Abstract
Synaptic development, function and plasticity are highly regulated processes requiring a precise coordination of pre- and postsynaptic events. Recent studies have begun to highlight Wingless–Int (Wnt) signaling as a key player in synapse differentiation and function. Emerging roles of Wnts include the differentiation of synaptic specializations, microtubule dynamics, architecture of synaptic protein organization, modulation of synaptic efficacy and regulation of gene expression. These processes are driven by a variety of Wnt transduction pathways. Combined with a myriad of Wnts and Frizzled receptor family members, these pathways highlight the versatility of Wnt signaling and the potential for combinatorial use of these pathways in different aspects of synapse development and function. The identification of neurons secreting Wnt and those containing molecular components downstream of Frizzled receptors indicates that Wnts can function both as anterograde and retrograde signals. These studies open new avenues for understanding how embryonic morphogens are utilized during the development and function of synaptic networks.
Wnt transduction pathways: a brief précis
Exciting roles for embryonic morphogens of the Wingless–Int family (Wnt) beyond those in early pattern formation are beginning to emerge. These include roles in axon guidance, dendrite morphology, synapse formation and plasticity [1,2]. These studies make a clear case for Wnt function in post-mitotic neurons, and highlight the multitude of mechanisms activated by Wnts within the nervous system.
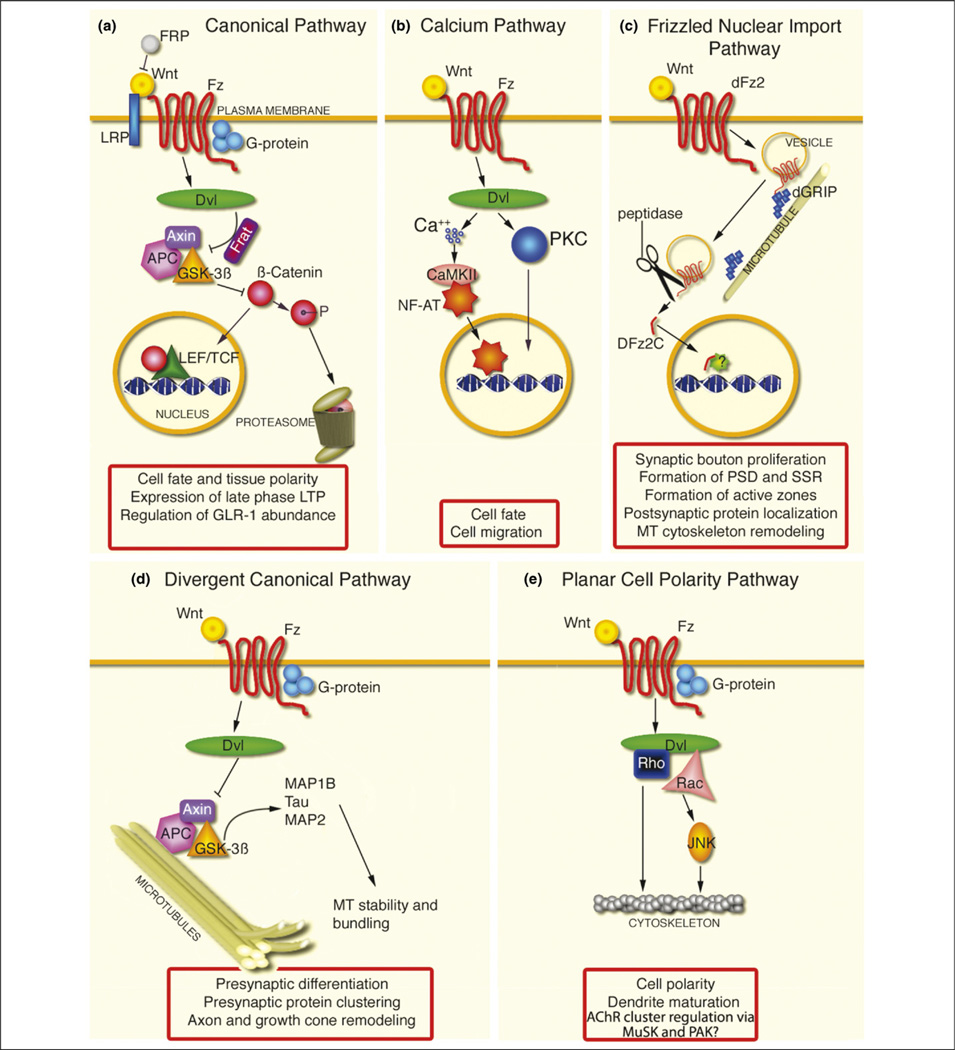
The Wnt pathway has pivotal roles in defining positional information in the embryo. Misregulation of this pathway is linked to cancer, and the pathogenesis of Alzheimer’s and Huntington disease [3,4]. The best studied Wnt transduction cascade is the canonical pathway, in which Wnt binds to its receptor Frizzled (Fz) in the receiving cell (Figure 1a,b). This binding activates the postsynaptic density-95/Discs-Large (DLG)/zona occludens-1 (PDZ) protein dishevelled (DVL), which disrupts a so-called ‘destruction complex’ containing glycogen synthase kinase-3β (GSK-3β), Axin and adenomatous polyposis coli (APC). In the absence of Wnt signaling, this complex constitutively phosphorylates β-catenin, leading to its proteasomal degradation. The binding of Wnt to Fz disrupts the destruction complex, resulting in cytoplasmic stabilization of β-catenin and its import into the nucleus [5]. In the nucleus, β-catenin associates with lymphoid enhancer factor (also known as T-cell factor) (LEF/TCF) transcription factors to regulate transcription. Additionally, β-catenin can bind to cadherins [6,7], but this function is not discussed here.
Figure 1.
Wnt transduction pathways and their functions during synapse development, function and plasticity. (a) Summary of the canonical Wnt signaling pathway. In this pathway, secreted Wnts bind to Fz receptors in the receiving cell. This binding activates the scaffolding PDZ protein DVL, which disrupts a so-called ‘destruction complex’ containing GSK-3β, axin and APC. In the absence of Wnt signaling, this complex constitutively phosphorylates β-catenin, leading to its degradation by ubiquitin-dependent proteasomal degradation. Wnt binding to Fz receptors disrupts the destruction complex, resulting in cytoplasmic stabilization of β-catenin and its import into the nucleus. In the nucleus, β-catenin associates with members of the LEF/TCF transcription factor family to regulate transcription. Also depicted are a Wnt antagonist FRP and an inhibitor of GSK-3, frequently rearranged in advanced T-cell lymphomas–GSK-3-binding protein (Frat–GBP), which can also bind to DVL. (b) The Wnt–Ca2+ signaling pathway. In this pathway, Fz activation by Wnts leads to increased intracellular Ca2+ and nuclear import of the transcription factor NF-AT. (c) The Frizzled nuclear import (FNI) pathway. In this signaling pathway, Wnts binds to Fz2 receptors in the receiving cell. Fz is endocytosed and back-transported towards the nucleus in a process that depends on its binding to the 7-PDZ protein GRIP. At some point during this trafficking, the C-terminal region of Fz2 is cleaved, and the cleaved fragment imported into the nucleus. (d) The divergent canonical Wnt pathway. In this signaling pathway, Wnt binding to Fz inhibits the destruction complex, and prevents the phosphorylation of microtubule-associated proteins such as MAP1B, Tau and MAP2, thereby regulating microtubule stability. (e) The PCP pathway. In this transduction pathway, Fz acts through JNK to regulate the cytoskeleton. Abbreviations: CAMKII, calcium–calmodulin-dependent protein kinase II; LRP, low-density lipoprotein receptor; PKC, protein kinase C.
Two non-canonical Wnt pathways have a role in development: (i) the planar cell polarity (PCP) pathway, in which Fz acts through Jun N-terminal kinase (JNK) to regulate the cytoskeleton (Figure 1e), and (ii) the Wnt–Ca2+ signaling pathway, in which Fz activation leads to increased intracellular Ca2+ and nuclear import of the transcription factor nuclear factor of activated T cells (NF-AT) [1] (Figure 1b). Thus, alternative Wnt pathways are utilized to specify pattern formation during development. Although the final output of the canonical and Wnt–Ca2+ pathways is the regulation of gene expression, the PCP pathway controls planar cell polarity by modulating the cytoskeleton.
Vertebrate Wnts as retrograde signals during synapse development
The first hint that Wnts might function in synapse development originated from the discovery that Wnt-3 is expressed in Purkinje cells in the developing cerebellum during neurite outgrowth and synapse formation [8]. Direct evidence emerged from work on cultured cerebellar granule cells (GCs), which express Wnt-7a in vivo during synaptogenesis with mossy fibers (MFs) and Purkinje cells [9]. Adding WNT-7a to cultured GCs increased axonal spreading and branching, as well as enhancing the clustering of the synaptic vesicle protein synapsin-I, particularly in spread axonal areas or in growth cones [9]. Thus, Wnt signaling seemed to regulate synapse development by modulating the cytoskeleton and presynaptic protein clustering.
It was subsequently reported that the secretion of WNT-7a by GCs regulated the formation of synapses between presynaptic MFs and postsynaptic GCs, which together make up multisynaptic glomerular rosettes [10]. MFs from pontine explants grown in GC-conditioned medium displayed increased axonal area and growth cone size, in addition to complexity, features typically observed after MFs enter the cerebellar cortex and contact GCs [11]. These changes were mimicked by the addition of soluble WNT-7a or the GSK-3β inhibitor lithium chloride (LiCl), and blocked by the Wnt antagonist secreted Frizzled-related protein (sFRP) [10].
The underlying mechanism of WNT-7a-induced axonal remodeling was elucidated by labeling microtubules (MTs), which revealed that WNT-7a caused MT unbundling at regions of axonal spreading and growth cone enlargement of MFs in culture [10]. This MT regulation might control the MF growth cone enlargement and axon spreading that occurs when they contact GCs in vivo [11]. Crucial in vivo evidence demonstrated that Wnt-7a−/− mice had decreased complexity and synapsin-I levels at glomerular rosettes, consistent with the observation that WNT-7a could induce synapsin-I clustering in cultured MFs [10]. However, these alterations in the mutant were only transient [10], indicating the presence of compensatory mechanisms. It was proposed that WNT-7a secretion by GCs regulated MF axonal remodeling and presynaptic differentiation retrogradely, and that this process, similar to the canonical Wnt signaling pathway, required GSK-3β inhibition.
Recent work has further elucidated the Wnt-7 genetic pathway regulating the development of MF synapses [12], suggesting thatWNT-7 secretion by GCs probably activates DVL1 in MFs, which in turn regulates presynaptic differentiation. Dvl1 was found to be present in adult synaptosomes, and acquired a presynaptic localization, as well as enhancing the clustering of presynaptic proteins when transfected into hippocampal cell cultures. Both Dvl1−/− and Wnt7a−/− mutants displayed defects in synapsin-I accumulation at glomerular rosettes, and this effect was enhanced in the double mutant. Furthermore, cultured Dvl1−/− neurons had alterations in the clustering of vesicle-associated membrane protein VAMP-II [12]. Ultrastructural analysis of glomerular rosettes showed that, whereas they were normal in Dvl1−/− or Wnt7a−/− mutants, in Wnt7a−/−–Dvl1−/− double mutants they had reduced complexity, suggesting that the two genes interact. Intriguingly, active zones and vesicle number, as well as distribution, seemed to be normal in the double mutants.
A similar retrograde role for Wnts was indicated at the sensory–motor neuron contacts of the lateral motor column. Wnt-3 is expressed in motor neurons during synapse formation with sensory neurons, and adding WNT-3 caused growth cone enlargement, as well as increased axonal branching of sensory neurons in culture. WNT-3 additionally induced clustering of synapsin-I [13], comparable to the effects of WNT-7 and DVL on MFs [10,12]. These effects were mimicked by LiCl, suggesting a role for GSK-3β. Unfortunately, Wnt-3−/− mutants are lethal, and thus an in vivo demonstration of retrograde signaling by Wnt-3 is lacking, although the remarkable similarities with Wnt-7 makes this scenario likely.
A host of studies supported the model that Wnts regulate axonal remodeling through a divergent canonical pathway that includes GSK-3β, DVL1, axin and the microtubule-associated protein MAP1B [14–16] (Figure 1d). GSK-3β can phosphorylate MAP1B [16], which is known to control the affinity of MAP1B for MTs and its ability to regulate MT dynamics [17,18]. Addition of WNT-7a to cultured neurons led to the loss of phosphorylated MAP1B from growth cones and spread axons [16], suggesting that the WNT-7a-mediated GSK-3β inhibition led to changes in MT dynamics through regulation of MAP1B. Further clues emerged from the finding that Dvl-1 associated with and stabilized MTs. Dvl-1 expression in cultured cells protected MTs from nocodazole-induced depolymerization [14], and this effect was mimicked by LiCl and blocked by GSK-3β expression, suggesting that Dvl-1 inhibits GSK-3β to regulate MT stability. Dvl-1 transfection into differentiated neuroblastoma-2a cells mimicked the axonal remodeling activity of Wnts on developing axons in a β-catenin-independent manner [15]. In addition, axin, through Dvl-1, stabilized MTs [15]. These observations support a model whereby a bifurcation of the canonical Wnt pathway regulates MT dynamics [15,2] (Figure 1d).
More recently, it has been demonstrated that Wnt-7b is expressed in the mouse hippocampus during dendrite maturation, and when transfected into hippocampal cultures, it increased dendrite complexity [19]. A similar effect was observed by transfecting Dvl-1, indicating that WNT-7b might signal through DVL-1 [19]. These observations were confirmed by studies of primary hippocampal neuron cultures from Dvl1−/− mutants, which displayed decreased dendritic branching. However, unlike WNT-7 signaling in the cerebellum and WNT-3 signaling in motor–sensory neurons, the action of DVL-1 on dendrite development did not require GSK-3β. Instead, DVL-1 seemed to signal through the non-canonical PCP pathway, using the small GTPase RAC and JNK to regulate actin and MT dynamics [19]. The effect of a Wnt member on postsynaptic morphogenesis raises the possibility that Wnt signaling might not only function in a retrograde fashion during synapse development, but that it might also operate as an anterograde, or even an autocrine, signal. This view is supported by studies in Drosophila, in which an anterograde signaling function at the neuromuscular junction (NMJ) has been demonstrated [20]. Thus, in the mammalian CNS, Wnts seem to function in a retrograde manner to regulate the differentiation of the presynaptic compartment, and they activate either a divergent canonical pathway or the PCP pathway (Box 1). However, evidence that Wnts might also modulate postsynaptic differentiation is beginning to emerge.
Box 1. Wnts during synapse differentiation, plasticity and function in mammals.
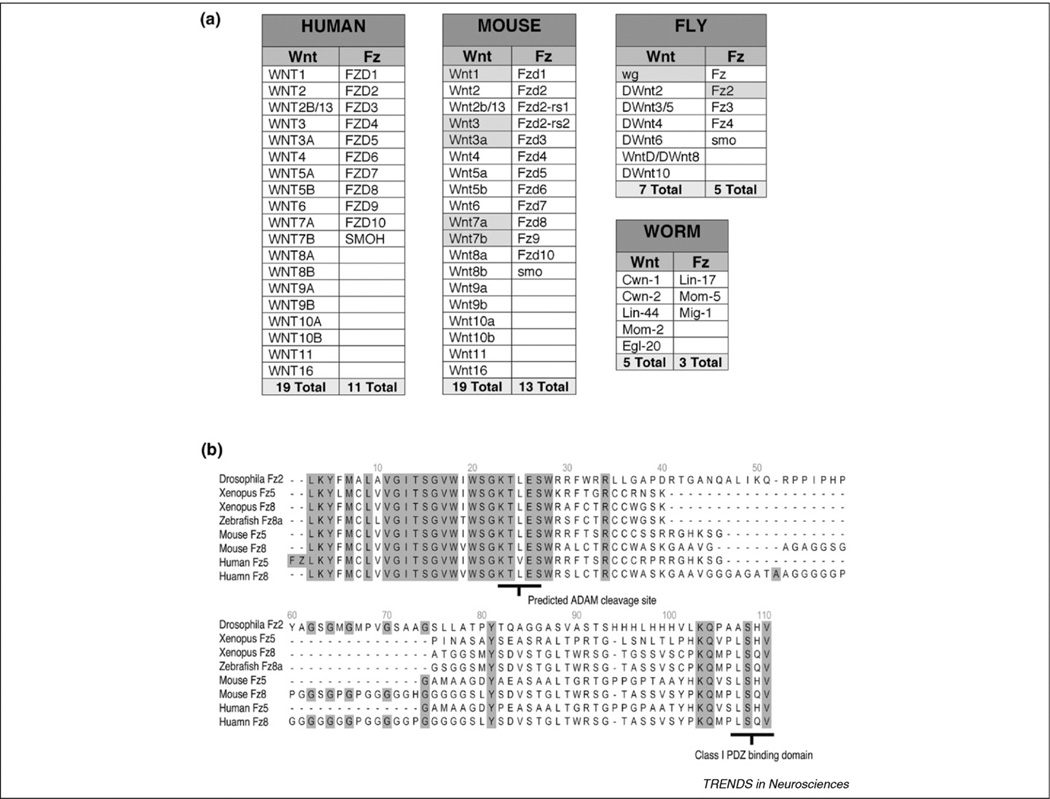
Studies of Wnt signaling during synapse development in mammals provide the following emerging landscape: (i) Wnts operate through multiple transduction pathways; (ii) although much of the evidence supports a retrograde signaling function for Wnts, some observations suggest that Wnts might have a more flexible mode of action, functioning either as anterograde or autocrine signals; (iii) the same Wnts can regulate synapse development in differing CNS regions through alternative downstream players; and (iv) alternative Wnts can regulate synaptic development in separate regions of the CNS through the same downstream signaling pathway. This apparent combinatorial use of Wnts and downstream effectors, coupled with the diversity of Wnts and Fz receptors (Figure 2a; http://www.stanford.edu/~rnusse/wntwindow.html), raises the intriguing possibility that Wnt signaling might be extensively used in a multitude of ways during synapse development. Moreover, analyses of Wnt and Fz in Drosophila [47] and in cell culture [48] suggest some degree of redundancy, which also seems to be present in the mammalian CNS.
Studies in slice preparations show that Wnt function is not restricted to synapse differentiation but is also associated with synaptic transmission and long-term synaptic plasticity. Wnt secretion can be elicited by L-LTP stimulation paradigms, activating a canonical Wnt pathway and leading to transcriptional modification in the presynaptic cell. Although these studies are tantalizing, they have only begun to scrape the surface of what will probably emerge as more widespread use of Wnts in the mature CNS. The construction of Cre recombinase drivers, enabling temporal and cell-specific knockouts to be constructed, will get around the essential early function of Wnts during pattern formation, so that studies of their independent synaptic roles can be conducted.
Wnt transduction pathways are potential regulators of postsynaptic receptor clustering and abundance
Another emerging role for Wnts in synapse development is in the regulation of postsynaptic receptor clustering and abundance. The best-studied pathway modulating postsynaptic receptor clustering is the regulation of acetylcholine (ACh) receptors by agrin and the tyrosine receptor kinase, muscle-specific kinase (MuSK) [21–23]. Agrin release from motor neurons functions through MuSK to maintain ACh receptor clusters at the vertebrate NMJ [21]. However, the intracellular cascades linking MuSK activation to receptor clustering are less well understood.
A link to Wnt regulation emerged from observations that DVL could bind to MuSK and p21-activated kinase (PAK1), and that this interaction was crucial for ACh receptor clustering downstream of agrin [24]. It should be noted, however, that agrin is now believed to be required for the maintenance of ACh receptor clusters rather than for the initial formation of clusters [21]. Consequently, DVL could also function in maintaining the clusters. The finding that agrin did not induce cytoplasmic accumulation of β-catenin makes a relationship to canonical Wnt signaling unlikely. Moreover, adding WNT-1 to myotubes had no effect on ACh receptor clustering, although other Wnts were not tested. Nevertheless, it was found that the neural agrin activated PAK1 in a DVL-dependent manner, and that PAK was downstream of cell division cycle protein 42 (CDC42) and RAC. Thus, it is possible that the PCP pathway is used because RAC functions in this pathway and PAK has the ability to regulate the cytoskeleton downstream of RAC (Figure 1e).
Although WNT-1 had no influence on ACh receptor clustering [24], it regulated MuSK expression in cultured myotubes [25], as demonstrated by an increase in MuSK promoter-reporter activity following the addition of WNT-1 and LiCl. Moreover, supplying WNT-1 increased endogenous MuSK mRNA levels in culture. MuSK expression in myotubes was shown to be regulated by an E-box promoter element, a sequence not normally bound by LEF/TCF or NF-AT transcription factors. Therefore, it is unlikely that the canonical or Wnt–Ca2+ pathways directly regulate MuSK expression. Nevertheless, these observations point to a potential role of WNT-1 in ACh receptor clustering or maintenance through regulation of MuSK expression, although an in vivo confirmation is still lacking. The apparent inconsistency between the above findings might be resolved if MuSK upregulation by WNT-1 requires agrin to influence ACh receptor clustering or maintenance.
Two additional studies report a role for APC in ACh receptor clustering [26,27]. In one, the underlying molecular mechanisms involved APC binding to postsynaptic density-93 (PSD-93), β-catenin and the MT end-binding protein EB1 to regulate nicotinic ACh receptor surface levels and clusters [27]. In the other, it was found that APC binds directly to the β-subunit of ACh receptors [26]. However, neither study looked for an upstream Wnt signal.
Recent work in Caenorhabditis elegans has described a canonical Wnt signaling pathway that regulates the abundance of the glutamate (Glu) receptor subunit GLR1 in the ventral nerve cord [28]. This pathway was found to utilize the worm homologs of β-catenin (bar-1), LEF/TCF (pop-1) and GSK-3β (gsk-3). Akin to the canonical Wnt pathway, LIN-23, the substrate-binding subunit of a Skp1–Cullin–F-box ubiquitin ligase, regulated BAR-1 levels to control GLR1 abundance. These results implicate a Wnt-mediated transcriptional control of GLR1 abundance, although the Wnt ligands and receptors have yet to be identified.
The above studies bolster the argument that Wnts are likely to function both in a retrograde and anterograde manner during synapse development. However, many of the molecular details involved in postsynaptic regulation of receptor clusters by Wnts remain to be explored.
Wnt signaling regulates synaptic function and activity-dependent synaptic plasticity
Misregulation of Wnt signaling has been implicated in schizophrenia [29,30] and Alzheimer’s disease [3], suggesting roles beyond synapse development. Two recent studies support this view [12,31]. Electrophysiological recordings in cerebellar slices from Wnt7a−/−–Dvl1−/− P14–P15 mice revealed a significant decrease in miniature excitatory postsynaptic current frequency at the MF–GC synapse. No defect was detected in either single mutant, probably owing to redundancy with Wnt-7a, Dvl-2 and Dvl-3. Ultrastructural studies of Wnt7a−/−–Dvl1−/− synapses suggested that this phenotype did not arise from changes in the number of synapses or a decrease in docked vesicles. Collectively, these observations support the existence of a retrograde Wnt signaling pathway in the regulation of neurotransmitter release.
One of the hallmarks of canonical Wnt signaling is the regulation of gene expression, a process that is also required for late-phase long-term potentiation (L-LTP) [32,33]. To identify molecular pathways underlying L-LTP, a microarray analysis of dentate gyrus slice preparations, in which LTP had been induced, was performed [31]. Notably, multiple Wnt signaling players were identified [31]. Furthermore, punctate WNT-3a and Frizzled-4 staining, both of which colocalized with the postsynaptic protein PSD-95, were found in the molecular layer of the dentate gyrus. Moreover, tetanic, but not basal, stimulation induced a significant decrease in WNT-3a signaling, suggesting that LTP-inducing stimulation drove WNT-3a release. These studies provided the first evidence for activity-dependent Wnt secretion (Box 1).
These studies also suggested that WNT-3a release probably activated a canonical Wnt pathway. LTP induction enhanced β-catenin nuclear import, and the microarray analysis revealed an upregulation of several canonical Wnt target genes. The nuclear accumulation of β-catenin could be suppressed by a Wnt-scavenger, Frizzled-8–Fc [34], and application of Frizzled-8–Fc or anti-Wnt-3a antibody reduced the magnitude of late-, but not of early-, LTP phases. Reciprocally, preincubation of slices with WNT-3a or LiCl potentiated LTP. Thus, these studies demonstrate a role of Wnts in the regulation of synaptic function and plasticity. The release of Wnts in an activity-dependent manner raises the exciting possibility that some of the activity-dependent modifications of neuronal circuits might result from Wnt signaling.
Wnt-1 has key roles during synapse differentiation in Drosophila
The initial discovery that the WNT-1 wingless (Wg) regulates synapse development in invertebrates emerged from studies at the Drosophila larval NMJ (Box 1) [35]. Wg was found within glutamatergic presynaptic boutons, and the Fz receptor DFz2, was concentrated at postsynaptic regions, as well as within motor neurons. Blocking Wg release by using a temperature-sensitive wg mutant (wgts) that prevents Wg exit from the endoplasmic reticulum, as well as the use of several molecular-genetic manipulations, suggested that Wg was secreted by presynaptic boutons but not by muscles. Furthermore, the use of the wgts mutant enabled the early roles of Wg to be bypassed, thereby facilitating the acute blockade of Wg release during a period of intense synaptic bouton proliferation. This perturbation led to drastic decreases in bouton numbers and to alterations in bouton morphology. These phenotypes could be rescued by presynaptic expression of a Wg transgene. Moreover, presynaptic Wg overexpression enhanced synaptic bouton proliferation. In wgts mutants, presynaptic boutons also had abnormal postsynaptic Discs-Large (DLG; a Drosophila member of the PSD-95 family) and Glu receptor localization. Most strikingly, loss of Wg signaling led to the formation of a subset of boutons (later termed ‘ghost boutons’) that contained synaptic vesicles but were devoid of active zones, PSDs, subsynaptic reticulum (SSR) and mitochondria, suggesting that Wg has central roles during synapse differentiation [36]. Similar effects were observed by manipulations that interfered with DFz2 function specifically in muscle cells.
Wgts mutants also displayed defects in presynaptic MT organization, as revealed by labeling for the MAP-1B-related protein Futsch [37]. Mutant boutons had enhanced unbundled MTs [35], suggesting that MTs remained in an abnormal dynamic state. Because alterations in MT dynamics are known to affect bouton proliferation [37,38], these results could explain the bouton outgrowth defect of wgts mutants. Given that DFz2 and GSK-3β (Shaggy) are present within presynaptic boutons, a disruption in presynaptic Wg signaling might be responsible for the above phenotype. Indeed, subsequent work demonstrated that presynaptic GSK-3β negatively regulates synaptic bouton proliferation, and that Futsch is downstream of GSK-3β function [39] and is phosphorylated by GSK-3β [40]. These observations support a role for Wg signaling in regulating presynaptic MTs similar to the observations in mammals [9,10,14–16] and, because Wg is secreted by boutons but not by muscles, these observations bring about the possibility that presynaptic DFz2 receptors might function in an autocrine loop during MT regulation.
These studies make a compelling case that Wnts can function in an anterograde, and perhaps in an autocrine fashion to establish some of the most basic synaptic structures, such as active zones and postsynaptic densities.
Frizzled finds its way to the nucleus
The search for the transduction cascade activated by Wg at the Drosophila NMJ led to the finding of a previously unrecognized alternative Wnt pathway, the Fz nuclear import pathway (FNI) [20] (Figure 1c and Box 2). Using antibodies recognizing the extracellular N- and intracellular C-terminus of DFz2 (DFz2N and DFz2C), it was found that, although both antibodies labeled the NMJ, the DFz2C antibody also labeled punctate structures within muscle nuclei, and DFz2N immunoreactivity was observed at the nuclear periphery. These intranuclear DFz2C foci localized to chromosomal regions, as assessed by propidium iodide staining, but were absent from heterochromatic regions based on heterochromatic protein 1 staining. Further biochemical and site-directed mutagenesis studies demonstrated that the C-terminal of DFz2 is cleaved at a glutamyl endopeptidase site that is conserved in some Fz receptors from flies to humans (Figure 2b), giving rise to an 8-kDa fragment that is imported into the muscle nuclei. It was proposed that DFz2C nuclear import, but not DFz2 cleavage, is Wg-dependent (Figure 1). Indeed, DFz2 nuclear import levels – but not DFz2 endocytosis, trafficking or cleavage – correlated with levels of Wg secretion.
Box 2. Wnts function as anterograde and perhaps autocrine signals during synapse differentiation in the fly.
The Drosophila larval muscles are innervated by glutamatergic motor neurons, and their presynaptic endings are composed of synaptic boutons resembling beads on a string. Each synaptic bouton contains many active zones [53] and is enveloped by the postsynaptic muscle membrane, which forms a complex structure, the SSR. Glutamate receptor clusters are localized at PSDs directly juxtaposed to presynaptic active zones. During larval development, the enormous increase in muscle cell size is accompanied by a corresponding enhancement in the number and size of synaptic boutons and release sites to maintain excitation–contraction efficacy, a process regulated by electrical activity [54].
Wg is secreted by presynaptic boutons, and DFz2 receptors are localized both at pre- and postsynaptic compartments, where they have crucial roles in the differentiation of synaptic specializations and the proliferation of synaptic boutons [35]. Wg release by synaptic boutons initiates an anterograde signaling cascade [the FNI pathway (Figure 1c)], involving the internalization, dGRIP-dependent trafficking, cleavage and nuclear import of DFz2. Nevertheless, postsynaptic disruption of the FNI pathway alters the development of both pre- and postsynaptic specializations. Thus, anterograde Wg signaling might trigger retrograde signals that regulate presynaptic development. A retrograde cascade at the fly NMJ is the transforming growth factor-β pathway, and it is tempting to envision a crosstalk between the two pathways, akin to their interactions during early pattern formation [55]. Alternatively, Wg signaling might activate an autocrine loop, which, in coordination with the FNI pathway, regulates presynaptic differentiation. Together with the observations in mammalian systems, it is likely that Wnts can function in several ways in cell-specific contexts. Many Wnt and Fz members exist in both organisms (Figure 2a), and a deep understanding of their operation at synapses will be required before attempting to construct a firm model.
Figure 2.
Wnt and Fz family members in different species, and alignment of the C-terminal region of those Fz members with a conserved glutamyl endopeptidase cleavage site. (a) List of Fz and Wnt members in humans, mice, flies and worms. Highlighted members have been implicated in synapse development, function or plasticity (based on information from Dr Role Nusse’s Wnt Homepage: http://www.stanford.edu/~rnusse/wntwindow.html). (b) C-terminal sequence alignment of several Fz receptors with a conserved glutamyl endopeptidase site [presumably a distintegrin and metalloprotease (ADAM) protease cleavage site] and a class I [Ser/Thr-X-Val-COO- (T/S-X-V)] PDZ-binding sequence. Alignments were performed with DS-gene software with the clustal W algorithm.
Nevertheless, DFz2 trafficking to the nucleus required endocytosis and back-transport of vesicles, as evidenced by the analysis of transgenic flies that interfered with these functions, and by examining the in vivo trafficking of endogenous DFz2 from the NMJ to the muscle nucleus [20]. The importance of this pathway was demonstrated by rescue experiments: whereas a full-length dfz2 transgene expressed in muscles rescued the dfz2 mutant defects in bouton number, a transgene lacking the cleavage site did not. Notably, expressing the DFz2C fragment did not bypass the requirement for Wg signaling, raising the possibility that DFz2C is modified in a Wg-dependent fashion before import [20].
Many receptors have been found to be cleaved and imported into the nucleus, including Notch, epidermal growth factor receptor and a C-terminal fragment of a voltage-gated Ca2+ channel [41–43], suggesting that this is a cellular strategy often used to establish communication between the plasma membrane and the nucleus. A common feature is that receptor fragments, often in combination with transcription factors, regulate transcription. Although this has not been demonstrated for DFz2, the above precedent makes this possibility likely.
An additional piece of the puzzle was provided by the finding that the Drosophila homolog of glutamate receptor-interacting protein (dGRIP) is required for DFz2 trafficking to the nucleus [36]. GRIP is a 7-PDZ protein, best known for its role in α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor trafficking in mammals, but it has also been associated with the transport of ephrin B receptors to dendrites, a process crucial for dendrite development [44,45]. At the Drosophila NMJ, dGRIP is localized in both pre- and postsynaptic vesicles associated with MTs [36]. Whereas a subpopulation of these vesicles colocalized with static Golgi bodies, others were highly mobile, reminiscent of GRIP localization in vertebrates [46]. Down-regulating dgrip in muscles mimicked the phenotypes arising from altering the FNI pathway and substantially decreased DFz2C nuclear import. Furthermore, both proteins colocalized in trafficking vesicles and physically interacted through the PDZ4–5 domain of dGRIP and the conserved PDZ-binding site in DFz2 (Figure 2b) [36]. Lowering muscle dGRIP levels inhibited the trafficking of DFz2 from the postsynaptic membrane to the nucleus. Although mammalian GRIP also seems to be crucial for postsynaptic development of cultured neurons [44], an association with Wnt pathways has not been established.
These studies highlight the notion that Wnt signaling is used at synapses in novel ways to coordinate the development of pre- and postsynaptic specializations. The elucidation of the function of DFz2C import into the nucleus is likely to enhance our understanding of this novel pathway.
Conclusions and future perspectives
Studies in animals as evolutionarily distant as worms and flies, as well as mammals, make a strong case for Wnt morphogens as crucial factors in the development and plasticity of synapses. Several notable findings stand out. (i) Various intracellular transduction pathways are activated by Wnts, and these seem to overlap only partially with those in early development. A common feature is the secretion of Wnt and its binding to a Fz receptor. Although the specificity of individual Wnt–Fz interactions has not been extensively investigated, certain Wnts show higher binding affinities for specific Fzs [47], and onlysomeWnt–Fz combinations signal efficiently through the canonical pathway in cell culture [48]. Additional specificity could arise from expression and differential subcellular localization of Fz receptors and Wnt co-receptors such as members of the low-density-lipoprotein related family. [49,50] and Ryk–Derailed [51,52]. The myriad of Wnt and Fz family members raises the speculation that different pathways might be activated in the same neuron for different processes, and that specificity is achieved by the combinatorial use of individual Wnts, their receptors or coreceptors, as well as the expression and/or localization of downstream components. (ii) Conserved processes regulated by Wnts are microtubule dynamics, synaptic protein organization and regulation of gene expression. Most intriguing will be the future identification of gene programs activated by Wnts, and their impact during synapse development and plasticity. (iii) An exciting development is the role of Wnts during activity-dependent plasticity, reinforcing the view that some of the developmental pathways involved in synapse development are also used for modifications in neuronal function in adult organisms. An activity-dependent regulation of Wg signaling also operates at the fly NMJ (B. Ataman and V. Budnik, unpublished) but the extent of the similarities remains to be determined. (iv) The finding that Fz receptors can translocate into the nucleus, presumably to regulate gene expression, highlights an increasingly apparent strategy used by cells directly to transmit signals from the extracellular milieu into the nucleus. It also underscores the diversity of intracellular processes that can be regulated by Wnts. (v) Alterations in Wnt function are linked to a variety of neurological disorders. Understanding Wnt function in the nervous system will accelerate the progress in treating and preventing these devastating human conditions.
References
- 1.Li F, et al. Vital elements of the Wnt–Frizzled signaling pathway in the nervous system. Curr. Neurovasc. Res. 2005;2:331–340. doi: 10.2174/156720205774322557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 3.Caricasole A, et al. Two sides of the same coin: Wnt signaling in neurodegeneration and neuro-oncology. Biosci. Rep. 2005;25:309–327. doi: 10.1007/s10540-005-2893-6. [DOI] [PubMed] [Google Scholar]
- 4.Terstappen GC, et al. The Wnt signaling pathway as a target for the treatment of neurodegenerative disorders. I Drugs. 2006;9:35–38. [PubMed] [Google Scholar]
- 5.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 6.Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat. Rev. Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- 7.Salinas PC, Price SR. Cadherins and catenins in synapse development. Curr. Opin. Neurobiol. 2005;15:73–80. doi: 10.1016/j.conb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Salinas PC, Nusse R. Regional expression of the Wnt-3 gene in the developing mouse forebrain in relationship to diencephalic neuromeres. Mech. Dev. 1992;39:151–160. doi: 10.1016/0925-4773(92)90042-i. [DOI] [PubMed] [Google Scholar]
- 9.Lucas FR, Salinas PC. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev. Biol. 1997;192:31–44. doi: 10.1006/dbio.1997.8734. [DOI] [PubMed] [Google Scholar]
- 10.Hall AC, et al. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 11.Hamori J, Somogyi J. Differentiation of cerebellar mossy fiber synapses in the rat: a quantitative electron microscope study. J. Comp. Neurol. 1983;220:365–377. doi: 10.1002/cne.902200402. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad-Annuar A, et al. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J. Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krylova O, et al. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- 14.Krylova O, et al. Dishevelled-1 regulates microtubule stability: a new function mediated by glycogen synthase kinase-3β. J. Cell Biol. 2000;151:83–94. doi: 10.1083/jcb.151.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciani L, et al. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J. Cell Biol. 2004;164:243–253. doi: 10.1083/jcb.200309096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas FR, et al. Inhibition of GSK-3β leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J. Cell Sci. 1998;111:1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- 17.Halpain S, Dehmelt L. The MAP1 family of microtubule-associated proteins. Genome Biol. 2006;7:224. doi: 10.1186/gb-2006-7-6-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goold RG, Gordon-Weeks PR. Glycogen synthase kinase 3β and the regulation of axon growth. Biochem. Soc. Trans. 2004;32:809–811. doi: 10.1042/BST0320809. [DOI] [PubMed] [Google Scholar]
- 19.Rosso SB, et al. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat. Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 20.Mathew D, et al. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kummer TT, et al. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr. Opin. Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Madhavan R, Peng HB. Molecular regulation of postsynaptic differentiation at the neuromuscular junction. IUBMB Life. 2005;57:719–730. doi: 10.1080/15216540500338739. [DOI] [PubMed] [Google Scholar]
- 23.McMahan UJ. The agrin hypothesis. Cold Spring Harb. Symp. Quant. Biol. 1990;55:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- 24.Luo ZG, et al. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron. 2002;35:489–505. doi: 10.1016/s0896-6273(02)00783-3. [DOI] [PubMed] [Google Scholar]
- 25.Kim CH, et al. Regulation of MuSK expression by a novel signaling pathway. J. Biol. Chem. 2003;278:38522–38527. doi: 10.1074/jbc.M305058200. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, et al. Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nat. Neurosci. 2003;6:1017–1018. doi: 10.1038/nn1128. [DOI] [PubMed] [Google Scholar]
- 27.Temburni MK, et al. Neuronal nicotinic synapse assembly requires the adenomatous polyposis coli tumor suppressor protein. J. Neurosci. 2004;24:6776–6784. doi: 10.1523/JNEUROSCI.1826-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dreier L, et al. LIN-23-mediated degradation of β-catenin regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Neuron. 2005;46:51–64. doi: 10.1016/j.neuron.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 29.De Ferrari GV, Moon RT. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene. 2006;25:7545–7553. doi: 10.1038/sj.onc.1210064. [DOI] [PubMed] [Google Scholar]
- 30.Ferrero A, et al. Vertex. 2006;17:165–171. [PubMed] [Google Scholar]
- 31.Chen J, et al. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J. Biol. Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- 32.Huang EP. Synaptic plasticity: going through phases with LTP. Curr. Biol. 1998;8:R350–R352. doi: 10.1016/s0960-9822(98)70219-2. [DOI] [PubMed] [Google Scholar]
- 33.Pittenger C, Kandel E. A genetic switch for long-term memory. C. R. Acad. Sci. III. 1998;321:91–96. doi: 10.1016/s0764-4469(97)89807-1. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh JC, et al. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- 35.Packard M, et al. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ataman B, et al. Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7841–7846. doi: 10.1073/pnas.0600387103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roos J, et al. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron. 2000;26:371–382. doi: 10.1016/s0896-6273(00)81170-8. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz-Canada C, et al. New synaptic bouton formation is disrupted by misregulation of microtubule stability in a PKC mutants. Neuron. 2004;42:567–580. doi: 10.1016/s0896-6273(04)00255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franco B, et al. Shaggy, the homolog of glycogen synthase kinase 3, controls neuromuscular junction growth in Drosophila. J. Neurosci. 2004;24:6573–6577. doi: 10.1523/JNEUROSCI.1580-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gogel S, et al. The Drosophila microtubule associated protein Futsch is phosphorylated by Shaggy/Zeste-white 3 at an homologous GSK3β phosphorylation site in MAP1B. Mol. Cell. Neurosci. 2006;33:188–199. doi: 10.1016/j.mcn.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Gomez-Ospina N, et al. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin SY, et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat. Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 43.Baron M, et al. Multiple levels of Notch signal regulation. Mol. Membr. Biol. 2002;19:27–38. doi: 10.1080/09687680110112929. [DOI] [PubMed] [Google Scholar]
- 44.Hoogenraad CC, et al. GRIP1 controls dendrite morphogenesis by regulating EphB receptor trafficking. Nat. Neurosci. 2005;8:906–915. doi: 10.1038/nn1487. [DOI] [PubMed] [Google Scholar]
- 45.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 46.Burette A, et al. Characterization of glutamate receptor interacting protein-immunopositive neurons in cerebellum and cerebral cortex of the albino rat. J. Comp. Neurol. 1999;411:601–612. [PubMed] [Google Scholar]
- 47.Wu CH, Nusse R. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J. Biol. Chem. 2002;277:41762–41769. doi: 10.1074/jbc.M207850200. [DOI] [PubMed] [Google Scholar]
- 48.Takada R, et al. Analysis of combinatorial effects of Wnts and Frizzleds on β-catenin/armadillo stabilization and Dishevelled phosphorylation. Genes Cells. 2005;10:919–928. doi: 10.1111/j.1365-2443.2005.00889.x. [DOI] [PubMed] [Google Scholar]
- 49.Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J. Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- 50.Wu J, et al. Subcellular localization of frizzled receptors, mediated by their cytoplasmic tails, regulates signaling pathway specificity. PLoS Biol. 2004;2:E158. doi: 10.1371/journal.pbio.0020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu W, et al. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 52.Yoshikawa S, et al. Wnt-mediated axon guidance through the Drosophila Derailed receptor. Nature. 2003;422:583–588. doi: 10.1038/nature01522. [DOI] [PubMed] [Google Scholar]
- 53.Prokop A. Organization of the efferent system and structure of neuromuscular junctions in Drosophila. Int. Rev. Neurobiol. 2006;75:71–90. doi: 10.1016/S0074-7742(06)75004-8. [DOI] [PubMed] [Google Scholar]
- 54.Griffith LC, Budnik V. Plasticity and second messengers during synapse development. Int. Rev. Neurobiol. 2006;75:237–265. doi: 10.1016/S0074-7742(06)75011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X, Cao X. BMP signaling and HOX transcription factors in limb development. Front. Biosci. 2003;8:s805–s812. doi: 10.2741/1150. [DOI] [PubMed] [Google Scholar]