Abstract
Increased dietary long-chain n-3 polyunsaturated fatty acid (LCn-3PUFA) intake stimulates muscle protein anabolism in individuals who experience muscle loss due to aging or cancer cachexia. However, it is not known whether LCn-3PUFA elicit similar anabolic effects in healthy individuals. To answer this question we evaluated the effect of 8 weeks of LCn-3PUFA supplementation (4 g·d−1 of Lovaza®) in nine 25–45 y old healthy subjects on the rate of muscle protein synthesis (by using stable isotope labelled tracer techniques) and the activation (phosphorylation) of elements of the mTOR-p70s6k pathway during basal, postabsorptive conditions and during a hyperinsulinemic-hyperaminoacidemic clamp. We also measured the concentrations of protein, RNA, and DNA in muscle to obtain indices of the protein synthetic capacity, translational efficiency and cell size. Neither the basal muscle protein fractional synthesis rate nor basal signalling element phosphorylation changed in response to LCn-3PUFA supplementation but the anabolic response to insulin and amino acid infusion was greater after LCn-3PUFA (i.e., the muscle protein fractional synthesis rate during insulin and amino acid infusion increased from 0.062 ± 0.004 to 0.083 ± 0.007 %·h−1 and the phospho mTORSer2448 and p70s6kThr389 concentrations increased by ~50%; all P < 0.05). In addition, the muscle protein concentration and the protein-to-DNA ratio (i.e., muscle cell size) were both greater (P < 0.05) after LCn-3PUFA supplementation. We conclude that LCn-3PUFA have anabolic properties in healthy young and middle aged adults.
Keywords: n-3 PUFA, fish oil, muscle protein synthesis
Introduction
Long-chain n-3 polyunsaturated fatty acids (LCn-3PUFA) are essential nutrients with many potential health benefits. The general consensus appears to be that LCn-3PUFA, particularly eicosapentaenoic acid (EPA; C20:5 n-3) and docosahexaenoic acid (DHA; C22:6 n-3), have anti-inflammatory properties [1] and reduce the risk for cardiovascular disease [1]. Furthermore, there is good evidence from studies in animals that LCn-3PUFA improve the sensitivity of whole body and muscle glucose metabolism to insulin [2–5]; although the results from studies in human subjects are equivocal (reviewed by Fedor and Kelley [6]).
There is also emerging evidence for a muscle anabolic effect of LCn-3PUFA. For example, low-dose LCn-3PUFA supplementation (i.e., 1 – 2% of total daily energy intake - as in our study), alone or in combination with amino acid supplementation, has been reported to help maintain whole-body protein synthesis, whole-body protein net balance, and muscle mass in burned rats and tumour-bearing mice [7, 8]. Furthermore, we have recently demonstrated that LCn-3PUFA supplementation (4 g·d−1 of Lovaza®) in older adults (≥65 y) significantly increased the rate of muscle protein synthesis during hyperinsulinemia-hyperaminoacidemia, most likely because of greater activation of the mTOR-p70s6k signalling pathway [9], and Ryan et al. [10] reported that increased LCn-3PUFA intake blunted the loss of total body and limb fat-free masses in patients with resectable, non-metastatic oesophageal cancer undergoing oesophageal cancer surgery. The exact mechanisms responsible for the beneficial effect of LCn-3PUFA on muscle protein metabolism are unknown but one might speculate that they are related to the anti-inflammatory properties of LCn-3PUFA [1] because burn injury, cancer and aging are all associated with increased inflammatory activity [11–14], which is known to induce muscle loss [15, 16]. On the other hand, it is possible that LCn-3PUFA have intrinsic muscle protein anabolic properties, in which case they should also stimulate muscle protein synthesis in healthy, young subjects. In fact, feed enriched in menhaden oil, a fish oil rich in EPA and DHA, doubled the insulin-stimulated non-oxidative whole-body disposal of amino acids (a marker of increased whole-body protein synthesis) and increased the activation of the mTOR-p70s6k signalling pathway in muscle of young and still growing steers [5] suggesting this may be the case. The effect of LCn-3PUFA intake on muscle protein metabolism in healthy young adults, however, has not been studied to date.
The purpose of the present study therefore was to determine the effect of LCn-3PUFA supplementation for 8 weeks on indices of muscle protein anabolism in human muscle in young/middle aged adults. To this end, we measured the fractional rate of muscle protein synthesis (by using stable isotope labelled tracer techniques) during basal, post-absorptive conditions and during hyperinsulinemia-hyperaminoacidemia (within the range normally seen after meal consumption [17, 18]), the concentrations of protein, RNA, and DNA in muscle (to obtain indices of the protein synthetic capacity, translational efficiency [19, 20] and cell size [21]), and the activation (as phosphorylation) of elements of intracellular signalling pathways involved in the regulation of muscle protein synthesis (Akt; mTOR; p70s6k; eEF2) [22, 23] in healthy men and women. We also measured markers of inflammation in plasma (C-reactive protein [CRP], interleukin 6 [IL-6], tumour necrosis factor alpha [TNF-α]) and the rate of appearance of glucose into plasma (an index of endogenous glucose production) and the rate of whole-body glucose uptake (glucose rate of disappearance) to gauge the relationship between the effect of LCn-3PUFA on glucose and muscle protein metabolism.
Methods
Subjects
Nine healthy individuals (5 men and 4 women; age: 39.7 ± 1.7 y; BMI 25.9 ± 1.0 kg/m2; body fat determined by dual X-ray absorptiometry: 25 ± 3 %; means ± SEM) participated in this study. All subjects were considered to be in good health after completing a comprehensive medical evaluation, which included a history and physical examination and standard blood tests. None of the subjects engaged in regular physical activities (i.e., they exercised ≤1.5 h·wk−1), consumed fish oil supplements, or took any medications; none reported excessive alcohol intake or consumed tobacco products. Written, informed consent was obtained from all subjects before their participation in the study, which was approved by the Human Subjects Research Protection Office and the Clinical Research Unit Advisory Committee at Washington University School of Medicine in St. Louis, MO.
Experimental protocol
Each subject completed two stable isotope labelled tracer infusion studies to determine the effect of LCn-3PUFA supplementation on the rate of muscle protein synthesis and anabolic signalling during basal, postabsorptive conditions and during insulin and amino acid infusion. The first study was performed within 1–3 weeks of screening (before the intervention); the second one took place after 8 weeks of dietary supplementation with 4 g·d−1 of Lovaza® (GlaxoSmithKline, Research Triangle Park, North Carolina, USA) containing 1.86 and 1.50 g·d−1, respectively of the ethylesters of eicosapentaenoic acid [EPA; 20:5n-3] and docosahexaenoic acid [DHA; 22:6n-3]). Compliance was evaluated by pill count and changes in the muscle phospholipid fatty acid composition. We gave each subject an excess number of pills and asked them to return any remaining pills at the end of the study.
Before each muscle protein metabolism study, subjects were instructed to adhere to their usual diet and to refrain from vigorous physical activities for three days. The evening before the study, subjects were admitted to the Clinical Research Unit at Washington University School of Medicine. At 2000 h, they consumed a standard meal providing 50.2 kJ per kg body weight (15% as protein, 55% as carbohydrates and 30% as fat). Subjects then rested in bed and fasted (except for water) until completion of the study the next day. At ~0600 h on the following morning, a cannula was inserted into an antecubital vein for the infusion of stable isotope labelled tracers (i.e., a phenylalanine tracer to measure the rate of muscle protein synthesis and a glucose tracer to measure the glucose rate of appearance in the systemic circulation); another cannula was inserted into a vein of the contralateral hand, which was warmed to 55 oC for blood sampling. At ~0800 h, primed, constant infusions of [ring-2H5]phenylalanine (priming dose: 2.8 μmol·kg fat-free mass [FFM]−1, infusion rate: 0.08 μmol·kg FFM−1·min−1) and [6,6-2H2]glucose (priming dose: 18 μmol·kg body wt−1, infusion rate: 0.22 μmol·kg body wt−1·min−1), all purchased from Cambridge Isotope Laboratories Inc. (Andover, MA, USA), were started and maintained for seven hours. Four hours after the start of the tracer infusions, a hyperinsulinemic-hyperaminoacidemic clamp was started and maintained for three hours. Human insulin (Novolin R, Novo Nordisk, Princeton, NJ) was infused at a rate of 20 mU·m−2 body surface area (BSA)·min−1 (initiated with two priming doses of 80 mU·m−2 BSA·min−1 for 5 minutes and then 40 mU·m−2 BSA·min−1 for additional 5 minutes). Plasma amino acid availability was increased by providing an intravenous amino acid mixture (Travasol 10%, Baxter, Deerfield, IL, USA) at a rate of 105 mg amino acids·kg FFM−1·h−1 (priming dose: 35 mg amino acids·kg FFM−1). Euglycemia (blood glucose concentration of ~5.5 mM) was maintained during the clamp procedure by variable rate infusion of 20% dextrose (Baxter, Deerfield, IL, USA) enriched to 2.5% with [6,6-2H2]glucose. To adjust for the increased plasma amino acid availability and reduced hepatic glucose production during the clamp procedure, the [ring-2H5]phenylalanine infusion rate was increased to 0.12 μmol·kg FFM−1·min−1 and the [6,6-2H2]glucose infusion rate was decreased to 0.11 μmol·kg body wt−1·min−1.
Blood samples (4 ml) were obtained before beginning the tracer infusions and then at 60, 90, 180, 210, 220, 230, 240, 270, 300, 330, 360, 390, 400, 410, and 420 min to determine the labelling of phenylalanine and glucose in plasma and plasma substrate, hormone and cytokine concentrations. Additional blood (~1 ml) was obtained every 10 minutes during the clamp to monitor plasma glucose concentration. Muscle tissue (~100 mg) was obtained under local anaesthesia (lidocaine, 2%) from the quadriceps femoris by using a Tilley-Henkel forceps [24] at 60 min and 240 min to determine the basal rate of muscle protein synthesis (labelled phenylalanine incorporation into muscle protein; see Calculations) and the basal concentrations of phosphorylated elements of intramuscular signal transduction proteins (Akt; mTOR; p70s6k; and eEF2) involved in the regulation of muscle protein synthesis. A third muscle biopsy was obtained at 420 min (i.e., 3 h after starting the clamp procedure) to determine both the rate of muscle protein synthesis and the intracellular signalling responses to hyperinsulinemia-hyperaminoacidemia. The second and third biopsies were obtained from the same incision on the leg contralateral to that biopsied first; the forceps was directed in proximal and distal direction so that the two biopsies were collected ~5–10 cm apart.
Sample processing and analyses
One millilitre of blood was collected in pre-chilled tubes containing heparin, plasma was separated immediately by centrifugation and glucose concentration measured immediately. The remaining blood (~3 ml) was collected in pre-chilled tubes containing EDTA, plasma was separated by centrifugation within 30 min of collection and then stored at −80 °C until final analyses. Muscle samples were rinsed in ice-cold saline immediately after collection, cleared of visible fat and connective tissue, frozen in liquid nitrogen and stored at −80 °C until final analysis.
Plasma glucose concentration was measured on an automated glucose analyzer (Yellow Spring Instruments, Yellow Springs, OH). Plasma insulin concentration was determined by radioimmunoassay (Linco Research, St. Louis, MO). Commercially available ELISA kits (R&D Systems Inc, Minneapolis, MN) were used to determine plasma concentrations of CRP, TNF-α and IL-6.
Muscle phospholipid fatty acid composition was determined after extracting lipids from ~30 mg of muscle tissue with 2 ml chloroform/methanol (2:1, v/v) containing 0.01% butylated hydroxytoluene. Phospholipids were then isolated by using thin layer chromatography (Whatman TLC LK 6D, Fischer Scientific, Pittsburgh, PA), fatty acids were converted to their methyl esters by reacting with 10% acetyl chloride in methanol and their peak areas measured by using GC-MS (MSD 5973 System, Hewlett-Packard).
To determine the labelling of plasma glucose, plasma proteins were precipitatedwith ice-cold acetone, and hexane was used to extract plasma lipids. The aqueous phase, containing glucose, was driedby speed-vac centrifugation (Savant Instruments, Farmingdale, NY), glucose was derivatised with heptafluorobutyric acid and the tracer-to-tracee ratio (TTR) was determined by using gas-chromatography/mass-spectrometry (GC-MS, Hewlett-Packard MSD 5973 system with capillary column) as previously described [25].
To determine the plasma concentrations of phenylalanine and leucine (thought to be a major regulator of muscle protein synthesis [26]) and the labelling of phenylalanine in plasma, known amounts of [1-13C]phenylalanine and nor-leucine were added to an aliquot of each plasma sample, plasma proteins were precipitated, and the supernatant, containing free amino acids, was collected to prepare the t-butyldimethylsilyl (t-BDMS) derivatives of phenylalanine and leucine to determine their TTRs by GC-MS (MSD 5973 System, Hewlett-Packard) [27, 28]. To determine phenylalanine labelling in muscle proteins and in tissue fluid, samples (~20 mg) were homogenised in 1 ml trichloroacetic acid solution (3 % w/v), proteins precipitated by centrifugation, and the supernatant, containing free amino acids, collected. The pellet containing muscle proteins was washed and then hydrolysed in 6 N HCl at 110 °C for 24 h. Amino acids in the protein hydrolysate and supernatant samples were purified on cation-exchange columns (Dowex 50W-X8–200, Bio-Rad Laboratories, Richmond, CA), and the t-BDMS derivative of phenylalanine prepared to determine its TTR by GC-MS (MSD 5973 System, Hewlett-Packard) analysis [27, 28]. The extent of phenylalanine labelling in plasma, muscle tissue fluid, and muscle protein was calculated based on the simultaneously measured TTR of standards of known isotope labelling.
Western analysis was used to measure the phosphorylation of Akt, mTOR, p70s6k, and eEF2. Briefly, frozen muscle tissue (~20 mg) was rapidly homogenised in ice-cold buffer (50 mM Tris-HCL pH 7.5, 1 mM EDTA, 1 mM EGTA, 10 mM glycerophosphate, 50 mM NaF, 0.1 % Triton-X, 0.1 % 2-mercaptoethanol, 1 protease and 1 phosphatase inhibitor tablet [Roche Diagnostics Ltd, Burgess Hill, UK]) at 10 μl·mg−1 tissue. Proteins were extracted by shaking for 15 min at 4°C and samples were then centrifuged at 13000 × g for 10 min at 4 oC and the supernatant, containing the proteins, was collected. The protein concentration in the supernatant was determined by the Bradford method with a commercial reagent (B6916, Sigma-Aldrich, St. Louis, MO) and adjusted to 3 mg·ml−1 in 3 × Laemmli buffer. Fifty micrograms of protein from each sample were loaded onto 12 % XT-Bis Tris gels, separated by SDS PAGE, and transferred on ice at 100 V for 45 min to methanol pre-wetted 0.2 μm PVDF membranes. Blots were then incubated sequentially with 5% (w/v) non-fat milk for 1 h, primary antibodies overnight at 4 °C, and then secondary antibody (1:2000 anti-rabbit; New England Biolabs, Ipswich, MA) for 1 h. The following primary antibodies were used at a concentration of 1:2000: AktThr308, mTORSer2448, p70s6kThr389, and eEF2Thr56 with pan-actin (as a loading control), all purchased from New England Biolabs. Membranes were developed using Immobilon Western Chemiluminescent HRP substrate (Millipore, Billerica, MA) and the protein bands were visualized and quantified by densitometry on a Chemidoc XRS (Bio-Rad Laboratories, Inc. Hercules, CA) ensuring no pixel saturation. Data were expressed in relation to GAPDH.
To determine the muscle protein concentration (per mg of wet weight), the protein-to-DNA ratio (an estimate of cell size), the RNA-to-protein ratio (an index of the ribosomal capacity for protein synthesis) and the RNA-to-DNA ratio (the cell capacity for protein synthesis) in muscle, tissue (~15 mg) was snipped before homogenisation in 0.2 M PCA. After centrifugation at 2,800 g, and washing with 0.2 M PCA, the pellet was resuspended in 0.3 M NaOH to quantify alkali soluble proteins using the Bradford assay and spectrophotometry. Next, proteins were precipitated out with 1M PCA before centrifugation and removal of the supernatant for RNA quantification by spectrophotometry. The remaining pellet was resuspended in 2 M PCA and incubated at 70 °C for 1 h before centrifugation and removal of the supernatant for DNA quantification by spectrophotometry.
Calculations
The fractional synthesis rate (FSR) of mixed muscle protein was calculated from the rate of incorporation of [ring-2H5]phenylalanine into muscle protein, using a standard precursor-product model as follows: FSR = ΔEp/Eic × 1/t × 100; where ΔEp is the change between two consecutive biopsies in extent of labelling (TTR) of protein-bound phenylalanine. Eic is the mean labelling over time of the precursor for protein synthesis and t is the time between biopsies. The free phenylalanine labelling in muscle tissue fluid was chosen to represent the immediate precursor for muscle protein synthesis (i.e., aminoacyl-t-RNA) [29]. In addition, we calculated the muscle protein FSR by using the average plasma phenylalanine enrichments between 60 min and 240 min (basal) and between 270 min and 420 min (clamp).
The translation efficiency (mg protein produced per μg RNA per hour) was calculated by dividing the product of the muscle protein FSR (in %·h−1) and the muscle protein concentration (in mg per g wet tissue) by the muscle total RNA concentration (in μg per g wet tissue) [19, 20].
Glucose rate of appearance in plasma during basal conditions and the clamp procedure was calculated by dividing the glucose tracer infusion rate by the average plasma glucose TTR during the last 30 min of the basal period and the last 30 min of the clamp. Glucose rate of appearance during basal conditions equals glucose rate of disappearance and represents endogenous glucose production whereas during the clamp procedure, glucose rate of appearance represents the sum of endogenous glucose production and the rate of infused glucose. Endogenous glucose production rate during the clamp was therefore calculated by subtracting the glucose infusion rate from the glucose rate of appearance; glucose rate of disappearance was assumed to be equal to the glucose rate of appearance plus the tracer infusion rate.
Statistical analysis
All data sets were tested for normality and skewed data sets were log-transformed for statistical analysis. Differences before and after LCn-3PUFA supplementation in single time-point measurements (e.g., systemic inflammatory markers, muscle phospholipid fatty acid composition) were evaluated by using Student’s t-test. Repeated measures analysis of variance (ANOVA) and Tukey’s post-hoc procedure was used to evaluate differences before and after LCn-3PUFA supplementation in plasma glucose, insulin, phenylalanine and leucine concentrations, mixed muscle protein FSR and muscle intracellular signalling elements during basal, post-absorptive conditions and during the clamp procedure. A P-value of ≤0.05 was considered statistically significant. Data are presented as means ± SEM or median with 25th and 75th percentiles in brackets for skewed data sets.
Results
Compliance with LCn-3PUFA supplementation and muscle phospholipid fatty acid composition
All subjects consumed ≥160 of the 224 pills assigned to them. Average compliance, as judged by the left-over pill count, was 94 ± 3%. This was confirmed by analysis of the muscle phospholipid fatty acid profile, which demonstrated a doubling of the proportion of LCn-3PUFA at the expense of n-6 PUFA and mono-unsaturated fatty acids with no changes in saturated fatty acid concentrations (Table 1).
Plasma substrate, insulin and cytokine concentrations
Plasma glucose, insulin, phenylalanine and leucine concentrations were not affected by LCn-3PUFA supplementation, neither during basal, postabsorptive conditions nor during the hyperinsulinemic-hyperaminoacidemic clamp (Table 2 and Supplement Table 1S). During the clamp, plasma glucose concentration was successfully maintained at ~5.4 mM and plasma insulin, phenylalanine and leucine concentrations rose by ~four-fold, 80% and 40% above basal values, respectively (all P < 0.001) both before and after LCn-3PUFA supplementation (Table 2).
Table 2.
Plasma glucose, insulin, leucine, and phenylalanine concentrations during basal, postabsorptive conditions and during the hyperinsulinemic-hyperaminoacidemic clamp procedure before and after eight weeks of long-chain n-3 polyunsaturated fatty acid supplementation
| Before | After | |||
|---|---|---|---|---|
| Basal | Clamp | Basal | Clamp | |
| Glucose (mM) | 4.9 ± 0.1 | 5.4 ± 0.1a | 4.9 ± 0.1 | 5.4 ± 0.1a |
| Insulin (μU·ml−1) | 5.2 ± 0.8 | 28.9 ± 1.8a | 5.6 ± 0.7 | 31.4 ± 2.2a |
| Phenylalanine (μM) | 56 ± 5 | 98 ± 8a | 56 ± 3 | 100 ± 6a |
| Leucine (μM) | 113 ± 5 | 162 ± 7a | 113 ± 5 | 165 ± 9a |
Values are mean ± SEM.
Value significantly different from corresponding basal value (P < 0.001).
As expected, the concentration of inflammatory markers in plasma was low in this healthy cohort of subjects and there were no differences (all P ≥0.33) before and after LCn-3PUFA supplementation in the plasma concentrations of CRP (0.61 [0.39, 1.03] vs. 0.73 [0.17, 1.23] mg·l−1, respectively), TNF-α (0.30 ± 0.02 vs. 0.31 ± 0.02 pg·ml−1, respectively) and IL-6 (1.49 [1.05, 3.25] vs. 1.34 [0.98, 1.47] pg·ml−1, respectively).
Plasma phenylalanine and glucose and muscle free phenylalanine enrichments
Plasma phenylalanine TTR was steady between 60 min and 420 min and plasma glucose TTR was steady between 210 and 240 min of the basal period and 390 and 420 min during the hyperinsulinemic-hyperaminoacidemic clamp, both before and after LCn-3PUFA supplementation (Supplement Table 1S). The muscle free phenylalanine enrichments before and after LCn-3PUFA supplementation were 0.066 ± 0.004 and 0.062 ± 0.004, respectively at the end of the basal, postabsorptive period and 0.074 ± 0.003 and 0.072 ± 0.004, respectively at the end of the hyperinsulinemic-hyperaminoacidemic clamp.
Muscle protein concentration and synthesis
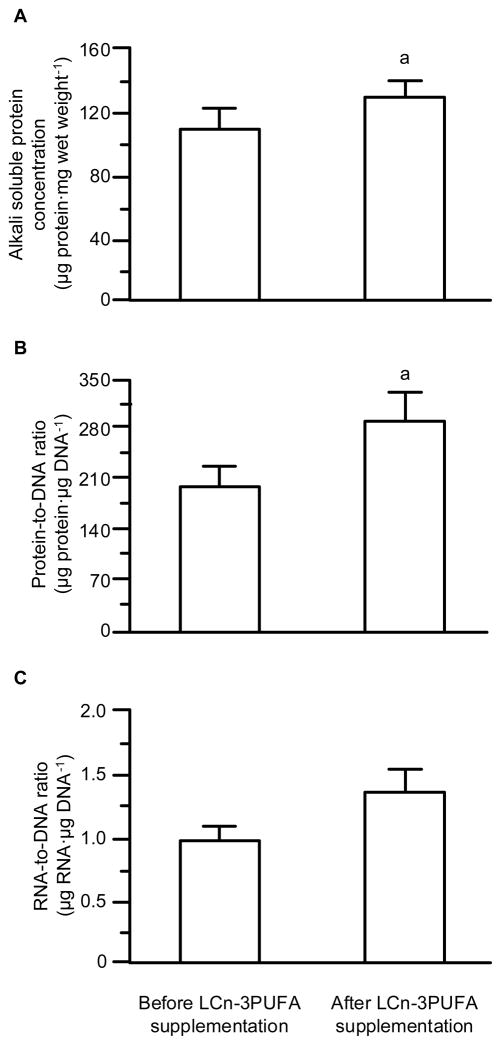
Both, the alkali soluble protein concentration and the protein-to-DNA ratio, a measure of cell size [21], increased (P ≤0.04) after LCn-3PUFA supplementation (Figure 1). However, neither the muscle RNA concentration (0.62 ± 0.07 vs. 0.68 ± 0.04 μg RNA·mg muscle wet weight −1; P = 0.45) nor the RNA-to-protein ratio, an index of the ribosomal capacity for protein synthesis, (5.8 ± 0.9 vs. 5.3 ± 0.5 μg RNA·mg protein−1; P = 0.65) in muscle were affected by LCn-3PUFA supplementation. There was a trend for an increase in the RNA-to-DNA ratio, the cell capacity for protein synthesis (Figure 1), but the difference did not reach statistical significance (P = 0.13).
Figure 1. Muscle protein concentration, cell size, and capacity for protein synthesis.
Muscle alkali soluble protein concentration (A), the protein-to-DNA ratio in muscle (an index of cell size; B), and the RNA-to-DNA ratio (an index for the cell capacity for protein synthesis; C) before and after eight weeks of long-chain n-3 polyunsaturated fatty acid (LCn-3PUFA) supplementation. Values are means ± SEM. a Value significantly (P < 0.05) different from corresponding value before LCn-3PUFA supplementation. The difference in the RNA-to-DNA ratio did not reach statistical significance (P = 0.13).
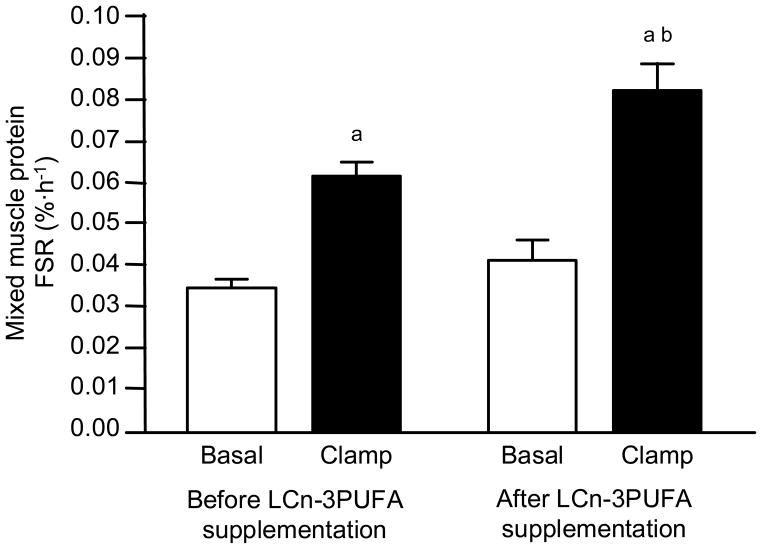
The basal muscle protein FSR (calculated by using the muscle free phenylalanine enrichment as the precursor enrichment) was not different before and after LCn-3PUFA supplementation, (Figure 2). Insulin and amino acid infusion led to a marked increase in the muscle protein FSR (P < 0.001) and the anabolic response (i.e., the increase from basal values) was ~50% greater after LCn-3PUFA supplementation (0.042 ± 0.005 %·h−1 vs. 0.027 ± 0.005 %·h−1; P = 0.01). Consequently, the muscle protein FSR during insulin and amino acid infusion was significantly greater (P < 0.01) after than before LCn-3PUFA supplementation (Figure 2). Using the plasma phenylalanine enrichment as the precursor enrichment in the muscle protein FSR calculation did not affect the results. The basal muscle protein FSR was not different before and after LCn-3PUFA supplementation (0.022 ± 0.002 %·h−1 vs. 0.025 ± 0.003 %·h−1, respectively; P = 0.18). Insulin and amino acid infusion led to a marked increase in the muscle protein FSR (P < 0.001) and the anabolic response (i.e., the increase from basal values) was ~50% greater after LCn-3PUFA supplementation (0.038 ± 0.005 %·h−1 vs. 0.025 ± 0.004 %·h−1; P = 0.04).
Figure 2. Muscle protein fractional synthesis rates.
Muscle protein fractional synthesis rates (FSR) during basal, post-absorptive conditions and during the hyperinsulinemic-hyperaminoacidemic clamp procedure before and after eight weeks of long-chain n-3 polyunsaturated fatty acid (LCn-3PUFA) supplementation. Values are means ± SEM. ANOVA revealed a significant main effect of hyperinsulinemia-hyperaminoacidemia (P < 0.001) and a hyperinsulinemia-hyperaminoacidemia x LCn-3PUFA interaction (P = 0.01). a Value significantly different (P < 0.01) from corresponding basal value. b Value significantly (P < 0.01) different from corresponding value before LCn-3PUFA supplementation.
LCn-3PUFA supplementation did not affect the translational efficiency during basal conditions (0.0068 ± 0.0008 vs. 0.0079 ± 0.0009 mg protein·μg RNA−1·h−1 before and after supplementation, respectively; P = 0.42); however, during insulin-amino acid infusion the translational efficiency was ~35% greater after than before LCn-3PUFA supplementation (0.0234 ± 0.0018 vs. 0.0174 ± 0.0012 mg protein·μg RNA−1·h−1, respectively; P < 0.01).
Phosphorylation of anabolic signalling transduction proteins
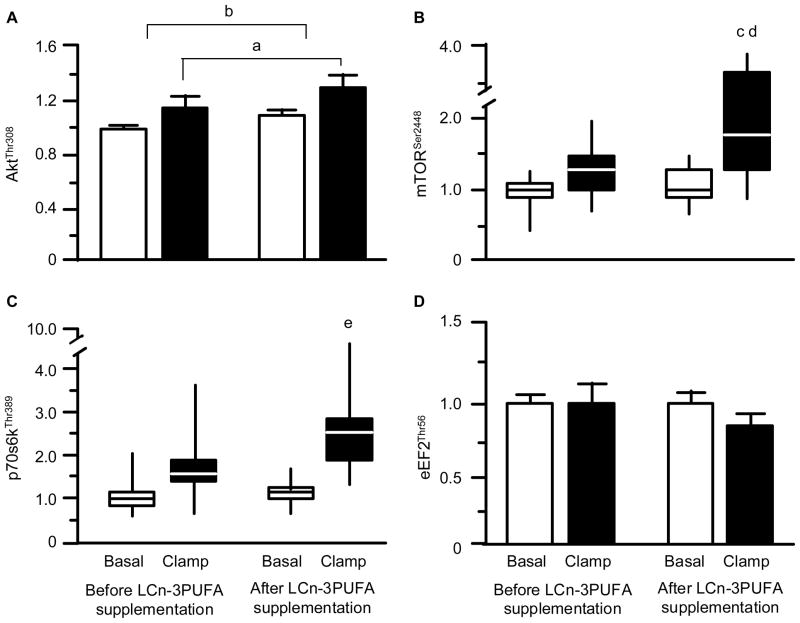
The concentration of AktThr308 in muscle was greater during insulin-amino acid infusion than during basal conditions (P = 0.042 for main effect of clamp) and was greater (although the difference was small) after than before LCn-3PUFA supplementation (P = 0.023). There was, however, no difference (P = 0.976) before and after LCn-3PUFA supplementation in the extent of the hyperinsulinemia-hyperaminoacidemia induced increase in AktThr308 phosphorylation (Figure 3A). The basal concentrations of mTORSer2448 and p70s6kThr389 in muscle were not different before and after LCn-3PUFA supplementation; ANOVA revealed a significant stimulatory effect of hyperinsulinemia-hyperaminoacidemia (P < 0.01 for main effect of clamp) and a significant hyperinsulinemia-hyperaminoacidema x time (pre-post intervention) interaction (P < 0.05). Tukey’s post-hoc analysis indicated that this was due to increased mTORSer2448 and p70s6kThr389 activation after LCn-3PUFA supplementation (Figures 3B and 3C). Neither hyperinsulinemia-hyperaminoacidemia (P = 0.27) nor LCn-3PUFA supplementation (P = 0.21) had an effect on the concentration of eEF2Thr56 in muscle (Figure 3D).
Figure 3. Skeletal muscle anabolic signalling responses.
Concentrations (arbitrary units) of phosphorylated AktThr308 (A), mTORSer2448 (B), p70s6kThr389 (C), and eEF2Thr56 (D) during basal, postabsorptive conditions and during the hyperinsulinemic-hyperaminoacidemic clamp procedure before and after eight weeks of long-chain n-3 polyunsaturated fatty acid (LCn-3PUFA) supplementation. Values are means ± SEM or medians (horizontal lines) with quartiles (boxes) and minimum and maximum values (vertical lines). a,b For AktThr308, ANOVA revealed a main effect of hyperinsulinemia-hyperaminoacidemia (P = 0.042) and LCn-3PUFA (P = 0.023) but no interaction (P = 0.976). For mTORSer2448 and p70s6kThr389, ANOVA revealed a main effect of hyperinsulinemia-hyperaminoacidemia (P < 0.01) and a significant interaction (P < 0.05); Tukey post-hoc analysis located the specific differences as follows: c value significantly different from corresponding basal value (P < 0.01); d value significantly different from corresponding value before LCn-3PUFA supplementation (P < 0.05). e value significantly different from corresponding basal value (P < 0.05).
Glucose kinetics
LCn-3PUFA supplementation had no effect on whole-body glucose kinetics. Basal glucose rate of appearance was 9.4 ± 0.3 μmol·kg body weight−1·min−1 before and 9.4 ± 0.3 μmol·kg body weight1·min−1 after LCn-3PUFA supplementation (P = 0.90). During the clamp, endogenous glucose rate of appearance decreased by ~70% from basal values (P < 0.001) to 2.7 ± 0.4 μmol·kg body weight−1·min−1 before and to 2.6 ± 0.5 μmol·kg body weight−1·min−1 after LCn-3PUFA supplementation (no difference in the extent of decrease before and after supplementation, P = 0.74). Glucose rate of disappearance during the clamp was 25.9 ± 2.3 μmol·kg body weight−1·min−1 before and 27.4 ± 2.8 μmol·kg body weight−1·min−1 after LCn-3PUFA supplementation (P = 0.46).
Discussion
We provide evidence that LCn-3PUFA supplementation causes a considerable increase in the muscle protein anabolic response to hyperinsulinemia-hyperaminoacidemia in healthy young and middle-aged adults. These data compliment and extend the results we recently obtained in older adults [9] and demonstrate that LCn-3PUFA supplementation not only alleviates the muscle protein anabolic resistance associated with old age [9, 30–32] but can actually boost the anabolic response to nutritional stimuli in healthy muscle from young and middle-aged adults.
The specific mechanism(s) by which LCn-3PUFA act on the muscle protein synthesis process remain mostly unknown. Our results indicate that LCn-3PUFA alone are not sufficient to elicit an anabolic effect (because the basal rate of muscle protein synthesis was not affected by LCn-3PUFA supplementation) but that they require additional anabolic stimuli such as amino acids and augment their anabolic effect by increasing the activation of the mTOR-p70s6k signalling pathway (which is considered an integral control point for muscle protein anabolism [33] and muscle cell growth [34–36]) and translational efficiency. What caused the greater activation of the mTOR-p70s6k signalling pathway after LCn-3PUFA supplementation remains unclear. It most likely was not increased Akt signalling because although LCn-3PUFA increased the concentration of AktThr308, the increase occurred both during basal conditions and during hyperinsulinema-hyperaminoacidemia whereas the stimulatory effect of hyperinsulinemia-hyperaminoacademia was the same before and after LCn-3PUFA supplementation. Thus, the effect was probably mediated via one or more alternative pathway(s), which have yet to be determined (but may include e.g., Rheb or vps34 [37, 38]). Considering the observed changes in skeletal muscle phospholipid composition, it is also possible that LCn-3PUFA supplementation modulated key substrates along the anabolic signalling cascades by affecting membrane lipid composition and/or fluidity [39, 40]. For example, increased membrane DHA content activates PKC [39], which stimulates translational activity [41]. It is unlikely that the beneficial effect of LCn-3PUFA on muscle protein synthesis was related to their anti-inflammatory properties [42, 43] because our subjects were young and healthy and we did not detect any treatment-induced changes in inflammatory cytokine concentrations in plasma – most likely because the concentrations were very low to begin with.
The increases in Akt, mTOR and p70s6k phosphorylation during the hyperinsulinemic-hyperaminoacidemic clamp in our study were small and did not always reach statistical significance at the p<0.05 level before intervention. This was most likely the result of a type-2 error associated with Tukey’s post-hoc analysis; in fact, when we applied Student’s t-test to evaluate the clamp-induced increases in mTOR and p70s6k phosphorylation before LCn-3PUFA supplementation, we obtained p-values of 0.01 and 0.06, respectively. It is unlikely that the small increase in signalling activation was due to the timing of the muscle biopsy (i.e., 3 h after the start of the insulin, amino acid and glucose infusion) because the phosphorylation of IRS-1, PI3K, Akt and mTOR in vivo in human muscle increases quickly and then remains steady (and elevated above basal values) for at least 180 min during constant insulin infusion and increased amino acid delivery to the muscle [44–46]. The small increases in anabolic signalling element phosphorylation were most likely due to the relatively low insulin and amino acid infusion rate, which we chose to avoid a potential “ceiling effect”. Specifically we infused amino acids and insulin at rates close to those used to achieve the half-maximal amino acid induced increase in muscle protein synthesis [47] and insulin mediated increase in Akt phopshorylation [48]. In fact, our results are well in line with the results obtained by other investigators [31], who found that during low-dose insulin infusion (similar to the one used in the present study) in conjunction with a high dose amino acid infusion (double the one used in this protocol) Akt, mTOR and p70s6k phosphorylation increased by ~30%, ~30% and ~90% respectively. The respective values in our study were ~25%, ~25% and ~50%. Furthermore, although it may seem as if there was a dissociation between mTOR and p70s6k activation because the magnitude of change in the phosphorylation of the two appeared to be different, the apparent discrepancy in signal activation is not surprising. The activation of mTOR and downstream signalling is known to be complex, involving not only phosphorylation but also regulation of interactions with many of its binding partners such as PRAS40, RAPTOR, DEPTOR, etc. [15, 49, 50]. Furthermore, although early work showed that the p70s6k was downstream of mTOR, it is now known that mTOR and p70s6k phosphorylate one another and the phosphorylation of mTOR on Ser2448 is mediated by p70s6k [51]. Thus, one cannot expect a simple 1:1 relationship in the extent of mTOR and p70s6k phosphorylation. However, we can be fairly sure on the basis of our data that mTOR-p70s6k signalling was increased by LCn-3PUFA supplementation.
We made our measurements of muscle protein synthesis during a 3-h infusion of insulin, amino acids and glucose because the rate of muscle protein synthesis rises quickly (within <30 min) in response to increased amino acid availability but then returns to basal values after ~2.5–3.0 h [52]. Therefore, we assume that the increase in the anabolic response after LCn-3PUFA supplementation was due to an increase in the magnitude of the anabolic response. However, we cannot rule out the possibility that the effect was due to an increase in the duration of the anabolic effect of nutritional stimuli. Similarly, it is possible, but unlikely, that the greater mTOR and p70s6k phosphorylation after LCn-3PUFA supplementation was due to prolonged activation rather than greater peak magnitude of activation because, as pointed out above, the phosphorylation of anabolic signalling elements increases quickly and then remains steady (and elevated above basal values) for at least 180 min during constant insulin infusion and increased amino acid delivery to the muscle [44–46].
The stimulation of the muscle protein anabolic response to hyperinsulinemia-hyperaminoacidemia by LCn-3PUFA supplementation occurred in the absence of significant changes in whole-body insulin-mediated glucose disposal. This is consistent with the lack of an effect of LCn-3PUFA on insulin-mediated Akt phosphorylation in muscle but contradicts the results from studies in animals [2–5] and also some studies in human subjects [53, 54]. For example, Delarue et al. [53] demonstrated that 1.8 g·d−1 of fish oil, which is rich in LCn-3PUFA, given for three weeks, diminished the insulin resistance of glucose metabolism as a consequence of dexamethasone treatment in healthy subjects. Also, Popp-Snijders et al. [54] demonstrated that 3 g·d−1 of fish oil, given for eight weeks, improved insulin sensitivity in subjects with type-2 diabetes mellitus. However, most studies in human subjects failed to discover a beneficial effect of LCn-3PUFA on insulin sensitivity (reviewed in detail by Fedor and Kelley [6]). It is possible that the simultaneous administration of glucose and amino acids and the increased mTOR signalling after LCn-3PUFA supplementation in our study masked a potential beneficial effect of LCn-3PUFA on glucose metabolism. There is evidence that increased amino acid-induced mTOR signalling inhibits insulin sensitivity [55, 56] and administration of rapamycin, a known inhibitor of mTOR, increases glucose uptake during a hyperaminoacidemic-hyperinsulinemic-euglycemic clamp in healthy men [57]. Nevertheless, protein/amino acids have a glucose lowering effect because co-ingestion of glucose and protein/amino acids increases plasma insulin concentration to a greater extent than glucose ingestion alone [58]. It is also possible that because our subjects were young and healthy, and had no signs of insulin resistance, LCn-3PUFA supplementation could not further increase their insulin sensitivity.
We elected to not measure potential changes in muscle mass during the eight weeks of LCn-3PUFA supplementation in our study because to do so would have required a much bigger sample size and most likely a longer duration of the intervention; changes in muscle protein metabolism, on the other hand, precede the corresponding changes in muscle mass and therefore ought to occur sooner after the start of the intervention. We also expected the effect of LCn-3PUFA on muscle protein synthesis to be greater (and thus more easily detectable with a small number of subjects) than their effect on muscle mass because the changes in muscle protein metabolism persist for only a few hours a day in sedentary individuals (during increased amino acid and insulin availability). In fact, with nine subjects we were able to demonstrate significant changes (in the order of ~30%) in the rate of muscle protein synthesis and the concentration of phosphorylated signaling elements in muscle during hyperinsulinemia-hyperaminoacidemia. Furthermore, we measured significant increases in both the muscle protein-to-DNA ratio (an index of muscle cell size [21]) and the muscle protein concentration (per mg wet weight of muscle) which suggest that LCn-3PUFA may have exerted an overall muscle anabolic effect in the order of 1–2% of muscle mass gain (i.e., the appendicular skeletal muscle mass in our subjects at the beginning of the study was 24 kg, the alkali soluble protein concentration in muscle was ~11% and increased by ~15% equivalent to a protein gain of ~0.4 kg). Therefore, it is unlikely that we would have obtained meaningful data, had we measured FFM or thigh muscle volume in our study. However, if confirmed in future studies, changes in muscle mass of this magnitude over such a short period of time would certainly be of clinical importance considering that the decline in muscle mass, which starts at about age 50 y is 0.2–0.5 % per year in healthy subjects [59, 60] and increased morbidity is demonstrable with as little as 5 % loss of muscle mass [61]. Improvements in muscle mass of this magnitude will also be clinically important in other muscle wasting conditions such as cancer cachexia and there is some evidence in the literature already that LCn-3PUFA supplementation may spare lean body mass in this population [62].
In summary, we have shown that LCn-3PUFA supplementation in healthy 25 – 45 y old individuals increases mTOR signalling and the anabolic response of muscle protein synthesis to hyperinsulinemia-hyperaminoacidemia, which resulted in increased muscle cell size (protein-to-DNA ratio) and protein concentration. These results confirm and expand upon our previous data obtained in older adults and support the notion of a direct muscle protein anabolic effect of LCn-3PUFA. Furthermore, these data provide a good basis for future research concerning the interaction between muscle protein and lipid metabolism.
Supplementary Material
Table 1.
Muscle phospholipid fatty acid profile before and after eight weeks of long-chain n-3 polyunsaturated fatty acid supplementation
| Before | After | P value | |
|---|---|---|---|
| Saturated FA | |||
| C14:0 | 0.48 ± 0.05 | 0.42 ± 0.03 | |
| C16:0 | 17.39 ± 0.53 | 17.69 ± 0.48 | |
| C18:0 | 18.35 ± 0.77 | 19.82 ± 0.70 | |
| Total | 36.22 ± 0.76 | 37.93 ± 0.96 | 0.21 |
| Mono-unsaturated FA | |||
| C16:1 n-7 | 0.58 ± 0.15 | 0.39 ± 0.05 | |
| C18:1 n-9 | 8.21 ± 1.13 | 6.35 ± 0.44 | |
| Total | 8.79 ± 1.27 | 6.74 ± 0.48 | 0.08 |
| n-6 PUFA | |||
| C18:2 n-6 | 32.09 ± 0.79 | 29.98 ± 0.71 | |
| C20:3 n-6 | 1.33 ± 0.10 | 1.27 ± 0.10 | |
| C20:4 n-6 | 17.19 ± 0.73 | 15.16 ± 0.48 | |
| Total | 50.61 ± 0.95 | 46.41 ± 0.59 | 0.01 |
| n-3 PUFA | |||
| C20:5 n-3 | 0.66 ± 0.11 | 2.57 ± 0.31 | |
| C22:5 n-3 | 1.81 ± 0.09 | 2.30 ± 0.08 | |
| C22:6 n-3 | 1.91 ± 0.17 | 4.05 ± 0.35 | |
| Total | 4.38 ± 0.33 | 8.93 ± 0.67 | <0.001 |
Values (percent of total fatty acids) are mean ± SEM. FA: fatty acid; PUFA: polyunsaturated fatty acid.
Acknowledgments
The authors wish to thank Hadia Jaffery and Rachel Burrows for help in subject recruitment, the staff of the Center for Applied Research Services for technical assistance, and the study subjects for their participation.
Funding
This publication was made possible by grant number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), NIH grants AR 49869, RR 00954 (Biomedical Mass Spectrometry Resource), and DK 56341 (Clinical Nutrition Research Unit), a grant from the Longer Life Foundation, the University of Nottingham, the UK Biotechnology and Biological Sciences Research Council grants BB/XX510697/1 and BB/C516779/1, and a European Union EXEGENESIS program grant. Gordon Smith was supported by an Ellison Medical Foundation/American Federation for Aging Research Postdoctoral Fellowship. Philip Atherton is a designated Research Councils UK fellow. Dominic Reeds was supported by an American Society of Nutrition Physician Nutrition Support Specialist Award.
Abbreviations
- ANOVA
analysis of variance
- BMI
body mass index
- BSA
body surface area
- CRP
C-reactive protein
- DHA
docosahexaenoic acid
- DNA
deoxyribonucleic acid
- eEF2
eukaryotic translation elongation factor 2
- EPA
eicosapentaenoic acid
- FFM
fat free mass
- FSR
fractional synthesis rate
- GC-MS
gas chromatography–mass spectrometry
- IL-6
interleukin 6
- LCn-3PUFA
long-chain n-3 polyunsaturated fatty acids
- mTOR
mammalian target of rapamycin
- PUFA
polyunsaturated fatty acids
- p70s6k
p70s6 kinase
- PKC
protein kinase C
- PRAS40
proline-rich Akt substrate of 40 kilodaltons
- Rheb
Ras homolog enriched in brain
- RNA
ribonucleic acid
- SDS PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- t-BDMS
tertiary-butyldimethylsilyl
- TNF-α
tumour necrosis factor alpha
- TTR
tracer tracee ratio
Footnotes
Author contributions
GIS was involved in conducting the study, processing the study samples, collecting data, performing the final data analyses, and writing the manuscript. PA and MJR were involved in processing the study samples, collecting data, and writing the manuscript. DNR and BSM were involved in conducting the study. DR was involved in sample processing and sample analyses. BM was involved in designing and conducting the study, processing the study samples, collecting data, performing the final data analyses, and writing the manuscript.
References
- 1.Fetterman JW, Jr, Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am J Health Syst Pharm. 2009;66:1169–79. doi: 10.2146/ajhp080411. [DOI] [PubMed] [Google Scholar]
- 2.Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 1987;237:885–8. doi: 10.1126/science.3303333. [DOI] [PubMed] [Google Scholar]
- 3.Taouis M, Dagou C, Ster C, Durand G, Pinault M, Delarue J. N-3 polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am J Physiol Endocrinol Metab. 2002;282:E664–71. doi: 10.1152/ajpendo.00320.2001. [DOI] [PubMed] [Google Scholar]
- 4.Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991;40:280–9. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- 5.Gingras AA, White PJ, Chouinard PY, Julien P, Davis TA, Dombrowski L, Couture Y, Dubreuil P, Myre A, Bergeron K, Marette A, Thivierge MC. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J Physiol. 2007;579:269–84. doi: 10.1113/jphysiol.2006.121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2009;12:138–46. doi: 10.1097/MCO.0b013e3283218299. [DOI] [PubMed] [Google Scholar]
- 7.van Norren K, Kegler D, Argiles JM, Luiking Y, Gorselink M, Laviano A, Arts K, Faber J, Jansen H, van der Beek EM, van Helvoort A. Dietary supplementation with a specific combination of high protein, leucine, and fish oil improves muscle function and daily activity in tumour-bearing cachectic mice. Br J Cancer. 2009;100:713–22. doi: 10.1038/sj.bjc.6604905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi N, Tashiro T, Yamamori H, Takagi K, Morishima Y, Otsubo Y, Sugiura T, Furukawa K, Nitta H, Nakajima N, Suzuki N, Ito I. Effect of intravenous omega-6 and omega-3 fat emulsions on nitrogen retention and protein kinetics in burned rats. Nutrition. 1999;15:135–9. doi: 10.1016/s0899-9007(98)00168-3. [DOI] [PubMed] [Google Scholar]
- 9.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93:402–412. doi: 10.3945/ajcn.110.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan AM, Reynolds JV, Healy L, Byrne M, Moore J, Brannelly N, McHugh A, McCormack D, Flood P. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009;249:355–63. doi: 10.1097/SLA.0b013e31819a4789. [DOI] [PubMed] [Google Scholar]
- 11.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–6. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 12.Gordon JN, Green SR, Goggin PM. Cancer cachexia. Q J M. 2005;98:779–88. doi: 10.1093/qjmed/hci127. [DOI] [PubMed] [Google Scholar]
- 13.Degens H. The role of systemic inflammation in age-related muscle weakness and wasting. Scand J Med Sci Sports. 2010;20:28–38. doi: 10.1111/j.1600-0838.2009.01018.x. [DOI] [PubMed] [Google Scholar]
- 14.Jeschke MG, Mlcak RP, Finnerty CC, Norbury WB, Gauglitz GG, Kulp GA, Herndon DN. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11:R90. doi: 10.1186/cc6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–9. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 16.Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, Combaret L, Dardevet D. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol. 2009;587:5483–5492. doi: 10.1113/jphysiol.2009.178319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86:451–6. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 18.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94:14930–5. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vary TC, Jefferson LS, Kimball SR. Amino acid-induced stimulation of translation initiation in rat skeletal muscle. Am J Physiol. 1999;277:E1077–86. doi: 10.1152/ajpendo.1999.277.6.E1077. [DOI] [PubMed] [Google Scholar]
- 20.Millward DJ, Garlick PJ, James WP, Nnanyelugo DO, Ryatt JS. Relationship between protein synthesis and RNA content in skeletal muscle. Nature. 1973;241:204–5. doi: 10.1038/241204a0. [DOI] [PubMed] [Google Scholar]
- 21.Forsberg AM, Nilsson E, Werneman J, Bergstrom J, Hultman E. Muscle composition in relation to age and sex. Clin Sci. 1991;81:249–56. doi: 10.1042/cs0810249. [DOI] [PubMed] [Google Scholar]
- 22.Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol. 2009;106:1374–84. doi: 10.1152/japplphysiol.91397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol. 2004;66:799–828. doi: 10.1146/annurev.physiol.66.052102.134444. [DOI] [PubMed] [Google Scholar]
- 24.Dietrichson P, Coakley J, Smith PE, Griffiths RD, Helliwell TR, Edwards RH. Conchotome and needle percutaneous biopsy of skeletal muscle. J Neurol Neurosurg Psychiatry. 1987;50:1461–7. doi: 10.1136/jnnp.50.11.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mittendorfer B, Horowitz JF, Klein S. Gender differences in lipid and glucose kinetics during short-term fasting. Am J Physiol Endocrinol Metab. 2001;281:E1333–9. doi: 10.1152/ajpendo.2001.281.6.E1333. [DOI] [PubMed] [Google Scholar]
- 26.Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575:305–15. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith GI, Villareal DT, Mittendorfer B. Measurement of human mixed muscle protein fractional synthesis rate depends on the choice of amino acid tracer. Am J Physiol Endocrinol Metab. 2007;293:E666–71. doi: 10.1152/ajpendo.00185.2007. [DOI] [PubMed] [Google Scholar]
- 28.Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metabolism. 1997;46:943–8. doi: 10.1016/s0026-0495(97)90084-6. [DOI] [PubMed] [Google Scholar]
- 29.Watt PW, Lindsay Y, Scrimgeour CM, Chien PA, Gibson JN, Taylor DJ, Rennie MJ. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: Use in studies of human tissue protein synthesis. Proc Natl Acad Sci U S A. 1991;88:5892–6. doi: 10.1073/pnas.88.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–4. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 31.Guillet C, Prod’homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004;18:1586–7. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–9. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–46. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276:C120–7. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- 35.O’Neil TK, Duffy LR, Frey JW, Hornberger TA. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol. 2009;587:3691–701. doi: 10.1113/jphysiol.2009.173609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–9. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 37.Goodman CA, Miu MH, Frey JW, Mabrey DM, Lincoln HC, Ge Y, Chen J, Hornberger TA. A phosphatidylinositol 3-kinase/protein kinase B-independent activation of mammalian target of rapamycin signaling is sufficient to induce skeletal muscle hypertrophy. Mol Biol Cell. 2010;21:3258–68. doi: 10.1091/mbc.E10-05-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem. 2005;280:33076–82. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- 39.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 40.Mansilla MC, Banchio CE, de Mendoza D. Signalling pathways controlling fatty acid desaturation. Subcell Biochem. 2008;49:71–99. doi: 10.1007/978-1-4020-8831-5_3. [DOI] [PubMed] [Google Scholar]
- 41.Grosso S, Volta V, Sala LA, Vietri M, Marchisio PC, Ron D, Biffo S. PKCbetaII modulates translation independently from mTOR and through RACK1. Biochem J. 2008;415:77–85. doi: 10.1042/BJ20080463. [DOI] [PubMed] [Google Scholar]
- 42.Browning LM. n-3 Polyunsaturated fatty acids, inflammation and obesity-related disease. Proc Nutr Soc. 2003;62:447–53. doi: 10.1079/pns2003252. [DOI] [PubMed] [Google Scholar]
- 43.Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6:461–7. doi: 10.1007/s11883-004-0087-5. [DOI] [PubMed] [Google Scholar]
- 44.Timmerman KL, Lee JL, Dreyer HC, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, Volpi E. Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial-dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J Clin Endocrinol Metab. 2010;95:3848–57. doi: 10.1210/jc.2009-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wojtaszewski JF, Hansen BF, Kiens B, Richter EA. Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes. 1997;46:1775–81. doi: 10.2337/diab.46.11.1775. [DOI] [PubMed] [Google Scholar]
- 46.Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939–45. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- 47.Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol. 2003;552:315–24. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–86. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–15. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem. 2005;280:25485–90. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- 52.Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–9. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delarue J, Li CH, Cohen R, Corporeau C, Simon B. Interaction of fish oil and a glucocorticoid on metabolic responses to an oral glucose load in healthy human subjects. Br J Nutr. 2006;95:267–72. doi: 10.1079/bjn20051631. [DOI] [PubMed] [Google Scholar]
- 54.Popp-Snijders C, Schouten JA, Heine RJ, van der Meer J, van der Veen EA. Dietary supplementation of omega-3 polyunsaturated fatty acids improves insulin sensitivity in non-insulin-dependent diabetes. Diabetes Res. 1987;4:141–7. [PubMed] [Google Scholar]
- 55.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–26. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–60. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- 57.Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, Roth E, Furnsinn C, Promintzer M, Anderwald C, Bischof M, Roden M. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007;56:1600–7. doi: 10.2337/db06-1016. [DOI] [PubMed] [Google Scholar]
- 58.Manders RJ, Wagenmakers AJ, Koopman R, Zorenc AH, Menheere PP, Schaper NC, Saris WH, van Loon LJ. Co-ingestion of a protein hydrolysate and amino acid mixture with carbohydrate improves plasma glucose disposal in patients with type 2 diabetes. Am J Clin Nutr. 2005;82:76–83. doi: 10.1093/ajcn.82.1.76. [DOI] [PubMed] [Google Scholar]
- 59.Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr. 2001;55:663–72. doi: 10.1038/sj.ejcn.1601198. [DOI] [PubMed] [Google Scholar]
- 60.Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83:229–39. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 61.Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care. 2003;6:295–9. doi: 10.1097/01.mco.0000068965.34812.62. [DOI] [PubMed] [Google Scholar]
- 62.Ryan AM, Reynolds JV, Healy L, Byrne M, Moore J, Brannelly N, McHugh A, McCormack D, Flood P. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009;249:355–63. doi: 10.1097/SLA.0b013e31819a4789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.