observation, and cutoff is the maximal length of stimulus allowed (10 s for 55 °C tail-withdrawal). Antinociceptive A50 values and 95% confidence intervals were determined using linear regression software (FlashCalc). Opioid activity of the test compounds was assessed by pre-treating animals with naloxone (10 mg/kg ip, −10 min) followed by an i.c.v. injection of an approximate A90 dose of test compound. If a compound did not produce a full agonist effect, then the dose that produced the greatest antinociceptive effect was used. Antinociception was assessed in the 55 °C warm-water tail-withdrawal test at 10, 20 and 30 min. A positive response to a fixed dose of naloxone was indicated when greater than 80% reduction in the antinociceptive effect of the agonist was observed.
Tolerance Regimen
Mice were injected twice daily (8 a.m. and 8 p.m.) with an approximate A90 dose of morphine or A90 dose of 17d for 3 days. Antinociceptive dose–response curves in the warm-water tail-withdrawal assay were generated on the morning of the fourth day using the procedures outlined above.
ACKNOWLEDGMENTS
This investigation was supported by NIH grant R01DA008883 from the National Institute on Drug Abuse (NIDA). Portions of this work were supported by the Intramural Research Program of NIDA. We thank Dr. James M. Riordan, Mr. Mark D. Richardson, Ms. Joan C. Bearden, and Ms. Jackie W. Truss for analytical and spectral data. We are grateful to Dr. John A. Secrist III for his encouragement, valuable comments and suggestions during the course of this work.
ABBREVIATIONS USED
- CHO
Chinese hamster ovary
- CPM
cyclopropylmethyl
- DADLE
[d-Ala2,d-Leu5]enkephalin
- DAMGO
[d-Ala2,Me-Phe,Gly-ol5]enkephalin
- DOR
δ opioid receptor
- [35S]GTP-γ-S
guanosine-5′-O-(3-[35S]thio-triphosphate
- KOR
κ opioid receptor
- MOR
µ opioid receptor
- TIPP[ψ]
H-Tyr-Ticψ[CH2-NH]-Phe-Phe-OH
REFERENCES
- 1.McCurdy CR, Prisinzano TE. Opiod Receptor Ligands. In: Abraham DJ, Rotella DP, editors. Burger's Medicinal Chemistry, Drug Discovery and Development. 7th ed. Vol. 8. New York, NY: John Wiley & Sons; 2010. pp. 569–735. [Google Scholar]
- 2.Marcus DA, Cope DK, Deodhar A, Payne R. Chronic Pain: An Atlas of Investigation and Management. Oxford: Clinical Publishing; 2009. Chronic pain management strategies; pp. 17–38. [Google Scholar]
- 3.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- 4.Zollner C, Stein C. Opioids. Handbook Exp Pharmacol. 2007:31–63. doi: 10.1007/978-3-540-33823-9_2. [DOI] [PubMed] [Google Scholar]
- 5.Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradley PB, Portoghese PS, Hamon M. International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol. Rev. 1996;48:567–592. [PubMed] [Google Scholar]
- 6.Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu. Rev. Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein A, Naidu A. Multiple opioid receptors: ligand selectivity profiles and binding site signatures. Mol. Pharmacol. 1989;36:265–272. [PubMed] [Google Scholar]
- 8.Minami M, Satoh M. Molecular biology of the opioid receptors: structures, functions and distributions. Neurosci. Res. 1995;23:121–145. doi: 10.1016/0168-0102(95)00933-k. [DOI] [PubMed] [Google Scholar]
- 9.Zimmerman DM, Leander JD. Selective opioid receptor agonists and antagonists: research tools and potential therapeutic agents. J. Med. Chem. 1990;33:895–902. doi: 10.1021/jm00165a002. [DOI] [PubMed] [Google Scholar]
- 10.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 11.Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr. Opin. Neurobiol. 2004;14:370–378. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Smith AP, Lee NM. Opioid receptor interactions: local and nonlocal, symmetric and asymmetric, physical and functional. Life Sci. 2003;73:1873–1893. doi: 10.1016/s0024-3205(03)00549-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Bao L, Guan JS. Role of delivery and trafficking of delta-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol. Sci. 2006;27:324–329. doi: 10.1016/j.tips.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, Wu SX, Hokfelt TG, Bao L, Zhang X. Coexpression of δ- and μ-opioid receptors in nociceptive sensory neurons. Proc. Natl. Acad. Sci. U.S.A. 2010;107:13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Pan ZZ. Synaptic mechanism for functional synergism between δ- and μ- opioid receptors. J. Neurosci. 2010;30:4735–4745. doi: 10.1523/JNEUROSCI.5968-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in δ opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T, Ikeda H, Tsuji M, Misawa M, Narita M, Tseng LF. Antisense oligodeoxynucleotide to delta opioid receptors attenuates morphine dependence in mice. Life Sci. 1997;61:PL 165–PL 170. doi: 10.1016/s0024-3205(97)00620-6. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Blazquez P, Garcia-Espana A, Garzon J. Antisense oligodeoxynucleotides to opioid mu and delta receptors reduced morphine dependence in mice: role of delta-2 opioid receptors. J. Pharmacol. Exp. Ther. 1997;280:1423–1431. [PubMed] [Google Scholar]
- 19.Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J. Pharmacol. Exp. Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- 20.Fundytus ME, Schiller PW, Shapiro M, Weltrowska G, Coderre TJ. Attenuation of morphine tolerance and dependence with the highly selective delta-opioid receptor antagonist TIPP[ψ] Eur. J. Pharmacol. 1995;286:105–108. doi: 10.1016/0014-2999(95)00554-x. [DOI] [PubMed] [Google Scholar]
- 21.Ananthan S. Opioid ligands with mixed μ/δ opioid receptor interactions: An emerging approach to novel analgesics. AAPS J. 2006;8:E118–125. doi: 10.1208/aapsj080114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiller PW. Bi- or multifunctional opioid peptide drugs. Life Sci. 2009;86:598–603. doi: 10.1016/j.lfs.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc. Natl. Acad. Sci. U.S.A. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ananthan S, Kezar HS, 3rd, Carter RL, Saini SK, Rice KC, Wells JL, Davis P, Xu H, Dersch CM, Bilsky EJ, Porreca F, Rothman RB. Synthesis, opioid receptor binding, and biological activities of naltrexone-derived pyrido- and pyrimidomorphinans. J. Med. Chem. 1999;42:3527–3538. doi: 10.1021/jm990039i. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Lu YF, Rice KC, Ananthan S, Rothman RB. SoRI 9409, a non-peptide opioid μ receptor agonist/δ receptor antagonist, fails to stimulate [35S]-GTP-γ-S binding at cloned opioid receptors. Brain. Res. Bull. 2001;55:507–511. doi: 10.1016/s0361-9230(01)00550-0. [DOI] [PubMed] [Google Scholar]
- 26.Ananthan S, Khare NK, Saini SK, Seitz LE, Bartlett JL, Davis P, Dersch CM, Porreca F, Rothman RB, Bilsky EJ. Identification of opioid ligands possessing mixed μ agonist/δ antagonist activity among pyridomorphinans derived from naloxone, oxymorphone, and hydromorphone. J. Med. Chem. 2004;47:1400–1412. doi: 10.1021/jm030311v. [DOI] [PubMed] [Google Scholar]
- 27.Feinberg AP, Creese I, Snyder SH. The opiate receptor: a model explaining structure-activity relationships of opiate agonists and antagonists. Proc. Natl. Acad. Sci. U.S.A. 1976;73:4215–4219. doi: 10.1073/pnas.73.11.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casy AF, Parfitt RT. Opioid Analgesics: Chemistry and Receptors. New York: Plenum Press; 1986. [Google Scholar]
- 29.Schmidhammer H, Burkard WP, Egstein-Aeppli L. Synthesis and biological evaluation of 14-alkoxymorphinans. 4. Opioid agonists and partial opioid agonists in a series of N-(cyclobutylmethyl)-14-methoxymorphinan-6-ones. Helv. Chim. Acta. 1989;72:1233–1240. [Google Scholar]
- 30.Schmidhammer H, Aeppli L, Atwell L, Fritsch F, Jacobson AE, Nebuchla M, Sperk G. Synthesis and biological evaluation of 14-alkoxymorphinans. 1. Highly potent opioid agonists in the series of (-)-14-methoxy-N-methylmorphinan-6-ones. J. Med. Chem. 1984;27:1575–1579. doi: 10.1021/jm00378a009. [DOI] [PubMed] [Google Scholar]
- 31.Schmidhammer H, Burkard WP, Eggstein-Aeppli L, Smith CF. Synthesis and biological evaluation of 14-alkoxymorphinans. 2. (-)-N-(cyclopropylmethyl)-4,14- dimethoxymorphinan-6-one, a selective mu opioid receptor antagonist. J. Med. Chem. 1989;32:418–421. doi: 10.1021/jm00122a021. [DOI] [PubMed] [Google Scholar]
- 32.Schmidhammer H. Opioid Receptor Antagonists. New York: Elsevier; 1998. pp. 83–132. [PubMed] [Google Scholar]
- 33.Greiner E, Spetea M, Krassnig R, Schullner F, Aceto M, Harris LS, Traynor JR, Woods JH, Coop A, Schmidhammer H. Synthesis and biological evaluation of 14-alkoxymorphinans. 18. N-substituted 14-phenylpropyloxymorphinan-6-ones with unanticipated agonist properties: extending the scope of common structure-activity relationships. J. Med. Chem. 2003;46:1758–1763. doi: 10.1021/jm021118o. [DOI] [PubMed] [Google Scholar]
- 34.Lattanzi R, Spetea M, Schullner F, Rief SB, Krassnig R, Negri L, Schmidhammer H. Synthesis and biological evaluation of 14-alkoxymorphinans. 22. Influence of the 14-alkoxy group and the substitution in position 5 in 14-alkoxymorphinan-6-ones on in vitro and in vivo activities. J. Med. Chem. 2005;48:3372–3378. doi: 10.1021/jm040894o. [DOI] [PubMed] [Google Scholar]
- 35.Schmidhammer H. 14-Alkoxymorphinans-A series of highly potent opioid agonists, antagonists, and partial agonists. Curr. Top. Med. Chem. 1993;1:261–276. [Google Scholar]
- 36.Li G, Aschenbach LC, He H, Selley DE, Zhang Y. 14-O-Heterocyclic-substituted naltrexone derivatives as non-peptide mu opioid receptor selective antagonists: design, synthesis, and biological studies. Bioorg. Med. Chem. Lett. 2009;19:1825–1829. doi: 10.1016/j.bmcl.2008.12.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moynihan H, Jales AR, Greedy BM, Rennison D, Broadbear JH, Purington L, Traynor JR, Woods JH, Lewis JW, Husbands SM. 14 β-O-Cinnamoylnaltrexone and related dihydrocodeinones are mu opioid receptor partial agonists with predominant antagonist activity. J. Med. Chem. 2009;52:1553–1557. doi: 10.1021/jm8012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fontana G, Savona G, Rodriguez B, Dersch CM, Rothman RB, Prisinzano TE. Synthetic studies of neoclerodane diterpenoids from Salvia splendens and evaluation of opioid receptor affinity. Tetrahedron. 2008;64:10041–10048. doi: 10.1016/j.tet.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothman RB, Murphy DL, Xu H, Godin JA, Dersch CM, Partilla JS, Tidgewell K, Schmidt M, Prisinzano TE. Salvinorin A: Allosteric interactions at the μ-opioid receptor. J. Pharmacol. Exp. Ther. 2007;320:801–810. doi: 10.1124/jpet.106.113167. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Partilla JS, Wang X, Rutherford JM, Tidgewell K, Prisinzano TE, Bohn LM, Rothman RB. A comparison of noninternalizing (herkinorin) and internalizing (DAMGO) μ-opioid agonists on cellular markers related to opioid tolerance and dependence. Synapse. 2007;61:166–175. doi: 10.1002/syn.20356. [DOI] [PubMed] [Google Scholar]
- 41.Ananthan S, Kezar HS, 3rd, Saini SK, Khare NK, Davis P, Dersch CM, Porreca F, Rothman RB. Synthesis, opioid receptor binding, and functional activity of 5'-substituted 17-cyclopropylmethylpyrido[2',3':6,7]morphinans. Bioorg. Med. Chem. Lett. 2003;13:529–532. doi: 10.1016/s0960-894x(02)00934-4. [DOI] [PubMed] [Google Scholar]
- 42.Ananthan S, Khare NK, Saini SK, Davis P, Dersch CM, Porreca F, Rothman RB. Novel ligands for the opioid receptors: synthesis and structure-activity relationships among 5'-aryl and 5'-heteroaryl 17-cyclopropylmethyl-4,5 alpha-epoxypyrido[ 2',3':6,7]morphinans. Bioorg. Med. Chem. 2003;11:4143–4154. doi: 10.1016/s0968-0896(03)00432-2. [DOI] [PubMed] [Google Scholar]
- 43.Sadee W, Wang D, Bilsky EJ. Basal opioid receptor activity, neutral antagonists, and therapeutic opportunities. Life Sci. 2005;76:1427–1437. doi: 10.1016/j.lfs.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 44.Rothman RB, Ananthan S, Bilsky EJ. Novel Opioid Antagonists with Mixed/Dual Selectivity. In: Negus SS, editor. Opiate Receptors and Antagonists. Humana Press; 2009. pp. 137–151. [Google Scholar]
- 45.Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wells JL, Bartlett JL, Ananthan S, Bilsky EJ. In vivo pharmacological characterization of SoRI 9409, a nonpeptidic opioid mu-agonist/delta-antagonist that produces limited antinociceptive tolerance and attenuates morphine physical dependence. J. Pharmacol. Exp. Ther. 2001;297:597–605. [PubMed] [Google Scholar]
- 47.Xu H, Wang X, Wang J, Rothman RB. Opioid peptide receptor studies. 17. Attenuation of chronic morphine effects after antisense oligodeoxynucleotide knockdown of RGS9 protein in cells expressing the cloned Mu opioid receptor. Synapse. 2004;52:209–217. doi: 10.1002/syn.20019. [DOI] [PubMed] [Google Scholar]
- 48.Bilsky EJ, Inturrisi CE, Sadee W, Hruby VJ, Porreca F. Competitive and noncompetitive NMDA antagonists block the development of antinociceptive tolerance to morphine, but not to selective mu or delta opioid agonists in mice. Pain. 1996;68:229–237. doi: 10.1016/s0304-3959(96)03185-5. [DOI] [PubMed] [Google Scholar]