Abstract
In flies, retrograde BMP signaling is an important mechanism by which postsynaptic cells regulate the structure and function of presynaptic terminals, ostensibly through changes in gene expression. Transcriptional targets, however, have remained mysterious. In this issue of Neuron, Haghighi and colleagues begin to unravel this puzzle by identifying the cytoskeletal regulator Trio.
A crucial aspect of synapse assembly is the coordinated sizing of pre- and postsynaptic structures. Studies in many systems suggest the presence of both anterograde and retrograde signals that mediate this intertwined process. A prevalent signaling mechanism that mediates retrograde control of presynaptic growth is the control of gene expression by the bone morphogenetic protein (BMP) family (Aberle et al., 2002; Marqués et al., 2002; McCabe et al., 2003; Rawson et al., 2003). However, until now, specific BMP transcriptional targets had remained elusive. In this issue of Neuron, Ball and colleagues (Ball et al., 2010) provide the first evidence for such a target, using the Drosophila larval neuromuscular junction (NMJ) as a model system. In this preparation, the release of a BMP by postsynaptic muscles regulates the extent of presynaptic growth. During larval development, the body wall muscles undergo a massive increase in size, leading to a rapid decrease in the input resistance of the muscle membrane. To maintain synaptic efficacy, presynaptic terminals must enhance presynaptic output. This is accomplished by a continuous increase in the number of presynaptic boutons and the number of neurotransmitter release sites within each bouton, events that occur in exact coordination with muscle growth. Thus, synaptic expansion serves as a homeostatic control of synaptic strength (Davis, 2006).
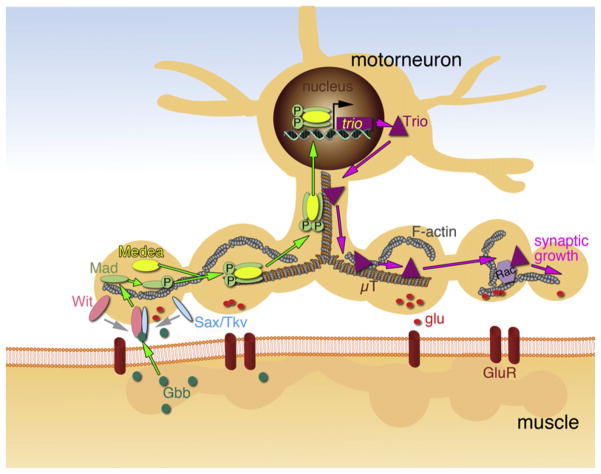
Several lines of evidence suggest that the coordinated expansion of presynaptic terminals is regulated by the release of the BMP Glass bottom boat (Gbb) from the growing muscle cells (Figure 1). Gbb binds to the type I BMP receptors Thick veins (Tkv) or Saxophone (Sax) and type II BMP receptor, Wishful thinking (Wit), at presynaptic terminals (Aberle et al., 2002; Marqués et al., 2002; McCabe et al., 2003; Rawson et al., 2003). In the BMP pathway, dimerization of type I and type II BMP receptors leads to phosphorylation of the intracellular transcription factor Receptor Activated Smad (R-Smad; Mothers Against Dpp [Mad] being an R-Smad in Drosophila), which then binds to a co-Smad (Medea in Drosophila) forming a transcriptionally active complex. The complex is imported into the nucleus where it regulates the transcription of BMP target genes (Moustakas and Heldin, 2009). Consistent with this model, mutations in sax, wit, and gbb result in NMJs with abnormally fewer synaptic boutons and a decrease in the size of evoked responses. Further, alterations in the above genes or disruption of retrograde transport in motor neuron axons eliminates the labeling of embryonic motor neuron nuclei by antibodies that cross-react with phosphorylated Mad (P-Mad) (Marqués et al., 2002; McCabe et al., 2003; Rawson et al., 2003). However, a mechanism by which the translocation of P-Mad into the nucleus is translated into changes in synaptic growth and function has not been identified despite intense research in this area.
Figure 1. Retrograde BMP Signaling at the Drosophila Larval NMJ.
Gbb is released by muscle cells, promoting the dimerization of presynaptic Wit and Tkv or Sax. The receptor then phosphorylates Mad within the motor neuron, which establish a complex with Medea. The P-Mad/Medea complex is translocated into the motor neuron nucleus, where it binds to target genes. One such target gene is the GEF Trio, a regulator of Rac, which is thought to regulate the synaptic cytoskeleton, promoting synaptic growth (see text for further details). μT, microtubule; glu, glutamate; GluR, glutamate receptor.
Ball and colleagues demonstrate that the Rho-type guanyl-nucleotide exchange factor (GEF), Trio, is under the transcriptional control of the BMP pathway and, together with Rac, is involved in presynaptic growth and regulation of neurotransmitter release. Small G proteins and their exchange factors have been long implicated in modulating actin polymerization. Further, studies at the Drosophila NMJ show that Trio regulates a population of microtubules important for synaptic growth (Pawson et al., 2008). Thus, these studies provide the first direct link between activation of the BMP pathway and the growth of presynaptic arbors.
The authors show that overexpressing either a GEF-dependent or GEF-independent form of Rac in motor neurons induced an increase in presynaptic expansion. Most importantly, the effects of the GEF-dependent, but not the GEF-independent, Rac relied on BMP signaling, as mutations in wit or mad prevented the action of GEF-dependent Rac. The identity of the GEF regulating Rac function was determined by a candidate gene approach. In particular, the authors showed that a mutation in trio reduced the number of presynaptic boutons and neurotransmitter release. Notably, expressing a Trio transgene in motor neurons suppressed the effect of mutations in mad and wit, suggesting that Trio was downstream of these BMP pathway signaling components. Consistent with a transcriptional control of trio by BMP, the authors found that in mad mutants, the level of Trio was reduced in the embryonic nervous system and the larval ventral ganglion. A likely direct interaction of Mad with the trio promoter was supported by two lines of evidence. First, an in vitro luciferase reporter assay of the trio promoter showed an increase in reporter activity when Mad or a constitutively active Tkv form was expressed in HEK293 cells. This enhancement in reporter activity was dissected to a 1688 bp region within the trio promoter. Second, a chromatin immunoprecipitation (ChIP) assay of Mad, conducted in animals in which a Myc-tagged Mad transgene was overexpressed in motorneurons, resulted in the amplification of the trio promoter, suggesting that trio is a direct transcriptional target of Mad.
These findings solidify the notion that retrograde BMP signaling is required for the regulation of cytoskeletal elements known to be important for synaptic growth and that part of this regulation includes the transcriptional activation of a cytoskeletal regulator. Although BMPs have been best studied for their role as retrograde regulators, several BMP receptors and P-Mad are also found in Drosophila larval muscles (Dudu et al., 2006), suggesting that BMP regulation is more complex and likely to control a variety of synaptic mechanisms beyond retrograde control. Indeed, recent studies have uncovered a role for the type I Activin-type BMP receptor, Baboon (Babo), in larval muscles in controlling the transcription of Gbb (Ellis et al., 2010). Although the exact source of the BMP ligand in this case, Dawdle, is not clear, these experiments suggest that BMP signaling in the muscles themselves regulate the retrograde activity of BMPs.
BMPs have also been implicated in synapse development and plasticity in mammals (e.g., Sun et al., 2007), but whether they operate in a retrograde manner is still undetermined. Instead, members of the Wnt family, which collaborate with BMPs during embryonic patterning, appear in part to play such a retrograde role (Salinas and Zou, 2008). Not surprisingly, both in the mammalian nervous system, as well as in the fly, Wnts also play anterograde and autocrine functions to regulate the development of both pre- and postsynaptic compartments (Korkut and Budnik, 2009; Salinas and Zou, 2008). Given the role of the above two well-characterized morphogens during synapse development, it is highly likely that other such morphogens will further increase the complexity of the signaling pathways that regulate synaptic growth.
Although the above studies provide a mechanism for BMP-mediated retrograde control of synaptic growth, the processes that regulate synaptic strength through BMP signaling pathways are less clear. For example, Gbb-dependent synaptic growth can be separated from BMP-dependent changes in synaptic strength (Goold and Davis, 2007). This is in contrast with the present study in which regulation of both synaptic growth and synaptic strength was shown. The dual control of synaptic growth and neurotransmitter release by Trio could be explained by a demonstrated interaction between Trio and the Receptor protein phosphatase Dlar, which controls the development of release sites (Kaufmann et al., 2002; Pawson et al., 2008). Whether this second function could also be under the control of BMPs remains to be investigated.
Finally, it is also expected that BMP-dependent retrograde control of synapse development will involve the transcriptional regulation of many other genes, which together will weave the fabric of synaptic growth and function. The identification of Trio as a target for BMP regulation constitutes a significant first building block.
References
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhães TR, Goodman CS. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. [DOI] [PubMed] [Google Scholar]
- Ball RW, Warren-Paquin M, Tsurudome K, Liao EH, Elazzouzi F, Cavanagh C, An BS, Wang TT, White JH, Haghighi AP. Neuron. 2010;66:536–549. doi: 10.1016/j.neuron.2010.04.011. this issue. [DOI] [PubMed] [Google Scholar]
- Davis GW. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- Dudu V, Bittig T, Entchev E, Kicheva A, Jülicher F, González-Gaitán M. Curr Biol. 2006;16:625–635. doi: 10.1016/j.cub.2006.02.061. [DOI] [PubMed] [Google Scholar]
- Ellis JE, Parker L, Cho J, Arora K. Dev Biol. 2010;24:24. doi: 10.1016/j.ydbio.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Goold CP, Davis GW. Neuron. 2007;56:109–123. doi: 10.1016/j.neuron.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann N, DeProto J, Ranjan R, Wan H, Van Vactor D. Neuron. 2002;34:27–38. doi: 10.1016/s0896-6273(02)00643-8. [DOI] [PubMed] [Google Scholar]
- Korkut C, Budnik V. Nat Rev Neurosci. 2009;10:627–634. doi: 10.1038/nrn2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués G, Bao H, Haerry TE, Shimell MJ, Duchek P, Zhang B, O’Connor MB. Neuron. 2002;33:529–543. doi: 10.1016/s0896-6273(02)00595-0. [DOI] [PubMed] [Google Scholar]
- McCabe BD, Marqués G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O’Connor MB. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Development. 2009;136:3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- Pawson C, Eaton BA, Davis GW. J Neurosci. 2008;28:11111–11123. doi: 10.1523/JNEUROSCI.0833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson JM, Lee M, Kennedy EL, Selleck SB. J Neurobiol. 2003;55:134–150. doi: 10.1002/neu.10189. [DOI] [PubMed] [Google Scholar]
- Salinas PC, Zou Y. Annu Rev Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- Sun M, Thomas MJ, Herder R, Bofenkamp ML, Selleck SB, O’Connor MB. J Neurosci. 2007;27:7740–7750. doi: 10.1523/JNEUROSCI.1604-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]