Summary
At vertebrate neuromuscular junctions (NMJs), Agrin deplays pivotal roles in synapse development, but molecules that activate synapse formation at central synapses are largely unknown. Members of the Wnt family are well established as morphogens, yet recently they have also been implicated in synapse maturation. Here we demonstrate that the Drosophila Wnt, Wingless (Wg), is essential for synapse development. We show that Wg and its receptor are expressed at glutamatergic NMJs, and that Wg is secreted by synaptic boutons. Loss of Wg leads to dramatic reductions in target-dependent synapse formation, and new boutons either fail to develop active zones and postsynaptic specializations or these are strikingly aberrant. We suggest that Wg signals the coordinated development of pre- and postsynaptic compartments.
Introduction
Although much is known about the molecular mechanisms of synapse differentiation, major gaps remain in our understanding of this process, particularly with regard to the signals mediating the coordinated structuring of the pre- and postsynaptic apparatus. Studies at both vertebrate and invertebrate synapses, have identified a number of scaffolding proteins, including Rapsyn, and proteins of the PSD-95 and Munc-13 families, as important elements in the proper spatial assembly of synaptic proteins (Garner et al., 2000; Sanes and Lichtman, 1999). In addition, several secreted or anchored factors such as BDNF, Neuroligin, and Agrin have been implicated in synapse differentiation (Kovalchuk et al., 2002; Sanes and Lichtman, 1999; Scheiffele et al., 2000). At the vertebrate neuromuscular junction (NMJ), Agrin plays a pivotal role in activating postsynaptic differentiation. However, the signals that coordinate the development of central synapses are still unknown, just as the role of Agrin at central synapses has remained elusive.
A recent study has suggested that Wnts may work as signaling factors that induce presynaptic differentiation (Burden, 2000; Davis, 2000; Hall et al., 2000). In culture, Wnt-7a enhanced growth cone complexity and microtubule dynamics, and these effects were antagonized by interfering with Wnt signaling. Consistent with these findings, wnt-7a mutants displayed delayed maturation of cerebellar synapses. In Drosophila embryos, overexpression of Dwnt-3 disrupted the formation of commissural tracts (Fradkin et al., 1995), suggesting that Wnts may be involved in synaptic connectivity in insects.
Wnt function during morphogenesis and pattern formation has been extensively studied (Dierick and Bejsovec, 1999). In these processes, Wnts provide a positional cue that determines cell differentiation and body axis formation. In Drosophila, this function involves formation of a Wg gradient across many cell diameters from Wg-expressing cells (Pfeiffer et al., 2000). There is also evidence suggesting that formation of this gradient depends on secretion and movement of Wg through the extracellular space, as well as by turnover via endocytosis (Dubois et al., 2001; Strigini and Cohen, 2000).
Several molecules involved in early pattern formation also function at later developmental stages to specify cell fate and positional identity (Chu-LaGraff and Doe, 1993). An attractive idea is that a subset of these molecules might also operate in the nervous system to signal positional cues for the juxtaposition of pre- and postsynaptic specializations. Here we tested this hypothesis by using the Drosophila larval NMJ, a well-characterized system with many molecular similarities to vertebrate central synapses (Koh et al., 2000). We find that Wg and its receptor are expressed at glutamatergic synapses, that Wg is secreted by synaptic boutons becoming deposited at postsynaptic sites, and that it can be taken up by muscles. We also show that blocking Wg secretion severely reduces muscle-dependent formation of new synaptic boutons. At the ultrastructural level, defective Wg function prevents formation of active zones and postsynaptic structures in many boutons, demonstrating a novel in vivo role for Wg in synapse differentiation. Mutant boutons that do develop active zones exhibit dramatic defects in active zone shape, and in the postsynaptic membrane directly apposed to the active zones. These studies show that Wg provides an essential in vivo cue for the coordinated development of pre- and postsynaptic structures.
Results
Wg Is Secreted by Type Ib Boutons
We used antibodies against Wg to examine its distribution at the larval NMJ. We found that Wg immunoreactivity was strongest at glutamatergic type Ib boutons (Figures 1A and 1D) from the earliest stages of larval development, but was absent, or at very low levels at type Is, II, and III boutons (Figures 1A–1C; Gramates and Budnik, 1999). Comparison of Wg immunoreactivity with presynaptic markers including anti-HRP, which stains the presynaptic membrane (Figures 1B, 1C, and inset), and vesicle markers, including Cysteine string protein (CSP; Figure 2G) and Synapsin revealed that Wg was localized both presynaptically and postsynaptically. Postsynaptic localization of Wg was observed around synaptic boutons; the postsynaptic region being defined as the NMJ region that failed to colocalize with any presynaptic marker (Figure 1C inset; Figures 2A and 2G). Postsynaptic Wg localization was also confirmed by double labeling anti-DLG and anti-Wg (Figures 1D–1F and inset). Previous studies show that DLG is both pre- and postsynaptic, but is particularly enriched at the muscle postsynaptic membrane complex (subsynaptic reticulum-SSR), a system of folded membranes that appears continuous with the plasma membrane (Guan et al., 1996; Lahey et al., 1994).
Figure 1. Wg and its Receptor, DFz2, Are Localized at the NMJ.
(A–C) NMJs at muscles 12 and 13 double-stained with (A) anti-Wg and (B) anti-HRP to stain the presynaptic arbor.
(C) Merged Wg and HRP images show that Wg is enriched at type Ib endings.
(D–F) NMJs at muscles 12 and 13 double-stained with (D) anti-Wg and (E) anti-DLG, which stains primarily the postsynaptic muscle region of type I boutons.
(F) Merged Wg and DLG images show that Wg occupies much of the DLG positive region. Insets: high magnification views of single confocal slices through type Ib boutons.
(G–I) NMJs labeled with (G) anti-DFz2, (H) anti-DLG, and (I) both merged to show their colocalization. Insets correspond to high magnification views of a single confocal slice through type Ib boutons. Calibration bar is 50 µm in (A–I) and 12 µm in insets.
Figure 2. Wg Is Secreted by Type Ib Boutons and Endocytosed by Muscles.
Images show single confocal slices of (A and G) wild-type NMJs at muscles 6 and 7 double-stained with (A) anti-Wg and anti-HRP, and (G) anti-Wg and anti-CSP.
(B and H) NMJs of larvae with presynaptically driven Wg double-stained with (B) anti-Wg and anti-HRP, and (H) anti-Wg and anti-CSP. Note the postsynaptic buildup of Wg.
(C and I) NMJs in Wg secretion-defective porc mutants double labeled with (C) anti-Wg and anti-HRP, and (I) anti-Wg and anti-CSP.
(D and J) NMJs in temperature-shifted wgts mutants double-stained with (D) anti-Wg and anti-HRP, and (J) anti-Wg and anti-CSP. Note that postsynaptic Wg is dramatically reduced in both porc and wgts mutants.
(E and K) NMJs in temperature-shifted shits1 double-stained with (E) anti-Wg and anti-HRP, and (K) anti-Wg and anti-CSP. Note the significant increase in postsynaptic Wg in this mutant.
(F) Merged anti-HRP and GFP at an NMJ of a wg-Gal4 larva driving expression of Wg-GFP.
(M) NMJ of a larva where UAS-Wg was expressed in muscle, double-stained with anti-Wg and anti-HRP. Note the accumulation of Wg around muscle nucleus (n), whereas levels of Wg at the NMJ are similar to wild-type.
(N) Summary of the distribution of Wg (green) at the NMJ of the various mutants shown in diagrammatic form.
(L and O) Single confocal slices through the dorsal region of the CNS showing motorneurons in (L) a C380 X UAS-LacZ larva double-stained with anti-Wg and anti-βgal, and (O) a wild-type larva double-stained with anti-Wg and anti-glutamate. Arrow indicates the CNS midline; anterior is left. Calibration bar is 20 µm in (A–K), (M), and 26 µm in (L and O).
Interestingly, we also found that the Wg receptor, DFrizzled2 (DFz2) (Chen and Struhl, 1999) was exactly colocalized with DLG (Figures 1G–1I). In the CNS, DFz2 was also localized in motorneurons and in the neuropil (Supplemental Figure S2 available at http://www.cell.com/cgi/content/full/111/3/319/DC1). These observations suggest that DFz2 is expressed both pre- and postsynaptically.
To determine if other Wg pathway components were also expressed at the NMJ, we used antibodies against GSK-3β/Zeste-white 3 and the β-catenin homolog, Armadillo (Arm). We found GSK-3β enriched presynaptically at type Ib boutons (Supplemental Figure S1 available at above website). In contrast, we were unable to detect Arm staining.
Studies in epithelial and brain tissue show that secreted Wg becomes deposited in the extracellular matrix and is internalized by surrounding cells (Dubois et al., 2001; Lin and Perrimon, 1999; Strigini and Cohen, 2000). To determine if Wg could be secreted by type Ib boutons and/or muscles, we used several approaches.
First, Wg was overexpressed at type I boutons using the UAS/Gal4 system with motorneuron Gal4 drivers C380 and C164, or muscle Gal4 drivers BG487 and C57 (Budnik et al., 1996). Presynaptic overexpression resulted in dramatic increase of Wg label at type Ib boutons (compare Figures 2A and 2G to 2B and 2H). However, this increase was not confined to presynaptic cells as expected, but was also found at the postsynaptic region. At this region, Wg label was significantly more intense and widespread than in wild-type, although still restricted to the area surrounding each synaptic bouton. In contrast, postsynaptic Wg expression resulted in perinuclear Wg accumulation and did not alter the intensity of NMJ staining (Figure 2M). We also used a wg-Gal4 strain in which the Wg promoter was fused to Gal4 to drive a GFP-tagged Wg (GFP-Wg). Analysis of wg-Gal4 using UAS-LacZ reveals that this strain replicates the endogenous Wg expression pattern and shows expression in neurons, but not in muscles. Consistent with the notion that Wg is secreted by synaptic boutons we found that in wg-Gal4 X UAS-Wg-GFP larvae, GFP was distributed both pre- and postsynaptically (Figure 2F). These observations suggest that postsynaptic Wg results from Wg secretion by type I boutons, and not by muscle cells. We also observed Wg in motor neuron cell bodies, which were identified by using anti-glutamate or by examining βgal staining in progeny fromC380 X UAS-LacZ (Figures 2L and 2O).
To block Wg secretion, mutations in porcupine (porc) and wg were used. porc encodes an endoplasmic reticulum(ER) protein required for Wg processing and trafficking (Kadowaki et al., 1996). In temperature sensitive wg mutants (wgts), Wg is trapped in the ER at restrictive temperature, dramatically reducing its secretion (van den Heuvel et al., 1993). wgts larvae were raised until early third instar at permissive temperature and then shifted to restrictive temperature until late third instar. We found that, in both porc (Figures 2C and 2I) and heat-pulsed wgts (Figures 2D and 2J), Wg was almost eliminated at postsynaptic sites and was severely reduced at presynaptic boutons (compare Figures 2A to 2C and 2D, and 2G to 2I and 2J). This result, together with Wg overexpression experiments, strongly suggests that Wg is secreted by presynaptic boutons.
Wg is known to be endocytosed in epidermal tissues by a Dynamin-dependent process (Dubois et al., 2001; Moline et al., 1999). We therefore blocked endocytosis using mutations in the Drosophila Dynamin, shibire (shi), (Chen et al., 1991). Shibire is most prominently expressed at synaptic boutons (Estes et al., 1996), but it is also expressed in hot spots in muscles. To test if mutations in shi had any influence on Wg distribution, we used a temperature sensitive shi allele, shits1, which was pulsed at restrictive temperature for 3 hr before fixation. These mutants exhibited a dramatic increase in postsynaptic Wg (Figures 2E and 2K), similar to that observed with presynaptic Wg overexpression. Identical results were obtained when a dominant negative form of Shibire, ShiD (Moline et al., 1999), was expressed only postsynaptically, but not when it was expressed presynaptically (not shown). Thus, interfering with Dynamin function results in postsynaptic Wg accumulation, and this effect is mimicked by blocking Dynamin function at the postsynaptic cell alone. An interpretation of this result is that secreted Wg is normally endocytosed by muscles, and that in the absence of endocytosis (in shi mutants) Wg accumulates.
Wg Is Involved in Target-Dependent Synapse Formation
To examine Wg function at the NMJ, we used wgts mutants. Null mutations in wg are embryonic lethal due to the requirement of Wg for morphogenesis (Nusslein Volhard et al., 1985). This requirement can be bypassed by raising wgts mutants at permissive temperature during embryonic and early larval stages. We shifted larvae to restrictive temperature to suppress Wg function during the 3rd instar stage.
During larval development, NMJs undergo continuous synaptic bouton formation to compensate for muscle growth while maintaining synaptic efficacy (Gorczyca et al., 1993; Lnenicka and Keshishian, 2000; Schuster et al., 1996). This target-dependent synapse formation includes an increase in the number and size of synaptic boutons as well as in the number of active zones. Analysis of NMJs in wgts mutants showed that loss of Wg function had dramatic consequences for target dependent-synapse formation. In wild-type, about half of type I boutons are formed during the last day of larval development (Gorczyca et al., 1993). In temperature-shifted wgts mutants, NMJ expansion was dramatically reduced resulting in over a 25% decrease in the number of boutons compared to wild-type controls (Figure 3A). This is greater than a 50% reduction in the number of boutons that should have formed during the last day of the third instar larval stage and could be completely rescued by presynaptic expression of Wg (Figure 3A), but not by expression of GFP alone or by postsynaptic expression of Wg. The defect in NMJ expansion in wgts mutants was not due to changes in muscle size (not shown). In contrast, the expansion of type II and III boutons was not affected in wgts mutants (not shown). These results suggest that Wg function is necessary for normal type I synapse formation during the larval stages, and that lack of Wg function severely impairs the ability of the presynaptic arbor to expand in correlation to muscle growth. This conclusion was supported by overexpressing Wg presynaptically, which resulted in the opposite effect, a significant increase in the number of boutons (Figure 3B).
Figure 3. Synaptic Bouton Number and the Proportion of Boutons Containing Unbundled Cytoskeleton Are Altered in wgts Mutants.
(A) Number of type Ib, Is, and total boutons in temperature-shifted wild-type, wgts, and wgts expressing Wg presynaptically (red) determined in preparations double-stained with Wg and HRP antibodies. N = 18 for CS, 15 for wgts, and 16 for rescue.
(B) Bouton number in non-heat pulsed wild-type and larvae overexpressing Wg presynaptically (pre-Wg+). N = 9 for CS and 11 for pre-Wg+.
(C) Number of Wg positive boutons, and the percentage of Wg positive boutons containing unbundled (unbund), looped (loop), or both splayed and punctate Futsch. Measurements were made at segments A2 and A3. For Futsch measurements N = 26 for CS A2, 20 for CS A3, 19 for wgts A2, 20 for wgts A3, 9 for rescue A2, and 9 for rescue A3. ** represent p < 0.0001; * represents p < 0.001.
(D and F) wild-type NMJs stained with (D) anti-Futsch and (F) double-stained with anti-Futsch and anti-Wg antibodies.
(E and G) wgts NMJs stained as above. In (D) and (E), boutons containing unbundled Futsch have been slightly overexposed (green squares) to facilitate visibility of less intense signal. Arrowheads (F and G) point to boutons with unbundled Futsch; t indicates trachea, which also stain with anti-Wg.
(H–J) Examples of unbundled Futsch filaments showing (H) a loop, (I) splayed filaments, and (J) diffuse and punctate Futsch. Calibration bar is 30 µm in (D–G), and 5 µm in (H–J).
In mammals, the Wnt pathway has been implicated in cytoskeletal reorganization during growth-cone extension (Salinas and Hall, 1999). Activation of Wnts results in inhibition of GSK-3β, which is believed to destabilize microtubules through phosphorylation of the microtubule associated protein MAP1B (Cook et al., 1996; Lucas et al., 1998; Goold et al., 1999). We found GSK-3β enriched at presynaptic boutons (Supplemental Figure S1 available at http://www.cell.com/cgi/content/full/111/3/319/DC1). In addition, the MAP1B-related Synapprotein, Futsch, has been shown to colocalize with microtubules at the NMJ and to be involved in NMJ expansion (Roos et al., 2000).
In wild-type, Futsch is observed as a filamentous bundle that traverses the center of NMJ processes (Figures 3D and 3F). Often at distal boutons (at the end of a branch), this bundle can form a loop (Figure 3H), have a splayed appearance, in which many thin filaments diverge from the central bundle (Figure 3I) or lose its filamentous appearance becoming diffuse and punctate (Figure 3J). During neurite extension, microtubule loops are associated with paused growth cones, while the transition to new axonal growth is accompanied by splaying of looped microtubules, and by invasion of the lamellipodia by microtubule fragments (Dent et al., 1999). In flies, mutations that affect NMJ expansion, such as dfxr and futsch, have an abnormal proportion of unbundled filaments (forming loops, splayed filaments, or disintegrated filaments) at terminal boutons (Roos et al., 2000; Zhang et al., 2001). Because our observations implicate Wg in NMJ expansion, we examined Futsch distribution in temperature-shifted wgts larvae and quantified the proportion of type Ib boutons containing unbundled filaments. We found that indeed the proportion of unbundled filaments was significantly higher in wgts mutants (Figures 3C, 3E, and 3G), with the most substantial increase in the percentage of splayed or disintegrated filaments, and with a decrease in boutons containing a loop. These results suggest that in wg mutants synaptic expansion is arrested, but the boutons retain cytoskeletal features correlated to dynamic growth.
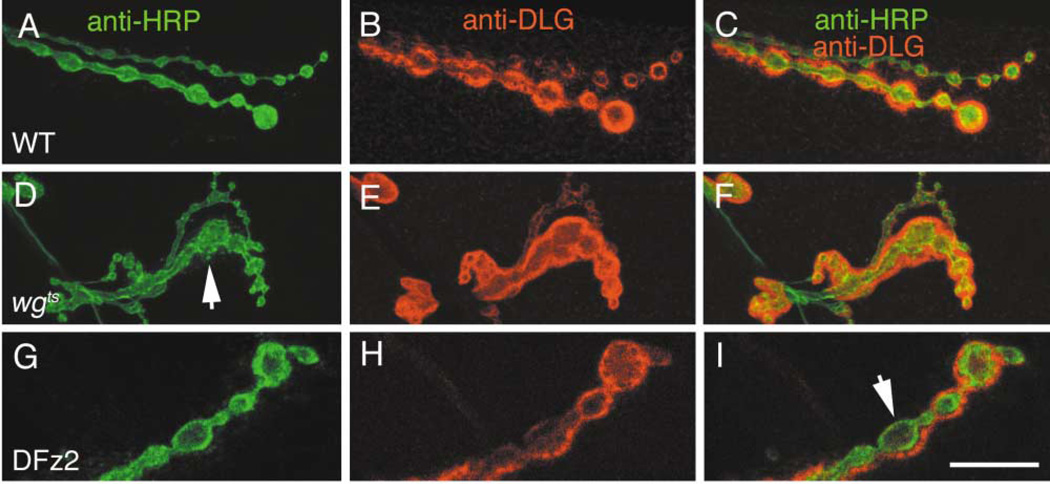
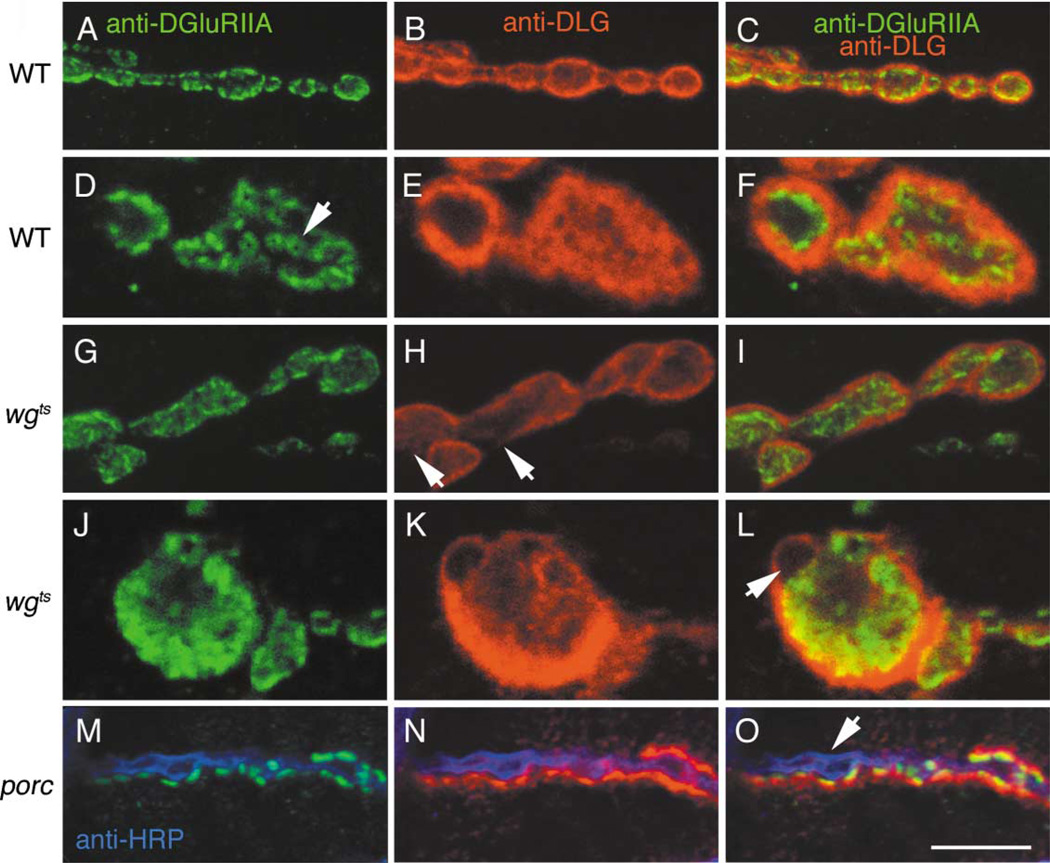
Mutations in wg Alter Bouton Morphology and Glutamate Receptor Distribution
We examined the general morphology of synaptic boutons in wgts mutants and the distribution of a number of pre- and postsynaptic proteins including CSP, Synapsin, Fasciclin II, DLG, and muscle glutamate receptors (DGluRIIA). We found that wgts boutons had a more irregular appearance (Figures 4A and 4D). Wild-type boutons are elliptical with a smooth outline, have a well-defined size range (Johansen et al., 1989), and are separated by neuritic processes (Figure 4A). In wgts mutants many boutons were noticeably larger, they greatly deviated from an elliptical shape, displayed a rough outline, and often did not show a clear separation from neighboring boutons (Figure 4D). We also found that distribution of glutamate receptors and DLG was altered in the mutants. In wild-type, DGluRIIA are often localized in well-defined hot spots that have a doughnut shaped profile (Figures 5A, 5D arrow), and which are directly apposed to active zones (Petersen et al., 1997). In wgts mutants, DGluRIIA immunoreactivity was more diffuse, and the regular distribution of hot spots was lost (compare Figures 5D to 5J). Although many hot spots remained in the mutants, they had an irregular size and shape. DLG immunoreactivity was also abnormal in many wgts boutons. In wild-type, DLG surrounds individual boutons where it forms a thick and well-defined rim (Figures 4B and 5B). In wgts boutons, DLG often appeared surrounding groups of boutons (Figures 4D–4F) or was absent from regions of the bouton periphery (Figure 5H, arrow). Synapsin and Shi, were also slightly altered (not shown).
Figure 4. Synaptic Morphology and DLG Distribution Are Altered in wgts Mutants.
(A–C) Wild-type type I boutons double-stained with (A) anti-HRP, (B) anti-DLG, and (C) both merged.
(D–F) Boutons in wgts double labeled as above. Note the abnormally shaped type I boutons in wgts mutants (arrow), and that unlike wild-type, DLG surrounds groups of boutons in the mutant.
(G–I) Type Ib boutons in a strain overexpressing DFz2 in postsynaptic muscles stained as above.
(I), (G), and (H) merged to show abnormal lack of DLG in areas surrounding boutons (arrow). A similar phenotype is observed in wgts mutants (see Figure 5). Calibration bar = 10 µm.
Figure 5. Glutamate Receptor Distribution Is Altered in wgts Mutants.
(A–C) Low and (D–F) high magnification of wild-type boutons stained with (A and D) anti-DGluRIIA, (B and E) anti-DLG, and (C and F) both merged. Note the presence of DGluRIIA immunoreactive clusters, some of which have a doughnut-like appearance (arrow in D).
(G–I) Low and (J–L) high magnification of boutons in wgts double labeled as above. Note that in wgts mutants, GluRIIA label appears diffuse and both the regularity and shape of immunoreactive clusters are aberrant. Arrow in (L) points to a lack of DGluRIIA in a bouton bud.
(M–O) porc mutant NMJ triple labeled with anti-HRP (Blue), anti-DGluRIIA (Green), and anti-DLG (Red).
(M) shows DGluRIIA and HRP labels, (N) shows DLG and HRP labels, and (O) shows the triple labeling. Note that both DLG and DGluRIIA are absent from almost an entire side of this string of boutons (arrow), but appear normal at the other side. Calibration bar = 10 µm in (A–C), (G–I), (M–O), and 4 µm in (D–F), (J–L).
To determine if these phenotypes resulted from specific alterations in Wg signaling, we examined NMJs in porc mutants and in larvae overexpressing DFz2 in muscles. Previous studies show that overexpression of DFz2 leads to phenotypes similar to mutations in Wg, presumably due to spatial dilution of the ligand (Moline et al., 1999, Cadigan et al., 1998). Both a mutation in porc and DFz2 overexpression caused phenotypes similar to mutations in wg, including bouton regions devoid of DLG, diffuse or absent DGluRIIA staining (e.g., Figures 4G–4I, 5M–5O), and irregular bouton morphology. Similar phenotypes were observed when we expressed a dominant negative DFz2, UAS-DFz2DN, in muscles (not shown).
Wg Provides a Signal for Pre- and Postsynaptic Differentiation
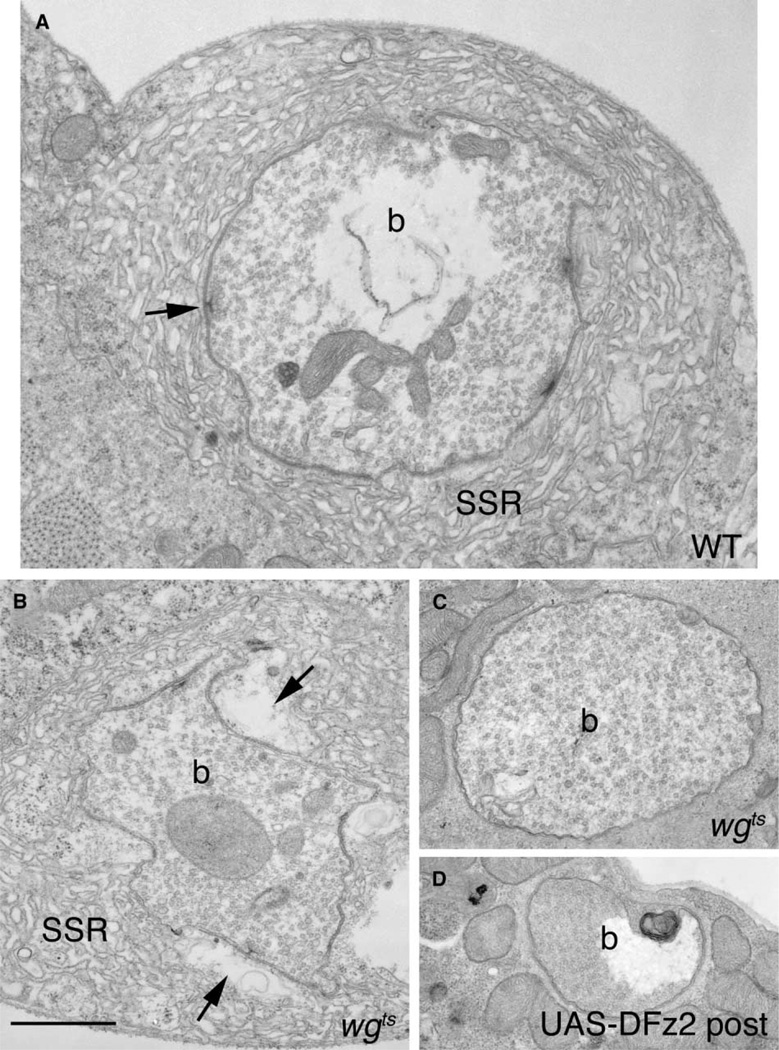
To examine mutant synapse ultrastructure we serially sectioned type I NMJs and performed a morphometric analysis (Table 1). Because during larval development NMJs expand in part by budding from existing boutons at the end of the terminal (Zito et al., 1999), we expected that if Wg is required for bouton development, then bouton morphology would be affected in only the subset of boutons that developed under partial or complete loss of Wg. We found that boutons in temperature-shifted wgts could be subdivided into three categories. The first category, (33% of the boutons; N = 36), exhibited the most remarkable abnormalities. Unlike wild-type (Figure 6A), these boutons were thoroughly filled with synaptic vesicles and were entirely devoid of both active zones (both the electron dense thickening of pre- and postsynaptic membranes and the T bar) and mitochondria (Figure 6C). Postsynaptically, these boutons also completely lacked SSR (Figure 6C). Analysis of the bouton positions within a branch demonstrated that they were located most frequently at the distal region of a branch, and that therefore they likely formed later in NMJ development, presumably in the absence of Wg function. Similar phenotypes were found in many boutons of larvae overexpressing DFz2 postsynaptically (Figure 6D), concordant with our observations that these larvae have synaptic abnormalities similar to those observed in wg mutants. These results uncover a novel role for Wg signaling, the formation of active zones.
Table 1.
Morphometric Analysis of Type I Synaptic Boutons in wg Mutants
| Wild-Type | wgts | p | |
|---|---|---|---|
| Total number of active zones | 1.43 ± 0.16 (N = 29) | 1.02 ± 0.23 (N = 36) | > 0.05 |
| Number of active zones in boutons with enlarged postsynaptic pockets | N/A | 2.2 ± 0.4 (N = 12) | < 0.05* |
| Number of active zones in boutons with normal postsynaptic pockets | 1.43 ± 0.16 (N = 29) | 0.92 ± 0.29 (N = 12) | > 0.05 |
| Cross-sectional bouton area | 2.42 ± 0.19 (N = 29) | 1.99 ± 0.2 (N = 36) | > 0.05 |
| Number of SSR layers | 12.6 ± 0.5 (N = 29) | 7.1 ± 1.1 (N = 36) | < 0.0001 |
| SSR density (Number of layers/length) [µm−1] | 29.6 ± 2.0 (N = 29) | 30.7 ± 2.14 (N = 36) | > 0.05 |
| Number of mitochondrial profiles | 5.7 ± 0.52 (N = 29) | 1.8 ± 0.35 (N = 36) | < 0.0001 |
compared to total number of active zones in wild-type.
Figure 6. Ultrastructure of Synaptic Boutons Is Abnormal in wg Mutants and in Larvae Overexpressing Dfz2 Postsynaptically.
Electron micrographs of type Ib boutons in (A) wild-type, (B and C) wgts mutants, and (D) in a strain overexpressing DFz2 in the postsynaptic muscles (UAS-DFz2 post). Note boutons lacking SSR, active zones, and mitochondria. In (B), a wgts bouton with abnormally enlarged pockets in the postsynaptic region apposed to active zones (compare arrows in A and B) is shown. b = bouton, SSR = Subsynaptic Reticulum. Calibration bar is 1.5 µm.
The second group of boutons, 33% of the boutons, were localized at intermediate positions within a branch, and therefore, presumably matured under partial loss of Wg function. Most of these boutons (about 60%) contained active zones with abnormally shaped T bars (Figure 7). In wild-type, T bars consist of a stem associated with the presynaptic membrane and a bar perpendicular to the distal end of the stem (Figures 7A–7C; Jia et al., 1993). In mutant boutons of this second category, many T bars appeared as amorphous dense areas or contained aberrantly shaped stems with little or no bar (Figures 7D–7F). These abnormalities were never observed in wild-type, regardless of the plane of sectioning. Remarkably, the postsynaptic area immediately apposed to active zones was also dramatically altered (Figure 6B; arrows). In wild-type, this region contains a shallow pocket of amorphous material (arrows in Figures 7B and 7C). In wgts mutant boutons of this type, the postsynaptic pocket was strikingly enlarged and often contained a number of membranous structures (Figure 6B arrows, Figure 7E asterisk). These large pockets gave boutons an irregular appearance, in which regions of the presynaptic compartment appeared detached from the SSR (Figure 6B). Unlike the postsynaptic region directly apposed to active zones, the rest of the postsynaptic area appeared relatively normal, although the number of SSR layers was significantly reduced (Table 1). In addition, these boutons had fewer mitochondrial profiles (Table 1).
Figure 7. Active Zones and Postsynaptic Region Are Altered in wgts Mutants.
(A and D) High and (B, C, E, and F) lower magnification views of active zones in (A–C) wild-type, and a range of aberrant morphologies of the mutant active zones. In (E) is a dramatically enlarged pocket (asterisk) apposed to an active zone in the mutant.
(G and H) Two models on the likely operation of Wg during the development of the NMJ.
(G) According to this model, the Wg receptor, DFz2, is localized both pre- and postsynaptically and interacts with Wg secreted by the presynaptic bouton. This triggers independent pre- and postsynaptic transduction cascades leading to the proper positioning and morphology of active zones and to proper development of the postsynaptic apparatus. In (H), DFz2 is localized exclusively at the postsynaptic junctional region. The interaction of DFz2 with Wg, results in proper formation and positioning of postsynaptic apparatus and elicits an unidentified retrograde message that signals the proper formation and positioning of active zones. In both models, secreted Wg is removed from the membrane by endocytosis. Calibration bar is 0.2 µm in (A and D) and 0.5 µm in (B, C, E, and F).
In the last category (33% of the boutons), boutons were indistinguishable from wild-type with regard to the above parameters (but see below; Table 1). Most of these boutons were located closer to the motorneuron’s initial point of contact with the muscle, and therefore likely developed under permissive Wg levels.
Thus, alterations in Wg levels severely reduce synapse formation, and synapses that still form are grossly abnormal, either lacking both active zones and postsynaptic SSR, or having defective active zones and postsynaptic specializations. Interestingly, despite these changes, the overall number of active zones in the entire NMJ was not significantly altered compared to wild-type, primarily due to a small increase in the mean number of active zones in those boutons containing large pockets (Table 1). This result confirms previous studies indicating the presence of compensatory mechanisms at this NMJ (Davis, 2000).
Discussion
Here we show that Wg has an essential role in pre- and postsynaptic development. We provide evidence that Wg is secreted by type Ib boutons of the NMJ, and that is it likely to interactwithDFz2,which is clustered around synaptic boutons, either postsynaptically or both pre- and postsynaptically. Our studies with shi mutants also suggest that secreted Wg is likely to be endocytosed by muscles. Under low Wg levels, synapse formation is severely impaired, with many boutons lacking active zones and postsynaptic structures. In those mutant boutons that develop active zones, release sites are abnormally shaped and the postsynaptic apparatus is markedly abnormal. We suggest that Wg provides an essential signal for active zone development, and that this signal is critical for proper development of the postsynaptic apparatus. We propose two models by which Wg may signal synaptic development (Figures 7G and 7H). Secretion of Wg and its interaction with pre- and postsynaptic DFz2 may initiate a cascade of events that signals both pre- and postsynaptic differentiation (Figure 7G). Alternatively, Wg secretion initiates a postsynaptic response, which elicits a retrograde signal required for proper formation of active zones and new synaptic boutons (Figure 7H). We further propose that endocytosis of Wg by muscles might regulate Wg concentration at the synapse. The presence of DFz2 in motorneurons suggests that the receptor is present pre- as well as postsynaptically and lends support to the first model.
Several lines of evidence support these models. First, mutations blocking Wg secretion dramatically reduced postsynaptic accumulation of Wg. In the absence of Shi function, postsynaptic Wg was dramatically increased and this could be mimicked by selectively blocking postsynaptic Shi. The physiological significance of Wg endocytosis might be to terminate the Wg signaling cascade by receptor-mediated endocytosis. Interestingly, in a previous ultrastructural study shi mutant synapses were shown to contain abnormally large pockets apposed to active zones (Estes et al., 1996). The presence of these pockets in wg mutant boutons of intermediate severity, suggests that endocytosis might be required for normal development of the postsynaptic apparatus. Dynamin’s role in endocytosis has been extensively documented (De Camilli et al., 1995; Delgado et al., 2000). A recent report, nonetheless, provides evidence that in disc epithelium Shibire may be required for Wg secretion (Strigini and Cohen, 2000). However, our results significantly differ from this observation, in that in the wing disc, mutations in porc had the same effect as mutations in shi, confining Wg immunoreactivity to Wg producing cells. In contrast, we found mutations in porc prevented postsynaptic Wg accumulation, while mutations in shi enhanced postsynaptic accumulation.
Additional support for Wg being secreted by the boutons emerged from our studies with Wg overexpression. Overexpressing Wg at type I boutons, but not within muscle cells, resulted in a substantial increase in postsynaptic Wg at the NMJ. Further, driving GFP-tagged Wg, using a strain containing the wg promoter fused to Gal4, resulted in postsynaptic GFP, even though the wg-Gal4 strain does not show reporter gene expression in muscle cells.
Our studies show that Wg has a dramatic influence on synaptic bouton formation and on determination of bouton structure. At the light level, mutations in wg resulted in a drastic reduction in target-dependent synapse formation, and in an increase in the proportion of boutons associated with a dynamic cytoskeleton. Muscle dependent NMJ expansion has been widely documented in Drosophila (Gorczyca et al., 1993; Koh et al., 2002; Roos et al., 2000; Zito et al., 1999) and precise correlation between muscle growth and synaptic bouton number suggests the presence of a complex signaling mechanism that couples synapse formation to muscle growth (Davis and Goodman, 1998). Our work provides compelling evidence that Wg is involved in this process. Our results also show that the influence of Wg on synapse formation has a degree of specificity for different neurons, since alterations in Wg levels affected only the glutamatergic type I endings.
The Wg pathway, through inactivation of GSK-3β (Cook et al., 1996), has been implicated in the regulation of microtubules in axons. In our studies, we found that the organization of Futsch, a MAP1B-related protein (Roos et al., 2000), is altered at growing regions of wg mutant NMJs. While direct phosphorylation of Futsch by GSK-3β has not yet been demonstrated, GSK-3β is enriched at presynaptic boutons, and alteration in Wg levels leads to alterations of the microtubular network. Based on previous observations of growth cones (Dent et al., 1999) and of Drosophila mutants that alter NMJ expansion (Roos et al., 2000; Zhang et al., 2001), these results are consistent with the idea that the defect in NMJ expansion in wg mutants is not the result of a premature stabilization of the arbor. In wg mutants, the synaptic cytoskeleton is undergoing dynamic changes, but factors required to promote formation of the entire boutons are lacking. This view is supported by the finding that a significant proportion of boutons that did develop under decreased Wg levels, lacked essential synaptic elements such as active zones and mitochondria, and had abnormal morphology. Thus, the cytoskeletal changes underlying NMJ expansion are probably necessary, but not sufficient to ensure synaptic growth.
Our results are consistent with the studies of Hall et al. (2000), in which activation of a Wnt-7a-dependent pathway resulted in an increase in growth cone complexity and in changes in microtubule bundling. This study also found that wnt-7a knockout mice had a delay in the development of multisynaptic glomerular rosettes in the cerebellum and in the accumulation of Synapsin. An apparent discrepancy is that, in the cerebellum, it was the postsynaptic cell which secreted Wnt-7, and which retrogradely affected the development of the presynaptic region. It would be interesting to determine whether different Wnt isoforms may operate in different cells (eg: pre- versus postsynaptic cells) to establish synapse morphology, or whether the difference with our study simply reflects the divergence of the two tissues examined.
While many of the molecular components of the active zone have been identified (Garner et al., 2000), the mechanisms that trigger their differentiation are unknown. One of the most remarkable phenotypes encountered in wgts mutants was the lack of active zones in about a third of the boutons examined. This result points to Wg as a pivotal factor during presynaptic differentiation. Interestingly, boutons lacking active zones were also devoid of postsynaptic specializations, suggesting that active zones might be required for postsynaptic development. This argument is supported by our finding that in most mutant boutons that developed active zones, these active zones were abnormal, and these boutons developed aberrant postsynaptic specializations. This model is also supported by studies showing that active zone formation precedes SSR development (Guan et al., 1996). Mutations in the Drosophila Rho dPix also lead to a severe reduction of the SSR. However, active zones were still present in dpix mutants and minimal SSR persisted (Parnas et al., 2001). In contrast, mutations in kakapo also display active sites that are lacking T bars, but in these mutants the structured electron dense apposition of pre- and postsynaptic membranes remains intact (Prokop et al., 1998). In Drosophila, active zones can form in the absence of target muscles (Prokop et al., 1996), suggesting that the absence of active zones in wg mutants is not due to defective muscles.
Recent studies point to a role for bone morphogenetic proteins (BMP) in synapse development (Aberle et al., 2002; Marques et al., 2002). Drosophila mutants lacking wishful thinking (wit), a type II BMP receptor, like mutations in wg, display reduced bouton number. In addition, although active zones do form in wit mutants, many have an abnormal morphology. A member of the BMP family in Drosophila is Decapentaplegic (Dpp). Significantly, mutations in dpp and wg have similar phenotypes in wing development. In the wing disc, Wg and Dpp are localized in orthogonal gradients, which collaborate in defining positional information during wing morphogenesis (Day and Lawrence, 2001). At the NMJ, wit is localized and appears to function in the presynaptic cell (Aberle et al., 2002), raising the intriguing possibility that the ligand is secreted by the postsynaptic cell. Thus, as in the wing disc, Wg and Dpp-like ligands may collaborate in the coordinated development of pre- and postsynaptic structures.
A subset of boutons in wg mutants appeared to have normal active zones, but the morphology of the postsynaptic membrane directly juxtaposed to active zones was dramatically altered. Strikingly, synaptic proteins, such as DLG and DGluRIIA, were abnormally localized in wg mutants, and this phenotype could be replicated by mutations in porc or by postsynaptic overexpression of either DFz2 or DFz2DN. The aberrant distribution of DGluRIIA and DLG observed in wg mutants may be the consequence of grossly abnormal postsynaptic structure. Alternatively, the diffuse appearance of glutamate receptors may point to a direct role of Wg in glutamate receptor clustering.
Taken together, these results are consistent with a model by which Wg is required for the formation of active zones, and that active zone formation is essential for proper development of the postsynaptic apparatus. In this context, Wg may serve a function similar to Agrin at the vertebrate NMJ, in which an extracellular matrix associated protein secreted by the presynaptic cell signals postsynaptic differentiation. Remarkably, mutant mice lacking Agrin have widespread abnormalities in the presynaptic terminal, and blocking Agrin function by antibodies blocks presynaptic differentiation (Gautam et al., 1996), providing compelling evidence that Agrin is also required for presynaptic development. A fly Agrin homolog does not appear to be present in the Drosophila genome, suggesting that a different set of secreted proteins, such as Wg, may subserve Agrin’s function.
Experimental Procedures
Flies
Flies were reared at 25°C, except where indicated. The following mutants were used: wgts (wgIL114; Nusslein-Volhard et al., 1985), porcG18 (Kadowaki et al., 1996), shits1 (Poodry and Edgar, 1979), wg-Gal4 (gift of Dr. S. Cohen), and wg-lacz (17en40; Kaphingst and Kunes, 1994). We used presynaptic Gal4 drivers C164 and C380 (Torroja et al., 1999), postsynaptic drivers BG487 and C57 (Budnik et al., 1996), and the following UAS strains: UAS-Wg (Binari et al., 1997), UAS-Wg-GFP (gift of Dr. S. Cohen), UAS-DFz2 and UAS-DFz2DN (Cadigan et al., 1998), and UAS-ShiD (K44A) (Moline et al., 1999). Wild-type flies were Canton Special (CS). For temperature pulses in wgts, balanced larvae reared at 17°C were shifted to 27–30°C for 8 to 16 hr prior to dissection. For shits1 mutant pulses, larvae raised at 18°C were pulsed at 32°C for 3 hr.
Immunocytochemistry
Wandering third instar larvae were dissected in Ca2+ free saline and fixed in Bouin’s fixative unless specified. The following antibodies were used: anti-Wg (1:200; Reichsman et al., 1996), anti-DLGPDZ (1:40, 000; Koh et al., 1999), mAb 22C10 (1:100; Roos et al., 2000), anti-βgal (4% paraformaldehyde; 1:1000; Cappel), mAb anti-βgal (1:250; Promega), anti-DFz2 (1:100; S.C., unpublished data), mAb anti-CSP (1:250; gift of Dr. K. Zinsmaier), mAb anti-Synapsin (1:25; synorf1; gift of Dr. E. Buchner), anti-Drosophila GSK-3β (4% paraformaldehyde; 1:25; Upstate Biotech), anti-glutamate (1:100; Incstar), and 1:200 anti-HRP-FITC or TxRd (Cappel). mAb anti-Armadillo (N2-7A1) and mAb anti-DGluRIIA (8B4D2, 1:10) were from the Developmental Studies Hybridoma Bank. Fluorescent conjugated antibodies were from Jackson Labs or Molecular Probes. Specificity of anti-Wg was demonstrated in Reichsman et al. (1996), and confirmed here by overexpressing Wg and by using mutations of porc and wgts, which resulted in alteration of the signal.
Quantification of bouton number was performed at muscles 6 and 7 (A3 and A4). Confocal images were acquired using a BioRad MRC 600 or a Leica TCS NT and processed using ImageJ 1.26.
Electron Microscopy
Body wall muscles were prepared for TEM as in Torroja et al. (1999). Boutons were serially sectioned and photographed at 10,000–30,000× using a JEOL 100S TEM. For morphometric analysis, the cross-section corresponding to the bouton midline (cross-section of largest diameter) was identified and used for quantification using NIH Image as in Budnik et al. (1996). The number of samples used were 3 temperature-shifted wild-type (29 boutons), 4 temperature-shifted wgts (36 boutons), and 3 UAS-DFz2 X C57 (16 boutons). Statistical analysis was performed using a two-tailed Student t test.
Supplementary Material
Acknowledgments
This work was supported by NIH grants RO1 NS 37061, 42629, and 3700 to V.B., R1 HD 36000 to S.C., and NSF BBS 8714235 to the Microscopy Facility. We would like to thank Drs. S. Cohen, E. Buchner, T. Godenschwege, R. Nusse, N. Perrimon, and K. Zinsmaier for kind gifts of antibodies and fly stocks, and Drs. C. Ruiz-Canada, J. Roche, and D. Mathew for careful reading of the manuscript and helpful discussions. We also thank L.R-S. Yin for help in the EM.
References
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS. Wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. [DOI] [PubMed] [Google Scholar]
- Binari RC, Staveley BE, Johnson WA, Godavarti R, Sasisekharan R, Manoukian AS. Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling. Development. 1997;124:2623–2632. doi: 10.1242/dev.124.13.2623. [DOI] [PubMed] [Google Scholar]
- Budnik V, Koh YH, Guan B, Hartmann B, Hough C, Woods D, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden SJ. Wnts as retrograde signals for axon and growth cone differentiation. Cell. 2000;100:495–497. doi: 10.1016/s0092-8674(00)80685-6. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Fish MP, Rulifson EJ, Nusse R. Wingless repression of Drosophila frizzled 2 expression shapes the wingless morphogen gradient in the wing. Cell. 1998;93:767–777. doi: 10.1016/s0092-8674(00)81438-5. [DOI] [PubMed] [Google Scholar]
- Chen C, Struhl G. Wingless transduction by the frizzled and frizzled2 proteins of Drosophila. Development. 1999;126:5441–5452. doi: 10.1242/dev.126.23.5441. [DOI] [PubMed] [Google Scholar]
- Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- Chu-LaGraff Q, Doe CQ. Neuroblast specification and formation regulated by wingless in the Drosophila CNS. Science. 1993;261:1594–1597. doi: 10.1126/science.8372355. [DOI] [PubMed] [Google Scholar]
- Cook D, Fry MJ, Hughes K, Sumathipala R, Woodgett JR, Dale TC. Wingless inactivates glycogen synthase kinase-3 via an intracellular signaling pathway which involves a protein kinase C. EMBO J. 1996;15:4526–4536. [PMC free article] [PubMed] [Google Scholar]
- Davis GW. The making of a synapse: target-derived signals and presynaptic differentiation. Neuron. 2000;26:551–554. doi: 10.1016/s0896-6273(00)81190-3. [DOI] [PubMed] [Google Scholar]
- Davis GW, Goodman CS. Genetic analysis of synaptic development and plasticity: homeostatic regulation of synaptic efficacy. Curr. Opin. Neurobiol. 1998;8:149–156. doi: 10.1016/s0959-4388(98)80018-4. [DOI] [PubMed] [Google Scholar]
- Day SJ, Lawrence PA. Measuring dimensions: the regulation of size and shape. Development. 2001;127:2977–2987. doi: 10.1242/dev.127.14.2977. [DOI] [PubMed] [Google Scholar]
- De Camilli P, Takei K, McPherson PS. The function of dynamin in endocytosis. Curr. Opin. Neurobiol. 1995;5:559–565. doi: 10.1016/0959-4388(95)80059-x. [DOI] [PubMed] [Google Scholar]
- Delgado R, Maureira C, Oliva C, Kidokoro Y, Labarca P. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron. 2000;28:941–953. doi: 10.1016/s0896-6273(00)00165-3. [DOI] [PubMed] [Google Scholar]
- Dent EW, Callaway JL, Szebenyi G, Baas PW, Kalil K. Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. J. Neurosci. 1999;19:8894–8908. doi: 10.1523/JNEUROSCI.19-20-08894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick H, Bejsovec A. Cellular mechanisms of wingless/Wnt signal transduction. Curr. Top. Dev. Biol. 1999;43:153–190. doi: 10.1016/s0070-2153(08)60381-6. [DOI] [PubMed] [Google Scholar]
- Dubois L, Lecourtois M, Alexandre C, Hirst E, Vincent JP. Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell. 2001;105:613–624. doi: 10.1016/s0092-8674(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Estes PS, Roos J, van der Bliek A, Kelly RB, Krishnan KS, Ramaswami M. Traffic of dynamin within individual Drosophila synaptic boutons relative to compartment-specific markers. J. Neurosci. 1996;16:5443–5456. doi: 10.1523/JNEUROSCI.16-17-05443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradkin LG, Noordermeer JN, Nusse R. The Drosophila Wnt protein DWnt-3 is a secreted glycoprotein localized on the axon tracts of the embryonic CNS. Dev. Biol. 1995;168:202–213. doi: 10.1006/dbio.1995.1072. [DOI] [PubMed] [Google Scholar]
- Garner CC, Kindler S, Gundelfinger ED. Molecular determinants of presynaptic active zones. Curr. Opin. Neurobiol. 2000;10:321–327. doi: 10.1016/s0959-4388(00)00093-3. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- Goold RG, Owen R, Gordon-Weeks PR. Glycogen synthase kinase 3β phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. J. Cell Sci. 1999;112:3373–3384. doi: 10.1242/jcs.112.19.3373. [DOI] [PubMed] [Google Scholar]
- Gorczyca M, Augart C, Budnik V. Insulin-like receptor and insulin-like peptide are localized at neuromuscular junctions in Drosophila. J. Neurosci. 1993;13:3692–3704. doi: 10.1523/JNEUROSCI.13-09-03692.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramates LS, Budnik V. Assembly and maturation of the Drosophila larval neuromuscular junction. Int. Rev. Neurobiol. 1999;43:93–117. doi: 10.1016/s0074-7742(08)60542-5. [DOI] [PubMed] [Google Scholar]
- Guan B, Hartmann B, Kho YH, Gorczyca M, Budnik V. The Drosophila tumor suppressor gene, dlg, is involved in structural plasticity at a glutamatergic synapse. Curr. Biol. 1996;6:695–706. doi: 10.1016/s0960-9822(09)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Jia XX, Gorczyca M, Budnik V. Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild type and mutants with increased excitability. J. Neurobiol. 1993;24:1025–1044. doi: 10.1002/neu.480240804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J, Halpern ME, Johansen KM, Keshishian H. Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J. Neurosci. 1989;9:710–725. doi: 10.1523/JNEUROSCI.09-02-00710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in wingless processing. Genes Dev. 1996;10:3116–3128. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- Kaphingst K, Kunes S. Pattern formation in the visual centers of the Drosophila brain: wingless acts via decapentaplegic to specify the dorsoventral axis. Cell. 1994;78:437–448. doi: 10.1016/0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Koh YH, Popova E, Thomas U, Griffith LC, Budnik V. Regulation of DLG localization at synapses by CaMKII-dependent phosphorylation. Cell. 1999;98:353–363. doi: 10.1016/s0092-8674(00)81964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh YH, Gramates LS, Budnik V. Drosophila larval neuromuscular junction: molecular components and mechanisms underlying synaptic plasticity. Microsc. Res. Tech. 2000;49:14–25. doi: 10.1002/(SICI)1097-0029(20000401)49:1<14::AID-JEMT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Koh YH, Ruiz-Canada C, Gorczyca M, Budnik V. The Ras1-mitogen-activated protein kinase signal transduction pathway regulates synaptic plasticity through fasciclin II-mediated cell adhesion. J. Neurosci. 2002;22:2496–2504. doi: 10.1523/JNEUROSCI.22-07-02496.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science. 2002;295:1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- Lahey T, Gorczyca M, Jia XX, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13:823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Perrimon N. Dally cooperates with Drosophila frizzled 2 to transduce wingless signaling. Nature. 1999;400:281–284. doi: 10.1038/22343. [DOI] [PubMed] [Google Scholar]
- Lnenicka GA, Keshishian H. Identified motor terminals in Drosophila larvae show distinct differences in morphology and physiology. J. Neurobiol. 2000;43:186–197. [PubMed] [Google Scholar]
- Lucas FR, Goold RG, Gordon-Weeks PR, Salinas PC. Inhibition of GSK-3β leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J. Cell Sci. 1998;111:1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- Marques G, Bao H, Haerry TE, Shimell MJ, Duchek P, Zhang B, O’Connor MB. The Drosophila BMP type II receptor wishful thinking regulates neuromuscular synapse morphology and function. Neuron. 2002;33:529–543. doi: 10.1016/s0896-6273(02)00595-0. [DOI] [PubMed] [Google Scholar]
- Moline MM, Southern C, Bejsovec A. Directionality of wingless protein transport influences epidermal patterning in the Drosophila embryo. Development. 1999;126:4375–4384. doi: 10.1242/dev.126.19.4375. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Kluding H, Jurgens G. Genes affecting the segmental subdivision of the Drosophila embryo. Cold Spring Harb. Symp. Quant. Biol. 1985;50:145–154. doi: 10.1101/sqb.1985.050.01.020. [DOI] [PubMed] [Google Scholar]
- Parnas D, Haghighi AP, Fetter RD, Kim SW, Goodman CS. Regulation of postsynaptic structure and protein localization by the Rho-type guanine nucleotide exchange factor dPix. Neuron. 2001;32:415–424. doi: 10.1016/s0896-6273(01)00485-8. [DOI] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S, Alexandre C, Calleja M, Vincent JP. The progeny of wingless-expressing cells deliver the signal at a distance in Drosophila embryos. Curr. Biol. 2000;10:321–324. doi: 10.1016/s0960-9822(00)00381-x. [DOI] [PubMed] [Google Scholar]
- Poodry CA, Edgar L. Reversible alteration in the neuromuscular junctions of Drosophila melanogaster bearing a temperature-sensitive mutation, shibire. J. Cell Biol. 1979;81:520–527. doi: 10.1083/jcb.81.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A, Landgraf M, Rushton E, Broadie K, Bate M. Presynaptic development at the Drosophila neuromuscular junction: assembly and localization of presynaptic active zones. Neuron. 1996;17:617–626. doi: 10.1016/s0896-6273(00)80195-6. [DOI] [PubMed] [Google Scholar]
- Prokop A, Uhler J, Roote J, Bate M. The kakapo mutation affects terminal arborization and central dendritic sprouting of Drosophila motorneurons. J. Cell Biol. 1998;143:1283–1294. doi: 10.1083/jcb.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichsman F, Smith L, Cumberledge S. Glycosaminoglycans can modulate extracellular localization of the wingless protein and promote signal transduction. J. Cell Biol. 1996;135:819–827. doi: 10.1083/jcb.135.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, Hummel T, Ng N, Klambt C, Davis GW. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron. 2000;26:371–382. doi: 10.1016/s0896-6273(00)81170-8. [DOI] [PubMed] [Google Scholar]
- Salinas PC, Hall AC. Lithium and synaptic plasticity. Bipolar Disord. 1999;1:87–90. doi: 10.1034/j.1399-5618.1999.010205.x. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996;17:641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Curr. Biol. 2000;10:293–300. doi: 10.1016/s0960-9822(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Torroja L, Packard M, Gorczyca M, White K, Budnik V. The Drosophila β-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J. Neurosci. 1999;19:7793–7803. doi: 10.1523/JNEUROSCI.19-18-07793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Harryman-Samos C, Klingensmith J, Perrimon N, Nusse R. Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. EMBO J. 1993;12:5293–5302. doi: 10.1002/j.1460-2075.1993.tb06225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- Zito K, Parnas D, Fetter RD, Isacoff EY, Goodman CS. Watching a synapse grow: noninvasive confocal imaging of synaptic growth in Drosophila. Neuron. 1999;22:719–729. doi: 10.1016/s0896-6273(00)80731-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.