Abstract
Introduction
Stem cells from the apical papilla (SCAP) are a type of mesenchymal stem cells found in the developing tissue, apical papilla, of immature permanent teeth. Studies have shown that SCAP are likely to be a source of primary odontoblasts that are responsible for the formation of root dentin. Basic fibroblast growth factor (bFGF) is a signaling molecule and pleiotropic growth factor involved in tooth root development, and it promotes proliferation of a variety of cell types. The effects of bFGF on SCAP, however, have not been examined.
Methods
We investigated the regulatory effects of bFGF on the proliferation and differentiation potential of human SCAP in vitro. Changes in the cell cycle and proliferation, colony-forming unit–fibroblastic formation, alkaline phosphatase (ALP) activity, osteogenic/dentinogenic differentiation, and stem cell gene makers of SCAP, cultured in the presence or absence of bFGF, were evaluated.
Results
Treatment with 5 ng/mL bFGF significantly increased SCAP proliferation and their colony-forming unit–fibroblastic formation efficiency. The growth factor also increased the expression of STRO-1 and the stem cell gene makers Nanog, Oct4, Sox2, and Rex1 in SCAP. In contrast, bFGF reduced the ALP activity, mineral nodule formation, and the expression of ALP, osteocalcin, bone sialoprotein, and dentin sialophosphoprotein. When SCAP cultures were expanded in the presence of bFGF for 1 week, subsequent stimulation of the osteogenic/dentinogenic condition resulted in enhanced differentiation.
Conclusions
Under certain conditions, bFGF enhances SCAP stemness by up-regulating stem cell gene expression, increasing proliferation ability, and potentiating differentiation potency.
Keywords: Basic fibroblast growth factor, cell differentiation, cell proliferation, osteo/dentinogenesis, stem cells from the apical papilla, stemness
Stem cells from the apical papilla (SCAP) are a population of mesenchymal stem cells (MSCs) residing in the apical papilla of incompletely developed teeth (1, 2). SCAP demonstrate characteristic features of stem/progenitor cells with the capability to differentiate into a variety of cell types (3). Recently, SCAP have been shown to possess the ability to regenerate vascularized human dental pulp–like tissues in emptied root canal spaces and to produce new dentin-like tissue on existing dentinal walls (4). These findings suggest that SCAP might be the source of primary odontoblasts that are responsible for the formation of root dentin (5, 6). The apical papilla is the precursor of the radicular pulp (3, 7). Although the immunophenotype of SCAP is similar to that of dental pulp stem cells (DPSCs) on the basis of the osteogenic/dentinogenic and growth factor receptor gene profiles, SCAP appear to have a greater dentinogenic potential than DPSCs (2, 7).
Basic fibroblast growth factor (bFGF) is a member of FGFs that plays an important role in the morphogenesis and histogenesis of tooth, and it effectively promotes tooth root elongation and periodontal tissue formation (8). Basic FGF is expressed in most cells of the root during development of both the maxillary and mandibular molars in mice (9).
Basic FGF is known to be a critically important factor for maintaining the self-renewal ability of human embryonic stem (hES) cells in cultures and is a potent mitogen for cultured neural progenitors (10, 11). The differentiated fibroblasts within hES cell colonies in cultures appear to play an important role in responding to bFGF, which supports hES cell self-renewal. For adult stem cells, the effects of bFGF on MSCs vary, depending on the tissue origin. Basic FGF enhances human MSC proliferation and osteogenic and chondrogenic differentiation (12–14) and maintains multilineage differentiation potential (12, 15). Basic FGF dramatically induces the mRNA expression of dentin sialophosphoprotein (DSPP) and bone sialoprotein (BSP) in immature adult rat incisor dental pulp cells (16), suppresses alkaline phosphatase (ALP) activity and osteogenesis differentiation in human dental pulp cells (hDPCs), and inhibits osteogenesis in mouse adipose tissue-derived stromal cells, whereas bFGF sustains their proliferative and osteogenic potential state (12, 17, 18).
The effects of bFGF on the biologic functions of SCAP have not been investigated. Considering that bFGF is expressed in the developing root as noted above, its role on dental stem cells might be evaluated by studying its effects on cultured SCAP. Therefore, we investigated the response of SCAP to various concentrations of bFGF in cultures in terms of their proliferation, colony-formation efficiency, stem cell marker expression, and differentiation potential.
Materials and Methods
Sample Collection and Cell Culture
Human third molars (n = 8) with immature developing roots (ie, roots developed to <2/3 their full size) were collected from 4 individuals (13–15 years old). The extraction procedure was approved by the Institutional Review Board of the Fourth Military Medical University and performed with the informed consent of the patients. The apical papilla was isolated as previously described (1, 2). Briefly, the apical papilla was minced and digested in a solution of 3 mg/mL collagenase type I (Invitrogen, Carlsbad, CA) and 4 mg/mL dispase (Invitrogen) for 40 minutes at 37°C. Single-cell suspensions were obtained by passing the solution through a 70-µm strainer. Cells were seeded at 1 × 104 cells/ well into 6-well plates and cultured with a standard medium defined as α-MEM (Invitrogen) supplemented with 15% fetal bovine serum (FBS) (Hyclone, Kerrville, TX), 100 mol/L L-ascorbic acid 2-phosphate, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C in 5% CO2. The medium was changed every 2–3 days. After 7–10 days, the attached cells were collected and prepared for limiting dilution procedures to obtain single-colony-derived strains. The cell suspensions were diluted such that each well of the 96-well plate was seeded with approximately 1 cell. A number of these single-colony-derived strains were pooled, cultured, and passaged at 1:3 ratio when they reached 80% confluence. The experiments were carried out by using cells at passages 3–12.
Multipotent differentiation of the cells was assessed as described previously (1). For the induction of adipogenesis, cells were exposed to adipogenic medium (α-MEM supplemented with 1 µg/mL insulin, 1 µmol/L dexamethasone, 0.5 mmol/L 3-isobutyl-1-methyl-xanthine, and 10% FBS) for 3 weeks. The oil droplets that accumulated inside the cells were visualized by staining with oil red O reagent.
Immunophenotypic Characterization
The immunophenotypes of isolated cells were confirmed by immunocytofluorescence staining by using rabbit antihuman antibodies against STRO-1 (R&D Systems Inc, Minneapolis, MN), CD24, and cytokeratin (Santa Cruz Biotechnology, Inc, Santa Cruz, CA). DAPI (Beyotime Institute of Biotechnology, China) was used as a nuclear counterstain.
SCAP from the 3rd, 6th, and 10th passages (1 × 105 cells/T25 flask) were grown in standard medium with or without the presence of recombinant human bFGF (5 ng/mL) (PeproTech Inc, Rocky Hill, NJ). After 4 days, the percentages of STRO-1–positive cells were determined by flow cytometry. The cells were immersed in 3% H2O2/methanol for 15 minutes to quench the endogenous peroxidase activity and then incubated with rabbit antihuman STRO-1 antibody (1:300) for 60 minutes. Parallel samples were treated with an isotype-matched control antibody under the same conditions. The samples were subsequently washed and stained with a secondary antibody mouse antirabbit–fluorescein isothiocyanate (FITC) for 40 minutes in the dark. The cells were then rinsed and resuspended in phosphate-buffered saline (PBS) for analysis by flow microfluorometry by using a FACSAria Flow Cytometer (Becton, Dickinson and Co, Franklin Lakes, NJ).
Evaluation of Cell Proliferation
Various concentrations of bFGF were tested (0, 1, 3, 5, 10, and 20 ng/mL) to determine the optimal effect on cell proliferation. SCAP (passage 3) were seeded at a density of 1 × 104 cells/well in 96-well plates. After 24 hours, the medium was replaced with fresh medium containing the above indicated concentrations of bFGF. At different times after treatment, the cultures were supplemented with 30 µL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 g/L; Sigma-Aldrich, St Louis, MO) and incubated for 4 hours. The supernatants were then removed and replaced with 150 µL dimethyl sulfoxide into the culture and incubated for 10 minutes. The resulting solution was spectrophotometrically measured (Power-wave 340; Bio-tek Instruments, Winooski, VT) at a wavelength of 490 nm. For each bFGF concentration tested, 5 treated and 5 control wells were analyzed.
Cell Cycle Analysis
SCAP were seeded in standard medium at a density of 5 × 103 cells/T25 flask. After 24 hours, cells were cultured with serum-free α-MEM for 24 hours. Subsequently, the medium was replaced with fresh medium in the presence or absence of bFGF (5 ng/mL), and the cells were cultured for 72 hours. Harvested SCAP were then fixed in cold 75% ethanol at 4°C for 12 hours. After further washing, the cell suspensions were stained with 100 mg/L RNase and 100 mg/mL propidium iodide (Sigma). The DNA content of stained nuclei was analyzed by a flow cytometry system (FACSCalibur). The frequency distribution of DNA fluorescence signal can be displayed to show the G1/G0, S, and G2/M cell cycle phases. The percentage of cells residing in the G1, S, and G2 phases was determined by using the Mod Fit software (Verity Software House, Topsham, ME).
Colony-Forming Assays
SCAP at 3rd, 6th, and 12th passages were seeded in triplicate at 200 cells/well into 6-well plates in standard medium for 24 hours. The medium was then replaced by fresh medium in the presence or absence of 5 ng/mL bFGF. After 8 days, the cell clones were fixed with 4% polyoxymethylene for 15 minutes, stained with Giemsa for 30 minutes, and washed with water. Aggregates of >50 cells were counted as a colony under microscopic observation, and colony-forming efficiency was determined. Experiments were carried out at least twice, and most were performed 3 times.
ALP Activity Assay
SCAP at 3rd passage were seeded at 1 × 104 cells/well in 96-well plates with standard medium. After 24 hours the medium was changed to osteogenic/dentinogenic differentiation medium (containing 50 µg/mL ascorbic acid and 10 mmol/L beta-glycerolphosphate) with or without bFGF. After being cultured for various time periods, the ALP activity was detected by using an ALP assay kit (JianCheng Co, Nanjing, China) according to the manufacturer’s instructions. The results were measured spectrophotometrically at 520 nm.
In Vitro Mineralization Assay
Cells at the 3rd passage (1 × 104 cells/well) were initially cultured to 80% confluence in 48-well plates. Cells were either pretreated or not pretreated by bFGF (5 ng/mL) for 7 days before the cultures were replaced with osteogenic/dentinogenic differentiation media with or without bFGF. At the end of differentiation stimulation, cells were washed twice (5 minutes each) in PBS and fixed with 4% polyoxymethylene for 15 minutes. After fixation, the cell layers were stained with alizarin red S (ARS) for 15 minutes and then washed with PBS. ARS staining was quantified by colorimetric assay, as previously reported (19). Aliquots (150 µL) of the supernatant were then determined by absorbance measurement at 405 nm. In each sample, measurements were performed at least in triplicate.
Quantitative Reverse Transcriptase Polymerase Chain Reaction
The expression of osteogenic/dentinogenic markers, including DSPP, ALP, BSP, and osteocalcin (OCN), were detected by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). SCAP were cultured in osteogenic/dentinogenic differentiation medium with or without the presence of bFGF. The cells were harvested 1, 2, and 4 weeks later, and total RNA was isolated by using RNAiso (Takara, Shiga, Japan). First-strand cDNA was synthesized by using a Takara RNA PCR kit. Synthesized cDNA was subjected to qRT-PCR. The subsequent PCR amplification step was performed by using SYBR Premix Ex Taq II (Takara). The reaction was performed by using an ABI PRISM 7500 Real-Time PCR system (Applied Biosystems, Benicia, CA) and the following thermal cycling conditions: 40 cycles of 95°C for 2 minutes, 95°C for 15 seconds, 60°C for 1 minute, and 72°C for 25 seconds. The PCR primer sequences listed in Table 1 were designed according to the report by Alongi et al (20).
TABLE 1.
Primer Sequences for Quantitative RT-PCR
| Gene name (GenBank accession number) |
Primer sequences (5′-3′) | Product size (base pairs) |
|---|---|---|
| DSPP | TCACAAGGGAGAAGGGAATG | 182 |
| (NM_14208) | TGCCATTTGCTGTCAGTTT | |
| BSP | AAAGTGAGAACGGGGAACCT | 161 |
| (NM_004967) | GATGCAAAGCCAGAATGGAT | |
| OCN | GGCAGCGAGGTAGTGAAGAG | 230 |
| (NM_199173) | CTGGAGAGGAGCAGAACTGG | |
| ALP | CCACGTCTTCACATTTGGTG | 196 |
| (NM_000478) | AGACTGCGCCrGGTAGTTGT | |
| Nanog | CTGTGATTTGTGGGCCTGAA | 151 |
| (NM_024865) | TGTTTGCCTTTGGGACTGGT | |
| Rexl | GCTGACCACCAGCACACTAGGC | 298 |
| (NM_174900) | TTTCTGGTGTCTTGTCTTTGCCCG | |
| Sox 2 | GCCTGGGCGCCGAGTGGA | 443 |
| (NM_003106) | GGGCGAGCCGTTCATGTAGGTCTG | |
| Oct4 | ACATGTGTAAGCTGCGGCC | 301 |
| (NM_00270l) | GTTGTGCATAGTCGCTGCTTG | |
| GAPDH | CAAGGCTGAGAACGGGAAGC | 191 |
| (NM_002046) | AGGGGGCAGAGATGATGACC |
Oct4, Nanog, Sox2, and Rex-1 mRNA levels were also determined. SCAP from the 3rd, 6th, and 10th passages were seeded in standard medium at 1 × 105 cells/dish. After 24 hours, cells were made quiescent by replacing the medium with serum-free α-MEM for 24 hours. They were then divided into 2 groups at every passage. One group was cultured with standard medium without bFGF, and the other was cultured with bFGF. Cell samples were harvested at different times after treatment for total RNA extraction. Reverse transcription and qRT-PCR were performed as described above. The samples were subjected to 40 cycles of amplification at 95°C for 1 minute, 95°C for 15 seconds, 58°C for 1 minute, and 72°C for 25 seconds by using the specific primers designed against the corresponding cDNA sequences found in Genbank (Table 1).
The results of the assays were normalized to the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Triplicate reactions and 3 separate experiments were performed.
Statistical Analysis
The data were analyzed by 1-way analysis of variance and Student’s t test by using SPSS 13.5 software (SPSS Inc, Chicago, IL). The difference between experimental groups was considered to be statistically significant at P < .05.
Results
Isolation and Characterization of SCAP
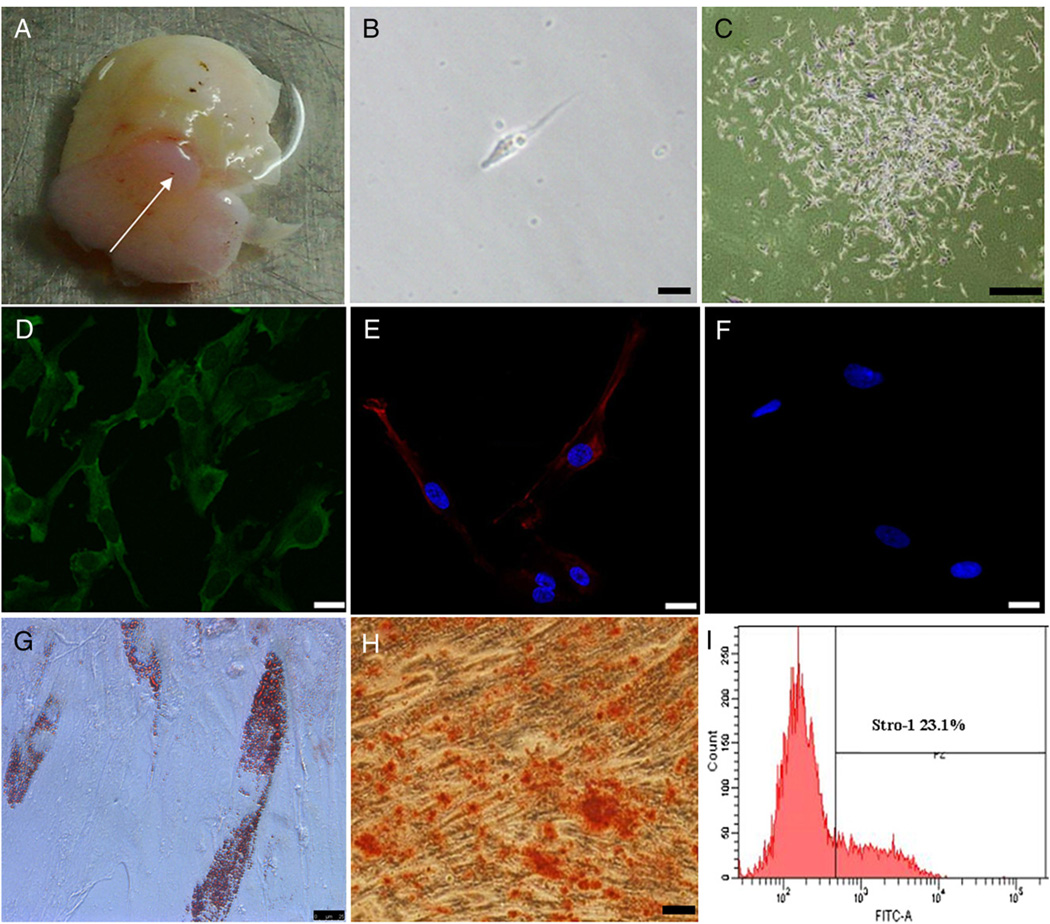
The SCAP isolated from apical papilla (Fig. 1A) were capable of forming adherent clonogenic cell clusters, and most of the cells retained a fibroblastic spindle shape (Fig. 1B and C). Ex vivo expanded SCAP were positive for the early mesenchymal stem/progenitor cell marker, STRO-1 (Fig. 1D). As shown in Figure 1E, SCAP were positive for CD24, which appears to be a specific marker for SCAP (2). SCAP were negative for cytokeratin immunofluorescence staining (Fig. 1F). By flow cytometry, approximately 23.1% of the SCAP were positive for STRO-1 (Fig. 1I). After 4 weeks of osteogenesis/dentinogenesis induction, SCAP formed extensive amounts of ARS-positive mineral deposits (Fig. 1H), indicating calcium accumulation in vitro. In addition, some SCAP developed into oil red O–positive lipid fat cells (Fig. 1G) after 3 weeks of culture under adipogenic conditions.
Figure 1.
Immunophenotypic characterization and multiple differentiation analysis of SCAP. (A) Immature tooth with apical papilla (arrow). (B) Single fibroblastic cell attached 1 day after cell seeding. (C) Formation of CFU-F of SCAP. (D) Immunocytofluorescence staining of STRO-1 (green) at passage 1. (E) Immunocytofluorescence staining of CD24 (red; blue is DAPI stain of nuclei). (F) Negative immunofluorescence staining for cytokeratin (blue: DAPI). (G) Oil red O staining after adipogenic induction for 3 weeks. (H) ARS staining after osteogenic induction for 4 weeks. (I) Flow cytometric analysis of ex vivo expanded SCAP at passage 1 for early mesenchymal progenitor marker STRO-1.
Basic FGF Increases the Proliferation of SCAP
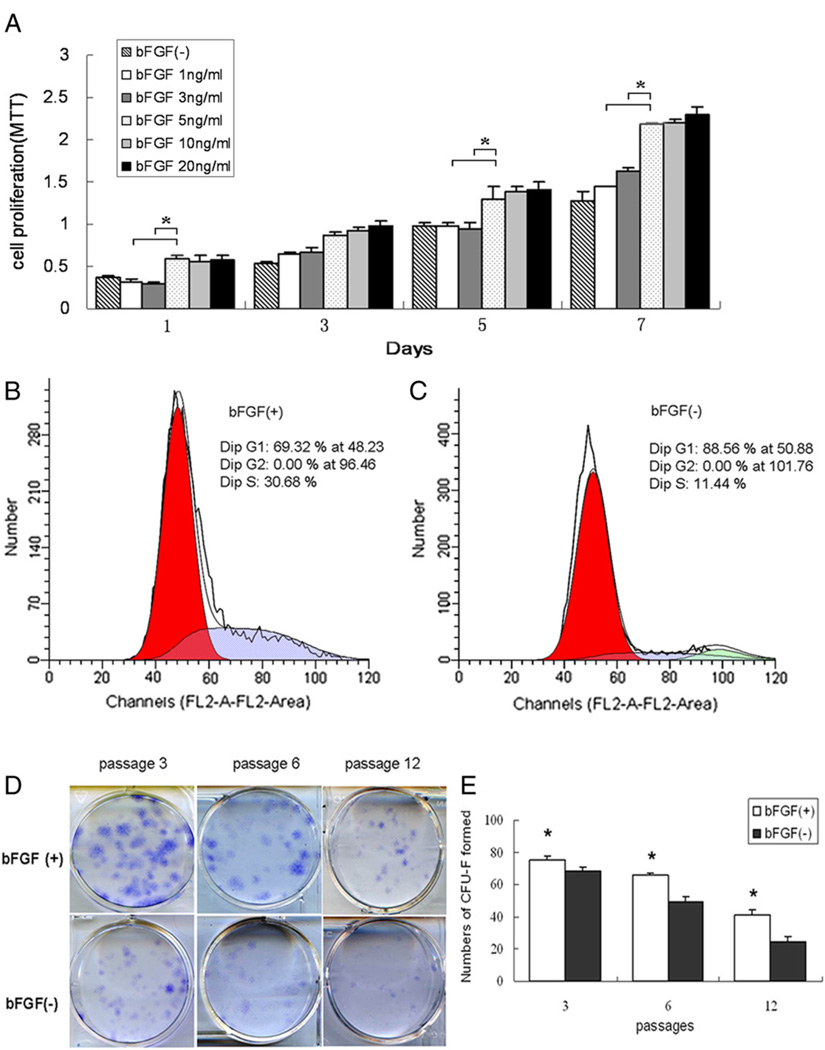
As shown in Figure 2A, bFGF promoted the proliferation markedly at 1, 5, and 7 days. The dose-response studies showed that 5 ng/mL of bFGF had the most optimal effect on proliferation, and higher amounts did not increase proliferation further.
Figure 2.
Effects of bFGF on SCAP proliferation. (A) SCAP at passage 3 were made quiescent by serum starvation for 24 hours and then cultured in the standard medium α-MEM (15% FBS) containing various concentrations of bFGF. At 1, 3, 5, and 7 days, cells were measured for proliferation by MTT (*P < .05). (B, C) Cell cycle analysis of SCAP showing cells escaped G0G1 arrest by shifting to S (30.68%) and G2M phases (0%) in the presence of bFGF (B), and 88.56% of the cells arrested in G0G1 phase without bFGF (C). (D, E) SCAP colony-forming analysis. (D) SCAP at passages 3, 6, and 12 were seeded at 200 cells/well in 6-well plates and cultured in α-MEM (15% FBS) with bFGF (5 ng/mL) for 8 days and stained with Giemsa. (E) Numbers of CFU-F with the presence of bFGF were significantly higher than those in the control group (without bFGF), especially at passages 6 and 12 (n = 6, *P < .05).
Increased cell growth is reflected by an increased number of cells in S phase and a decreased representation of cells arrested in the G0/G1 phase. To show the relationship between SCAP proliferation and bFGF treatment, we used flow cytometry to examine the cell cycle distribution of SCAP cultured in the presence or absence of bFGF. A higher percentage of bFGF-treated cells (30.68%) were in S phases as compared with the untreated controls (Fig. 2B and C), indicating that an increased number of SCAP proceeded into the G2/S phase after bFGF treatment. In control SCAP, most cells (88.56%) stayed in the G1 phase.
Basic FGF Enhances Colony-Forming Efficiency of SCAP
Treatment with bFGF enhanced the colony-forming unit–fibroblastic (CFU-F) forming efficiency of all passages, as evidenced by the increased number and the increased size of colonies (Fig. 2D). The increased numbers of CFU-F formation of SCAP at passages 3, 6, and 12 in the bFGF-treated groups were 75.33 ± 2.68 (P < .05), 66 ± 1.29 (P < .05), and 41.33 ± 21.66 (P < .05), respectively, and were significantly higher than those in control groups (Fig. 2E).
Effects of bFGF on Osteogenic/Dentinogenic Differentiation of SCAP
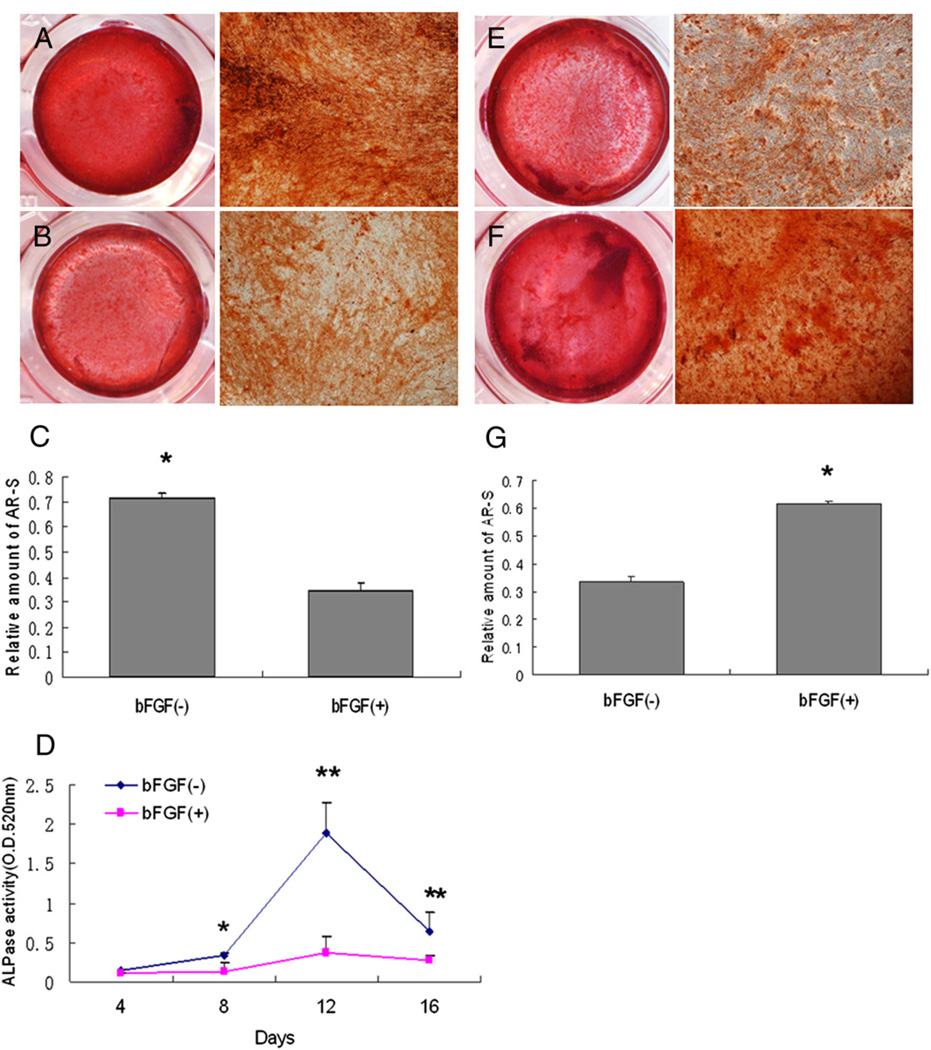
ALP assays and ARS staining were performed to investigate the influence of bFGF on cytodifferentiation of SCAP. Mineralization appeared to be affected by the presence of bFGF. As shown in Figure 3A through C, SCAP cultured with osteogenic differentiation medium containing bFGF for 4 weeks led to a decrease in mineralized nodule formation compared with the controls. Basic FGF also significantly suppressed the levels of ALP activity in SCAP over time in cultures (P < .05) (Fig. 3D). In contrast, when the expanding SCAP were pretreated with bFGF for 1 week before the differentiation stimulation, they showed increased mineralized nodule formation under subsequent osteogenic stimulation (Fig. 3E through G).
Figure 3.
Effects of bFGF on osteogenic/dentinogenic differentiation of SCAP. (A–D) SCAP at passage 3 were induced with differentiation medium in the absence (A) or presence (B) of bFGF (5 ng/mL) for 4 weeks and cultures stained with ARS. (C) Quantitative measurement of ARS stain showing significant higher mineralization in non–bFGF-treated group (*P < .05). (D) Under the same osteogenic/dentinogenic induction conditions, ALP activity was measured, and the untreated group was higher than that in the treated group after 8 days (*P < .05, **P < .01). (E–G) SCAP were cultured with standard medium in the absence (E) or presence of bFGF (5 ng/mL) (F) for 1 week before the 2-week osteogenic/dentinogenic differentiation stimulation and cultures stained with ARS. (G) Quantitative analysis of the amount of mineralization in bFGF-treated group was significantly higher than in the non-treated group (*P < .05).
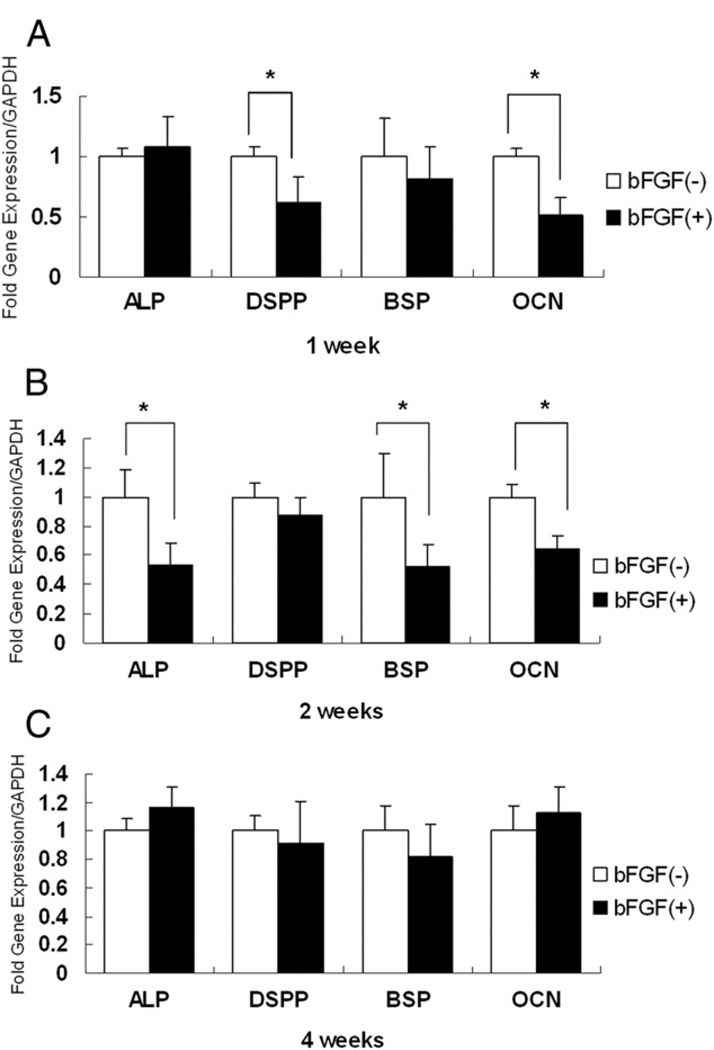
Quantitative RT-PCR was used to detect marker expression such as BSP, DSPP, OCN, and ALP. As shown in Figure 4A, SCAP grown in differentiation medium containing bFGF for 1 week showed lower expression of DSPP, BSP, and OCN, especially DSPP and OCN. At 2 weeks, the expression of ALP, BSP, and OCN was markedly lower than that of controls (Fig. 4B). Of note, after 4 weeks, the levels of these 4 osteogenic/dentinogenic genes were not significantly different from those of the untreated groups (Fig. 4C).
Figure 4.
Effects of bFGF on osteogenic/dentinogenic gene expression after differentiation induction. SCAP at passage 3 never exposed to bFGF were cultured with osteogenic/dentinogenic induction medium with or without the presence of bFGF (5 ng/mL) for 1, 2, and 4 weeks. Expression of DSPP and OCN was decreased at 1 week (A), expression of ALP, BSP, and OCN was reduced at 2 weeks (B) when bFGF was present in the cultures. No significant change of expression of ALP, DSPP, OCN, and BSP was detected at 4 weeks (C). Bar charts represent the relative expression levels of each gene normalized against GADPH (*P < .05).
Basic FGF Increases Stem Cell Marker Expression in SCAP
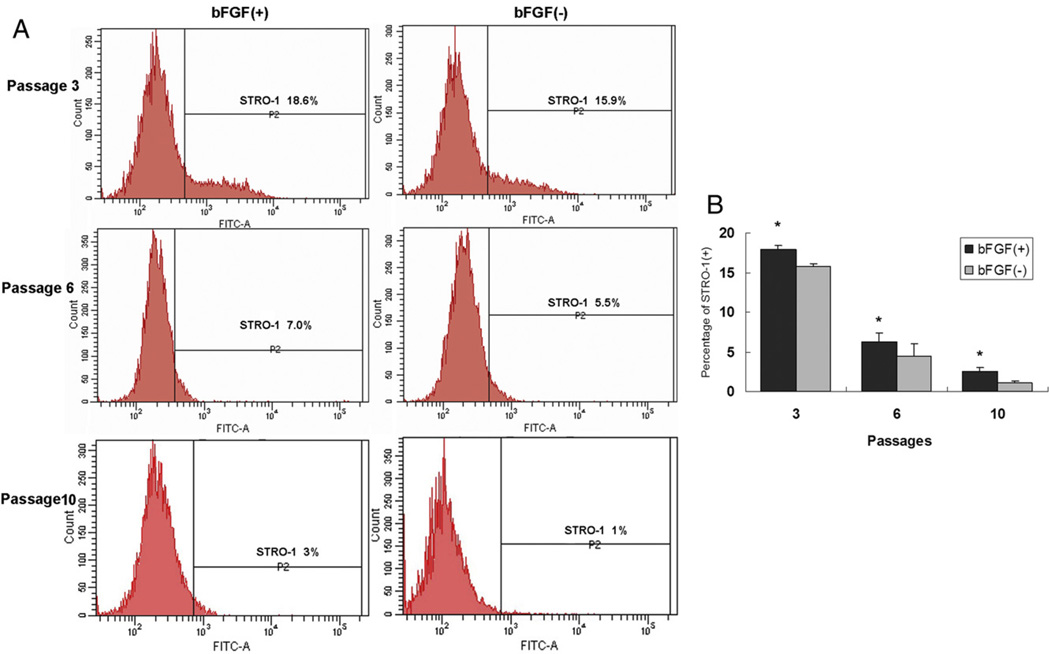
STRO-1 has been used as a marker for most mesenchymal stem/progenitor cells, including SCAP (3). As shown in Figure 5, the expression of STRO-1+ cells at passages 3, 6, and 10 was significantly higher in bFGF-treated cells than in untreated cells, implicating that bFGF treatment is important for sustaining SCAP stemness.
Figure 5.
Effects of bFGF on STRO-1 expression by SCAP. (A) SCAP from passages 3, 6, and 10 were cultured in the presence or absence of bFGF for 48 hours and subjected to flow cytometry analysis. (B) Percentage in bFGF-treated group was higher than that in untreated group. Values are expressed as mean ± standard deviation (n = 6, *P < .05).
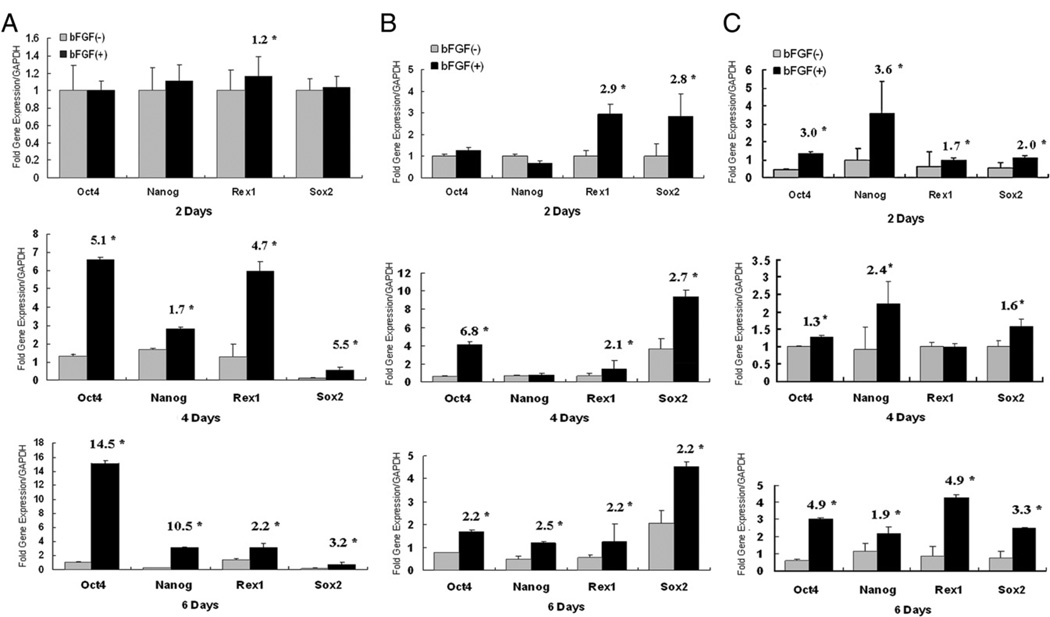
To further determine this effect of bFGF on SCAP, we examined the expression levels of Oct4, Nanog, Sox2, and Rex1. These 4 genes are highly expressed by ES cells and are associated with pluripotency, which is essential for the maintenance of the undifferentiated state of these cells (21, 22). MSCs also express some levels of these pluripotent genes (23, 24), and their expression diminishes or disappears after longtime culturing and passaging (21). Our results showed that regardless of the passage numbers, bFGF increased these pluripotent gene expression levels, especially after 4 or 6 days (passages 3 and 6) and 2 days (passage 10) of treatment (Fig. 6).
Figure 6.
Effects of bFGF on stem cell marker expression. Each passage of SCAP (3, 6, and 10) was cultured in standard medium with or without the presence of bFGF (5 ng/mL) for 2, 4, and 6 days. (A) At passage 3, there was no difference at the mRNA levels of Nanog, Oct4, and Sox2 at the 2 days, whereas all 4 genes were up-regulated in bFGF-treated groups at 4 and 6 days. At 6 and 10 days, the levels of expression of the 4 genes remained significantly different between bFGF-treated and untreated groups. The expressions of all 4 genes were mostly significantly higher in bFGF-treated than untreated for passages 6 (B) and 10 (C). Numbers in the bar charts are fold difference between bFGF-treated and nontreated (n = 12, *P < .05).
Discussion
In the present study, we demonstrated that bFGF enhanced a number of stem cell properties of SCAP, including increased proliferation, CFU-F forming capacity, and expression of STRO-1, Nanog, Sox2, Oct4, and Rex1. In addition, bFGF suppressed osteogenic/dentinogenic differentiation. When cells were exposed to the factor during expansion before osteogenic/dentinogenic stimulation, the differentiation was enhanced. Therefore, in this system, bFGF seems to function as a factor to maintain the cell behaviors that define stem cell stemness, ie, strengthening self-renewal ability and regulating cell differentiation of SCAP in vitro. The data help establish a basis for future in vivo verification of its functions in the maintenance of SCAP stemness.
Basic FGF has been known to induce cell proliferation (12, 14, 16). Our present findings in regard to increased proliferation of SCAP by bFGF are in agreement with published reports on other MSCs. Although treatment with bFGF (2.5 ng/mL) for 28 days does not affect the colony-forming efficiency of bone marrow stromal cells, it does increase the size of colonies (25). Our findings indicated that bFGF treatment for 8 days enhanced the cell colony-forming efficiency and the size of the colonies of SCAP, especially at higher passages (6 and 12) (Fig. 2D).
Basic FGF has important roles in cell differentiation. Some reports have shown that bFGF reduces differentiation and abolishes the mineralization of the extracellular matrix in osteoblastic cells, chondrocytes, DPCs, and adipose tissue–derived stromal cells (17, 18). Basic FGF decreased ALP activity and the formation of calcified nodules in hDPCs, but the suppression was abolished when hDPCs were cultured subsequently in the absence of bFGF (17, 26). Our in vitro studies showed that different timing of the presence of bFGF might either suppress osteogenic/dentinogenic differentiation of SCAP or enhance osteogenic/dentinogenc differentiation. These findings indicate that the effects of bFGF are differentiation stage specific and that bFGF might modulate cell differentiation by acting at distinct stages of cell maturation (27).
STRO-1 expression is known to decrease as the number of cell passages increases (28–30). Previous studies showed that the expression of STRO-1 in DPCs was enhanced when DPCs were cultured in the presence of bFGF (31). Similar to the reported studies, our present work showed that bFGF can sustain STRO-1 expression in SCAP up to passage 10. Transcription factors Oct4, Nanog, Sox2, and Rex1 are involved in the maintenance of the undifferentiated state of pluripotent stem cells. Their expression diminishes when ES cells undergo differentiation and during normal embryonic development (21, 22). Basic FGF is normally used to maintain ES cell cultures and to prevent cell differentiation (11). Withdrawing bFGF results in reduced expression of Oct4 and Nanog in pluripotent stem cells (32). Like ES cells, DPCs maintain the expression of Oct4 and Nanog for up to 5 passages, and the expression of these factors is absent at 17 passages (29). Our data showed that SCAP express Nanog, Sox2, Oct4, and Rex1 and that bFGF inhibited the reduced expression of Nanog, Sox2, Oct4, and Rex1 in SCAP at higher passages, indicating that it plays a role in maintaining the stemness of SCAP.
Our present in vitro studies implicate the potential role of bFGF during root development. During tooth development, bFGF is expressed in odontoblasts of the root of the tooth during the phase of root development and is localized in a more apical direction with the progression of time (9). SCAP in cultures express fibroblast growth factor receptor 1, fibroblast growth factor receptor 3, and bFGF (1). Basic FGF at later stage of tooth development might be expressed by SCAP in vivo and, therefore, might function as an autocrine self-regulation for their stemness and differentiation potency.
Because of recent interest in translational research that uses dental stem cells for the regeneration of tissues, bFGF might be used to maintain stem cell potency of SCAP in cultures, thereby facilitating the long-term usage of these cells for various tissue regeneration applications, including pulp/dentin regeneration, bioroot formation (2), and even for neural tissue regeneration (33).
Acknowledgments
The authors acknowledge the assistance from the staff of the Laboratory of Department of Endodontics, Fourth Military Medical University.
Footnotes
The authors deny any conflicts of interest related to this study
References
- 1.Sonoyama W, Liu Y, Yamaza T, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonoyama W, Liu Y, Fang D, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–796. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang GT, Yamaza T, Shea LD, et al. Stem/progenitor cell–mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010;16:605–615. doi: 10.1089/ten.tea.2009.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang GT. A paradigm shift in endodontic management of immature teeth: conservation of stem cells for regeneration. J Dent. 2008;36:379–386. doi: 10.1016/j.jdent.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645–651. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tziafas D, Kodonas K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J Endod. 2010;36:781–789. doi: 10.1016/j.joen.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Ota MS, Nakatomi M, Iseki S, Nakahara T, Eto K. Sonic hedgehog and FGF signaling are important for tooth root development. Eur Cell Mater. 2007;14(Suppl):45. [Google Scholar]
- 9.Madan AK, Kramer B. Immunolocalization of fibroblast growth factor-2 (FGF-2) in the developing root and supporting structures of the murine tooth. J Mol Histol. 2005;36:171–178. doi: 10.1007/s10735-005-2684-1. [DOI] [PubMed] [Google Scholar]
- 10.Kitchens DL, Snwyder EY, Gottlieb DI. FGF and EGF are mitogens for immortalized neural progenitors. J Neurobiol. 1994;25:797–807. doi: 10.1002/neu.480250705. [DOI] [PubMed] [Google Scholar]
- 11.Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 12.Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 13.Ito T, Sawada R, Fujiwara Y, Tsuchiya T. FGF-2 increases osteogenic and chondrogenic differentiation potentials of human mesenchymal stem cells by inactivation of TGF-b signaling. Cytotechnology. 2008;56:1–7. doi: 10.1007/s10616-007-9092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JH, Lee MC, Seong SC, Park KH, Lee S. Enhanced proliferation and chondrogenic differentiation of human synovium-derived stem cells expanded with basic fibroblast growth factor. Tissue Eng Part A. 2011;23:991–1002. doi: 10.1089/ten.TEA.2010.0277. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsumi S, Shimazu A, Miyazaki K, et al. Retention of multilineage differentiation potential of mesenchymal stem cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288:413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- 16.Nakao K, Itoh M, Tomita Y, Tomooka Y, Tsuji T. FGF-2 potently induces both proliferation and DSP expression in collagen type I gel cultures of adult incisor immature pulp cells. Biochem Biophys Res Commun. 2004;325:1052–1059. doi: 10.1016/j.bbrc.2004.10.136. [DOI] [PubMed] [Google Scholar]
- 17.Shimabukuro Y, Ueda M, Ozasa M, et al. Fibroblast growth factor-2 regulates the cell function of human dental pulp cells. J Endod. 2009;35:1529–1535. doi: 10.1016/j.joen.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Quarto N, Longaker MT. FGF-2 inhibits osteogenesis in mouse adipose tissue-derived stromal cells and sustains their proliferative and osteogenic potential state. Tissue Eng. 2006;12:1405–1418. doi: 10.1089/ten.2006.12.1405. [DOI] [PubMed] [Google Scholar]
- 19.Gregory CA, Gunn WG, Peister A, Prockop DJ. An alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Alongi DJ, Yamaza T, Song Y, et al. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med. 2010;5:617–631. doi: 10.2217/rme.10.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitsui K, Tokuzawa Y, Itoh H, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 22.Niwa H. Molecular mechanism to maintain stem cell renewal of ES cells. Cell Struct Funct. 2001;26:137–148. doi: 10.1247/csf.26.137. [DOI] [PubMed] [Google Scholar]
- 23.Kerkis I, Kerkis A, Dozortsev D, et al. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105–116. doi: 10.1159/000099617. [DOI] [PubMed] [Google Scholar]
- 24.Mansukhani A, Ambrosetti D, Holmes G, Cornivelli L, Basilico C. Sox2 induction by FGF and FGFR2 activating mutations inhibits Wnt signaling and osteoblast differentiation. J Cell Biol. 2005;168:1065–1076. doi: 10.1083/jcb.200409182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh S, Jefferiss C, Stewart K, Jordan GR, Screen J, Beresford JN. Expression of the developmental markers STRO-1 and alkaline phosphatase in cultures of human marrow stromal cells: regulation by fibroblast growth factor (FGF)-2 and relationship to the expression of FGF receptors 1–4. Bone. 2000;27:185–195. doi: 10.1016/s8756-3282(00)00319-7. [DOI] [PubMed] [Google Scholar]
- 26.Shiba H, Nakamura S, Shirakawa M, et al. Effects of basic fibroblast growth factor on proliferation, the expression of osteonectin (SPARC) and alkaline phosphatase, and calcification in cultures of human pulp cells. Dev Biol. 1995;170:457–466. doi: 10.1006/dbio.1995.1229. [DOI] [PubMed] [Google Scholar]
- 27.Debiais F, Hott M, Graulet AM, Marie PJ. The effects of fibroblast growth factor-2 on human neonatal calvaria osteoblastic cells are differentiation stage specific. J Bone Miner Res. 1998;13:645–654. doi: 10.1359/jbmr.1998.13.4.645. [DOI] [PubMed] [Google Scholar]
- 28.Harting M, Jimenez F, Pati S, Baumgartner J, Cox C Jr. Immunophenotype characterization of rat mesenchymal stromal cells. Cytotherapy. 2008;10:243–253. doi: 10.1080/14653240801950000. [DOI] [PubMed] [Google Scholar]
- 29.Yang R, Chen M, Lee CH, Yoon R, Lal S, Mao JJ. Clones of ectopic stem cells in the regeneration of muscle defects in vivo. PLoS One. 2010;5:e13547. doi: 10.1371/journal.pone.0013547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itaya T, Kagami H, Okada K, et al. Characteristic changes of periodontal ligament-derived cells during passage. J Periodontal Res. 2009;44:425–433. doi: 10.1111/j.1600-0765.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- 31.Morito A, Kida Y, Suzuki K, et al. Effects of basic fibroblast growth factor on the development of the stem cell properties of human dental pulp cells. Arch Histol Cytol. 2009;72:51–64. doi: 10.1679/aohc.72.51. [DOI] [PubMed] [Google Scholar]
- 32.Honda A, Hirose M, Ogura A. Basic FGF and Activin/Nodal but not LIF signaling sustain undifferentiated status of rabbit embryonic stem cells. Exp Cell Res. 2009;315:2033–2042. doi: 10.1016/j.yexcr.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Abe S, Yamaguchi S, Amagasa T. Multilineage cells from apical pulp of human tooth with immature apex. Oral Science International. 2007;4:45–58. [Google Scholar]