Abstract
The effects of ultraviolet-B (UV-B) radiation on water relations, leaf development, and gas-exchange characteristics in pea (Pisum sativum L. cv Meteor) plants subjected to drought were investigated. Plants grown throughout their development under a high irradiance of UV-B radiation (0.63 W m−2) were compared with those grown without UV-B radiation, and after 12 d one-half of the plants were subjected to 24 d of drought that resulted in mild water stress. UV-B radiation resulted in a decrease of adaxial stomatal conductance by approximately 65%, increasing stomatal limitation of CO2 uptake by 10 to 15%. However, there was no loss of mesophyll light-saturated photosynthetic activity. Growth in UV-B radiation resulted in large reductions of leaf area and plant biomass, which were associated with a decline in leaf cell numbers and cell division. UV-B radiation also inhibited epidermal cell expansion of the exposed surface of leaves. There was an interaction between UV-B radiation and drought treatments: UV-B radiation both delayed and reduced the severity of drought stress through reductions in plant water-loss rates, stomatal conductance, and leaf area.
Photosynthetic rate and productivity in many plant species can be reduced by increased exposure to UV-B radiation (Teramura and Ziska, 1996). To date there is no consensus on the mechanistic basis of UV-B-induced inhibition of CO2 assimilation in mature leaves. A reduction in Rubisco activity has been suggested as a cause of the reduced CO2 assimilation rate in leaves exposed to increased UV-B radiation. Prolonged exposure to elevated levels of UV-B radiation has been demonstrated to result in decreases of both Rubisco activity and content (Strid et al., 1990; Jordan et al., 1992; Kulandaivelu and Nedunchezhian, 1993). A primary cause of the decrease in the light-saturated rate of CO2 assimilation induced by exposure to elevated UV-B radiation in leaves of oilseed rape has been shown to be a loss of Rubisco (Allen et al., 1997), which may also be associated with the loss of activity of other Calvin cycle enzymes (Baker et al., 1997).
UV-B-induced inhibition of photosynthesis has also been attributed to a reduction in the activity of PSII photochemistry (Fiscus and Booker, 1995). However, UV-B radiation has been shown to inhibit photosynthesis without an appreciable effect on PSII photochemistry in pea (Pisum sativum L.; Nogués and Baker, 1995), oilseed rape (Allen et al., 1997), soybean (Middleton and Teramura, 1993), rice (Ziska and Teramura, 1992), and algae (Lesser, 1996). Therefore, it would appear that UV-B inhibition of PSII photochemistry is not a ubiquitous primary effect on photosynthesis. It is not clear whether changes in stomatal function play a major role in the UV-B-induced inhibition of photosynthesis. Exposure to UV-B radiation can modify the speed of stomatal opening and closing and reduce the rate of leaf transpiration (Negash, 1987; Middleton and Teramura, 1993; Day and Vogelmann, 1995), although stomatal effects have not been found to affect photosynthesis in other studies (Murali and Teramura, 1986; Sullivan and Teramura, 1989; Teramura et al., 1991; Ziska and Teramura, 1992). It is possible that UV-B-induced effects on stomata could modify plant-water relations and growth but have negligible effects on leaf photosynthetic capacity.
The majority of the studies of the mechanistic basis of plant responses to increased UV-B radiation have been conducted under controlled environmental conditions on plants grown from seed in low or negligible flux densities of UV-B radiation and then exposed to UV-B radiation. Such conditions might be expected to increase plant sensitivity to damage by elevated UV-B radiation (Tevini and Teramura, 1989). In such experiments, UV-B radiation is generally the only significant environmental stress, although in natural conditions plants will experience many other stresses, such as drought, which greatly reduce plant growth and productivity and may alter the susceptibility to UV-B-induced damage. Studies of the combined effects of UV-B and water stress have identified reductions in growth (Teramura et al., 1983, 1984a, 1984b; Tevini et al., 1983; Balakumar et al., 1993), but the mechanistic bases of such responses have not been identified.
The objective of this study was to determine the mechanisms by which UV-B radiation affects water relations, leaf development, and gas-exchange characteristics in droughted pea plants grown and developed with UV-B radiation in a greenhouse. To identify the mechanisms involved in the UV-B response, a high ratio of UV-B to PAR was used, as is common in such mechanistic studies (He et al., 1993; Jordan et al., 1994; Liu et al., 1995; Nogués and Baker, 1995; Jansen et al., 1996; Mackerness et al., 1996; Allen et al., 1997). Measurements of leaf gas exchange and chlorophyll fluorescence were made in conjunction with in situ measurements of adaxial and abaxial gs and estimates of cell frequencies. We demonstrate that long-term growth in UV-B light affects leaf characteristics, primarily through a reduction in leaf size. UV-B light was also found to have a direct effect on gs in the absence of any modification to photosynthesis.
MATERIALS AND METHODS
Pea (Pisum sativum L. cv Meteor) plants were grown from seed in a mixture of perlite:Levington F2 compost (2:1, v/v) in a greenhouse as described by Nogués and Baker (1995), except that 1.5-dm3 pots (depth of 33.5 cm) were used. Minimum PPFD during a 16-h photoperiod was maintained at approximately 500 μmol m−2 s−1 by supplementary lighting. Temperature and vapor pressure deficit were maintained at approximately 23/19°C and 1.7/1.3 kPa day/night, respectively. The youngest fully expanded leaves were used for all measurements unless otherwise stated.
UV-B Radiation and Drought Treatments
After the seeds were sown, pots were placed in a transparent UV-exposure cabinet within the greenhouse according to the method of Allen et al. (1997). The UV spectrum at the top of the plants was measured with a scanning spectroradiometer (model SR 991-PC, Macam Photometrics, Livingston, UK) and was similar to that previously described (Allen et al., 1997). Greenhouse and cabinet transmission of UV-A radiation, supplemented by UV fluorescent lamps, ensured that UV-A exposure was maintained for photorepair and for flavonoid biosynthesis (Teramura and Ziska, 1996). The biologically weighted UV-B dosages, according to the generalized plant-action spectrum (normalized to 300 nm; Caldwell, 1971) for the UV-B and control treatments, were 0.63 W m−2 (32 kJ m−2 d−1) and 0.001 W m−2, respectively. The UV-exposure cabinet was divided into four independent sections (i.e. two without UV-B and two with UV-B radiation), and these sections were regularly exchanged to minimize any between-section differences other than in the UV-B treatment.
Plants were watered to saturation on alternate days with Hoagland solution. After 12 d from sowing one-half of the plants were subjected to progressive drought by withholding water. Well-watered and droughted plants were divided equally between all four sections in a split-plot design. Consequently, plants were subjected to one of four treatments: (a) without UV-B light and well watered, (b) increased UV-B light and well watered, (c) without UV-B light and droughted, and (d) increased UV-B light and droughted. ψw was measured on alternate days at 8 am using a pressure chamber (PMS Instrument Co., Corvallis, OR), with damp paper at the bottom to avoid too much evaporation during the measurements. RWC was determined as: (fresh weight − dry weight)/(turgid weight − dry weight) × 100, where turgid weight is the weight of the leaf after equilibration in distilled water for 24 h. E of the whole plant was calculated from the change in weight of the pots (water loss by the soil was minimized by using cellulose film sealed on top of the pots).
At the end of the experiment (24 d from the beginning of the drought treatment) plants were harvested and oven dried at 80°C for 2 d, and analyses of biomass of shoots and roots were carried out. Total plant leaf area was estimated prior to drying using a flat-bed ScanJet scanner (model Iicx, Hewlett-Packard) and analyzed with an IMPIXL image-processing package. Analysis of variance with Bonferroni adjusted pairwise comparisons was done using SYSTAT software. All proportional data were subjected to an arcsine square-root transformation prior to analysis.
Analysis of Leaf Gas Exchange and Fluorescence
Plants were removed from the cabinet for measurements of CO2 assimilation in at least six attached leaves per treatment every 3rd d, using an IR gas analyzer (CIRAS-1, PP Systems, Hitchin, UK) with a PPFD source of 1200 μmol m−2 s−1. Measurements were made between 10 am and 4 pm. Net CO2 assimilation rate and intercellular CO2 concentration were calculated according to the method of von Caemmerer and Farquhar (1981). l, which is the proportionate decrease in light-saturated net CO2 assimilation attributable to stomata, was calculated by the method of Farquhar and Sharkey (1982). Estimations of Vc,max and Jmax were made by fitting a maximum likelihood regression below and above the inflexion of the net CO2 assimilation rate/intercellular CO2 concentration response using the method of McMurtrie and Wang (1993).
The adaxial and abaxial leaf conductances were measured in situ at approximately midday every 3rd d, using an automatic transit-time porometer (AP4, Delta-T Devices, Cambridge, UK) and measurements were taken from at least six leaves per treatment. In the interest of safety the UV-B fluorescent lamps were switched off immediately prior to leaf-conductance measurements.
Steady-state-modulated chlorophyll fluorescence of the adaxial surface of attached leaves was measured using a fluorimeter (PAM-2000, H. Walz GmbH, Effeltrich, Germany) following the gas-exchange measurements. Fluorescence signals were analyzed as described by Andrews et al. (1993) to provide estimates of the Fv/Fm, measured after 15 min of dark adaptation, and φPSII (Genty et al., 1989), measured at a PPFD of 500 μmol m−2 s−1, which was similar to the minimum mean growth PPFD.
Stomata and Cell Frequency
Determinations of stomata and epidermal cell numbers on both sides of the sixth pair of leaves were made at different stages of development, 5 to 12 d after the beginning of the drought treatment using nail-polish impressions (Poole et al., 1996). Three stages of leaf development were studied: (a) stage I corresponds to leaves immediately prior to emergence from stipules, i.e. before leaves were exposed to any significant UV-B radiation; (b) stage II corresponds to leaves immediately prior to unfolding, i.e. before the adaxial surface was exposed to UV-B radiation; and (c) stage III corresponds to maximum leaf expansion. Plants were examined daily to determine developmental stage. A systematic sampling strategy was adopted whereby three pairs of sites were selected at either side of the main vein at the tip, in the middle, and at the base of the leaves to account for variation within leaves (Poole et al., 1996). At each site the numbers of fields of view chosen were sufficient to count more than 100 epidermal cells and the associated stomata using a microscope linked to a television monitor via a video camera (Leica). Mean frequencies of epidermal cells and stomata per leaf were calculated from the average of the six sites.
Chlorophyll a fluorescence was imaged from the outermost layer of mesophyll (palisade) cells using a standard fluorescence microscope (model DMRX, Leica) with an attached Peltier-cooled charge-coupled device camera (Wright Instruments, Ltd., Middlesex, UK) as estimate by Oxborough and Baker (1997), to estimate the number of palisade mesophyll cells in the leaves. The sampling strategy that we adopted was similar to that described above, except that only one image was taken per field of view (six sites per leaf).
Pigment Analysis
Water-soluble pigments (flavonoid and anthocyanin) were extracted from leaves 24 d after the beginning of the drought treatment using the method of Jordan et al. (1994). Four leaves were ground to a powder in liquid N2 before extraction in 10 mL of acidified methanol (HCl:methanol, 1:99, v/v). Absorption spectra of the extracts were determined using a Cary 210 spectrophotometer (Varian, Palo Alto, CA), and the flavonoid and anthocyanin contents were estimated from A300 and A530, respectively (Mepsted et al., 1996).
RESULTS
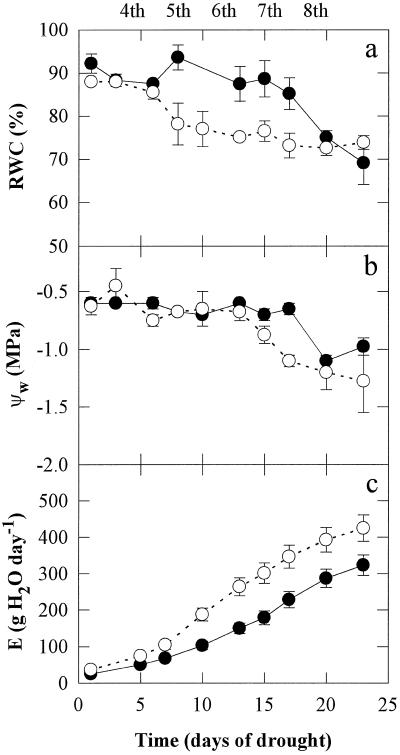
The changes in plant water status during 24 d of drought treatment are shown in Figure 1. The plants were grown throughout their development without UV-B or under UV-B irradiation. Well-watered leaves had an RWC of about 90% and a ψw of approximately −0.5 MPa throughout the treatment period (data not shown). No major changes in the RWC of plants irradiated with UV-B light were observed for more than 15 d of drought, but after 24 d RWC had decreased to about 70% (Fig. 1a). In the non-UV-B-treated plants, RWC decreased from the 7th d of drought treatment, and after 24 d RWC had decreased by about 20% to a level similar to that in UV-B-treated leaves. No changes in ψw were observed in plants irradiated or not irradiated with UV-B for 13 d, but subsequently, ψw decreased rapidly (from approximately −0.5 to −1.3 MPa over 10 d) in the non-UV-B-treated plants. In the UV-B-treated plants, ψw remained at approximately −0.6 MPa until 17 d from the beginning of the drought treatment, and then decreased to approximately −1.0 MPa. The increase in E as the plants grew was substantially smaller in UV-B-irradiated plants (Fig. 1c).
Figure 1.
Changes in the RWC, ψw, and E during 24 d of drought treatment. The plants were grown throughout their development without UV-B radiation (○) or with 0.63 W m−2 UV-B (•). Leaves of well-watered plants had an RWC of about 90% and a ψw of about −0.5 MPa throughout the treatment period. Measurements began on the fourth pair and were finished on the eighth pair of fully mature leaves, as indicated at the top of the figure. Data are the means of six replicates and the ses are shown when larger than the symbols.
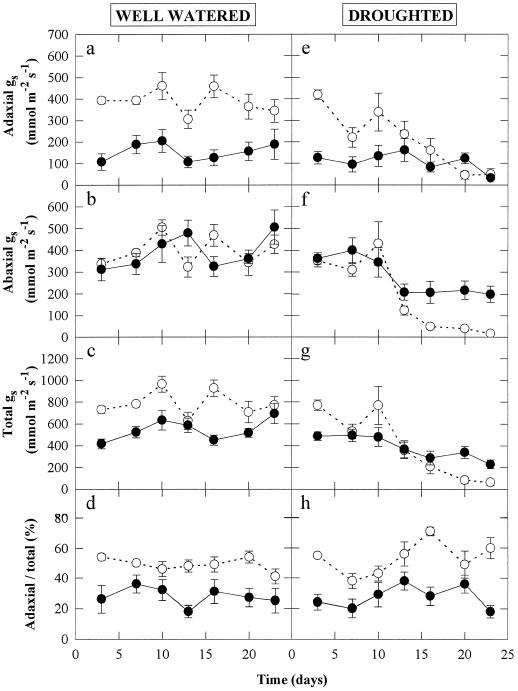
The effects of UV-B radiation from sowing and from drought for 24 d on gs are shown in Figure 2. Growth of well-watered pea plants under UV-B radiation greatly reduced adaxial gs by approximately 65% (Fig. 2a), but no significant effect was found on abaxial gs (Fig. 2b). Therefore, total gs (adaxial plus abaxial gs, Fig. 2c) decreased, and the ratio of adaxial to total gs (Fig. 2d) was decreased from approximately 50 to 30% by UV-B radiation. After 24 d of drought treatment, adaxial gs of non-UV-B-irradiated plants had decreased by approximately 85%, to a level similar to that in UV-B-treated plants (Fig. 2e). Drought decreased abaxial gs in both non- and UV-B-irradiated plants, but the magnitude of the decrease was greater in the non-UV-B-treated plants after 24 d (Fig. 2f). As with well-watered plants, the ratio of adaxial to total gs was maintained at approximately 50% for non-UV-B-treated plants, but UV-B radiation reduced it to about 30% (Fig. 2h).
Figure 2.
Changes in the adaxial (a and e), abaxial (b and f), and total (adaxial plus abaxial; c and g) gs, and the ratio of adaxial to total gs (d and h) during 24 d of well-watered (a–d) or droughted (e–h) treatments. The plants were grown throughout their development without UV-B radiation (○) or with 0.63 W m−2 UV-B (•). Data are the means of six replicates and the ses are shown when larger than the symbols.
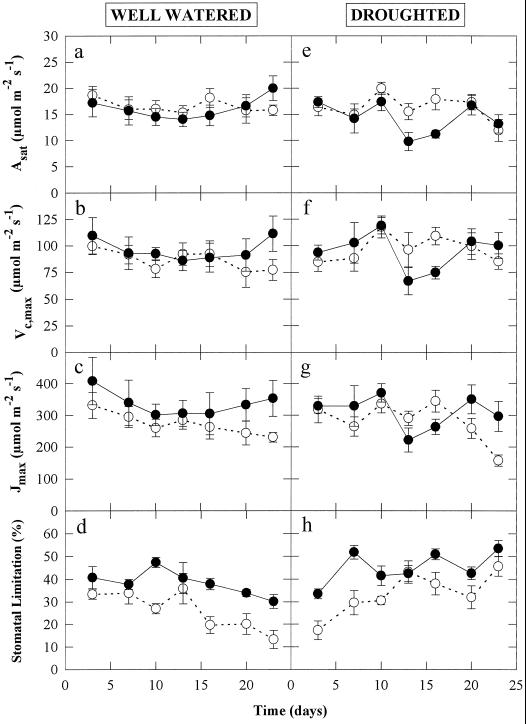
To evaluate the effects of UV-B irradiation and drought on photosynthesis, the changes in the Asat, Vc,max, Jmax, and l were measured on attached leaves of plants from all treatments throughout the droughting period (Fig. 3). There were no significant effects of UV-B irradiation or drought on the Asat, Vc,max, or Jmax (Fig. 3, a–g). The only photosynthetic parameter that was significantly affected by UV-B was l, which was increased by about 10 to 15% throughout the measurement period in both well-watered and droughted plants (Fig. 3, d and h). Drought also increased l by 10 to 15% after 15 d in plants grown with and without UV-B (Fig. 3h). Furthermore, at no time during the experiment was there a significant effect of UV-B or drought on the Fv/Fm or on the φPSII at a PPFD of 500 μmol m−2 s−1. Fv/Fm and φPSII remained constant at about 0.78 ± 0.03 and 0.52 ± 0.04, respectively, throughout the measurement period. Imaging of Fv/Fm and φPSII from leaves from any treatment did not show any heterogeneity of these PSII quantum efficiencies at the palisade mesophyll cellular level (data not shown).
Figure 3.
Changes in the Asat, Vc,max, Jmax, and l during 24 d of well-watered (a–d) or droughted (e–h) treatments. The plants were grown throughout their development without UV-B radiation (○) or with 0.63 W m−2 UV-B (•). Leaf temperature was maintained at 25 ± 0.5°C, with 1200 μmol m−2 s−1 incident PPFD. Data are the means of six replicates and the ses are shown when larger than the symbols.
Table I gives the stomatal frequencies and indices of the adaxial and abaxial surfaces of the sixth leaf pair at three stages of development. Stomatal index is the frequency of stomata expressed as a percentage of the frequency of all epidermal cells. At the time of sampling, after only 5 to 12 d of drought, there was no significant effect of this treatment on any of the parameters studied, because water stress had not yet developed (Fig. 1b); therefore, results from well-watered and droughted plants were pooled. Stomatal development progressed at different rates on adaxial and abaxial surfaces, with the abaxial stomatal index at stage I being approximately 7 times that of the adaxial surface (Table I). At this stage, stomata were poorly differentiated on the adaxial surface, whereas one-third of the stomata were already differentiated on the abaxial surface. By stage II the stomatal index was similar on both surfaces and subsequently showed little change at full leaf expansion (stage III). At stage II all stomata were differentiated, although cell frequencies were changing because of cell expansion (only 60–70% of the cell expansion was completed as judged from epidermal cell frequencies at stage III). Palisade mesophyll cell frequencies changed little after stage II. Data from spongy mesophyll cell frequencies are not presented because of the difficulties in counting accurately the number of cells interspersed with air spaces. At stage II, UV-B irradiation significantly increased the adaxial stomatal frequency and index by about 14%, and this effect may also have occurred at stage III. However, no effects of UV-B treatment on the frequencies of abaxial stomata or other cell types were found at any stage. UV-B irradiation significantly reduced leaf area at stage II (by 19%) and stage III (by 39%) as a result of 21 to 39% reductions in the estimated number of stomatal, epidermal, and palisade mesophyll cells per leaf (the product of cell frequency and leaf area) at stage III (Table I). The same effects of UV-B irradiation are evident at stages I and II, but only the numbers of palisade cells and abaxial stomata at stage II were significantly affected (Table I).
Table I.
The effects of 0.63 W m−2 UV-B radiation on the frequencies and number per leaf of stomata and epidermal and mesophyll (palisade) cells of the sixth leaf pair at different stages of development
| Parameter | Growth Stage
I
|
Growth Stage II
|
Growth Stage III
|
|||
|---|---|---|---|---|---|---|
| UVB− | UVB+ | UVB− | UVB+ | UVB− | UVB+ | |
| Adaxial stomatal frequency (mm−2) | 27.6 ± 4.1 | 36.2 ± 10.2 | 229.2 ± 24.1 | 262.0 ± 18.5* | 146.4 ± 8.4 | 173.2 ± 5.1 |
| Adaxial epidermal cell frequency (mm−2) | 3723 ± 268 | 3559 ± 141 | 758 ± 73 | 862 ± 55 | 540 ± 35 | 610 ± 23 |
| Adaxial stomatal index (%) | 0.78 ± 0.13 | 1.01 ± 0.29 | 21.07 ± 1.52 | 23.42 ± 0.40* | 21.20 ± 0.35 | 22.20 ± 0.30 |
| Mesophyll (palisade) cell frequency (mm−2) | 6491 ± 189 | 6769 ± 238 | 3417 ± 118 | 2980 ± 88 | 2138 ± 343 | 2393 ± 229 |
| Abaxial stomatal index (%) | 7.85 ± 1.11 | 6.30 ± 1.34 | 25.22 ± 1.71 | 26.79 ± 0.58 | 22.98 ± 0.52 | 22.81 ± 1.03 |
| Abaxial epidermal cell frequency (mm−2) | 4558 ± 230 | 4831 ± 364 | 740 ± 63 | 766 ± 32 | 616 ± 33 | 626 ± 29 |
| Abaxial stomatal frequency (mm−2) | 368 ± 45 | 306 ± 63 | 284 ± 24 | 280 ± 15 | 183 ± 12 | 182 ± 7 |
| Leaf area (cm2) | 0.81 ± 0.12 | 0.61 ± 0.14 | 6.30 ± 0.60 | 5.09 ± 0.42* | 11.36 ± 0.67 | 6.89 ± 0.61* |
| No. of adaxial stomata (× 103) | 2.46 ± 0.65 | 2.90 ± 1.32 | 146.7 ± 21.8 | 129.4 ± 9.0 | 156.3 ± 9.7 | 123.0 ± 10.0* |
| No. of adaxial epidermal cells (× 103) | 288 ± 33 | 255 ± 47 | 488 ± 62 | 427 ± 27 | 582 ± 38 | 429 ± 32* |
| No. of palisade mesophyll cells (× 103) | 525 ± 80 | 403 ± 76 | 2388 ± 100 | 1520 ± 130* | 2320 ± 187 | 1668 ± 211* |
| No. of abaxial epidermal cells (× 103) | 352 ± 39 | 307 ± 61 | 473 ± 56 | 383 ± 24 | 692 ± 39 | 430 ± 41* |
| No. of abaxial stomata (× 103) | 33.1 ± 8.2 | 25.9 ± 9.5 | 179.6 ± 19.5 | 139.8 ± 9.7* | 204.8 ± 10.8 | 124.1 ± 10.4* |
Results from well-watered and droughted plants were pooled, because after only 5 to 12 d of drought, water stress had not yet developed, and there were no significant effects of this treatment on any of the parameters studied. Growth stages I, II, and III correspond to leaves immediately prior to emergence from stipules (i.e. before leaves were exposed to direct UV-B), immediately prior to unfolding (i.e. before the adaxial surface was exposed to direct UV-B), and at maximum leaf expansion, respectively. Leaf area is also given. Values are the means ± SE of eight replicate leaves. * indicates significant difference at P < 0.05 between UV-B treatments (UVB+) and controls (UVB−) at each growth stage.
The effects of increased UV-B exposure for over 36 d and after 24 d of drought on several plant growth characteristics are shown in Table II. UV-B exposure alone significantly reduced plant height, leaf area (by 47%), total dry weight (by 43%), leaf dry weight, and root dry weight, but did not significantly affect the number of leaves. Drought alone significantly reduced plant height, leaf area (by 57%), number of leaves per plant, specific leaf area, leaf area ratio, total dry weight (by 33%), leaf dry weight, and plant and soil water content. The combination of UV-B and drought produced an additive effect on most of the parameters studied, although only the decrease in soil water content was significantly different (Table II), confirming that the reduced leaf area in the UV-B treatment resulted in reduced plant water loss (Fig. 1c) and a slower reduction in soil water content.
Table II.
The effects of 0.63 W m−2 UV-B exposure from sowing for over 36 d of growth and for 24 d of drought on several plant-growth and leaf-pigment characteristics
| Parameter | WWa | UVB + WW | Db | UVB + D |
|---|---|---|---|---|
| Plant height (cm) | 39.0a | 31.8b,c | 26.3b | 23.1b,c |
| Leaf area (cm2) | 429a | 228b | 186b | 128b |
| Leaf no. | 16.1a | 11.4a,b | 8.5b | 8.2b |
| Specific leaf area (m2 kg−1) | 34.7a | 32.4a,b | 26.8b | 29.3a,b |
| Leaf weight ratio (kg kg−1) | 0.367a | 0.368a | 0.314a,b | 0.253b |
| Leaf area ratio (m2 kg−1) | 12.8a | 11.9a,b | 8.4b,c | 7.4c |
| Total dry wt (g) | 3.38a | 1.92b | 2.25b | 1.73b |
| Leaf dry wt (g) | 1.24a | 0.71b | 0.71b | 0.44b |
| Shoot dry wt (g) | 1.01a | 0.55a,b | 0.65a,b | 0.48b |
| Root dry wt (g) | 0.37a | 0.19b | 0.30a,b | 0.20b |
| Root/shoot ratio | 0.122a | 0.112a | 0.154a | 0.127a |
| Plant water content (%) | 85.0a | 86.0a | 77.5b | 81.1b |
| Soil water content (%) | 70.8a | 74.1a | 41.9b | 54.3c |
| Flavonoid content | 24.9a | 35.7a | 36.1a | 52.7b |
| Anthocyanin content | 2.54a | 3.90a | 4.73a | 7.24b |
Anthocyanin and flavonoid contents are expressed as absorbance per gram fresh weight of tissue at 530 and 300 nm, respectively. Leaf area values include stipules. Values are the means of four replicates and those in each row with the same letter are not significantly different according to analysis of variance pairwise comparisons (Bonferroni adjustment) at P < 0.05.
WW, Well-watered plants.
D, Drought-stressed plants.
Pigments that absorb UV-B radiation strongly are considered to play a major role in protecting plants from UV-B damage. Flavonoid and anthocyanin concentrations of plants subjected to UV-B radiation and drought are given in Table II. Flavonoid concentration was increased 43 and 45% by UV-B radiation and drought, respectively, and the combination of UV-B radiation and drought produced an additive and significant increase (112%) compared with the well-watered controls. Anthocyanin was also significantly increased by UV-B radiation and drought. Differences in the pigment concentrations with UV-B radiation or drought treatments were similar whether expressed on the basis of leaf area (data not shown) or fresh weight.
DISCUSSION
Large UV-B-induced reductions in plant biomass, similar to those seen in Table II, have been widely reported (Teramura et al., 1991; Barnes et al., 1993; Caldwell et al., 1994; Ålenius et al., 1995; Mepsted et al., 1996). However, the mechanisms involved in this response have not been identified. The amount of photosynthesis that can contribute to biomass is a product of both the area of leaf and the photosynthetic rate per unit area. Reductions in leaf area have been recorded for crops as diverse as rice, sunflower, rhubarb, and brussels sprouts (Teramura and Ziska, 1996), although few studies have made a distinction between leaf size and leaf number as components of leaf area. The mechanisms responsible for the reduction of leaf area have so far received comparatively little attention, particularly in dicotyledonous plants. In this study leaf number was not significantly affected, whereas leaf size was (Tables I and II), indicating UV-B inhibition of either cell division or cell expansion.
UV-B inhibition of cell expansion has been observed in cucumber cotyledons (Ballaré et al., 1991), tomato hypocotyls (Ballaré et al., 1995), and wheat (Hopkins, 1997) and barley leaves (Liu et al., 1995). UV-B irradiation could reduce cell expansion by changing turgor pressure or cell wall extensibility, and Tevini and Iwanzik (1986) suggested that direct oxidation of indole acetic acid by UV-B irradiation reduces cell wall expansion. The effects of UV-B irradiation on cell size at full expansion were small, at least in the sixth leaf, with cell frequencies largely unchanged in the mesophyll and lower epidermis (Table I). Stomatal frequency did increase in the upper surface in response to UV-B radiation without changes in stomatal index, which indicates a small (although not statistically significant at P = 0.05) reduction in cell expansion (Table I). This may be the cause of the typical slight curling of the leaf surface, which is often seen under high UV-B irradiances (Teramura and Ziska, 1996). However, it is clear that in the palisade mesophyll and both epidermal layers the largest contributor to the 39% reduction in leaf size was fewer cells per leaf (26–38% lower than control plants) rather than smaller cells (2–13% reduction in size, Table I). These results show that the primary cause of the reduced individual area at all three stages of the development of the sixth leaf was UV-B-induced inhibition of cell division. This observation was confirmed by the overall approximately 25% reduction in mean leaf size in either well-watered or droughted plants at the end of the experiment (Table II).
UV-B-induced inhibition of cell division has been reported in cucumber cotyledons (Tevini and Iwanzik, 1986), petunia leaf protoplasts (Staxén et al., 1993), and parsley (Logemann et al., 1995) and wheat leaves (Hopkins, 1997). Repair of UV-B damage to DNA before replication and direct UV-B-induced oxidation of tubulin, delaying microtubule formation, have been suggested as mechanisms for direct slowing of cell division (Staxén et al., 1993). UV-B radiation may also affect the key stages of cell division through transcriptional repression of the genes encoding for a mitotic cyclin and a p34cdc2 protein kinase (Logemann et al., 1995). Reductions in leaf area and cell division in all measured cell types were observed at all three stages of leaf development, with the effects becoming more pronounced approaching full expansion (Table I). This indicates that it is unlikely that UV-B radiation acts directly on the dividing cells because leaves at stage I were still enclosed by the folded bracts and, therefore, would have experienced a very low UV-B radiation exposure.
Indirect UV-B effects on leaf area could be a result of reduced photosynthate supply; however, we found no effects on Asat (Fig. 3). The lack of effect of UV-B radiation on Asat and other photosynthetic parameters contrasts with reports in which short-term exposure of mature leaves to similar UV-B irradiances in pea (Nogués and Baker, 1995) and oilseed rape (Allen et al., 1997) resulted in a rapid loss of photosynthetic competence primarily through effects on Rubisco. Clearly, when plants develop from seed, even under these high UV-B doses, they are protected from the loss of photosynthetic ability. A likely protective mechanism is the 43% increase in flavonoid content found here in UV-B-treated plants (Table II), as has been observed before in peas (Strid and Porra, 1992; Jordan et al., 1994) and many other species. Whereas Asat was not significantly affected by UV-B radiation throughout plant development (Fig. 3a), UV-B radiation did increase stomatal limitation by about 10% (Fig. 3d). This differs from reports that UV-B radiation can inhibit photosynthesis without changes in stomatal function (Ziska et al., 1992; Middleton and Teramura, 1993). The absence of a reduction in Asat in association with the increase in stomatal limitation (Fig. 3, a and d) suggests that there may have been a biochemical compensation within the photosynthetic apparatus. There is no evidence that this involved Rubisco, because there was no UV-B effect on Vc,max at any stage of plant development (Fig. 3b). However, there was a small but consistent increase in Jmax, indicating an increase in the rate of ribulose 1,5-bisphosphate regeneration in UV-B-treated plants (Fig. 3c). Given the complexity of the feedback in the control of photosynthesis, it is not possible from these gas-exchange data to identify the underlying mechanism for such a compensation. In addition, it is possible that in situ photosynthetic rates may have been affected by the UV-B radiation, even if the effects were not detected by the Asat assessment.
In situ measurements throughout the growth of the plants revealed an approximately 65% inhibition of adaxial gs by UV-B radiation, with no effect on the abaxial surface (Fig. 2, a and b), which reduced total gs by approximately 30% (Fig. 2c). This inhibitory effect on gs was clearly through changes in stomatal aperture, since stomatal frequency was either unchanged or increased (Table I). The adaxial stomata are the cells that are most exposed to UV-B radiation, because they are not screened by flavonoids in the epidermal layer. The reduction in stomatal aperture must be a direct response to UV-B radiation that was not mediated through photosynthesis, because this was unchanged and ci was slightly (1.5–2.0 Pa) lower in UV-B-treated plants than in the controls. Furthermore, the in situ inhibition of gs persisted when plants were temporarily removed from the UV-B cabinet for assessment of stomatal limitation. Wright and Murphy (1982) have shown that UV-B radiation can induce stomatal closure directly by inhibiting K+ accumulation by guard cells, and the mechanisms underlying the stomatal effect of UV-B warrant further investigation.
The drought treatment used here resulted in water stress developing slowly, with the first effects on RWC evident only after 8 d and on other parameters after 13 to 15 d (Figs. 1 and 2). Photosynthetic competence was maintained throughout this mild drought experiment (when RWC decreased to about 70% and ψw decreased to about −1.3 MPa) with no change in Asat, Vc,max, or Jmax being observed (Fig. 3, e–g). This supports previous results (Cornic, 1994) that mild water stress does not result in inhibition of photosynthetic capacity. However, the drought treatment was sufficient to result in 33 and 57% reductions in plant biomass and leaf area, respectively, at the end of the experiment (Table II).
Field (Teramura et al., 1990; Petropoulou et al., 1995) and controlled environment studies (Balakumar et al., 1993) have suggested an interaction between UV-B irradiation and water stress, and this was confirmed in this study with the reduction in final biomass due to UV-B irradiation being only 23% in the droughted plants compared with 43% in the well-watered ones (Table II). As discussed above, UV-B radiation exposure reduced both leaf area and gs. Under drought these effects reduced the total plant transpiration rate (Fig. 1c) and therefore slowed soil drying. At the final harvest this difference in plant transpiration rate was fully accounted for by the reduced leaf area, with daily transpiration per unit leaf area being very similar (2.3 and 2.5 g cm−2 d−1 for droughted controls and UV-B plants, respectively), even though UV-B plants had a slightly higher soil water content (Table II). The slower soil drying under UV-B irradiation resulted in a delay in the development of drought stress with declines in leaf RWC, ψw (Fig. 1, a and b), and gs (Fig. 2, e–g) occurring 5 to 12 d after those observed in the droughted controls. It is tempting to conclude that drought reduced the effect of UV-B irradiation on biomass accumulation, because UV-B irradiation had little effect in droughted conditions (only a 10% decline) compared with a 33% decline in biomass in well-watered conditions (Table II). However, in the droughted plants the soil water status in control and UV-B treatments was not the same; therefore, such a conclusion should not be drawn. Water stress can induce accumulation of UV-B-absorbing compounds (Murali and Teramura, 1986), which is likely to offer some increased protection from UV-B. In this study both UV-B and drought increased the concentration of flavonoids when imposed alone and had a synergistic effect when imposed together (UV-B radiation increased flavonoid concentration by 43%, drought by 45%, and UV-B radiation with drought by 112%; Table II).
Summary
This study has shown that irradiation with UV-B resulted in a decrease in adaxial gs by about 65%, increasing stomatal limitation of CO2 uptake by 10 to 15%. There was no loss of mesophyll photosynthetic activities with UV-B irradiation. The reductions in leaf area and plant biomass under long-term exposure to UV-B irradiation were associated with a decline in leaf cell numbers and cell division. There was an interaction between UV-B radiation and drought treatments: UV-B radiation delayed and reduced the severity of drought stress through a reduction in plant water-loss rates and through reductions in gs and leaf area.
ACKNOWLEDGMENTS
We are grateful to Andy McLeod (Institute of Terrestrial Ecology, Monks Wood) for the use of a spectroradiometer and to Kevin Oxborough for assistance with the fluorescence imaging system.
Abbreviations:
- Asat
light-saturated net CO2 assimilation rate
- E
daily evaporation rate
- Fv/Fm
ratio of variable to maximal fluorescence yield
- gs
stomatal conductance
- Jmax
maximum potential rate of electron transport contributing to ribulose 1,5-bisphosphate regeneration
- l
stomatal limitation to Asat
- φPSII
relative quantum efficiency of PSII photochemistry
- ψw
leaf water potential
- RWC
relative leaf water contentVc,
- max
maximum carboxylation velocity of Rubisco
Footnotes
This research was supported by a research grant to N.R.B. from the UK Natural Environment Research Council and to S.N. from the Generalitat de Catalunya (1996BEAI300222). D.J.A. was the recipient of a research studentship from the UK Biotechnology and Biological Sciences Research Council.
LITERATURE CITED
- Ålenius CM, Vogelmann TC, Bornman JF. A three-dimensional representation of the relationship between penetration of UV-B radiation and UV-screening pigments in leaves of Brassica napus. New Phytol. 1995;131:297–302. [Google Scholar]
- Allen DJ, McKee IF, Farage PK, Baker NR. Analysis of the limitation to CO2 assimilation on exposure of leaves of two Brassica napus cultivars to UV-B. Plant Cell Environ. 1997;20:633–640. [Google Scholar]
- Andrews JR, Bredenkamp GJ, Baker NR. Evaluation of the role of state transitions in determining the efficiency of light utilisation for CO2 assimilation in leaves. Photosynth Res. 1993;38:15–26. doi: 10.1007/BF00015057. [DOI] [PubMed] [Google Scholar]
- Baker NR, Nogués S, Allen DJ. Photosynthesis and photoinhibition. In: Lumsden P, editor. Plants and UV-B: Responses to Environmental Change. Cambridge, UK: Cambridge University Press; 1997. pp. 95–111. [Google Scholar]
- Balakumar T, Vincent VHB, Paliwal K. On the interaction of UV-B radiation (280–315 nm) with water-stress in crop plants. Physiol Plant. 1993;87:217–222. [Google Scholar]
- Ballaré CJ, Barnes PW, Flint SD, Price S. Inhibition of hypocotyl elongation by ultraviolet radiation in de-etiolated tomato seedlings. II. Time-course, comparisons with flavonoid responses, and adaptive significance. Physiol Plant. 1995;93:593–601. [Google Scholar]
- Ballaré CJ, Barnes PW, Kendrick RE. Photomorphogenic effects of UV-B radiation on hypocotyl elongation in wild type and stable-phytochrome-deficient mutant seedlings of cucumber. Physiol Plant. 1991;83:652–658. [Google Scholar]
- Barnes PW, Maggard SR, Holman SR, Vergara BS. Intraspecific variation in sensitivity to UV-B radiation in rice. Crop Sci. 1993;33:1041–1046. [Google Scholar]
- Caldwell MM (1971) Solar UV irradiation and the growth and development of higher plants. In AC Giese, ed, Photophysiology, Vol 6. Academic Press, New York, pp 131–177
- Caldwell MM, Flint SD, Searles PS. Spectral balance and UV-B sensitivity of soybean: a field experiment. Plant Cell Physiol. 1994;17:267–276. [Google Scholar]
- Cornic G. Drought stress and high light effects on leaf photosynthesis. In: Baker NR, Bowyer JR, editors. Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field. Oxford, UK: Bios Scientific Publishers; 1994. pp. 297–313. [Google Scholar]
- Day TA, Vogelmann TC. Alterations in photosynthesis and pigment distribution in pea leaves following UV-B exposure. Physiol Plant. 1995;94:433–440. [Google Scholar]
- Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annu Rev Plant Physiol. 1982;33:317–345. [Google Scholar]
- Fiscus EL, Booker FL. Is increased UV-B a threat to crop photosynthesis and productivity? Photosynth Res. 1995;43:81–92. doi: 10.1007/BF00042965. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- He J, Huang L-K, Chow WS, Whitecross MI, Anderson JM. Effects of supplementary ultraviolet-B radiation on rice and pea plants. Aust J Plant Physiol. 1993;20:129–142. [Google Scholar]
- Hopkins L (1997) The effects of elevated ultraviolet-B radiation on the growth and development of the primary leaf of wheat (Triticum aestivum L. cv Maris Huntsman). PhD thesis. University of St. Andrews, UK
- Jansen MAK, Gaba V, Greenburg BM, Mattoo AK, Edelman M. Low threshold levels of ultraviolet-B in a background of photosynthetically active radiation trigger rapid degradation of the D2 protein of photosystem II. Plant J. 1996;9:693–699. [Google Scholar]
- Jordan BR, He J, Chow WS, Anderson JM. Changes in mRNA levels and polypeptide subunits of ribulose 1,5-bisphosphate carboxylase in response to supplementary ultraviolet-B radiation. Plant Cell Environ. 1992;15:91–98. [Google Scholar]
- Jordan BR, James PE, Strid A, Anthony RG. The effect of ultraviolet-B radiation on gene expression and pigment composition in etiolated and green pea leaf tissue: UV-B induced changes are gene-specific and dependant upon the development stage. Plant Cell Environ. 1994;17:45–54. [Google Scholar]
- Kulandaivelu G, Nedunchezhian N. Synergistic effects of ultraviolet-B enhanced radiation and growth temperature on ribulose 1,5-bisphosphate and 14CO2 fixation in Vigna sinensis L. Photosynthetica. 1993;29:377–383. [Google Scholar]
- Lesser MP. Acclimation of phytoplankton to UV-B radiation: oxidative stress and photoinhibition of photosynthesis are not prevented by UV-absorbing compounds in the dinoflagellate Prorocentrum micans. Mar Ecol Prog Ser. 1996;132:287–297. [Google Scholar]
- Liu L, Gitz DC, McClure JW. Effects of UV-B on flavonoids, ferulic acid, growth and photosynthesis in barley primary leaves. Physiol Plant. 1995;93:725–733. [Google Scholar]
- Logemann E, Wu S-C, Schröder J, Schmelzer E, Somssich IE, Hahlbrock K. Gene activation by UV light, fungal elicitor or fungal infection in Petroselinum crispum is correlated with repression of cell cycle-related genes. Plant J. 1995;8:865–876. doi: 10.1046/j.1365-313x.1995.8060865.x. [DOI] [PubMed] [Google Scholar]
- Mackerness SAH, Butt PJ, Jordan BR, Thomas B. Amelioration of ultraviolet-B-induced down-regulation of mRNA levels for chloroplast proteins, by high irradiance, is mediated by photosynthesis. J Plant Physiol. 1996;148:100–106. [Google Scholar]
- McMurtrie RE, Wang Y-P. Mathematical models of the photosynthetic responses of tree stands to rising CO2 concentrations and temperatures. Plant Cell Environ. 1993;16:1–13. [Google Scholar]
- Mepsted R, Paul ND, Stephen J, Corlett JE, Nogués S, Baker NR, Jones HG, Ayres PG. Effects of enhanced UV-B radiation on pea (Pisum sativum L.) grown under field conditions in the UK. Global Change Biol. 1996;2:325–334. [Google Scholar]
- Middleton EM, Teramura AH. The role of flavonol glycosides and carotenoids in protecting soybean from ultraviolet-B damage. Plant Physiol. 1993;103:741–752. doi: 10.1104/pp.103.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali NS, Teramura AH. Effectiveness of UV-B radiation on the growth and physiology of field-grown soybean modified by water-stress. Photochem Photobiol. 1986;44:215–219. [Google Scholar]
- Negash L. Wavelength-dependence of stomatal closure by UV radiation in attached leaves of Eragrostis tef: action spectra under backgrounds of red and blue lights. Plant Physiol Biochem. 1987;25:753–760. [Google Scholar]
- Nogués S, Baker NR. Evaluation of the role of damage to photosystem II in the inhibition of CO2 assimilation in pea leaves on exposure to UV-B. Plant Cell Environ. 1995;18:781–787. [Google Scholar]
- Oxborough K, Baker NR. An instrument capable of imaging chlorophyll a fluorescence from intact leaves at very low irradiance and at the cellular and sub-cellular levels of organisation. Plant Cell Environ. 1997;20:1473–1483. [Google Scholar]
- Petropoulou Y, Kyparissis A, Kikolopoulos D, Manetas Y. Enhanced UV-B radiation alleviates the adverse effects of summer drought in two Mediterranean pines under field conditions. Physiol Plant. 1995;94:37–44. [Google Scholar]
- Poole I, Weyers DB, Lawson T, Raven JA. Variations in stomatal density and index: implications for paleoclimatic reconstructions. Plant Cell Environ. 1996;19:705–712. [Google Scholar]
- Staxén L, Bergounioux C, Bornman JF. Effect of ultraviolet radiation on cell division and microtubule organization in Petunia hybrida protoplasts. Protoplasm. 1993;173:70–76. [Google Scholar]
- Strid Å, Chow WS, Anderson JM. Effects of supplementary ultraviolet-B radiation on photosynthesis in Pisum sativum. Biochim Biophys Acta. 1990;1020:260–268. [Google Scholar]
- Strid Å, Porra RJ. Alterations in pigment content in leaves in Pisum sativum after exposure to supplementary UV-B. Plant Cell Physiol. 1992;33:1015–1023. [Google Scholar]
- Sullivan JH, Teramura AH. The effects of ultraviolet-B radiation on loblolly pine. 1. Growth, photosynthesis and pigment production in greenhouse-grown seedlings. Physiol Plant. 1989;77:202–207. [Google Scholar]
- Teramura AH, Forseth IN, Lydon J. Effects of ultraviolet-B irradiation on plants during mild water-stress. IV. The insensitivity of soybean internal water relations to ultraviolet-B radiation. Physiol Plant. 1984a;62:384–389. [Google Scholar]
- Teramura AH, Perry MC, Lydon J, McIntosh MS, Summers EG. Effects of ultraviolet-B irradiation on plants during mild water-stress. III. Effects on photosynthetic recovery and growth in soybean. Physiol Plant. 1984b;60:484–492. [Google Scholar]
- Teramura AH, Sullivan JH, Lydon J. Effects of UV-B radiation on soybean yield and seed quality: a 6-year field study. Physiol Plant. 1990;80:5–11. [Google Scholar]
- Teramura AH, Tevini M, Iwanzik W. Effects of ultraviolet-B irradiation on plants during mild water-stress. I. Effects on diurnal stomatal resistance. Physiol Plant. 1983;57:175–180. [Google Scholar]
- Teramura AH, Ziska LH. Ultraviolet-B radiation and photosynthesis. In: Baker NR, editor. Photosynthesis and the Environment. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 435–450. [Google Scholar]
- Teramura AH, Ziska LH, Sztein AE. Changes in growth and photosynthetic capacity of rice with increased UV-B radiation. Physiol Plant. 1991;83:373–380. [Google Scholar]
- Tevini M, Iwanzik W. Effects of UV-B radiation on growth and development of cucumber seedlings. In: Worrest RC, Caldwell MM, editors. Stratospheric Ozone Reduction, Solar Ultraviolet Radiation and Plant Life, Vol G8. Berlin: Springer-Verlag; 1986. pp. 271–285. [Google Scholar]
- Tevini M, Iwanzik W, Teramura AH. Effects of UV-B radiation on plants during mild water-stress. II. Effects on growth, protein and flavonoid content. Z Pflanzenphysiol. 1983;110:459–467. [Google Scholar]
- Tevini M, Teramura AH. UV-B effects on terrestrial plants. Photochem Photobiol. 1989;50:479–487. [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relations between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Wright LA, Murphy TM. Short-wave ultraviolet light closes leaf stomata. Am J Bot. 1982;69:1196–1199. [Google Scholar]
- Ziska LH, Teramura AH. CO2 enhancement of growth and photosynthesis in rice (Oryza sativa). Modification by increased ultraviolet-B radiation. Plant Physiol. 1992;99:473–481. doi: 10.1104/pp.99.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziska LH, Teramura AH, Sullivan JH. Physiological sensitivity of plants along an elevational gradient to UV-B radiation. Am J Bot. 1992;79:863–871. [Google Scholar]