Abstract
The formation of synaptic connections requires a dialogue between pre and postsynaptic cells to coordinate the assembly of the presynaptic release machinery and the postsynaptic receptive complexes. Signaling molecules of the Wnt family of proteins are central to this trans-synaptic dialogue. At the neuromuscular junction and central synapses, Wnts promote synaptic assembly by signaling to the developing pre and postsynaptic compartments. In addition, new studies reveal that expression of Wnt proteins and localization of their Fz receptors are regulated by neuronal activity. Importantly, Wnts mediates the synaptic changes induced by patterned neuronal activity or sensory experience in mature neurons. Here we review recent findings into the function of Wnt signaling at the synapse and its link to activity-dependent synaptic growth and function.
Introduction
Wnts are secreted glycoproteins with key roles in tissue patterning [1•]. Studies have also established a role for Wnts in postmitotic neurons, in axon pathfinding, dendritic development and synaptogenesis [2,3]. Indeed, altered Wnt signaling is linked to a number of neurological disorders, such as Alzheimer’s disease [4], Williams syndrome [5], schizophrenia [6] and mood disorders [7].
The Wnt family is diverse, ranging from 5 members in worms to 19 members in mammals [1•]. This complexity is further increased by the presence of a number of Wnt receptors of the Frizzled (Fz) family. In addition, non-conventional receptors such as the tyrosine kinase-like receptor Derailed (Drl)/Ryk, and the receptor tyrosine kinase Ror2 have been identified [1•]. Binding of Wnts to their receptors activates a number of intracellular cascades, and some of these are also used during synapse development and plasticity. The most characterized signaling cascade is the “Canonical Wnt Pathway”, in which binding of Wnts to their receptors activates the scaffolding protein Dishevelled (Dvl), which in turn inhibits a “destruction complex” including Axin, Adenomatous Polyposis Coli (APC), and the serine/threonine kinase GSK-3β. The destruction complex promotes the phosphorylation of β-catenin/Armadillo, which is then targeted for proteasome-dependent degradation. Inhibition of this complex by Wnt signaling leads to a rise in cytoplasmic β-catenin levels, and in its translocation into the nucleus, where it associates with the transcription factor TCF/LEF/ Pangolin to regulate Wnt target genes. In a “Divergent Wnt Canonical Pathway”, inhibition of GSK-3β modulates the phosphorylation of microtubule-associated proteins, such as the mammalian MAP1B, and the related Drosophila Futsch, promoting changes in microtubule stability and organization [8,9]. Alternatively, in the “Planar Cell Polarity (PCP) Wnt Pathway” both actin and microtubule cytoskeletons are regulated by activation of the small GTPases, RhoA or Rac1, and the c-Jun N-terminal kinase (JNK) by Dvl. Wnts are also known to increase intracellular Ca2+ levels, in the so-called “Calcium Wnt Pathway”, which activates protein kinase C (PKC) and Ca2+/calmodulin-dependent protein kinase II (CaMKII). Downstream of these events is the nuclear import of the transcription factor Nuclear Factor of Activated T-cells (NFAT).
Recently, the Frizzled Nuclear Import (FNI) pathway has been uncovered [3,10], and the unconventional Wnt receptor Ryk has been shown to use a similar pathway [11,12]. In this pathway Frizzled/Ryk receptors are internalized and cleaved, and a fragment is imported into the nucleus [10,12,13]. Although the function of Frizzled/Ryk receptor fragments in the nucleus is not known, it is speculated that they participate in the regulation of gene expression.
The knowledge of these diverse Wnt signaling pathways elucidated particularly during early embryogenesis has provided a framework for unraveling the mechanisms by which Wnts promote synapse formation and growth. However, studies of Wnts at synapses have also revealed new molecular mechanisms for Wnt action.
Wnt signaling at central synapses
Axon remodeling
A role for Wnt signaling in synapse formation was first demonstrated in the vertebrate nervous system. In the mouse cerebellum, Wnt7a regulates axon terminal remodeling and synaptic assembly [14]. Mossy fiber axons form terminal and en-passant synapses with cerebellar granule cell dendrites resulting in the assembly of a complex multisynaptic structure [15]. Granule cells secrete factors that induce the typical morphology of mossy fibers, which can be blocked by Wnt antagonists [14]. Axon remodeling is characterized by the formation of large growth cones and lamellipodia in the axon shaft, a process that is linked to the formation of synaptic boutons. Loss and gain of function studies in mice demonstrate that Wnt7a acts as a retrograde signal to regulate the remodeling of mossy fibers and synaptic assembly [14].
Remodeling is achieved through profound changes in the organization and stability of the cytoskeleton. Wnt7a or expression of Dvl1 increases the stability of microtubules in the axon shaft and at enlarged growth cones, through a divergent canonical pathway that requires GSK-3β inhibition but not transcription [16]. Inhibition of GSK-3β decreases the phosphorylation of the microtubule-associated protein MAP1B [9]. Thus, Wnt signaling promotes microtubule stability by directly regulating the cytoskeleton through changes in MAPs.
Wnt signaling also regulates the organization of microtubules by inducing the formation of looped microtubules within enlarged growth cones [14,17]. Looped microtubules have been observed at paused growth cones or at synaptic boutons of the Drosophila neuromuscular junction (NMJ) [8,18] where they contribute to proper development of the presynaptic terminal [19]. Time-lapse recordings of neurons expressing the microtubule plus end protein EB3-GFP show that Wnt induces a loss of directionality of microtubule growth, resulting in the formation of looped microtubules [17]. These changes in microtubule behavior are also associated with decreased growth cone translocation and increased growth cone size. Before looped MTs form, Wnt induces the loss of APC from the microtubule plus end, and APC knockdown mimics this effect [17]. Thus, Wnt signaling directly modulates the directionality of microtubule growth through the loss of APC from microtubule plus ends.
The behavior elicited by Wnt7a or Wnt3/Wnt3a is very different from typical axon guidance molecules, as Wnts decrease growth cone translocation but increase growth cone size and branching [14,17]. These effects are consistent with their role as signals that regulate the terminal remodeling of axons before synapse formation. Indeed, Wnt7a and Wnt3, which are expressed by postsynaptic targets, promote the assembly of presynaptic sites at early stages of synapse formation [14,20] in areas where microtubules unbundle or form loops.
Wnts in synaptic assembly
Wnts promote the assembly of central synapses by stimulating the recruitment of pre and postsynaptic components. In the cerebellum, loss of Wnt7a and/or Dvl1 function results in a strong deficit in the accumulation of synaptic markers at the mossy fiber-granule cell synapse. Conversely, Wnt7a or expression of Dvl1 stimulates the recruitment of synaptic vesicles, active zone proteins and the formation of numerous presynaptic recycling sites [14,21]. Importantly, Wnt7a; Dvl1 double mutants exhibit decreased miniature excitatory postsynaptic current frequency at the mossy fiber-granule cell synapse, consistent with a deficit in synapse formation [21]. In addition, other Wnts like Wnt5a preferentially stimulate postsynaptic assembly [22] suggesting that different Wnts might regulate synapse formation by promoting either presynaptic or postsynaptic assembly.
Different signaling pathways are activated at the presynapse and postsynapse. In axons, Wnt7a stimulates pre-synaptic assembly by inhibiting GSK-3β [14] but does not require transcription (Dickins and Salinas, unpublished) (Figure 1). In dendrites, in contrast, Wnt5a activates a non-canonical pathway that stimulates PSD95 clustering through JNK [22] (Figure 1). These different responses could be triggered by the presence of distinct Wnt receptors in dendrites and axons.
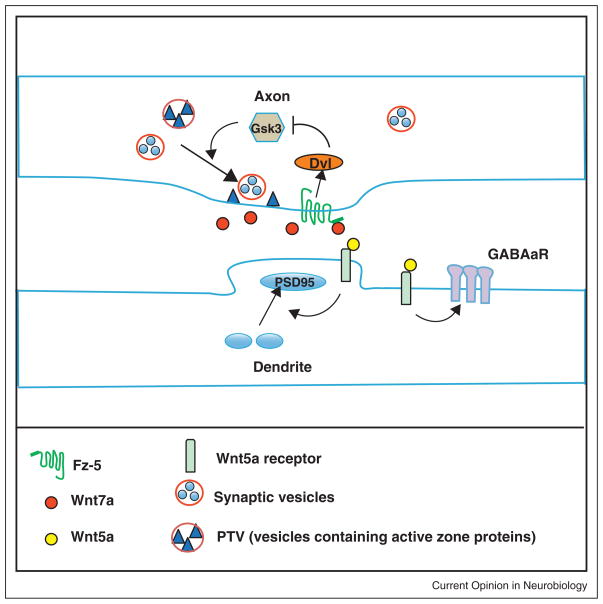
Figure 1.
Illustration of the role of Wnts in the assembly of synapses. Wnt7a through Fz5 and Dvl1 inhibits GSK-3β to promote the assembly of the presynaptic terminal. Inhibition of GSK-3β stimulates the recruitment of synaptic vesicles and active zone proteins such as Bassoon. Wnt5a, in contrast, acts on the postsynaptic side to increase clustering of PSD95. Wnt5a also increases the expression and clustering of GABAaR receptor on the dendritic shaft. The receptors for Wnt5a remain to be identified.
In the hippocampus, Frizzled-5 (Fz5) localizes to synaptic sites and its expression coincides with the peak of synapse formation. Importantly, expression of Fz5 in axons increases whereas Fz5 ShRNA knockdown significantly decreases the number of presynaptic sites [23••]. Moreover, loss of Fz5 function abolishes the ability of Wnt7a to promote the assembly of presynaptic sites [23••]. In hippocampal neurons, therefore, Fz5 is required for Wnt7a-mediated synapse formation (Figure 1).
Wnts also regulate the assembly of inhibitory synapses. In cultured hippocampal neurons, Wnt5a increases the level and retention of surface GABAa-Rs as well as the amplitude of GABA current. Increased recycling of the receptor without changes in endocytosis seems to mediate these changes [24] (Figure 1). As Wnt5a also promotes the formation of excitatory synapses [22], these results raise the question as to whether Wnts are pan-synaptogenic factors or whether they exhibit synaptic specificity.
Wnt function during activity-dependent morphological and functional plasticity
Neuronal activity plays a central role in the formation, refinement and function of neuronal circuits by modulating the number, morphology and efficacy of synapses. Moreover, several studies provide evidence for a link between neuronal activity and Wnt signaling. Firstly, depolarization stimulates the release or expression of Wnt proteins [25,26,27••]. Importantly, activation of NMDA receptors increases the expression of Wnt2 in hippocampal neurons [25], which then acts to promote dendritic development.
In hippocampal neurons, high frequency stimulation (HFS), which can induce long-term potentiation and promotes synapse formation, increases the level of surface Fz5 and its localization to synapses without affecting the total levels of the receptor [23••]. The effect of HFS in Fz5 mobilization is blocked by Wnt scavengers such as the extracellular domain (CRD) of Fz5 or the Wnt antagonist Sfrp [23••]. These results indicate that endogenous Wnts mediate the effect of HFS on Fz5 mobilization. Moreover, Fz5 CRD blocks the ability of HFS to stimulate synapse formation [23••]. Together these results indicate that neuronal activity regulates Wnt levels, which promotes Fz5 trafficking to synapses, a process that contributes to activity-mediated synapse assembly (Figure 2).
Figure 2.
Neuronal activity regulates synaptic localization of surface Fz5. Under control condition, a fraction of surface Fz5 is present at synaptic sites labelled with vGlut1 and NR1. High frequency stimulation (HFS) promotes the trafficking of Fz5 to the cell surface and also its localization to synapses (arrows). Blockade of endogenous Wnts with the CRD domain of Fz5 suppresses the mobilization of Fz5 to the cell surface and to synapse. Remarkable, blockade of endogenous Wnts also suppresses activity-mediated synaptogenesis.
Further evidence for a role of Wnt signaling in activity-mediated synaptic connectivity came from studies at the mossy fiber-CA3 synapse in the hippocampus. Exposure to an enriched environment (EE) increases the complexity and number of large mossy fiber terminals in the CA3 region [28••]. This remodeling is reminiscent of that induced by Wnt7a at the cerebellar mossy fiber-granule cell synapse [14]. Indeed, EE significantly increases the levels of Wnt7a/7b protein in the hippocampus. Importantly, local Wnt blockade with Sfrp1 suppresses EE-induced remodeling [28••]. Thus, behavioral experience regulates synapse remodeling through changes in Wnt signaling.
A role for Wnt signaling in synaptic plasticity is beginning to emerge. Electrophysiological recordings of hippocampal brain slices or cultured neurons suggest that Wnts regulate synaptic transmission [29–31]. Moreover, blockade of Wnt signaling impairs whereas activation of Wnt signaling increases long-term potentiation (LTP) in brain slices [32]. Although in vivo loss of function approaches are needed to fully establish a function for Wnt signaling in the plasticity of central synapses, these studies support the view that Wnt signaling can modulate synaptic transmission and plasticity in the central nervous system.
Wnt signaling at the neuromuscular synapse
Synaptic plasticity at the Drosophila neuromuscular junction
At the NMJ, Wnts regulate the localization of receptors, the differentiation of synaptic structure and synaptic plasticity [33,34]. While in both vertebrates and Drosophila, Wnts positively regulate synapse development, in Caenorhabditis elegans the binding of Wnt/Lin-44 to Fz/Lin-17 inhibits NMJ formation [46].
Drosophila larval body wall muscles are innervated by excitatory glutamatergic boutons. During larval development synaptic boutons, active zones, and postsynaptic GluR clusters proliferate to compensate for a large increase in muscle size while maintaining synaptic efficacy [35,36]. Wnt1, Wingless (Wg), is present at, and is thought to be released by synaptic boutons [19]. The release mechanism involves an interaction between Wg and the 8-pass transmembrane protein Evi/Wntless [37] present in exosome-like vesicles which are released into the extracellular space [38••] (Figure 3). This release mechanism provides a means by which hydrophobic Wnt proteins can traffic through the extracellular space [38••]. The Wg receptor, DFz2, is also present at the NMJ, both in motorneurons and in muscles [19]. Temporal block of Wg secretion decreases the proliferation of synaptic boutons and induces disruptions in the presynaptic microtubule cytoskeleton [19,39]. In addition, the localization of postsynaptic Discs-Large (DLG), a PSD95 family member, and GluR clusters is abnormal [19]. Most strikingly, a subset of the boutons (ghost boutons) are devoid of active zones and postsynaptic specializations, highlighting the role of Wg during synapse differentiation [13,19].
Figure 3.
Wg secreted from presynaptic motor neuron endings, binds to Fz-2 and co-receptor Arrow, which are localized presynaptically and postsynaptically. In the presynaptic cell, Wg activates a Divergent Canonical Wnt Pathway, involving Dvl activation, inhibition of GSK-3β activity and the regulation of the microtubule cytoskeleton through Futsch. In the postsynaptic cell, Wg activates the Frizzled Nuclear Import Pathway, which involves the cleavage and nuclear import of Fz-2. GRIP is required for the trafficking of receptors from the postsynaptic membrane towards the nucleus. WNT5 is also released from the presynaptic boutons and binds to its receptor Derailed (DRL) on the postsynaptic membrane to regulate synaptic bouton growth.
In muscles Wg activates the FNI pathway [10,13,27••,40•] (Figure 3). Overexpressing Wg in motor-neurons, which increases Wg secretion and proliferation of synaptic boutons, results in an increased nuclear import of the DFz2-C fragment [10,40•]. In contrast, downregulating the DFz2-interacting PDZ protein dGRIP, which is required for the transport of DFz2 to the nucleus, in muscles leads to a reduction in bouton number and DFz2-C nuclear import (Figure 3). A similar phenotype is observed in dfz2 mutants and this phenotype can be partially rescued by expression of a full-length dfz2 transgene in muscles, but not by a dfz2 transgene containing a mutation in the cleavage site [10]. Downregulation of dGRIP in muscles, mutations in dfz2, and mutations in importin-β11 (imp-β11) as well as in imp-α2, which are required for DFz2-C nuclear import, also lead to the formation of ghost boutons [10,13,40•], to a reduction in the size of postsynaptic membrane specializations, and to abnormal localization/levels of postsynaptic DLG and GluRs [19,40•]. Thus, the FNI pathway appears to play roles in both presynaptic and postsynaptic development. Nevertheless, mutations in imp-α2 have normal bouton number and the reduction in bouton number observed in imp-β11 mutants can be completely rescued by expressing Imp-β11 in motorneurons [40•]. In addition, restoring Wg signaling in motorneurons alone is sufficient to completely rescue bouton number and microtubule defects [39,40•]. Thus, it has been proposed that presynaptic Wnt signaling (see below) is required for presynaptic development and that the FNI pathway is only involved in postsynaptic development [40•]. However, interfering with the FNI pathway in the muscles alone is sufficient to reduce bouton number [13], to generate boutons lacking presynaptic active zones [13,40•], and dfz2 mutant phenotypes can be partially rescued by expressing DFz2 in muscle alone [10]. Part of the problem is that larval NMJs are capable of strong compensation, having several redundant mechanisms to control bouton number in both the presynaptic and postsynaptic cells. In addition, many of the studied genes have strong maternal contribution and the lack of a bouton number defect might be due to perdurance of maternally derived product.
In motorneurons Wg activates a divergent canonical pathway [39] (Figure 3). GSK-3β overexpression in motor-neurons, like mutations in wg, disrupts presynaptic microtubules [39]. The Fz co-receptor, Arrow (LRP5/ 6), and Dvl, but not β-catenin, are present at the NMJ [39]. Mutations in arrow mimic wg mutant phenotypes. However, Arrow appears to have both presynaptic and postsynaptic functions as some phenotypes are rescued by expressing an arrow transgene in either the presynaptic or postsynaptic cell [39]. Disruption of Dvl in neurons, by expressing a dominant-negative transgene, phenocopies disruption of Wg and Arrow [39]. In contrast, disrupting the function of the TCF homolog Pangolin or Armadillo/β-catenin produces no such phenotypes [39]. The bidirectional activation of alternative pathways by the same Wnt represents a mechanism to precisely match the development of presynaptic and postsynaptic structures, a critical process during synapse development.
Besides the role of Wnts in the scaling of innervation, Wg is also involved in activity-dependent plasticity [27••]. Spaced stimulation of motorneurons induces the formation of new ghost boutons as well as a potentiation of spontaneous neurotransmitter release [27••]. Time-lapse imaging of intact larvae shows that some of the ghost boutons mature, acquiring active zones and post-synaptic proteins [27••]. The de novo formation of bouton precursors requires 4–5 cycles of spaced simulation and is blocked by transcriptional and translational inhibitors. Reducing a single wg gene dose is sufficient to block the activity-dependent ghost bouton formation [27••]. In contrast, increasing Wg secretion, by overexpressing Wg in the motorneurons, bypasses some of the requirements for activity — while activity-dependent synaptic growth requires at least five cycles of spaced stimulation, NMJs with increased Wg secretion require only three cycles [27••].
Spaced and chronic increase in motorneuron activity result in a significant increase in DFz2-C nuclear import, and reduced activity leads to the opposite effect [27••]. Expressing a GSK-3β dominant-negative transgene in motorneurons also decreases the requirement for five cycles of spaced stimulation during activity-dependent bouton formation. In contrast, overexpressing GSK-3β markedly decreases the formation of new boutons [27••]. Thus, both the FNI and the divergent canonical Wnt pathways appear to regulate activity-dependent synaptic growth.
Wnt5 and its atypical receptor Derailed (Drl) also function as positive regulators of NMJ development [41]. Drl is present at the NMJ and drl mutants have a significant reduction in synaptic bouton number. In addition, in wnt5 mutants, the density of active zones is decreased, although they remain unaffected in drl mutants. Functional defects in wnt5 mutants include a reduction in the amplitude of evoked excitatory junctional currents (EJCs) as well as the frequency of spontaneous miniature EJCs (mEJCs) similar to the defects in gsk-3β mutants. However, both inhibition and overexpression of GSK-3β lead to a reduction in the amplitude of EJCs. Wnt5 appears to function in part in an anterograde manner, as overexpressing Wnt5 in motorneurons suppresses the drl phenotype and Drl is required in muscle for normal NMJ growth. Further, expressing Wnt5 in neurons but not in muscles, rescues the reduced synaptic bouton number of the wnt5 mutant, and overexpressing Wnt5 in motorneurons leads to synaptic overgrowth [27••]. However, the active zone phenotype is restored either by neuronal or muscle Wnt5 expression. The different effects of motorneuron-derived compared to muscle-derived Wnt5 raise the possibility of locally restricted signaling mechanisms that influence active zone induction versus synaptic bouton proliferation. For instance, active zone formation might require a local increase in secreted Wnt5 levels near the sites of active zone formation, while the regulation of bouton proliferation might require Wnt signaling at a site distant from synaptic endings. It is important to note that Wnts, although secreted proteins, are not freely diffusible owing to their hydrophobicity, and thus their traffic from release sites might be tightly controlled by the localization of carrier proteins such as Evi/Wls [38••].
Wnt signaling at the vertebrate NMJ
At the vertebrate NMJ, Wnt signaling regulates the prepatterning of AChR receptors before the arrival of axons and later by collaborating with the motorneurons-derived synaptogenic factor Agrin. Before the arrival of motor axons, aneural AChRs clusters form in the central domain of the muscle, a process called prepatterning. Although tyrosine kinase receptor MuSK expressed in the muscle is crucial in this process [42], its ligand Agrin is not required [42]. Thus, MuSK appears to be activated by an alternative ligand to regulate prepatterning. Indeed, in zebrafish embryos Wnt signaling through MuSK regulates prepatterning. Knockdown of Wnt11r, a Wnt expressed in tissues surrounding the spinal cord, results in severe defects in the clustering of aneural AChRs [43••]. Wnt11r binds to Unplugged/MuSK receptors and requires unplugged for its function. Thus, binding of Wnt11r to MuSK activates a signaling cascade that stimulates the clustering of aneural AChRs in the central region of the muscle before the arrival of the motor axons (Figure 4).
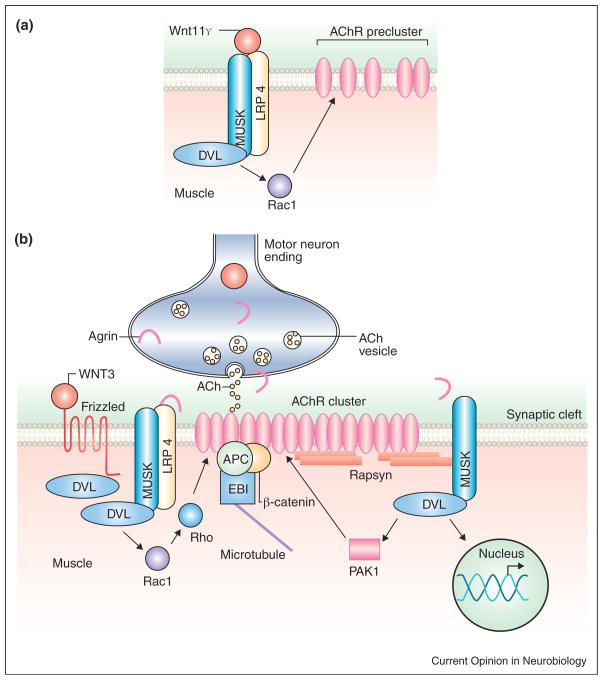
Figure 4.
(a) Wnt11r induces AChR preclustering before innervation through interactions with MuSK and LRP4. In mice, Wnt3 might also regulate this process. (b) Wnt3 collaborates with Agrin in the formation of AChR clusters after muscle innervation.
Wnt signaling also contributes to the assembly of NMJs by collaborating with Agrin. Gain and loss of function studies reveal that Wnt promotes the formation of AChR clusters during NMJ development in the chick limb [44••]. Moreover, Dvl1 knockout mice exhibit defects in the clustering of AChRs in the diaphragm [44••]. In cultured myotubes, Wnt3, which is expressed by moto-neurons, increases the formation of small but unstable AChR clusters. These clusters become stable and larger in the presence of Agrin. Thus, Wnt3 collaborates with Agrin by increasing the formation of microclusters, which can be converted into stable large AChR clusters (Figure 4).
Agrin induces the formation of large AChR clusters through the activation of both Rac and Rho and requires Dvl [45]. Interestingly, Wnt3 alone activates Rac1 whereas blockade of Rac suppresses the effect of Wnt3 microcluster formation [44••] (Figure 4). Although it remains to be determined whether motoneuronal Wnt3 or other Wnts play a role in Agrin-mediated synaptic assembly in vivo, these studies demonstrate that Wnt signaling collaborates with Agrin to regulate the assembly of the vertebrate NMJ.
Conclusions
Our knowledge on the role of Wnts in synaptic connectivity is rapidly growing. New studies demonstrate that Wnt do not only regulate synapse formation but also they promote the remodeling of mature terminals elicited by changes in electrical activity and environmental experience. Wnts can signal to the pre and postsynaptic terminals to promote assembly of the synapse. However, it remains unclear how Wnt signaling regulates the recruitment of synaptic components to future synaptic sites. Given that some Wnts specifically regulate pre or post-synaptic assembly, how is the coordinated assembly achieved? Studies in C. elegans demonstrate that Wnts can also inhibit synapse formation [46], raising the question as to what determines whether Wnts promote or inhibit synaptic assembly.
Several studies now indicate that Wnt mediates changes in synaptic number and plasticity elicited by neuronal activity. Indeed, neuronal activity does not only regulate Wnt release but also promotes the mobilization of Fz receptors thus enhancing the responsiveness of neurons to further neuronal activity. Although a role of Wnts in activity-dependent plasticity has been established at the Drosophila larval NMJ, in vivo loss of function experiments are still needed to establish the role of Wnt signaling in synaptic plasticity at central synapses. Detailed analyses of these questions should not only provide further understanding on how neuronal circuits are regulated but also they will shed light into possible approaches for the treatment of neurological disorders to which abnormal Wnt signaling has been linked.
Acknowledgments
We would like to thank to members of our laboratories for critical reading our manuscript. Our work has been funded by NIH (VB) and by BBSRC, MRC, Wellcome Trust EU F7 and ART (PCS).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1•.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. This is a comprehensive review on Wnt signaling that considers the integration of different cascades rather than the old view of distinct linear pathways. [DOI] [PubMed] [Google Scholar]
- 2.Salinas PC. Retrograde signalling at the synapse: a role for Wnt proteins. Biochem Soc Trans. 2005;33:1295–1298. doi: 10.1042/BST0331295. [DOI] [PubMed] [Google Scholar]
- 3.Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007;30:268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Ferrari GV, Papassotiropoulos A, Biechele T, Wavrant De-Vrieze F, Avila ME, Major MB, Myers A, Saez K, Henriquez JP, Zhao A, et al. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:9434–9439. doi: 10.1073/pnas.0603523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao C, Aviles C, Abel RA, Almli CR, McQuillen P, Pleasure SJ. Hippocampal and visuospatial learning defects in mice with a deletion of frizzled 9, a gene in the Williams syndrome deletion interval. Development. 2005;132:2917–2927. doi: 10.1242/dev.01871. [DOI] [PubMed] [Google Scholar]
- 6.Singh KK, Ge X, Mao Y, Drane L, Meletis K, Samuels BA, Tsai LH. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron. 2010;67:33–48. doi: 10.1016/j.neuron.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovestone S, Killick R, Di Forti M, Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci. 2007;30 :142–149. doi: 10.1016/j.tins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Roos J, Hummel T, Ng N, Klambt C, Davis GW. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron. 2000;26:371–382. doi: 10.1016/s0896-6273(00)81170-8. [DOI] [PubMed] [Google Scholar]
- 9.Lucas FR, Goold RG, Gordon-Weeks PR, Salinas PC. Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J Cell Sci. 1998;111(Pt 10):1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- 10.Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fradkin LG, Dura JM, Noordermeer JN. Ryks: new partners for Wnts in the developing and regenerating nervous system. Trends Neurosci. 2010;33:84–92. doi: 10.1016/j.tins.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Lyu J, Yamamoto V, Lu W. Cleavage of the Wnt receptor Ryk regulates neuronal differentiation during cortical neurogenesis. Dev Cell. 2008;15:773–780. doi: 10.1016/j.devcel.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Ataman B, Ashley J, Gorczyca D, Gorczyca M, Mathew D, Wichmann C, Sigrist SJ, Budnik V. Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP. Proc Natl Acad Sci U S A. 2006;103:7841–7846. doi: 10.1073/pnas.0600387103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 15.Hamori J, Somogyi J. Differentiation of cerebellar mossy fiber synapses in the rat: a quantitative electron microscope study. J Comp Neurol. 1983;220:365–377. doi: 10.1002/cne.902200402. [DOI] [PubMed] [Google Scholar]
- 16.Ciani L, Krylova O, Smalley MJ, Dale TC, Salinas PC. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J Cell Biol. 2004;164:243–253. doi: 10.1083/jcb.200309096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purro SA, Ciani L, Hoyos-Flight M, Stamatakou E, Siomou E, Salinas PC. Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for adenomatous polyposis coli. J Neurosci. 2008;28 :8644–8654. doi: 10.1523/JNEUROSCI.2320-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dent EW, Callaway JL, Szebenyi G, Baas PW, Kalil K. Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. J Neurosci. 1999;19:8894–8908. doi: 10.1523/JNEUROSCI.19-20-08894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krylova O, Herreros J, Cleverley KE, Ehler E, Henriquez JP, Hughes SM, Salinas PC. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farias GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, Inestrosa NC. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem. 2009;284:15857–15866. doi: 10.1074/jbc.M808986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Sahores M, Gibb A, Salinas PC. Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogenesis. Development. 2010;137:2215–2225. doi: 10.1242/dev.046722. This study demonstrates that Fz5 mediates the synaptogenenic function of Wnt7a in the hippocampus. Endogenous Wnts mediate the mobilization of surface Fz5 receptor to synapses. Remarkable, blockade of Fz5 mobilization during high frequency stimulation supresses the formation of synapses induced by neuronal activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuitino L, Godoy JA, Farias GG, Couve A, Bonansco C, Fuenzalida M, Inestrosa NC. Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. J Neurosci. 2010;30:8411–8420. doi: 10.1523/JNEUROSCI.5736-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- 27••.Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V. Rapid activity-dependent modifications in synaptic structure and function require bidirectional wnt signaling. Neuron. 2008;57:705–718. doi: 10.1016/j.neuron.2008.01.026. This paper characterizes a role of Wnt signaling in synaptic growth in response to patterned acute stimulation at the Drosophila NMJ. It shows that Wg release is promoted by activity, and that released Wg then activates alternative Wg transduction pathways in the pre and postsynaptic membrane to regulate the growth of synaptic boutons and the organization of the postsynaptic apparatus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Gogolla N, Galimberti I, Deguchi Y, Caroni P. Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron. 2009;62:510–525. doi: 10.1016/j.neuron.2009.04.022. A remarkable study demonstrating for the first time that exposure to an enriched environment increases Wnt expression in the hippocampus concomitantly with extensive remodeling of presynaptic structures. Importantly, Wnt contributes to this activity mediated remodeling. [DOI] [PubMed] [Google Scholar]
- 29.Avila ME, Sepulveda FJ, Burgos CF, Moraga-Cid G, Parodi J, Moon RT, Aguayo LG, Opazo C, De Ferrari GV. Canonical Wnt3a modulates intracellular calcium and enhances excitatory neurotransmission in hippocampal neurons. J Biol Chem. 2010;285:18939–18947. doi: 10.1074/jbc.M110.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerpa W, Godoy JA, Alfaro I, Farias GG, Metcalfe MJ, Fuentealba R, Bonansco C, Inestrosa NC. Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem. 2008;283:5918–5927. doi: 10.1074/jbc.M705943200. [DOI] [PubMed] [Google Scholar]
- 31.Beaumont V, Thompson SA, Choudhry F, Nuthall H, Glantschnig H, Lipfert L, David GR, Swain CJ, McAllister G, Munoz-Sanjuan I. Evidence for an enhancement of excitatory transmission in adult CNS by Wnt signaling pathway modulation. Mol Cell Neurosci. 2007;35:513–524. doi: 10.1016/j.mcn.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- 33.Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 2010;137:1017–1033. doi: 10.1242/dev.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korkut C, Budnik V. WNTs tune up the neuromuscular junction. Nat Rev Neurosci. 2009;10:627–634. doi: 10.1038/nrn2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins CA, DiAntonio A. Synaptic development: insights from Drosophila. Curr Opin Neurobiol. 2007;17:35–42. doi: 10.1016/j.conb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Peng X, Cooper RL. Development of Drosophila larval neuromuscular junctions: maintaining synaptic strength. Neuroscience. 2002;115:505–513. doi: 10.1016/s0306-4522(02)00380-9. [DOI] [PubMed] [Google Scholar]
- 37.Ching W, Nusse R. A dedicated Wnt secretion factor. Cell. 2006;125:432–433. doi: 10.1016/j.cell.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 38••.Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. A demonstration that in the fly, Wnt1 is released in association with exosome-like vesicles through interactions with Evi/Wntless. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miech C, Pauer HU, He X, Schwarz TL. Presynaptic local signaling by a canonical wingless pathway regulates development of the Drosophila neuromuscular junction. J Neurosci. 2008;28:10875–10884. doi: 10.1523/JNEUROSCI.0164-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Mosca TJ, Schwarz TL. The nuclear import of Frizzled2-C by Importins-beta11 and alpha2 promotes postsynaptic development. Nat Neurosci. 2010;13:935–943. doi: 10.1038/nn.2593. This study demonstrates that a classical nuclear import mechanism mediates the entry of Frizzled2 fragments during NMJ development in Drosophila. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liebl FL, Wu Y, Featherstone DE, Noordermeer JN, Fradkin L, Hing H. Derailed regulates development of the Drosophila neuromuscular junction. Dev Neurobiol. 2008;68:152–165. doi: 10.1002/dneu.20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- 43••.Jing L, Lefebvre JL, Gordon LR, Granato M. Wnt signals organize synaptic prepattern and axon guidance through the zebrafish unplugged/MuSK receptor. Neuron. 2009;61:721–733. doi: 10.1016/j.neuron.2008.12.025. An important paper implicating Wnt11r in the formation of aneural AChR clusters prior to innervation and suggesting that Wnt11r is a MuSK ligand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Henriquez JP, Webb A, Bence M, Bildsoe H, Sahores M, Hughes SM, Salinas PC. Wnt signaling promotes AChR aggregation at the neuromuscular synapse in collaboration with agrin. Proc Natl Acad Sci U S A. 2008;105:18812–18817. doi: 10.1073/pnas.0806300105. This paper demonstrates that Wnt3 collaborates with Agrin during the assembly of the vertebrate NMJ. The study shows that Wnt3 promotes the formation of small but unstable AChR clusters, which are stabilized and enlarged in the presence of Agrin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo ZG, Wang Q, Zhou JZ, Wang J, Luo Z, Liu M, He X, Wynshaw-Boris A, Xiong WC, Lu B, et al. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron. 2002;35 :489–505. doi: 10.1016/s0896-6273(02)00783-3. [DOI] [PubMed] [Google Scholar]
- 46.Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]