Abstract
Decompressive craniectomy (DC) is a useful technique for the treatment of traumatic brain injuries (TBI) with intracranial hypertension (ICHT) resistant to medical treatment, increasing survival, although its role in the functional prognosis of patients is not defined. It is also a technique that is not without complications, and may increase the patient's morbidity and mortality. We report two cases of patients with TBI who required DC and suffered complications from the technique
Keywords: Decompressive craniectomy, injury, traumatic brain, complications
INTRODUCTION
Intracranial hypertension (ICHT) management in patients who have suffered a severe TBI remains a challenge. Despite improvements in monitoring and medical treatment, morbidity and mortality remains high in patients with ICHT refractory to therapy.[1,2]
Initially one should try to manage the patient's ICHT after a severe TBI with medical treatment (elevation of the bed side, sedation, relaxation, osmolar therapy, hyperventilation, cerebrospinal fluid drainage, and barbiturate coma). However, in 10 to 15% of the patients, ICHT is refractory to medical treatment, being in these cases a DC, an effective measure for reducing intracranial pressure, in up to 80% of the cases.[3] There is a growing body of literature supporting the efficacy of DC, especially after TBI. It has been significantly demonstrated that in patients with ICHT, craniectomy alone lowered it, but opening the dura in addition to the bony skull resulted in an average decrease in intracranial pressure. Although it is associated with a shorter stay in Intensive Care Units, it is unclear whether DC improves the functional outcome in adult patients with severe TBI and ICHT refractory to treatment.[4,5] Moreover, it should be noted that this technique is not without complications, and those can appear following a sequence, in time. One of the first things that can occur is the expansion of a hemorrhagic contusion, followed by the appearance of a new subdural hematoma on the contralateral side, seizures, loss of cerebrospinal fluid through the scalp, and external brain herniation. The appearance of a subdural hematoma and postoperative infections are complications that can develop even four weeks after surgery.[6] Over time, some changes have been implemented in the manner in which the technique is carried out, by trying to find better surgical approaches, to avoid further complications. Comparing the effect of standard trauma craniectomy (STC) versus limited craniectomy (LC), the STC significantly improves the outcome in severe TBI, with refractory ICHT resulting from unilateral frontotemporoparietal contusion, with or without intracerebral or subdural hematoma. This suggests that STC, rather than LC, be recommended for such patients.[7]
At the moment, there is no clear indication for the use of a definite technique. It seems that the tendency has been to recommend unilateral frontotemporoparietal DC for the treatment of cerebral hemisphere injury or edema, where bifrontal DC is indicated if there is diffuse swelling.[8] We present two cases of patients with TBI, who required DC and who suffered complications secondary to the technique.
CASE REPORTS
Case 1
A 15-year-old female patient was admitted after a severe TBI. The axial computed tomography (CT) scan showed a frontoparietal subarachnoid hemorrhage, right frontal subdural hematoma, cerebral edema, and important bilateral uncal herniation. A wide bifrontal DC was carried out and subdural hematoma evacuation was done.
Seventeen days after admission, when objectified 11 points in Glasgow Coma Score (GCS) (4-1-6), and obeying simple commands, the patient suffered a neurological deterioration to 3 points on the GCS and pupil anisocoria. The CT scan showed that frontal intraparenchymal hemorrhagic lesions opened to the ventricular system [Figure 1]. The patient died in a state of severe brain damage, within the context of nosocomial infection, 35 days after admission.
Figure 1.
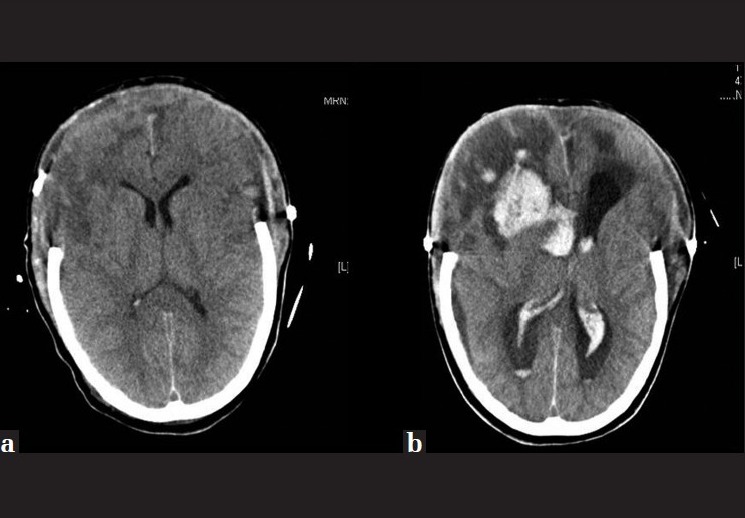
(a) Decompressive craniectomy performed after head injury and (b) the subsequent appearance of a frontal hemorrhage
Case 2
A 60-year-old man who suffered a severe TBI with a right subdural effusion, with a mass effect on the midline of 19 mm, ventricular compression on the same side, uncal and transfalcine herniation with effacement of cisterns and compression on the temporal horn, had a right frontotemporoparietal DC performed, and hematoma evacuation was done. The patient remained with 10 points (3-2-5) in the GCS, but with no connection to the environment.
After 20 days of admission, a right subdural hygroma appeared, which required surgery and titanium cranioplasty [Figure 2]. The patient was discharged home with a 3-point GCS, with severe disability, conscious, but with basic family-support for daily activities, after 28 days of admission.
Figure 2.
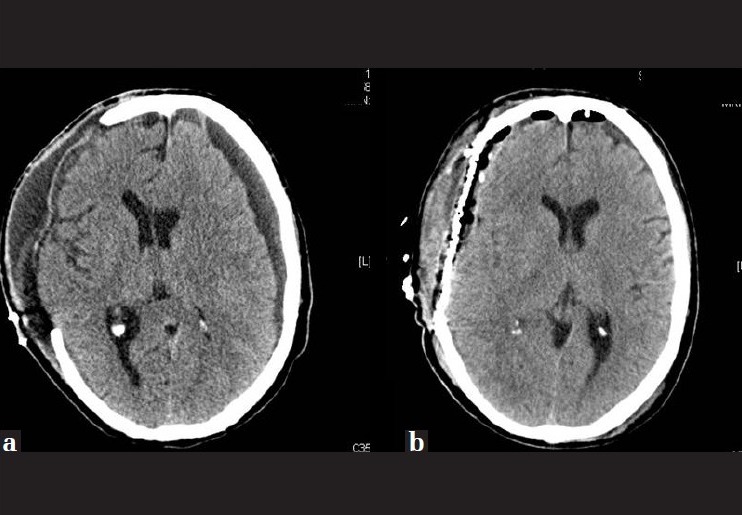
(a) Subdural hygroma next to the craniectomy area and (b) after evacuation of the hygroma and titanium cranioplasty
DISCUSSION
The age and GCS of the patient are major factors influencing DC effectiveness. It is generally reported that the outcome of patients younger than 50 years or with an initial GCS score of 6 or more is significantly better than that for older patients or those with an initial GCS score lower than 6. The factor having the greatest influence on the outcome is the time from injury to surgery. Patients who undergo DC within the first four hours following trauma have a better outcome compared to those who undergo DC after four hours.
Although the DC is a simple technique, it can be risky, and risks must be considered, as they can alter the prognosis. Complications are more frequent in patients with a low score on the Glasgow scale and in those over 60 years. Up to 50% of the patients had at least one complication.[7]
The expansion of hemorrhagic contusions is the most common complication of neurological impairment and death in patients who have had a period of lucidity after a severe TBI, as in the cases that we have reported. The explanation for this phenomenon is that when performing the DC, the hemostatic effect is lost when removing the bone and this facilitates the expansion of the effusion on the same side, and is contralateral in a few cases. The DC seems to be useful in ICHT treatment and in reducing the mortality rate. However, it seems to increase the incidence of late subdural hematoma.[9]
Hygroma is caused due to an alteration between production and reabsorption of cerebrospinal fluid. It is relatively common and it can be developed in up to 50% of the cases. The main cause is a disruption between the dura and arachnoids, when performing the DC. Among their most common locations are the subdural, subgaleal or the interhemispheric, being more common where the craniectomy it is site.[10] Its incidence can be reduced by performing duraplasty. It is rare that effusions present clinical expression and most of them are reabsorbed spontaneously, but when its expansion produces neurological impairment it is necessary to have an aggressive treatment such as surgical excision – cranioplasty being a definitive solution, as in the case that we have reported.[10] The presence of interhemispheric, subdural or subgaleal hygroma is a predictive radiographic sign of hydrocephalus in patients undergoing DC.[11] Hemorrhagic collections with mass effect are usually extra-axial collections, and are usually located at some distance from the craniectomy. Its etiology can be explained by the sudden decrease in intracranial pressure, so they rapidly appear.[12] It is a rare complication, but it significantly increases the morbidity. Cerebral herniation usually appears during the first week after surgery. Its incidence is up to 25% and it is caused due to edema induced by cerebral high perfusion and due to the increase of the hydrostatic gradient from the capillaries, after decompression.[13] When this complication occurs, it can generate brain parenchymal lacerations and can compromise the cortical venous flow against the edges of the bone defect, causing ischemia and necrosis.[12] Performing a wide craniectomy minimizes this complication.
The DC is also a risk factor for the development of hydrocephalus in up to 30% of cases, and can complicate the prognosis. Its appearance may be because this technique may induce alterations in the circulation of the cerebrospinal fluid (CSF). It is usually solved after placing the bone; however, it can persist for a long time despite the replacement. Sometimes, placement of a ventriculoperitoneal shunt is required.
The impairment of cutaneous healing has an incidence of up to 10%. The surgical emergency of the procedure can damage the superficial temporal artery, and reduce flow to the flap, or also can accidentally open the frontal sinus, contaminating the area. The wound infection decreases its incidence due to the administration of antibiotics during surgery, which remains at around 7%. Moreover, the sealing of the dura prevents CSF leaks, which can cause meningitis. Replacing the bone is also associated with higher rates of infection, increasing when the bone flap is preserved for long periods of time under freezing conditions.
CONCLUSIONS
The DC is a useful technique for the treatment of TBI with ICHT resistant to medical treatment. It increases the patient's survival, but its role in the functional prognosis of patients is still not defined. Early bi-frontotemporoparietal DC decreases the intracranial pressure and the length of stay in the ICU, but is associated with more unfavorable outcomes.[4]
Undertaking this decision requires that the clinicians involved take appropriate account of having used all the conventional weapons against ICHT and the absence of data indicating an already negative outcome.[14]
In addition, it is a technique that is not free of complications, which themselves can further increase the worst prognosis of these patients. Just as more studies are needed to define the type of patient who can benefit from a DC, and to decide which technique is most appropriate, all the complications derived from this technique must be evaluated.
We also recommend further studies to monitor brain oxygenation, because the normalization of intracranial pressure does not mean that the brain perfusion has been adequate. Strategies to improve cerebral oxygenation suggest the benefit of multiple approaches to monitor these patients.[15]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, S’calea TM, Eisenberg HM. Decompressive craniectomy for Outcome Following malignant swelling due to severe head injury. J Neurosurg. 2006;104:469–79. doi: 10.3171/jns.2006.104.4.469. [DOI] [PubMed] [Google Scholar]
- 2.Lubillo S, Blanco J, López P, Molina I, Dominguez J, Carreira L, et al. The role of decompressive craniectomy in the neurocritical patient. Intensive Care Med. 2009;33:74–83. doi: 10.1016/s0210-5691(09)70685-0. [DOI] [PubMed] [Google Scholar]
- 3.Flint AC, Manley GT, Gean AD, Hemphill JC, Rosenthal G. Post-operative expansion of after-sided contusions hemorrhagic decompressive hemicraniectomy in severe traumatic brain injury. J Neurotrauma. 2008;2:503–12. doi: 10.1089/neu.2007.0442. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493–502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- 5.Lubillo S, Blanco J, López P, Dominguez J, Ruiz R, Molina J, et al. Decompressive craniectomy Does Improve ICP Beside other parameters. Effects of the decompressive craniectomy on tissue pressure? Intensive Care Med. 2011;35:166–9. doi: 10.1016/j.medin.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Ban SP, Son YJ, Yang HJ, Chung YS, Lee SH, Han DH. Analysis of complications following decompressive craniectomy for traumatic Brain injury. J Korean Neurosurg Soc. 2010;48:244–50. doi: 10.3340/jkns.2010.48.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang JY, Xu W, Li WP, Xu WH, Zhang J, Bao YH, et al. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: A multicenter, prospective, randomized controlled study. J Neurotrauma. 2005;22:623–8. doi: 10.1089/neu.2005.22.623. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Wen L. Technical Considerations in decompressive craniectomy in the Treatment of traumatic brain injury. Int J Med Sci. 2010;7:385–90. doi: 10.7150/ijms.7.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu W, Guo C, Chen HO, Chen KE, Wen L, Huang H, et al. Decompressive craniectomy Effects of unilateral Patients with unilateral acute on post-traumatic brain swelling after-severe traumatic brain injury. Crit Care. 2009;13:R185. doi: 10.1186/cc8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang XF, Wen L, Shen F, Li G, Lou R, Liu WG, et al. Surgical decompressive craniectomy to secondary Complications in Patients With A head injury: A series of 108 consecutive cases. Acta Neurochir (Wien) 2008;150:1241–7. doi: 10.1007/s00701-008-0145-9. [DOI] [PubMed] [Google Scholar]
- 11.Paredes I, Cicuendez M, Delgado MA, Martinez-Perez R, Munarriz PM, Lagares A. Normal pressure subdural hygroma with mass effect as a complication of decompressive craniectomy. Surg Neurol Int. 2011;2:88. doi: 10.4103/2152-7806.82370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schirmer CM, Ackil AA, Jr, Malek AM. Decompressive Craniectomy. Neurocrit Care. 2008;8:456–70. doi: 10.1007/s12028-008-9082-y. [DOI] [PubMed] [Google Scholar]
- 13.Stiver SI. Complications of Decompressive craniectomy for traumatic brain injury. Neurosurg Focus. 2009;26:E7. doi: 10.3171/2009.4.FOCUS0965. [DOI] [PubMed] [Google Scholar]
- 14.Citerio G, Peter JD. Refractory elevated intracranial pressure: Intensivist's role in solving the dilemma of decompressive craniectomy. Intensive Care Med. 2007;33:45–8. doi: 10.1007/s00134-006-0381-5. [DOI] [PubMed] [Google Scholar]
- 15.Maloney-Wilensky E, Gracias V, Itkin A, Hoffman K, Bloom S, Yang W, et al. Brain tissue oxygen and outcome after severe traumatic brain injury: A systematic review. Crit Care Med. 2009;37:2057–63. doi: 10.1097/CCM.0b013e3181a009f8. [DOI] [PubMed] [Google Scholar]


