Abstract
Classical apoptotic cell death is now sufficiently well understood to be interrogated with mathematical modeling and to be skillfully manipulated with targeted drugs for clinical benefit. However, a biological black hole has emerged with the realization that apoptosis regulators are functionally multipolar. BCL-2 family proteins appear to have much greater effects on cells than can be explained by their known roles in apoptosis. While these effects may be observable simply because the cell is not dead, the general assumption is that BCL-2 proteins have yet undiscovered biochemical activities. Conversely, these yet uncharacterized day-jobs may underlie their profound effects on cell survival, challenging current assumptions about classical apoptosis. Even their sub-mitochondrial localizations remain controversial. Here we attempt to integrate seemingly conflicting information with the prospect that BCL-2 proteins themselves may be the critical crosstalk between life and death.
Keywords: apoptosis, Bcl-2, Bcl-xL, mitochondria, inner mitochondrial membrane, energetics
BCL-2 family proteins control more than apoptosis
The mammalian BCL-2 family is traditionally recognized as a collection of anti-apoptotic (e.g. BCL-2, BCL-xL, MCL-1) and pro-apoptotic proteins (e.g. BAX, BAK, BOK), plus the more distantly related BH3-only proteins (e.g. BID, BAD and others) that contain only one of the four BCL-2 homology (BH) motifs [1] (Fig. 1). In this capacity, BCL-2 family proteins determine whether cells live or die by controlling the permeability of the outer mitochondrial membrane following a cell death stimulus. If the pro-apoptotic family members prevail, cytochrome c exits mitochondria and stimulates caspase activation in the cytoplasm to cause characteristic apoptotic morphologies and cell death.
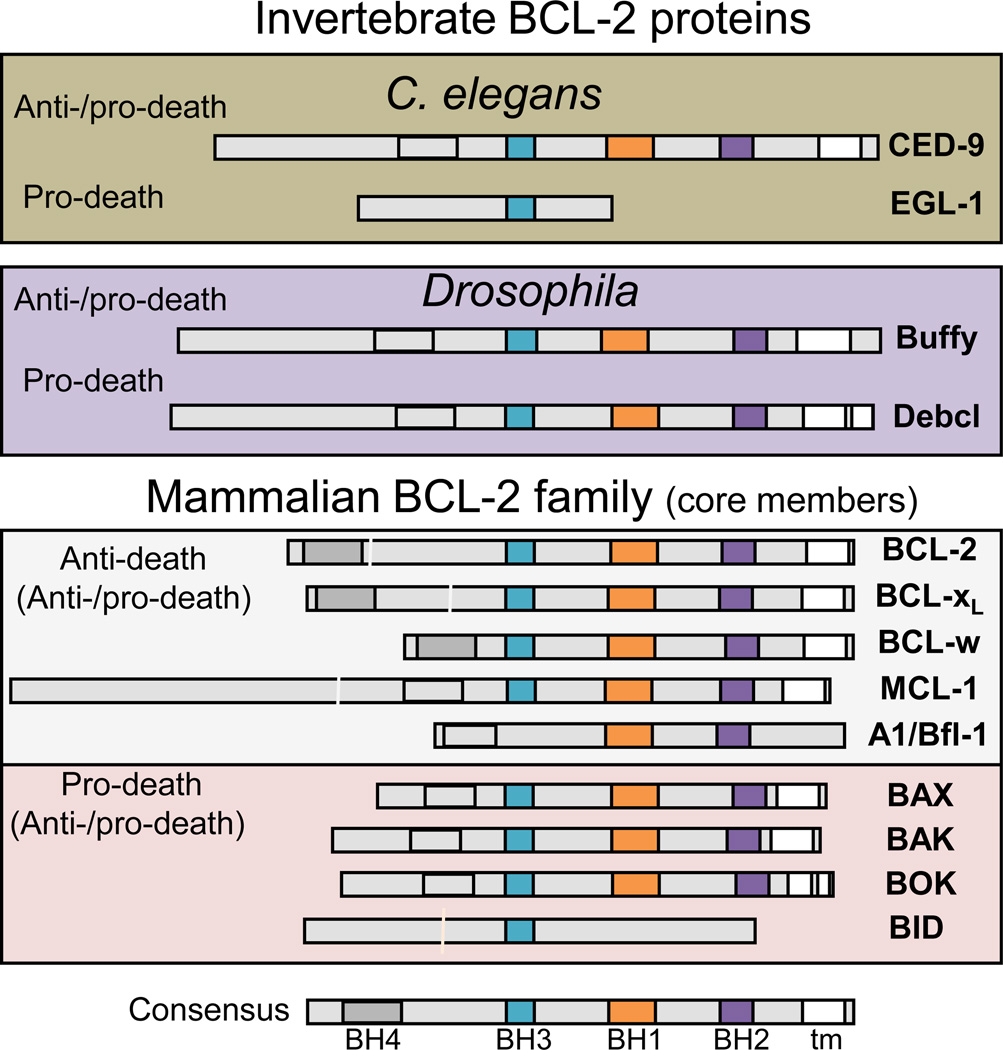
Figure 1.
Invertebrate and mammalian BCL-2 family proteins. Core conserved, multi-BH motif-containing members of the BCL-2 family often exhibit both anti- and pro-death activities depending on the circumstances (see text). Conserved caspase cleavage sites (vertical white lines), BH (BCL-2 homology) motifs, C-terminal transmembrane domain (tm), color key in consensus map, light gray boxes mark position of helix 1 but lacks amino acid homology to BH4. BID lacks significant overall amino acid sequence similarity, but retains the BCL-2 helical bundle structure.
The importance of BCL-2 family proteins during embryonic development, in healthy adult tissues and in cancer has been overwhelmingly demonstrated in genetically manipulated mouse models that abnormally accumulate extra (undead) cells, and is further supported by early findings from pre-clinical and clinical trials of BCL-2 inhibitors [2–4]. The detailed mechanisms of apoptosis are studied extensively in cultured cells, and are being further dissected in reconstituted biochemical reactions [5]. However, BCL-2 family proteins also have effects in long-lived cells that likely extend beyond thwarting classical apoptosis. BCL-2 proteins appear to have substantial effects on cellular metabolism [6], mitochondrial morphology [7], redox status [8], levels of acetyl-coA [9], calcium homeostasis [10], glucose sensing [11], autophagy [12], neuronal activity [13] and other fundamental processes of healthy cells. Some of these functions may be better conserved evolutionarily than classical apoptosis [14].
One trivial explanation for the seemingly profound effects of BCL-2 family proteins on healthy cells is that we are simply observing functions of normal cells that would otherwise be dead. For example, mice lacking pro-apoptotic BAX are widely exploited to investigate the effects of required signaling pathways in the nervous system, under the assumption that deletion of BAX simply facilitates neuron survival without other consequences [15]. The same logic could be applied to challenge our assumption that BCL-xL has additional non-apoptotic functions, when in fact these pleotropic effects of BCL-xL simply reflect surviving cell activities otherwise lost from the experiment. Conversely, if BAX directly alters neuronal activity through non-apoptotic mechanisms as suggested [16], then a non-apoptotic function of BAX on NGF signaling also needs reconsideration. Clearly these are both circular arguments that cannot be easily untangled.
Despite entangled logic and prevailing controversies, growing evidence supports the existence of non-apoptotic functions of BCL-2 proteins. While awaiting definitive biochemical mechanisms to resolve this issue, we consider some of the available evidence. However, even the most basic questions remain, for example whether non-apoptotic functions involve interactions between anti- and pro-apoptotic BCL-2 family members similar to apoptosis. Also unknown is whether non-apoptotic functions of BCL-2 proteins stem from their interactions with a multiplicity of unrelated binding partners reported in the literature [17], or if both apoptotic and non-apoptotic functions ascribed to BCL-2 proteins are manifestations of a biophysical mechanism that is yet undiscovered, or all of the above.
The non-apoptotic mechanisms of BCL-2 proteins could reflect a core property inherent to the helical bundle structure of BCL-2 family proteins that apparently pre-dates classical apoptosis [18, 19]. If apoptosis is an acquired or even ancillary role of BCL-2-shaped proteins to facilitate disposal of mammalian cell corpses, then their seemingly omnipotent effects on living cells remain unexplained. Here, we consider recent advances striving to delineate the underlying functions of BCL-2 family proteins.
Evolutionary distinctions between BCL-2 family proteins
BCL-2 proteins are not unique to mammals. Obvious homologues of mammalian BCL-2 proteins are encoded by flies (Arthropoda, e.g. Drosophila), round worms (Nematoda, e.g. C. elegans), flatworms (Platyhelminthes, e.g. schistosomes and planaria), hydra (Cnidaria, e.g. Hydra, jelly-related), and the simpler metazoans Porifera (sponges) and Placozoa, but are not known to exist in fungi, plants or prokaryotes [18, 20, 21]. Therefore, BCL-2 family proteins (defined by significant shared amino acid sequence similarity) are restricted to multi-cellular animals (and some of their viruses). These species also encode homologues of mammalian caspases, together implying that BCL-2-regulated apoptosis evolved with metazoans. However, this cannot be construed to mean that all types of cell death programs evolved with metazoans. To the contrary, non-apoptotic death programs apparently exist in single-cell species such as fungi and bacteria (as well as mammals and plants) to ensure survival of their species over individual cells [22, 23].
Metazoan BCL-2 genealogy also cannot automatically be extrapolated to infer functional corollaries throughout metazoans. For example, C. elegans BCL-2 proteins, and possibly some vertebrate family members, do not appear to regulate MOMP (mitochondrial outer membrane permeability), the quintessential role of BCL-2 proteins in mammalian apoptosis. Additionally, only a subset of vaccinia virus proteins that adopt a BCL-2-like structure have amino acid sequence similarity to BCL-2, and most do not regulate apoptosis [24–26]. Yet these viruses maintain multiple genes encoding BCL-2-shaped factors, implying critical roles in the virus life cycle. Whether the more distant BCL-2-like structural similarity of bacterial toxins, such as the Diphtheria toxin translocation domain, will share mechanistic similarities to mammalian BCL-2 homologs is not yet known [27]. The helical translocation domain of Diphtheria toxin, which undergoes pH-dependent conformational changes leading from shallow to full insertion into membranes, and together with other cell factors, translocates its catalytic domain across the membrane [28]. Perhaps some of these events share analogies with BCL-2 proteins.
The inclusion of BH3-only proteins as BCL-2 family members complicates the definition of this protein family. While BID folds similarly to other BCL-2 sequence homologues, despite lack of overall amino acid sequence similarity, other core BH3-only proteins are, thus far, unstructured and may or may not adopt a BCL-2 fold when engaged by its partners [29]. Another group of clearly unrelated proteins have also been reported to contain the poorly defined BH3 motif, including autophagy regulators Beclin 1/ATG6 and ATG12 [30, 31]. Therefore, these proteins can be classified as BH3-only proteins but are not BCL-2 family members nor adopt a BCL-2 structural fold [32]. Structure determinations have confirmed the bona fide BH3-like motif of Beclin bound to several viral and cellular BCL-2 homologues. However, a number of other proteins have been reported to contain BH3 motifs, but lack similar supporting evidence. The potential existence of BH3 motifs in species that lack BCL-2 proteins in some ways defies the definition of a BH3-only protein. Given the poorly defined sequence of the BH3 motif, there is considerable room for varied interpretations. The existence of a functional BH3-only protein in worms (EGL-1) that is involved in both apoptosis and non-apoptosis functions argues that at least some aspects of both the death and day-job mechanisms will overlap (see below). However, it is not yet known if worm BCL-2 proteins control other mitochondrial functions analogous to the metabolic functions of mammalian BCL-2 proteins [33, 34]. Again, the difficulty is to experimentally separate fundamental cell survival functions from apoptotic functions.
Worms provide novel insights for mammalian BCL-2
Historically, CED-9 paved the way to understand the function of mammalian BCL-2 proteins. Compelling genetic and biochemical evidence, have firmly established that C. elegans BCL-2-related protein CED-9, and a BH3-only protein EGL-1 (Fig. 1), regulate apoptosis defined as caspase-dependent (although MOMP-independent) cell death [35]. High resolution protein structures further support the conclusion that pro-death EGL-1 binds and inhibits CED-9 (BCL-2), thereby preventing CED-9 from binding and inhibiting CED-4, the activator of worm caspase CED-3 [36, 37]. However, the subcellular localization of these factors in worms is debated (Box 1). The mechanism of apoptosis in worms is analogous to the mammalian “indirect activation” model in that a BH3-only protein inhibits anti-death BCL-2 proteins. However, worms differ from mammals (and perhaps Drosophila) as mammalian BCL-2 proteins are not known to directly engage the caspase activation machinery, but may have intermediary factors [3, 38].
Box 1. Resolving inner versus outer mitochondrial membrane localization of BCL-2 proteins.
Controversies. Many studies have sought to delineate sub-mitochondrial localizations of BCL-2 family proteins, as this information is critical for deciphering function. New evidence that includes knock-out controls has confirmed earlier reports indicating that BCL-2 proteins can be on the inner mitochondrial membrane [33, 34, 82–86]. However, the prevailing opinion for over a decade has been that both multi-domain and BH3-only BCL-2 family proteins localize strictly to the outer, not inner, mitochondrial membrane [87–90]. Opposite results obtained with different antibodies has led to general distrust of immuno-gold electron microscopy results.
Conformation-specific antibodies may be more common. Taking a closer look at the evidence suggests that some discrepancies could be due to conformation-dependent antibody specificities (see Fig. 3). Given the well-characterized N-terminal conformation-specific antibodies that distinguish inactive from activated BAX and BAK (see text), it is worth considering this possibility for other BCL-2 family members. In fact, the majority of currently available antibodies are directed to N-terminal regions of BCL-2 proteins. Conformation-dependent epitopes are more likely to influence microscopy data where native epitopes are better preserved, but may also affect biochemical fractionation studies, which also depend on antibodies for Western blots, which are not immune from conformational and charge effects (see Box 3). In addition, there are countless antibodies that detect posttranslationally modified proteins only, and vice versa. Nevertheless, there is a general tendency to completely dismiss results with antibodies that recognize different patterns for the same protein by assuming non-specific cross-reactivity. While this is justifiable to an extent, there are additional considerations. It is challenging to distinguish conformation-dependent epitopes from cross-reacting artifacts. Using Western blots to make decisions about the suitability of different antibodies for microscopy is not well founded if the antibodies are conformationdependent. If knock-down cells or knock-out tissues exhibit a significant reduction in the signal, it may be worth considering the possibility of epitope-specific conformations. Finally, all antibodies will exhibit nonspecific interactions when used at inappropriate concentrations, and non-specific interactions of different antibodies are suppressed by different blocking solutions, emphasizing the need for genetic tools in evaluating BCL-2 family antibody specificities. In our experience, no two anti-BCL-xL or anti-BCL-2 antibodies behave exactly the same.
Worm BCL-2 family proteins appear to have additional non-apoptotic functions that are shared with mammals but are best characterized in worms. CED-9 regulates mitochondrial shape changes in healthy cells by physically interacting with the large dynamin-like GTPases that directly mediate organelle fusion (FZO-1/MFN1-2) and fission (DRP1) [39–41]. Similar findings have been reported for mammalian BCL-2 family proteins, as both pro- and anti-death BCL-2 family proteins regulate mitochondrial fission and fusion. BCL-xL interacts with Drp1 and can increase the absolute rates of mitochondrial fission and fusion in neurons [7, 42], and BAX can stimulate MFN2-dependent mitochondrial fusion in vitro [43]. Ironically, the pro-apoptotic family member BAX is required for normal mitochondrial fusion in healthy mammalian cells, while it promotes excessive mitochondrial fission during cell death [44].
The mitochondrial receptor involved in recruiting cytosolic Drp1 to sites of fission on mitochondria has been debated. In yeast the conserved Fis1 protein can recruit Dnm1 (yeast homolog of Drp1) to promote mitochondrial fission through a direct interaction, but this has been challenged in mammals with the identification of additional candidate receptors [45]. Interestingly, recruitment of worm DRP1 to mitochondria requires the BCL-2 family proteins [41]. Although the BH3-only protein EGL-1 inhibits CED-9 to promote cell death, EGL-1 can also form a complex with CED-9 to serve as a mitochondrial receptor for DRP1, apparently for the purpose of promoting normal organelle fission in healthy cells [41]. Thus, the interaction of a BCL-2 protein with its BH3-only binding partner is functional in both cell death and non-apoptotic functions. Although the factors CED-9, EGL-1 and DRP1 have all been linked to both mitochondrial fission in healthy cells and to apoptosis, a potential role for these factors in regulating a life-death switch is intriguing.
Fly BCL-2 proteins provide yet another perspective
Drosophila melanogaster encodes two BCL-2 homologous proteins, Buffy and Debcl, both of which are most like mammalian BOK, a poorly characterized putative pro-death protein (Fig. 1). Unlike worms, but similar to mammals, Drosophila mitochondria can become permeabilized to cytochrome-c during cell death [46, 47]. However, this permeabilization may not to involve BCL-2 family proteins, in contrast to mammals. Instead of BCL-2 proteins, permeability of Drosophila mitochondria requires a different set of pro-death factors that includes Reaper and Hid, which also localize to mitochondria [48]. There are no obvious overall sequence homologues of Reaper or Hid in mammals or worms, though distant orthologues have been reported in other insects [49].
Although cytochrome-c can be released from Drosophila mitochondria during apoptosis, a prodeath function for Drosophila cytochrome-c was not detected in some models [47, 48]. However, a pro-death role of Drosophila cytochrome-c-d was detected in the eye [50], during spermatogenesis and in specific cells affecting a scutellar bristle in the adult [51, 52]. Similar to mammals, Drosophila caspase Dronc and its activator Ark/Dark (homologue of mammalian Apaf-1) also contribute to this death, consistent with the mammalian pathway. Whether mitochondrial permeability is fundamental to Drosophila apoptosis in general is not fully explored, but for now, it appears unlikely that release of cytochrome c has a global role in flies and worms. However, the same is true for mammals based on phenotypes of a clever knock-in mouse encoding respiratory-competent but death-incompetent cytochrome c, which predominantly ablates cell death in the nervous system [53]. Analogous approaches may be required in other species to separate the conserved respiratory chain function of cytochrome c (the electron carrier between complexes III and IV), and potentially other functions such as redox regulation, from any direct pro-apoptotic role in catalyzing caspase activation. However, unlike mammals, the Ark/CED-4 apoptosome of flies and worms apparently is not activated by cytochrome c proteins, despite conservation of apoptosome-mediated caspase-dependent developmental cell death in all three species. Thus, the molecular mechanism by which release of Drosophila cytochrome c–d (or other mitochondrial factors) promotes death in Drosophila and most other species is unclear.
Buffy was originally reported to exhibit only anti-death activity [54]. Thus, it was somewhat unexpected that flies lacking Buffy seemed normal, in striking contrast to embryonic lethality in mice lacking anti-death family members BCL-xL or MCL-1, (although deletion of BCL-2 or BCL-w produce viable adults) [55, 56]. Similarly, worms lacking CED-9 are inviable [57]. Drosophila geneticists have downplayed a potential role for Drosophila BCL-2 homologues in regulating physiological cell death, claiming primarily minor phenotypes and controversies [48, 58, 59]. However, Drosophila BCL-2 family proteins may have a greater role in cell death regulation than currently appreciated. In contrast to earlier reports, an engineered genetic knock-out of pro-death Debcl revealed a few extra surviving cells in subregions of the embryonic nervous system [59]. This is potentially analogous to BAX knock-out mice, which have a few more neuroprogenitors and have low numbers of extra (undead) neurons that accumulate over time in the adult to significantly improve cognitive functions [60, 61]. Although these effects of BAX are assumed to be due to its pro-apoptotic function by triggering cytochrome c release (MOMP) and caspase activation, alternative functions for BAX (and BAK) in synaptic activity that appear to be independent of cell death cannot be ruled out [13, 16].
Drosophila BCL-2 family proteins may have a greater role in cell death than is currently appreciated. A recent twist arose with the discovery that the supposedly anti-death Buffy protein can also promote cell death, perhaps analogous to the bipolar functions of worm CED-9 and mammalian BCL-2 family proteins [62]. For example, in the absence of Buffy, Grim-mediated death is suppressed during Drosophila development causing increased numbers of glial cells [63]. Furthermore, a potent age-dependent pro-death function for Buffy and Debcl was recently reported in the ovary during nutrient deprivation [64]. Although mice lacking both BAX and BAK rarely yield viable pups, the lack of a lethal phenotype in double Buffy/Debcl-deficient files does not diminish the importance of Drosophila BCL-2 protein-mediated death, as lethality can reflect the death of key cells subsets. In the Drosophila model, single and double mutants of Buffy and Debcl retain undead egg chambers in mid-oogenesis when flies are deprived of dietary amino acids/protein. This process is not dependent on Grim, Reaper or Hid, and therefore may not involve MOMP or cytochrome-c. However, this death is dependent on the Drosophila caspase Dcp-1, and the new evidence suggests that fly BCL-2 proteins can promote caspase-dependent remodeling and degradation of mitochondria during cell death [64]. Intriguingly, autophagy regulators and mitochondrial fission and fusion factors are also required in this death pathway, although the details are not yet known [64]. Thus, Drosophila BCL-2 proteins appear to have mitochondrial functions in controlling cell death in the ovary that presumably occurs under physiological starvation conditions. Delineation of these mechanisms may be revealing about evolutionarily conserved non-MOMP mechanisms linking cell death/survival with mitophagy, and possibly even to mitochondrial energetics.
Regulation of mitochondrial dynamics in healthy cells is often considered a non-canonical or non-apoptotic function of mammalian and worm BCL-2 proteins. Taking together this study of Drosophila BCL-2 proteins in the ovary with the evidence from mammals and worms, suggests dual control of normal mitochondrial dynamics and cell death by BCL-2 family proteins may extend to Drosophila.
Linking apoptosis and alternative BCL-2 protein functions
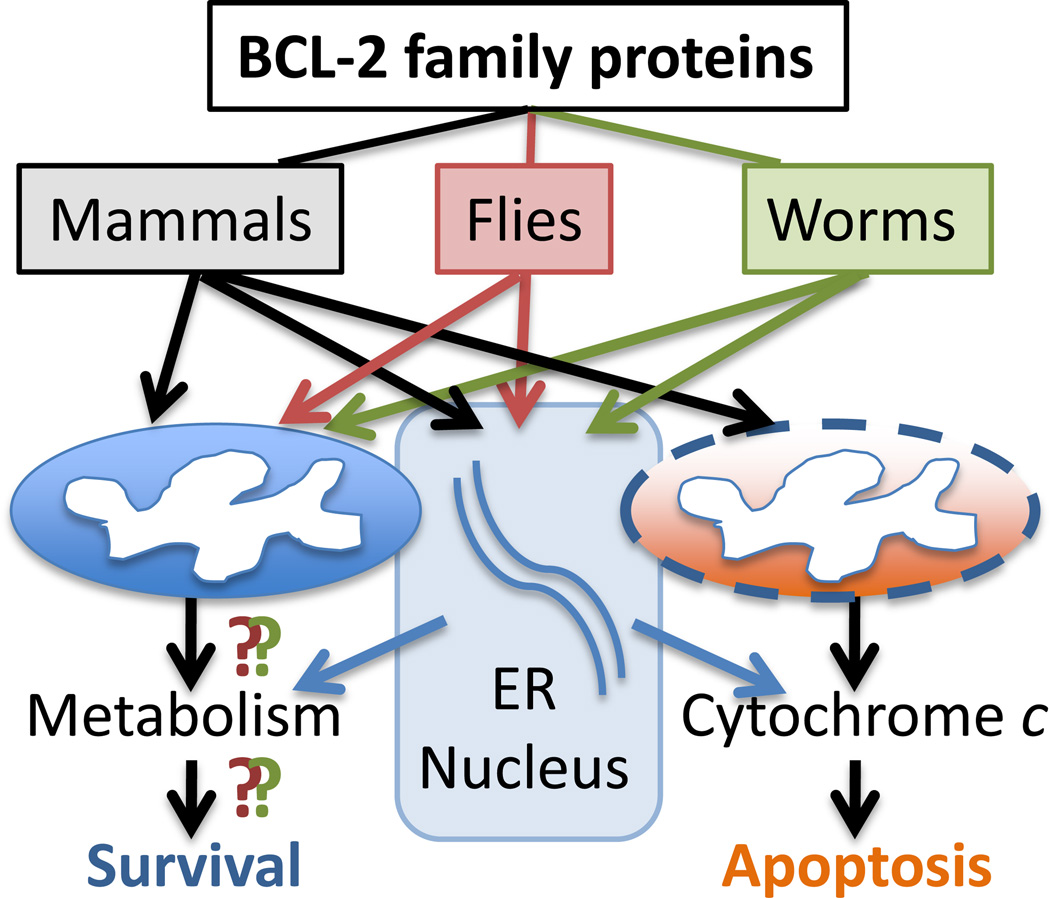
A general theme has emerged from comparing mammalian and invertebrate BCL-2 family proteins as described above. This evidence suggests that the control of mitochondrial membrane structure is an evolutionarily conserved function of BCL-2 proteins during cell death, but seemingly for different purposes. There is not a consistent theme regarding their roles in regulating apoptosis by controlling cytochrome c release or MOMP in general (Figure 2). This is further supported by comparing the ability of cytochrome c from different superphyla (ecdysozoans, platyzoans and lophotrochozoans) of the animal kingdom, leading to the conclusion that MOMP is an ancient function that was lost by some phyla such as Nematoda (e.g. C. elegans) during evolution [65]. This does not explain why flies and worms retained their BCL-2 family proteins after losing MOMP during evolution, and implies that their BCL-2 proteins have a function other than MOMP. However, it is difficult to definitively determine if this is related to an original function of primordial BCL-2 or to an acquired function. Despite inconsistent roles during cell death, BCL-2 proteins in worms flies and mammals have a role in altering mitochondrial morphology, opening the possibility of a shared yet unclarified function of BCl-2 proteins, though potentially adapted for specific purposes. Any conserved biophysical functions, when revealed, may also explain why BCL-2 proteins are evolutionarily conserved.
Figure 2.
Contrasting functions of invertebrate and mammalian BCL-2 family proteins. Seemingly disparate findings in mammals, flies and worms have not yielded a consensus regarding the function or subcellular localization of BCL-2 family orthologues.
Like the apoptotic functions of BCL-2 family proteins, their non-apoptotic functions in mitochondria may be directly related to their ability to control the permeability and structure of mitochondrial outer and inner membranes (see below). The physical and/or functional interactions between BCL-2 family proteins and the mitochondrial fission (e.g. Drp1) and fusion (e.g. Mfn/Fzo1) machineries is an emerging theme across species in both surviving and dying cells (see above). These mechanisms potentially link the studies in worms, mammals and now flies, in which non-canonical functions of BCL-2 proteins through unknown mechanisms control the mitochondrial dynamin-like GTPases that mediate the birth of new mitochondria through fission, their ΔΨ-dependent fusion events and potentially their degradation through mitophagy. For example, studies from several groups suggest that the mitochondrial membrane potential determines whether mitochondria undergo mitophagy or they are recycled by fusion to restore their capacity [66]. Thus, it is conceivable that BCL-2 proteins influence mitochondrial bioenergetic capacity in this manner, which in turn explains their pleotropic effects on cells. Then in response to some crisis, they are triggered to convert from their day-jobs to carry out their apoptosis functions in the final hour of the cell's life. However, this explanation seems woefully insufficient to explain more than a handful of all the effects ascribed to BCL-2 proteins, even those with known links to global metabolism such as the role of BCL-xL in suppressing cellular acetyl-coA and N-terminal alpha-acetylation of many cellular proteins to inform the apoptosis machinery about the metabolic state of the cell, or the role of BAD in glucose-sensing and feeding behaviors [9] and [11].
Electron microscopy reveals that mitochondria are tethered near neuronal synapses and that inner membrane cristae are oriented perpendicular to the synapse presumably for a purpose [67, 68]. Furthermore, caspases are needed to produce LTD (long term depression) in specific synapses and not in others, implying that subcellular remodeling (e.g. removal of a synapse or spine) is activated by highly localized caspase release [69]. This idea is further supported by evidence that death receptor DR6 recruits the BAX-caspase pathway to cause axonal degeneration [70].
The work in flies also could imply that DRP1 or other factors that regulate dynamics might localize the mitochondria at specific sites for activation of caspase-dependent mitochondrial remodeling at these sites [71]. The formation of the BAX pore in conjunction with Drp1 translocation to the mitochondria supports this idea. These events may ultimately be found to link non-canonical BCL-2 functions to bioenergetics. Mitochondrial depolarization and MOMP may activate or signal mitophagy in response to different stimuli, especially if mitochondria are not as long-lived as generally assumed.
Where do BCL-2 proteins localize in healthy cells?
The core anti- and pro-death (multi-BH motif) members of the BCL-2 family from mammals, flies and worms are helical proteins typically 20–35 kDa in size (Fig. 1). Membrane-targeting sequences have been mapped to the N-terminus, C-terminus and the central helical hairpin of BCL-2 family proteins (helices 5 and 6) located between BH1 and BH2 [1]. Although their classical apoptosis functions in mammals are played out on the outer mitochondrial membrane, BCL-2 family proteins are commonly found on the endoplasmic reticulum (ER), and have also been reported inside the nucleus, free in the cytoplasm, attached to cytoskeletal components, and in many distinct protein complexes. While some of these localizations have been connected to the regulation of apoptotic cell death, they may also be involved in non-apoptotic functions of BCL-2 family proteins.
The best-characterized membrane-targeting domain of BCL-2 family proteins is the C-terminal hydrophobic helix. This hydrophobic tail is often flanked by basic residues that contribute to outer mitochondrial membrane localization, as observed for BCL-xL [72, 73]. Fewer basic residues favors ER localization as observed for BCL-2. The hydrophobic tail also traverses the membrane to serve as a transmembrane (tm) anchor. Cytosolic versions of at least some family members have their C-terminal tail folded back into a prominent groove on one side of the molecule, the same groove that binds BH3 motifs of partner proteins during cell death [1]. In this manner, BAX is retained in the cytoplasm and away from mitochondria, helping to prevent apoptosis.
Re-localization to mitochondria is not the only mechanism for activation of BAX to kill. For example, pro-apoptotic BAK resides constitutively at the mitochondrial membrane [74]. Furthermore, unlike some family members, exposure of the C-terminal tail of BAX is not sufficient to target a heterologous protein (e.g. GFP) to mitochondria, unless the central Ser (S184) is deleted (or mutated) [75, 76]. A conformational change in the N-terminal helix of BAX represents an early required step in BAX activation to cause cell death [77–79]. Interestingly, specific monoclonal antibodies can distinguish unactivated and activated BAX. For example, the widely used monoclonal 6A7 directed against amino acids 13–19 specifically detects only activated BAX, presumably because a hidden epitope becomes exposed upon activation [75]. Similar activation-specific antibodies have been developed for BAK [74]. Ultimately, BAX is thought to oligomerize into either a proteinaceous or lipid-lined pore in the outer mitochondrial membrane, which is suggested to be a passageway through which cytochrome c exits mitochondria during apoptosis [80]. Inner membrane localization of BCL-2 proteins has also been reported but remains controversial (see below).
An alternative model suggests that BAX remodels membranes in a different way. In this scenario BAX is suggested to induce a lipidic pore at a hemifission site that leads to rupture of the outer mitochondrial membrane and cell death [81]. Considering the early evidence from the Drosophila ovary suggesting that BCL-2 proteins are involved in mitochondrial remodeling (see above), together with the idea that mammalian BAX remodels membranes near fission sites in cooperation with the fission factor Drp1, suggests an important link between mitochondrial dynamics and caspase-dependent apoptotic cell death. Thus, this link between mitochondrial dynamics and apoptosis may be conserved in some invertebrates (Fig. 2).
Outer versus inner mitochondrial membrane localization of BCL-2 proteins
Controversy surrounds the subcellular and submitochondrial localizations of BCL-2 proteins, particularly with regard to sub-mitochondrial compartments. This controversy is driven by the fact that different groups using biochemical fractionation experiments and immuno-electron microscopy, or both, have reported opposite results (Fig. 3 and Table 1). By revisiting these seemingly contradictory findings, several potential explanations emerge. Founded and unfounded concerns about antibody specificities, conformation-dependent epitopes (Box 1), and inherent assumptions about fractionation results and mitochondrial structure (Box 2) have resulted in information that could potentially be better integrated rather than selectively adopted or dismissed.
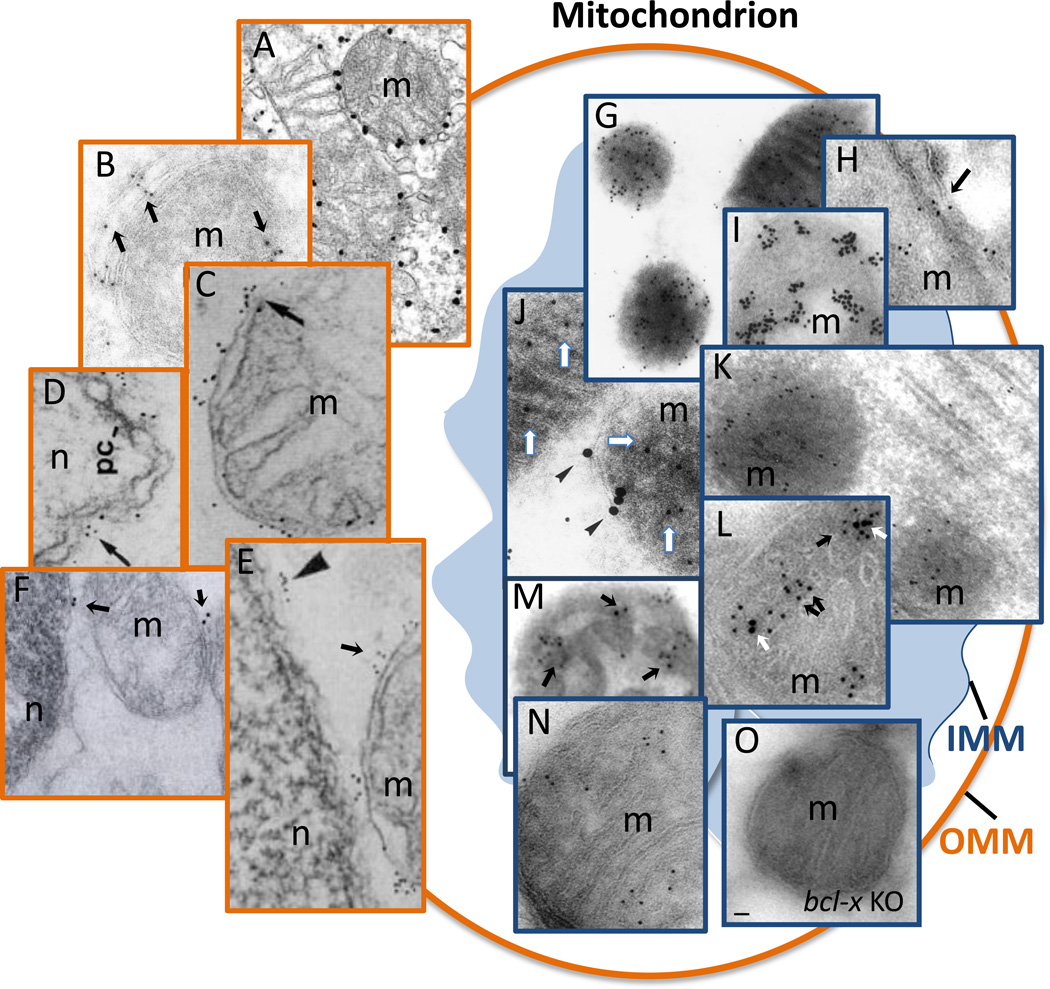
Figure 3.
Examples of published immuno-gold electron microscopy results that detect outer mitochondrial membrane (OMM) localization (A–F) or inner mitochondrial membrane (IMM) localization of BCL-2 family proteins (G–N). (A) Overexpressed BAX; (B–F) endogenous BCL-2; (G,J, K, M) endogenous BCL-2; (H and N) endogenous BCL-xL; (I) stably expressed BCL-2; (L) overexpressed BCL-xL; (O) conditional knock-out stained for BCL-xL in parallel with panels H and N. (All figures republished with permission, details in Table 1).
Table 1.
Immuno-gold labeling of BCL-2 family proteins.
|
Fig. 3 panel |
Sample source | Antibody used for immuno-gold labeling |
Refs. |
|---|---|---|---|
| Evidence for OUTER mitochondrial membrane labeling | |||
| A | BAX transfected Cos-7 cells | Anti-Bax | [91] |
| B | SU-DHL-4 cells | Anti-Bcl-2 monoclonal MAb #100 | Fig. 4 of [92] |
| C | Human lymph node biopsy/ lymphoma | Monoclonal mouse anti-human Bcl-2 (Dako 124) | Fig. 1D of [93] |
| D | Human lymph node biopsy/ lymphoma | Monoclonal mouse anti-human Bcl-2 (Dako 124) | Fig. 1E of [93] |
| E | Human lymph node biopsy/ lymphoma | Monoclonal mouse anti-human Bcl-2 (Dako 124) | Fig. 1C of [93] |
| F | FDC-P1 myeloid cells +bcl-2/neo virus | Monoclonal antibody Bcl-2–100 | Fig. 3A of [94] |
| Evidence for INNER mitochondrial membrane labeling | |||
| G | HL60 (human promyelocytic leukemia) | Anti-BCL-2 “pAb” (rabbit anti-human BCL-2 polyclonal against amino acids 4–21 of human/mouse/rat on 10 nm gold) | Fig. 2B of [84] |
| H | Mouse brain (hippocampus) | Rabbit anti-BCL-xL (Biocarta) | [33] |
| I | HeLa (human epithelial cancer) stably expressing human BCL-2 | Anti-BCL-2ΔC21 “mAb” (Santa Cruz) where 60% of mitochondrial label is inside mitochondria | [86] |
| J | HL60 | Anti-BCL-2 “pAb” (10 nm gold) co-labeled with anti-monoamine oxidase (20 nm gold, black arrows) to mark outer mitochondrial membrane | Fig. 3A of [84] |
| K | Rat liver | Rabbit anti-human BCL-2 polyclonal antibody “pAb” (10 nm gold), which is enriched in mitochondria rather than ER | Fig. 2A of [84] |
| L | Rat brain | Rabbit anti-BCL-xL (Biocarta, 10 nm gold, 5 nm gold, white arrows), co-labeled with and with anti-SOD (5 nm gold) | [34] |
| M | Isolated PC12 mitochondria | Anti-Bcl-2 [anti-rat Bcl-2, PharMingen], cryothin-section immunogold | Fig. 4B of [85] |
| N | Mouse brain (hippocampus) | Rabbit anti-BCL-xL (Biocarta) | [33] |
| O | bcl-x conditional knockout mouse brain (hippocampus) | Rabbit anti-BCL-xL (Biocarta) prepared in parallel with panels H and N | [33] |
Box 2. Are any mitochondrial proteins on both the inner and outer membrane?
The traditionally preferred method for distinguishing inner from outer membrane localization of cellular proteins is biochemical fractionation followed by Western blot analysis. It is widely accepted that even the best-optimized fractionation protocol results in imperfectly separated sub-fractions. This inevitable cross contamination is not a major problem, if any given protein is assumed to localize exclusively either to the inner or the outer mitochondrial membranes, but not to both. This assumption has held for the majority if not all mitochondrial proteins analyzed thus far, but a precedence for dual localization was recently established for the mitochondrial kinase PINK1, which is mutated in patients with Parkinson’s disease [117]. PINK1 is a kinase with a bona fide N-terminal mitochondrial import signal peptide that is cleaved during ΔΨ-dependent translocation to the inner membrane where it is promptly degraded [118]. However, upon mitochondrial depolarization, PINK1 accumulates in a large complex with the translocator of the outer membrane TOM [119]. If import into mitochondria does not resume and PINK1 is left on the mitochondrial surface, the result is recruitment and activation of Parkin, an E3 ubiquitin ligase that facilitates degradation of mitochondria via mitophagy [66]. Defects in this mechanism reduce turnover of impaired mitochondria, which is thought to contribute to Parkinson’s disease. Thus, PINK1 can localize to either inner or outer mitochondrial membranes to serve as a monitor of mitochondrial competence. This finding may help to ease opposition to the possibility that members of the BCL-2 family could normally localize to both the inner and outer mitochondrial membranes. If true, then the problem of cross contamination of mechanically separated sub-organelle fractions becomes a greater problem. Further complicating this issue is the intimate linkage between the outer mitochondrial membrane and the ER, and between the inner and outer mitochondrial membranes. The use of membrane extraction strategies and proteases are compromised by whether a given protein is inside or outside the membrane, and its relative extractability and protease susceptibility relative to selected control proteins. Despite the caveats associated with microscopy (Box 1), microscopy may be required to confirm fractionation results.
The original report argued for inner mitochondrial membrane localization of BCL-2 based on biochemical approaches [82]. This conclusion was supported by several subsequent immuno-electron microscopy studies [83–86]. However, another series of early biochemical and electron microscopy studies failed to detect inner membrane localization, overturning the original report and leading to the dominant opinion that BCL-2 family proteins are localized strictly to the outer, not inner, mitochondrial membrane [87–94]. Recently, the sub-mitochondrial localization debate has been revived by new findings indicating that at least some BCL-2 family members localize to both the outer and inner mitochondrial membranes, a dual-localization phenomenon with little precedence. Both immuno-gold electron microscopy and biochemical stripping of isolated mitochondria reveal that endogenous BCL-xL in mouse brains is prominently localized to the inner membrane/matrix, in addition to the outer mitochondrial membrane and the endoplasmic reticulum (ER) [33, 34] (Box 3). These results were confirmed by compairing bcl-x knockout brain in parallel (Figure 3).
Box 3. Grappling with the data for submitochondrial membrane localization.
After considering the issues discussed in Boxes 1 and 2, it is interesting to revisit the study by Motoyama et al. [84] in which two different antibodies against BCL-2 were used for immuno-gold EM labeling in a rigorous analysis of rat liver. The rabbit polyclonal antibody pAb raised against human BCL-2 amino acids 4–21 (100% identity to mouse and rat) labeled the inner mitochondrial membrane more than the outer, while a monoclonal antibody against rat BCL-2 (mAb 83-8B) favored the outer membrane [ER:OMM:IMM:cytosol ratio of ~5:3:4:<1 for pAb, versus <1:7:2:0 for mAb 83-8B) [84]. An additional monoclonal mAb 124 (DAKO) against amino acids 41–54 of human BCL-2 (not identical to rat) detected an OMM:IMM ratio of 2:1 in HL60 cells [84], while the rabbit polyclonal pAb yielded an OMM:IMM ratio of 1:4 in HL60 (see Fig. 3). Even when the same cell line (e.g. human lymphoma RS11846) was analyzed by different groups using different antibodies, opposite results were obtained. Examples of different antibodies detecting exclusively inner, exclusively outer (or ER), or both membranes are shown in Fig. 3. Unfortunately, these early studies could not make use of RNAi or gene knock-outs to confirm their results. Thus, more definitive evidence is required to prove that these antibodies detect conformationally distinct species of endogenous BCL-2 protein. Due to lack of antibodies, there are no studies on the localization of endogenous Drosophila BCL-2 proteins. Though some antibody reagents are being developed for C. elegans, [35, 41], again the results are controversial [120].
Given that BID and other BCL-2-related proteins prefer cardiolipin-enriched membranes, and that cardiolipin is required in some in vitro models of tBID-induced BAX oligomerization [95–98], it is somewhat surprising that these proteins would not find their way to the cardiolipin-enriched inner mitochondrial membrane during cell death, if not in healthy cells. Indeed, BID has been implicated in cristae remodeling [99]. In vitro studies suggest that BID and BAX may prefer cardiolipin concentrations that exceed those found in the outer membrane or at contact sites between inner and outer membranes [98, 100]. Interestingly, Drp1 was also reported to bind cardiolipin and this interaction was required for BAX-induced cytochrome c release in vitro [98].
The N-terminal 33 amino acids of MCL-1 were recently reported to serve as a mitochondrial import sequence [101]. Consistent with MCL-1 being a bona fide matrix-targeted protein, import requires a mitochondrial inner membrane potential (ΔΨ) and is accompanied by cleavage of an N-terminal signal peptide by a mitochondrial processing peptidase (MPP) [101]. An interesting alternative model has been proposed by another group [102]. In this model, MCL-1 first localizes to mitochondria via its C-terminal tail. From this anchored position, the cytosolic N-terminus of MCL-1 can then engage the outer membrane [and possibly inner membrane] machinery resulting in the same ΔΨ–dependent cleavage, but in this case, MCL-1 is not imported while anchored by its C-terminus in the outer membrane [102]. However, it is conceivable that the N-terminal import sequence of MCL-1 could engage mitochondria before MCL-1 becomes anchored to the outer membrane by its C-terminal tail. N-terminal clipping of MCL-1 is suggested to enhance the anti-apoptotic activity of MCL-1 in one report, while the other study reports the opposite effect of cleavage. Nevertheless, these studies suggest a novel mitochondrial localization pathway. Recent findings with the mitochondria-targeted kinase PINK1 extend the concept that mitochondrial proteins can purposefully localize to both the inner and outer mitochondrial membranes (Box 2).
New evidence connecting mammalian caspases and BCL-2 proteins
Studies of endogenous and exogenous BCL-2 family proteins in mouse brains have demonstrated striking bipolar functions of BCL-2 family proteins. For example, classical pro-death family members BAX, BAK and BAD (BH3-only) can potently protect against cell death depending on the developmental stage and specific death stimulus [13, 103, 104]. Conversely, anti-death BCL-2 family members can be converted into pro-death factors in cultured cell and in vitro, for example upon caspase or calpain cleavage [105–108].
To determine the importance of caspase cleavage of BCL-xL, a knock-in mouse was generated with its two caspase cleavage sites in BCL-xL (Asp61 and Asp76) mutated to alanine to render BCL-xL caspase-resistant [109]. This mouse is strikingly resistant to transient global ischemia, a model thought to reflect events following cardiac arrest [110]. This protective effect of uncleavable BCL-xL could potentially be explained either by preventing the loss of full-length BCL-xL, or by blocking the generation of a pro-apoptotic cleavage fragment based on evidence that cleaved BCL-xL can exhibit pore-forming properties similar to BAX under some conditions in vitro [111, 112]. These two possibilities are experimentally difficult to distinguish. While both may be true, evidence that cleaved BCL-xL is indeed pro-death is evident from unexpected results using ABT-737, a small molecule designed to inhibit BCL-xL by inserting into its BH3-binding cleft. Opposite to its effects in cancer models, ABT-737 strikingly protects wild type mice from ischemic injury, even when administered after the ischemic insult [109]. This finding further implies that ABT-737 can also inhibit the pro-death form of BCL-xL. This finding supports the hypothesis that cleavage of BCL-xL in mouse brains leads to generation of a fragment capable of promoting neuronal death following ischemic injury.
Several anti- and pro-death BCL-2 family members contain protease cleavage sites located between helix 1 and their BH3 motif (e.g. BCL-2, BCL-xL, MCL-1, BIM, BAD and BID) and may be regulated by phosphorylation, potentially to expose their BH3. The best characterized caspase target is the BH3-only protein BID, which is cleaved by caspase-8 following activation of cell surface death receptors [1]. Cleavage of BID in the extrinsic apoptosis pathway provides crosstalk with the intrinsic mitochondrial death apoptosis pathway, because caspase-truncated BID (tBID) can directly activate BAX to induce MOMP and the release of cytochrome c to facilitate further caspase activation and apoptosis. However, the biophysical functions of the membrane-associated helical bundle structures of BCL-2 proteins could have alternative functions rather than pore formation (and its inhibition), but how these proteins change membrane shape to control cell viability is not understood.
Bcl-xL inhibits inner mitochondrial membrane leakiness to save cells
If we accept that endogenous BCL-xL is localized on the inner as well as outer mitochondrial membranes, then functional evidence for the inner membrane role of BCL-xL must follow. A pro-survival role for inner membrane-localized BCL-xL is consistent with changes in mitochondrial membrane potential observed in bcl-x -deficient cultured neurons. In the absence of BCL-xL, there is a striking fluctuation in the potential across the mitochondrial inner membrane detected with potentiometric dyes TMRM or TMRE [33]. Thus, BCL-xL prevents the observed large membrane potential fluctuations. The simplest interpretation is that BCL-xL directly or indirectly closes an inner membrane channel. Patch clamp recordings of mitochondrial inner membrane vesicles further support this hypothesis [34]. In addition, single cell measurements of oxygen consumption in cultured neurons with and without transfected BCL-xL indicate that BCL-xL-expressing cells consume less oxygen to produce ATP [34]. Lower oxygen consumption is consistent with overall less ion flux across the inner membrane with BCL-xL, consistent with increased energetic efficiency and with earlier reports suggesting that BCL-xL can alter the permeability of mitochondrial membranes to regulate energetics [113].
Neither the detailed molecular mechanisms nor the molecular identity of this BCL-xL-inhibited inner membrane channel are known. However, a direct interaction between BCL-xL and the F1FO ATP synthase has been observed by several groups [33, 34, 114]. In one study, at least 10 subunits of the F1FO ATP synthase were pulled down with BCL-xL [114], while others observed an interaction with the F1FO–associated adenine nucleotide transporter/carrier ANT [86, 115]. However, Bcl-xL is not present in 3-dimensional structures of the F1Fo ATP synthase. Nevertheless, reconstituted vesicles bearing the F1Fo ATP synthase are sufficient to recapitulate the effects of BCL-xL on membrane permeability [34]. There are many other potential mechanisms that are yet untested, and the involvement of BAX and BAK cannot be ruled out. A Diphtheria toxin-like translocation function of BCL-xL could be involved in altering membrane conductances at both the inner and outer membranes. Assembly of the yeast F1FO ATP synthase requires a number of additional factors for which homologues in mammals are not known [116], though there is no evidence to date this role is filled by BCL-2 proteins. These ideas also do not incorporate the important role of BCL-xL on ER membranes, where BCL-2 proteins can also exert their anti- and pro-death activities [73].
Regardless of the mechanism, the mitochondrial membrane of bcl-x-deficient mammalian neurons, where BCL-xL is abundantly expressed throughout life, appears to be leakier than control cells, setting the stage for an energetic crisis where increased metabolic demand under stress conditions cannot be met. The result is premature collapse of the mitochondrial potential and subsequent premature death [33]. It is currently not known if C. elegans or Drosophila BCL-2 proteins have related functions or localizations in mitochondria, but new evidence for mitochondrial membrane potential-dependent effects on MCL-2 and the affinity of BCL-2 proteins for cardiolipin are intriguing.
Concluding remarks
BCL-2 family proteins are critical regulators of cell survival in times of stress, yet basic information about their conserved subcellular localizations, their folded structures when fully membrane-inserted, and their underlying biochemical activities are still unknown. Piecing together insight from the available model systems has not been straightforward but is poised to drive new ideas to ultimately resolve observed species differences. Without more information, it is difficult to assert which molecular events could ultimately be found to link the emerging energetic effects of BCL-2 proteins with their apoptotic functions. For example, direct effects of BCL-xL on mitochondrial membrane structures could conceivably drive the observed effects on redox control or acetyl-coA levels that result in N-acetylation of a range of cellular proteins. Nevertheless, a common theme preserved in flies, worms and mammals is the effect of BCL-2 proteins on mitochondrial morphology and cell survival/death.
Acknowledgements
This work was supported by NIH NS37402 and GM077875 (JMH) and NS045876 (E.A.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 2.Ranger AM, et al. Mouse models of cell death. Nature genetics. 2001;28:113–118. doi: 10.1038/88815. [DOI] [PubMed] [Google Scholar]
- 3.Ni Chonghaile T, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi L, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leber B, et al. Still embedded together binding to membranes regulates Bcl-2 protein interactions. Oncogene. 2010;29:5221–5230. doi: 10.1038/onc.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vander Heiden MG, Thompson CB. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nature cell biology. 1999;1:E209–E216. doi: 10.1038/70237. [DOI] [PubMed] [Google Scholar]
- 7.Berman SB, et al. Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. The Journal of cell biology. 2009;184:707–719. doi: 10.1083/jcb.200809060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowaltowski AJ, Fiskum G. Redox mechanisms of cytoprotection by Bcl-2. Antioxidants & redox signaling. 2005;7:508–514. doi: 10.1089/ars.2005.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi CH, et al. Metabolic regulation of protein N-alpha-acetylation by Bcl-xL promotes cell survival. Cell. 2011;146:607–620. doi: 10.1016/j.cell.2011.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scorrano L, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 11.Danial NN, et al. Homeostatic functions of BCL-2 proteins beyond apoptosis. Advances in experimental medicine and biology. 2010;687:1–32. doi: 10.1007/978-1-4419-6706-0_1. [DOI] [PubMed] [Google Scholar]
- 12.Liang XH, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 13.Fannjiang Y, et al. BAK alters neuronal excitability and can switch from anti- to pro-death function during postnatal development. Developmental cell. 2003;4:575–585. doi: 10.1016/s1534-5807(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 14.Autret A, Martin SJ. Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Molecular cell. 2009;36:355–363. doi: 10.1016/j.molcel.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Guo T, et al. An evolving NGF-Hoxd1 signaling pathway mediates development of divergent neural circuits in vertebrates. Nature neuroscience. 2011;14:31–36. doi: 10.1038/nn.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonas EA, et al. Actions of BAX on mitochondrial channel activity and on synaptic transmission. Antioxidants & redox signaling. 2005;7:1092–1100. doi: 10.1089/ars.2005.7.1092. [DOI] [PubMed] [Google Scholar]
- 17.Beverly LJ. Regulation of anti-apoptotic BCL2-proteins by non-canonical interactions: the next step forward or two steps back? J Cellular Biochemistry. 2012;113:3–12. doi: 10.1002/jcb.23335. [DOI] [PubMed] [Google Scholar]
- 18.Aouacheria A, et al. Phylogenomics of life-or-death switches in multicellular animals: Bcl-2, BH3-Only, and BNip families of apoptotic regulators. Mol Biol Evol. 2005;22:2395–2416. doi: 10.1093/molbev/msi234. [DOI] [PubMed] [Google Scholar]
- 19.Westphal D, et al. Molecular biology of Bax and Bak activation and action. Biochimica et biophysica acta. 2011;1813:521–531. doi: 10.1016/j.bbamcr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Lasi M, et al. The molecular cell death machinery in the simple cnidarian Hydra includes an expanded caspase family and pro- and anti-apoptotic Bcl-2 proteins. Cell Res. 2010;20:812–825. doi: 10.1038/cr.2010.66. [DOI] [PubMed] [Google Scholar]
- 21.Zmasek CM, et al. Surprising complexity of the ancestral apoptosis network. Genome biology. 2007;8:R226. doi: 10.1186/gb-2007-8-10-r226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellermeier CD, et al. A three-protein signaling pathway governing immunity to a bacterial cannibalism toxin. Cell. 2006;124:549–559. doi: 10.1016/j.cell.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 23.Teng X, et al. Gene-dependent cell death in yeast. Cell Death Dis. 2011;2:e188. doi: 10.1038/cddis.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kvansakul M, et al. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell death and differentiation. 2008;15:1564–1571. doi: 10.1038/cdd.2008.83. [DOI] [PubMed] [Google Scholar]
- 25.Bellows DS, et al. Epstein-Barr virus BALF1 is a BCL-2-like antagonist of the herpesvirus antiapoptotic BCL-2 proteins. Journal of virology. 2002;76:2469–2479. doi: 10.1128/jvi.76.5.2469-2479.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham SC, et al. Vaccinia virus proteins A52 and B14 Share a Bcl-2-like fold but have evolved to inhibit NF-kappaB rather than apoptosis. PLoS pathogens. 2008;4 doi: 10.1371/journal.ppat.1000128. e1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Current opinion in cell biology. 2006;18:685–689. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Murphy JR. Mechanism of Diphtheria Toxin Catalytic Domain Delivery to the Eukaryotic Cell Cytosol and the Cellular Factors that Directly Participate in the Process. Toxins (Basel) 2011;3:294–308. doi: 10.3390/toxins3030294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinds MG, et al. Bim, Bad and Bmf: intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets. Cell death and differentiation. 2007;14:128–136. doi: 10.1038/sj.cdd.4401934. [DOI] [PubMed] [Google Scholar]
- 30.Pattingre S, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Rubinstein AD, et al. The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members to promote mitochondrial apoptosis. Molecular cell. 2011;44:698–709. doi: 10.1016/j.molcel.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Huang W, et al. Crystal structure and biochemical analyses reveal Beclin 1 as a novel membrane binding protein. Cell Res. 2012;22:473–489. doi: 10.1038/cr.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YB, et al. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. The Journal of cell biology. 2011;195:263–276. doi: 10.1083/jcb.201108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alavian KN, et al. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nature cell biology. 2011;13:1224–1233. doi: 10.1038/ncb2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conradt B. Genetic control of programmed cell death during animal development. Annual review of genetics. 2009;43:493–523. doi: 10.1146/annurev.genet.42.110807.091533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan N, et al. Structure of the CED-4-CED-9 complex provides insights into programmed cell death in Caenorhabditis elegans. Nature. 2005;437:831–837. doi: 10.1038/nature04002. [DOI] [PubMed] [Google Scholar]
- 37.Yan N, et al. Structural, biochemical, and functional analyses of CED-9 recognition by the proapoptotic proteins EGL-1 and CED-4. Molecular cell. 2004;15:999–1006. doi: 10.1016/j.molcel.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Chau BN, et al. Aven, a novel inhibitor of caspase activation, binds Bcl-xL and Apaf-1. Molecular cell. 2000;6:31–40. [PubMed] [Google Scholar]
- 39.Jagasia R, et al. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature. 2005;433:754–760. doi: 10.1038/nature03316. [DOI] [PubMed] [Google Scholar]
- 40.Rolland SG, et al. The BCL-2-like protein CED-9 of C. elegans promotes FZO-1/Mfn1,2- and EAT-3/Opa1-dependent mitochondrial fusion. The Journal of cell biology. 2009;186:525–540. doi: 10.1083/jcb.200905070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Y, et al. A molecular switch that governs mitochondrial fusion and fission mediated by the BCL2-like protein CED-9 of Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E813–E822. doi: 10.1073/pnas.1103218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, et al. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2169–2174. doi: 10.1073/pnas.0711647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoppins S, et al. The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Molecular cell. 2011;41:150–160. doi: 10.1016/j.molcel.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karbowski M, et al. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- 45.Zhao J, et al. Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission. The EMBO journal. 2011;30:2762–2778. doi: 10.1038/emboj.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Challa M, et al. Drosophila Omi, a mitochondrial-localized IAP antagonist and proapoptotic serine protease. The EMBO journal. 2007;26:3144–3156. doi: 10.1038/sj.emboj.7601745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdelwahid E, et al. Mitochondrial disruption in Drosophila apoptosis. Developmental cell. 2007;12:793–806. doi: 10.1016/j.devcel.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Abdelwahid E, et al. Mitochondrial involvement in cell death of non-mammalian eukaryotes. Biochimica et biophysica acta. 2011;1813:597–607. doi: 10.1016/j.bbamcr.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bryant B, et al. A lepidopteran orthologue of reaper reveals functional conservation and evolution of IAP antagonists. Insect Mol Biol. 2009;18:341–351. doi: 10.1111/j.1365-2583.2009.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mendes CS, et al. Cytochrome c–d regulates developmental apoptosis in the Drosophila retina. EMBO reports. 2006;7:933–939. doi: 10.1038/sj.embor.7400773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arama E, et al. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Developmental cell. 2003;4:687–697. doi: 10.1016/s1534-5807(03)00120-5. [DOI] [PubMed] [Google Scholar]
- 52.Arama E, et al. The two Drosophila cytochrome C proteins can function in both respiration and caspase activation. The EMBO journal. 2006;25:232–243. doi: 10.1038/sj.emboj.7600920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hao Z, et al. Specific ablation of the apoptotic functions of cytochrome C reveals a differential requirement for cytochrome C and Apaf-1 in apoptosis. Cell. 2005;121:579–591. doi: 10.1016/j.cell.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Quinn L, et al. Buffy, a Drosophila Bcl-2 protein, has anti-apoptotic and cell cycle inhibitory functions. The EMBO journal. 2003;22:3568–3579. doi: 10.1093/emboj/cdg355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Motoyama N, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 56.Rinkenberger JL, et al. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes & development. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- 57.Hengartner MO, et al. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992;356:494–499. doi: 10.1038/356494a0. [DOI] [PubMed] [Google Scholar]
- 58.Sevrioukov EA, et al. Drosophila Bcl-2 proteins participate in stress-induced apoptosis, but are not required for normal development. Genesis. 2007;45:184–193. doi: 10.1002/dvg.20279. [DOI] [PubMed] [Google Scholar]
- 59.Galindo KA, et al. The Bax/Bak ortholog in Drosophila, Debcl, exerts limited control over programmed cell death. Development (Cambridge, England) 2009;136:275–283. doi: 10.1242/dev.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindsten T, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Molecular cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamb HM, Hardwick M. Noncanonical functions of BCL-2 proteins in the nervous system. Advances in experimental medicine and biology. 2010;687:115–129. doi: 10.1007/978-1-4419-6706-0_7. [DOI] [PubMed] [Google Scholar]
- 63.Wu JN, et al. grim promotes programmed cell death of Drosophila microchaete glial cells. Mech Dev. 2010;127:407–417. doi: 10.1016/j.mod.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanner EA, et al. Bcl-2 proteins and autophagy regulate mitochondrial dynamics during programmed cell death in the Drosophila ovary. Development (Cambridge, England) 2011;138:327–338. doi: 10.1242/dev.057943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bender CE, et al. Mitochondrial pathway of apoptosis is ancestral in metazoans. Proceedings of the National Academy of Sciences of the United States of America. 2012 doi: 10.1073/pnas.1120680109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perkins GA, et al. The micro-architecture of mitochondria at active zones: electron tomography reveals novel anchoring scaffolds and cristae structured for high-rate metabolism. J Neurosci. 2010;30:1015–1026. doi: 10.1523/JNEUROSCI.1517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spirou GA, et al. Ultrastructure of neurons and large synaptic terminals in the lateral nucleus of the trapezoid body of the cat. The Journal of comparative neurology. 1998;398:257–272. doi: 10.1002/(sici)1096-9861(19980824)398:2<257::aid-cne7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 69.Li Z, et al. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nikolaev A, et al. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Tanner EA, McCall K. Mitochondrial regulation of cell death in the Drosophila ovary. Autophagy. 2011;7:793–794. doi: 10.4161/auto.7.7.15695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaufmann T, et al. Characterization of the signal that directs Bcl-x(L), but not Bcl-2, to the mitochondrial outer membrane. The Journal of cell biology. 2003;160:53–64. doi: 10.1083/jcb.200210084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu W, et al. Bcl-2 mutants with restricted subcellular location reveal spatially distinct pathways for apoptosis in different cell types. The EMBO journal. 1996;15:4130–4141. [PMC free article] [PubMed] [Google Scholar]
- 74.Griffiths GJ, et al. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. The Journal of cell biology. 1999;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nechushtan A, et al. Conformation of the Bax C-terminus regulates subcellular location and cell death. The EMBO journal. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolter KG, et al. Movement of Bax from the cytosol to mitochondria during apoptosis. The Journal of cell biology. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim H, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Molecular cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lalier L, et al. Bax activation and mitochondrial insertion during apoptosis. Apoptosis. 2007;12:887–896. doi: 10.1007/s10495-007-0749-1. [DOI] [PubMed] [Google Scholar]
- 79.Crow MT, et al. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 80.Kinnally KW, Antonsson B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis. 2007;12:857–868. doi: 10.1007/s10495-007-0722-z. [DOI] [PubMed] [Google Scholar]
- 81.Landes T, Martinou JC. Mitochondrial outer membrane permeabilization during apoptosis: the role of mitochondrial fission. Biochimica et biophysica acta. 2011;1813:540–545. doi: 10.1016/j.bbamcr.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 82.Hockenbery D, et al. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 83.Kharbanda S, et al. Role for Bcl-xL as an inhibitor of cytosolic cytochrome C accumulation in DNA damage-induced apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6939–6942. doi: 10.1073/pnas.94.13.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Motoyama S, et al. Bcl-2 is located predominantly in the inner membrane and crista of mitochondria in rat liver. Biochemical and biophysical research communications. 1998;249:628–636. doi: 10.1006/bbrc.1998.9205. [DOI] [PubMed] [Google Scholar]
- 85.Gotow T, et al. Selective localization of Bcl-2 to the inner mitochondrial and smooth endoplasmic reticulum membranes in mammalian cells. Cell death and differentiation. 2000;7:666–674. doi: 10.1038/sj.cdd.4400694. [DOI] [PubMed] [Google Scholar]
- 86.Belzacq AS, et al. Bcl-2 and Bax modulate adenine nucleotide translocase activity. Cancer research. 2003;63:541–546. [PubMed] [Google Scholar]
- 87.Nakai M, et al. The bcl-2 protein is inserted into the outer membrane but not into the inner membrane of rat liver mitochondria in vitro. Biochemical and biophysical research communications. 1993;196:233–239. doi: 10.1006/bbrc.1993.2239. [DOI] [PubMed] [Google Scholar]
- 88.Nguyen M, et al. Targeting of Bcl-2 to the mitochondrial outer membrane by a COOH-terminal signal anchor sequence. The Journal of biological chemistry. 1993;268:25265–25268. [PubMed] [Google Scholar]
- 89.Nguyen M, et al. Role of membrane anchor domain of Bcl-2 in suppression of apoptosis caused by E1B-defective adenovirus. The Journal of biological chemistry. 1994;269:16521–16524. [PubMed] [Google Scholar]
- 90.Goping IS, et al. Regulated targeting of BAX to mitochondria. The Journal of cell biology. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nechushtan A, et al. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. The Journal of cell biology. 2001;153:1265–1276. doi: 10.1083/jcb.153.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monaghan P, et al. Ultrastructural localization of bcl-2 protein. J Histochem Cytochem. 1992;40:1819–1825. doi: 10.1177/40.12.1453000. [DOI] [PubMed] [Google Scholar]
- 93.Riparbelli MG, et al. Localization of the Bcl-2 protein to the outer mitochondrial membrane by electron microscopy. Exp Cell Res. 1995;221:363–369. doi: 10.1006/excr.1995.1386. [DOI] [PubMed] [Google Scholar]
- 94.Lithgow T, et al. The protein product of the oncogene bcl-2 is a component of the nuclear envelope, the endoplasmic reticulum, and the outer mitochondrial membrane. Cell Growth Differ. 1994;5:411–417. [PubMed] [Google Scholar]
- 95.Lutter M, et al. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nature cell biology. 2000;2:754–761. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- 96.Gonzalvez F, et al. tBid interaction with cardiolipin primarily orchestrates mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell death and differentiation. 2005;12:614–626. doi: 10.1038/sj.cdd.4401571. [DOI] [PubMed] [Google Scholar]
- 97.Kuwana T, et al. Bid, Bax, lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 98.Montessuit S, et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scorrano L, et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Developmental cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 100.Ardail D, et al. Mitochondrial contact sites. Lipid composition and dynamics. The Journal of biological chemistry. 1990;265:18797–18802. [PubMed] [Google Scholar]
- 101.Huang CR, Yang-Yen HF. The fast-mobility isoform of mouse Mcl-1 is a mitochondrial matrix-localized protein with attenuated anti-apoptotic activity. FEBS letters. 2010;584:3323–3330. doi: 10.1016/j.febslet.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 102.Warr MR, et al. Mitochondrion-dependent N-terminal processing of outer membrane Mcl-1 protein removes an essential Mule/Lasu1 protein-binding site. The Journal of biological chemistry. 2011;286:25098–25107. doi: 10.1074/jbc.M111.218321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lewis J, et al. Inhibition of virus-induced neuronal apoptosis by Bax. Nature medicine. 1999;5:832–835. doi: 10.1038/10556. [DOI] [PubMed] [Google Scholar]
- 104.Seo SY, et al. BAD is a pro-survival factor prior to activation of its pro-apoptotic function. The Journal of biological chemistry. 2004;279:42240–42249. doi: 10.1074/jbc.M406775200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheng EH, et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 106.Clem RJ, et al. Modulation of cell death by Bcl-XL through caspase interaction. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kirsch DG, et al. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. The Journal of biological chemistry. 1999;274:21155–21161. doi: 10.1074/jbc.274.30.21155. [DOI] [PubMed] [Google Scholar]
- 108.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. The Journal of cell biology. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jonas EA, et al. Nature neuroscience. 2012 (in press). [Google Scholar]
- 110.Oguro K, et al. Global ischemia-induced increases in the gap junctional proteins connexin 32(Cx32) and Cx36 in hippocampus and enhanced vulnerability of Cx32 knock-out mice. J Neurosci. 2001;21:7534–7542. doi: 10.1523/JNEUROSCI.21-19-07534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Basanez G, et al. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. The Journal of biological chemistry. 2002;277:49360–49365. doi: 10.1074/jbc.M206069200. [DOI] [PubMed] [Google Scholar]
- 112.Jonas EA, et al. Proapoptotic N-truncated BCL-xL protein activates endogenous mitochondrial channels in living synaptic terminals. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13590–13595. doi: 10.1073/pnas.0401372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yi CH, et al. Integration of Apoptosis and Metabolism. Cold Spring Harb Symp Quant Biol. 2011 doi: 10.1101/sqb.2011.76.010777. LXXVI, (in press) [DOI] [PubMed] [Google Scholar]
- 114.Vento MT, et al. Praf2 is a novel Bcl-xL/Bcl-2 interacting protein with the ability to modulate survival of cancer cells. PLoS ONE. 2010;5:e15636. doi: 10.1371/journal.pone.0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brenner C, et al. Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene. 2000;19:329–336. doi: 10.1038/sj.onc.1203298. [DOI] [PubMed] [Google Scholar]
- 116.Rak M, et al. Modular assembly of yeast mitochondrial ATP synthase. The EMBO journal. 2011 doi: 10.1038/emboj.2010.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Van Laar VS, Berman SB. Mitochondrial dynamics in Parkinson's disease. Exp Neurol. 2009;218:247–256. doi: 10.1016/j.expneurol.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jin SM, et al. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. The Journal of cell biology. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lazarou M, et al. Role of PINK1 Binding to the TOM Complex and Alternate Intracellular Membranes in Recruitment and Activation of the E3 Ligase Parkin. Developmental cell. 2012;22:320–333. doi: 10.1016/j.devcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pourkarimi E, et al. Evidence that CED-9/Bcl2 and CED-4/Apaf-1 localization is not consistent with the current model for C. elegans apoptosis induction. Cell death and differentiation. 2011 doi: 10.1038/cdd.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]