Abstract
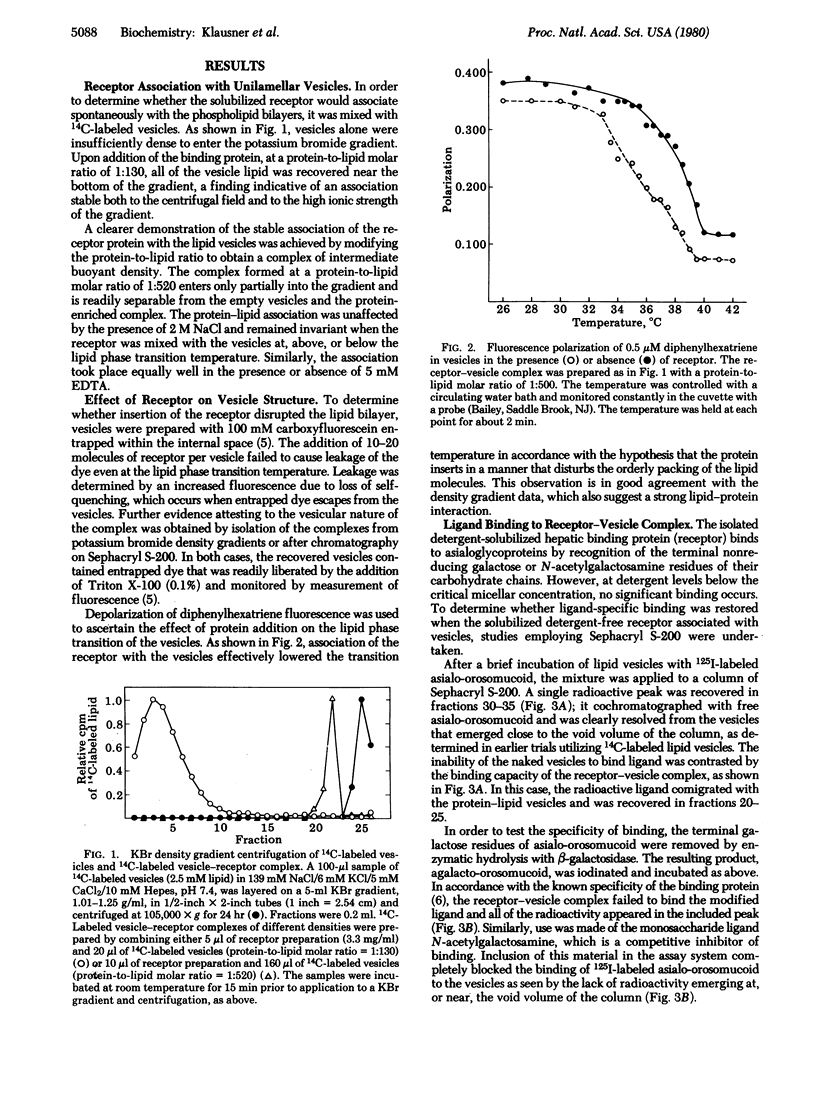
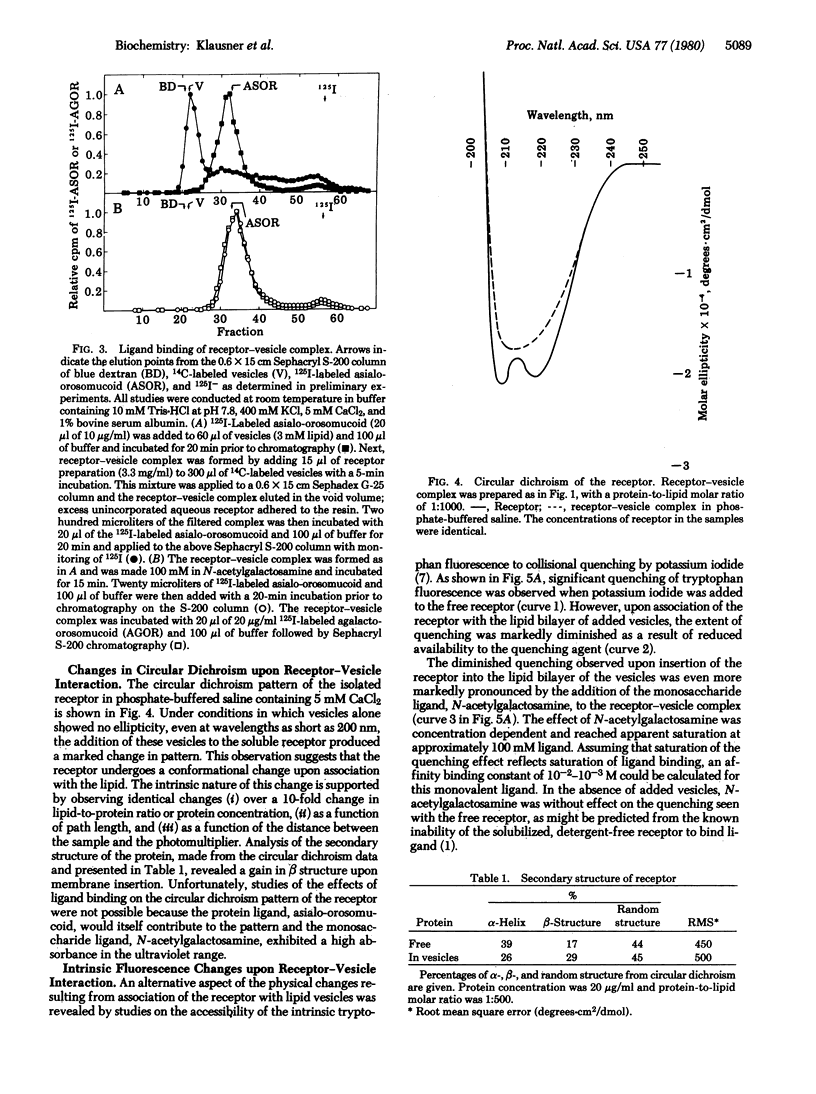
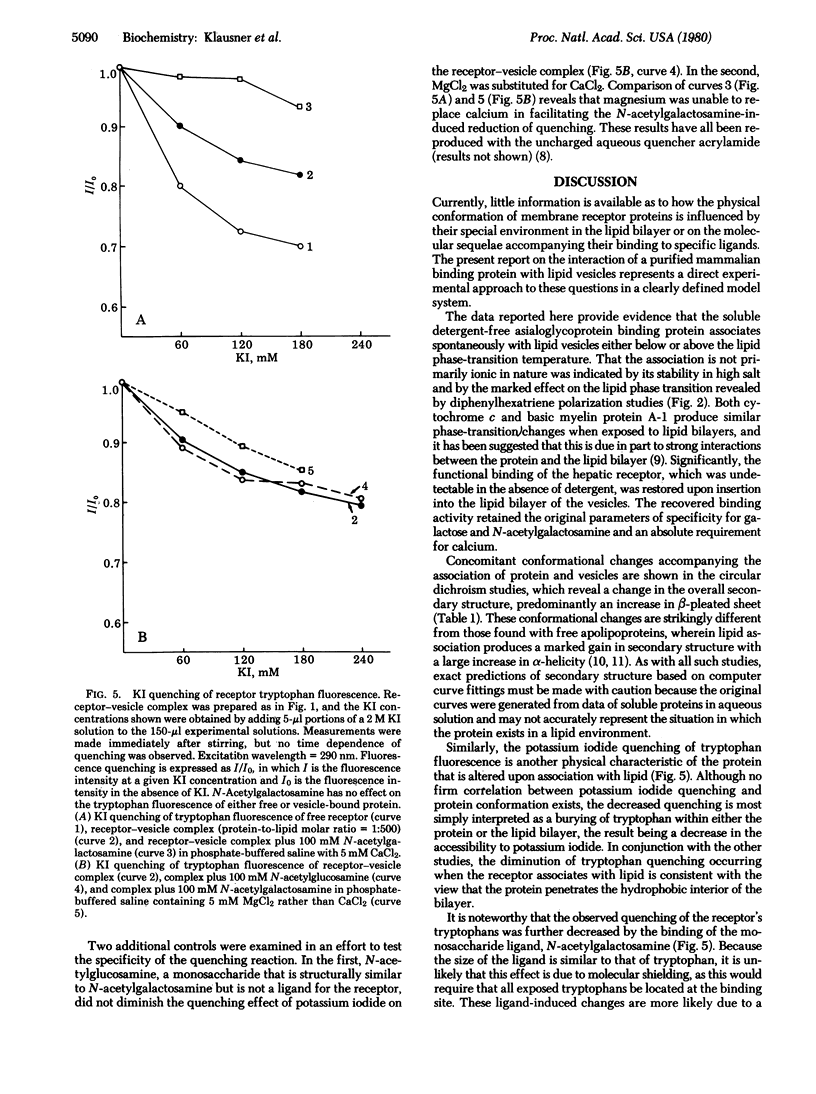
A solubilized detergent-free preparation of the hepatic binding protein specific for asialoglycoproteins associates spontaneously with small unilamellar lipid vesicles. This process is independent of the phase transition of the lipid and effectively restores the specific binding activity of the receptor protein. The insensitivity of the resulting lipid-protein complex to ionic strength provides evidence for a hydrophobic interaction. There is a perturbation of the lipid phase transition concomitant with addition of the protein. Circular dichroism studies indicate that the protein undergoes a conformational change on association with lipid. Binding of specific ligand produces further physical changes in the receptor as indicated by alterations in the tryptophan fluorescence quenching pattern.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assmann G., Brewer H. B., Jr Lipid-protein interactions in high density lipoproteins. Proc Natl Acad Sci U S A. 1974 Mar;71(3):989–993. doi: 10.1073/pnas.71.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Martinez H. M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972 Oct 24;11(22):4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- Eftink M. R., Ghiron C. A. Exposure of tryptophanyl residues in proteins. Quantitative determination by fluorescence quenching studies. Biochemistry. 1976 Feb 10;15(3):672–680. doi: 10.1021/bi00648a035. [DOI] [PubMed] [Google Scholar]
- Hudgin R. L., Pricer W. E., Jr, Ashwell G., Stockert R. J., Morell A. G. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974 Sep 10;249(17):5536–5543. [PubMed] [Google Scholar]
- Jonas A., Krajnovich D. J. Interaction of human and bovine A-1 apolipoproteins with L-alpha-dimyristoyl phosphadicylcholine and L-alpha-myristoyl lysophosphatidylcholine. J Biol Chem. 1977 Apr 10;252(7):2194–2199. [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Isolation and characterization of an avian hepatic binding protein specific for N-acetylglucosamine-terminated glycoproteins. J Biol Chem. 1977 Sep 25;252(18):6536–6543. [PubMed] [Google Scholar]
- Lehrer S. S. Solute perturbation of protein fluorescence. The quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry. 1971 Aug 17;10(17):3254–3263. doi: 10.1021/bi00793a015. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Moscarello M., Eylar E. H., Isac T. Effects of proteins on thermotropic phase transitions of phospholipid membranes. Biochim Biophys Acta. 1975 Sep 2;401(3):317–335. doi: 10.1016/0005-2736(75)90233-3. [DOI] [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. The binding of desialylated glycoproteins by plasma membranes of rat liver. Development of a quantitative inhibition assay. J Biol Chem. 1972 Jul 25;247(14):4633–4640. [PubMed] [Google Scholar]
- Weinstein J. N., Yoshikami S., Henkart P., Blumenthal R., Hagins W. A. Liposome-cell interaction: transfer and intracellular release of a trapped fluorescent marker. Science. 1977 Feb 4;195(4277):489–492. doi: 10.1126/science.835007. [DOI] [PubMed] [Google Scholar]


