Abstract
Aims
The molecular mechanisms controlling heart function and rhythmicity are incompletely understood. While it is widely accepted that the type 2 ryanodine receptor (Ryr2) is the major Ca2+ release channel in excitation–contraction coupling, the role of these channels in setting a consistent beating rate remains controversial. Gain-of-function RYR2 mutations in humans and genetically engineered mouse models are known to cause Ca2+ leak, arrhythmias, and sudden cardiac death. Embryonic stem-cell derived cardiomyocytes lacking Ryr2 display slower beating rates, but no supporting in vivo evidence has been presented. The aim of the present study was to test the hypothesis that RYR2 loss-of-function would reduce heart rate and rhythmicity in vivo.
Methods and results
We generated inducible, tissue-specific Ryr2 knockout mice with acute ∼50% loss of RYR2 protein in the heart but not in other tissues. Echocardiography, working heart perfusion, and in vivo ECG telemetry demonstrated that deletion of Ryr2 was sufficient to cause bradycardia and arrhythmia. Our results also show that cardiac Ryr2 knockout mice exhibit functional and structural hallmarks of heart failure, including sudden cardiac death.
Conclusion
These results illustrate that the RYR2 channel plays an essential role in pacing heart rate. Moreover, we find that RYR2 loss-of-function can lead to fatal arrhythmias typically associated with gain-of-function mutations. Given that RYR2 levels can be reduced in pathological conditions, including heart failure and diabetic cardiomyopathy, we predict that RYR2 loss contributes to disease-associated bradycardia, arrhythmia, and sudden death.
Keywords: Heart rate (variability), Bradycardia, Ca2+ channel, Arrhythmia (mechanisms), Excitation–contraction coupling
1. Introduction
Heart failure is associated with arrhythmias and ends in sudden cardiac death for many patients. The molecular mechanisms linking these pathologies remain to be elucidated fully. One candidate is the type 2 ryanodine receptor (RYR2), a large Ca2+ channel in the sarcoplasmic reticulum (SR) membrane. Mutations in the human RYR2 gene cause catecholaminergic polymorphic ventricular tachycardia type 1 (CPVT1) and arrhythmogenic right ventricular dysplasia (ARVD) type 2, rare sudden cardiac death syndromes often manifesting abnormal heart rate.1–3 Mutations leading to overactive Ca2+ channels and protracted calcium transients have been implicated in most cases.3–9 Gain-of-function ‘knock-in’ mouse models4,10–13 confirm non-redundant roles for the RYR2 channel in arrhythmias and sudden cardiac death.6,14,15 While these studies suggest that leaky RYR2 channels underlie many arrhythmias, it is controversial whether or not RYR2 mutations alter heart rate. For example, Ryr2R4496C mutant mice develop CPVT1-like pathology without a change in basal heart rate.15 However, mice with point mutations do not permit analyses of other parts of the Ryr2 protein or generalized loss-of-function. Recently loss-of-function mutations have been described that are associated with decreased RYR2 open probabilities.16,17 Ryr2 was directly implicated in myocyte beating rate using in vitro differentiated mouse embryonic stem cells,18 but the in vivo relevance of these experiments has remained unclear in the absence of inducible Ryr2 knockout mice.
The exact mechanisms which set and maintain heart rate remain controversial.15 One prevalent hypothesis states that heart rate is dictated exclusively by inwardly rectifying channels of the HCN family found in pacemaker cells.19 A complementary hypothesis suggests that the electrical events underlying heart rate are governed by two ‘interlocking ionic clocks’.20 In this model, heart rate is dependent on an ensemble of interacting ion channels that can be split into two groups, channels of the plasma membrane and channels of the ER/SR, each with their own periods of function and refraction.20 In this latter model, RYR2 is predicted to be a key regulator of heart rate and rhythmicity in vivo.18,21,22 While vigorous debate remains,15 there is wide agreement that an in vivo loss-of-function genetic approach is required to test this hypothesis.
Several studies have shown that the levels and/or activity of RYR2 are reduced by up to 50% in disease states such as heart failure and diabetes.23–26 Evidence has also been presented that RYR2 levels diminish with normal ageing.27,28 Pathological changes in the expression and/or function of RYR2 channels are usually considered to be downstream of other events in heart disease. Nevertheless, unbiased genome-wide association studies show that RYR2 polymorphisms increase susceptibility to hypertension,29 meaning that variation in RYR2 expression/function may represent an underlying causal factor in heart disease. Unfortunately, little is known about the in vivo effects of RYR2 loss-of-function. Recently, mice with lifelong Ryr2 haploinsufficiency were found to be resistant to pressure-overload induced cardiac hypertrophy, but also exhibited altered calcium handling, increased cardiomyocyte cell death, and decreased contractility.30 Unfortunately, that study of Ryr2+/− mice did not directly examine heart rate or rhythmicity. The lifelong Ryr2 reduction also carries the caveat of possible compensation by other SR calcium channels.
In the present study, we tested whether RYR2 has an in vivo role in regulating heart rate and rhythmicity, while confirming the expected role in function. We used inducible, tissue-specific Ryr2 knockout (cRyr2KO) mice, which allowed us to reduce RYR2 in cardiac tissue in vivo in adult animals in a manner that was not associated with compensation from other Ca2+ channels. Our results suggest that RYR2 plays a non-redundant role in the control of heart rate and provide in vivo evidence that RYR2 loss-of-function can lead to arrhythmia.
2. Methods
2.1. Experimental animals
Mice bearing the Ryr2 floxed allele were generated using R1 embryonic stem cells (129SvJ × 129Sv) containing an Ryr2 floxed allele,18 injected into C57BL/6 blastocysts and transferred to pseudopregnant females at the NIA transgenic facility. Agouti mice were identified and genotyping confirmed transmission of the floxed allele to offspring, which were backcrossed over 10 generations to the C57BL/6 background.
Tamoxifen-inducible, cardiomycyte-specific Ryr2 knockout mice were generated by crossing the mice harbouring conditional Ryr2 alleles (C57Bl6 Ryr2flox/flox mice) with mice expressing inducible Cre-recombinase under the control of the α-MHC promoter (C57Bl6-mhy6-mer-Cre-mer mice).31 Tamoxifen was injected into the intraperitoneal cavity of 8–20-week-old Ryr2flox/flox:mhy6-mer-Cre-mer mice (referred to as cRyr2KO mice) or littermate controls for three consecutive days at 3 mg per 40 g body weight. It is important to note that since both groups received tamoxifen, none of the effects reported herein can be attributed to this drug. Mice were handled according to protocols approved by the University of British Columbia Animal Care Committee, in accordance with the NIH Guide for the Care and Use of Animals.
2.2. In vivo and ex vivo analysis of cardiac function
Cardiac function was examined by echocardiography utilizing a Vevo 770 high-resolution image system (Viewsonics).1 Images were obtained through the anterior and posterior left ventricular walls at the papillary muscle level. Left ventricular mass and systolic function, left ventricular end-systolic and end-diastolic dimensions, interventricular septum thickness and posterior wall were measured from M-mode traces. Shortening and ejection fraction were calculated as described.1 We assessed heart rate before and after tamoxifen injection. Mice assessed by echocardiography were anaesthetized with isoflurane at the minimum gas concentration (1–2% isoflurane delivered in 1.5 L/min O2) necessary to maintain light anaesthesia (rodent remained immobile and unconscious). Heart function was further measured using the working heart model.32 These mice were euthanized by having their hearts excised under a dose of inhaled isoflurane (5% isoflurane delivered in 3 L/min O2) sufficient to maintain deep anaesthesia (assessed by toe-pinch method and breathing pattern). In vivo ECG was assessed utilizing ETA-F10 implantable telemetry devices according to the manufacturer's instructions (Data Sciences International). Inhaled isoflurane was used as an anaesthetic (1–5% isoflurane delivered in 1 L/min O2, assessed by toe-pinch method) during implantation surgery and injected analgesic, Buprenorphine (0.1 mg/kg), was administered daily as necessary. Heart rate and rhythmicity were analyzed following recovery from surgery and after the discontinuation of all analgesics.
2.3. Gene and protein expression analysis
Tissues used for RNA and protein analysis were isolated from mice euthanized with CO2 and cervical dislocation, and were rapidly extracted and snap frozen in liquid nitrogen. RNA was isolated from heart tissue using Trizol, followed by cleanup (RNeasy; Qiagen). After reverse transcription (SuperScript III; Invitrogen), TaqMan quantitative RT–PCR was conducted using probes from Applied Biosystems and PerfeCTa qPCR SuperMix (Quanta) on a StepOnePlus qPCR thermocycler (Applied Biosystems). SYBR Green quantitative RT–PCR was conducted using PerfeCTa SYBR Green qPCR SuperMix (Quanta). Relative gene expression changes were analyzed by the 2−ΔCt method. Hypoxanthine-guanine phosphoribosyltransferase and cyclophilin were used as internal controls, after ensuring that they were not altered in cRyr2KO cardiomyocytes.
Immunoblots were performed on lysates from mechanically disrupted heart, homogenized and sonicated in ice-cold lysis buffer. A total of 100 µg of protein, boiled with loading dye, was used in gel electrophoresis. Proteins over 120 kDa were transferred to polyvinylidene difluoride membranes using overnight wet transfer and subsequently treated with horseradish peroxidase-conjugated antibodies (Cell Signalling). Bands were visualized using an enhanced chemiluminescence and quantified by densitometry. Anti-HSP90 (Cell Signaling) and anti-EEA1 (Abcam) were used as loading controls for high kiloDalton immunoblots. Rabbit polyclonal anti-RYR2 antibodies were provided by Dr Anthony Lai.3
2.4. Statistical analysis
Data are expressed as mean ± SEM unless otherwise indicated. Results were considered statistically significant when P < 0.05 using the Student's t-test or two-factor mixed design ANOVA with repeated measures and the Bonferroni post-test, where appropriate. All experiments were repeated on at least three cRyr2KO mice and at least three of their littermates.
3. Results
3.1. Acute reductions in Ryr2 mRNA and protein in conditional knockout mice
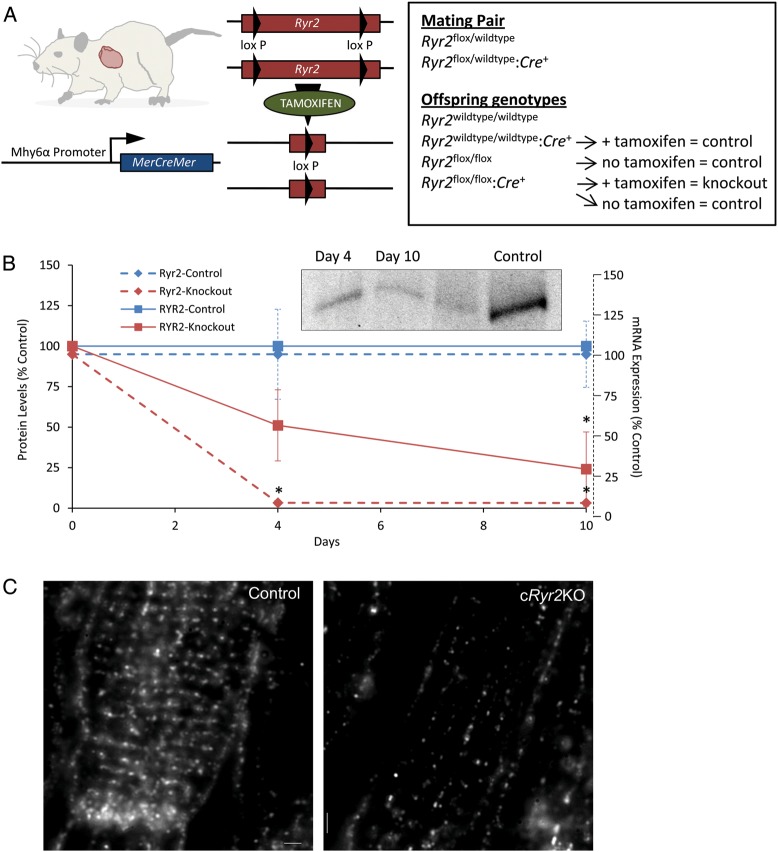
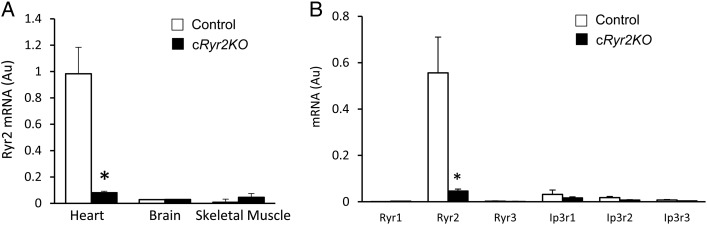
To create a tissue-specific, inducible model of Ryr2 gene loss-of-function, mice bearing Ryr2flox alleles were crossed with cardiomyocyte-specific, tamoxifen-activated Cre mice (myosin II heavy chain-α promoter) resulting in Ryr2flox/flox:Mer-Cre-Mer mice (cRyr2KO; Figure 1A). Prior to induction, Ryr2 expression was completely normal. However, when treated with the Mer-Cre-Mer agonist tamoxifen, genetic recombination occurred causing Ryr2 gene deletion in adult cardiomyocytes (Figure 1A). Tamoxifen injection for three consecutive days resulted in >90% decrease in Ryr2 mRNA, quantified by Taqman RT–qPCR in whole heart, 4 or 10 days after the first tamoxifen dose (Figure 1B). These data suggest a virtually complete knockout of the Ryr2 in cardiomyocytes, probably in most regions of these hearts including sinoatrial nodes.19 Four days after the initiation of tamoxifen, RYR2 protein was decreased ∼50% (Figure 1B). RYR2 protein levels in whole heart declined to ∼25% of control values after 10 days (Figure 1B). These observations were further confirmed using immunofluorescence staining, where dramatic changes in the abundance, and the orientation of RYR2 clusters along axial tubules, were evident (Figure 1C). Ryr2 gene knockout was tissue specific (Figure 2A) and we did not observe compensatory increases in other ER calcium release channels (Figure 2B), validating our use of an inducible loss-of-function model to study the role of Ryr2 reduction in heart rate, arrhythmia, and failure.
Figure 1.
Acute, cardiac-specific Ryr2 gene ablation in mice. (A) Schemata detailing tamoxifen-inducible, cardiomyocyte-specific Ryr2 knockout (cRyr2KO) mice and treatment groups. (B) Ryr2 mRNA and RYR2 protein levels in tissues post-induction of Ryr2 deletion. Western blot is representative and plot is quantified against control proteins EEA1 and HSP90. (Ryr2 mRNA: Control n = 6, cRyr2KO n = 6; RYR2 protein: Control n = 3, cRyr2KO n = 3; *P≤0.05). (C) Representative images of disrupted RYR2 protein distribution and re-organization in cryofixed sections of Ryr2 knockout hearts (Control n = 3, cRyr2KO n = 3).
Figure 2.
Conditional Ryr2 knockout is tissue specific and not compensated for by other SR Ca2+ release channels. (A) Ryr2 mRNA levels in heart, brain, and skeletal muscle 4 days following induction of Ryr2 deletion (Control n = 6, cRyr2KO n = 6; *P≤0.05). (B) SR Ca2+ release channel mRNA expression 10 days following induction of Ryr2 deletion (Control n = 6, cRyr2KO n = 6; *P ≤ 0.05).
3.2. Sudden cardiac death in cRyr2KO mice
Following induction of Ryr2 gene deletion, all cRyr2KO mice developed cardiomyopathy. Four days after the first tamoxifen injection, virtually all cRyr2KO mice were lethargic, but their tamoxifen-injected littermate controls were not. After this timepoint, some cRyr2KO mice rapidly reached their humane endpoint while others showed a dramatic improvement in health that could last as long as 48 days before eventually reaching their humane endpoint. Interestingly, the final stage had many features of sudden cardiac death. cRyr2KO mice reaching an early humane endpoint showed profound atrial clotting, suggesting the possibility of atrial fibrillation.
3.3. Heart function in cRyr2KO mice
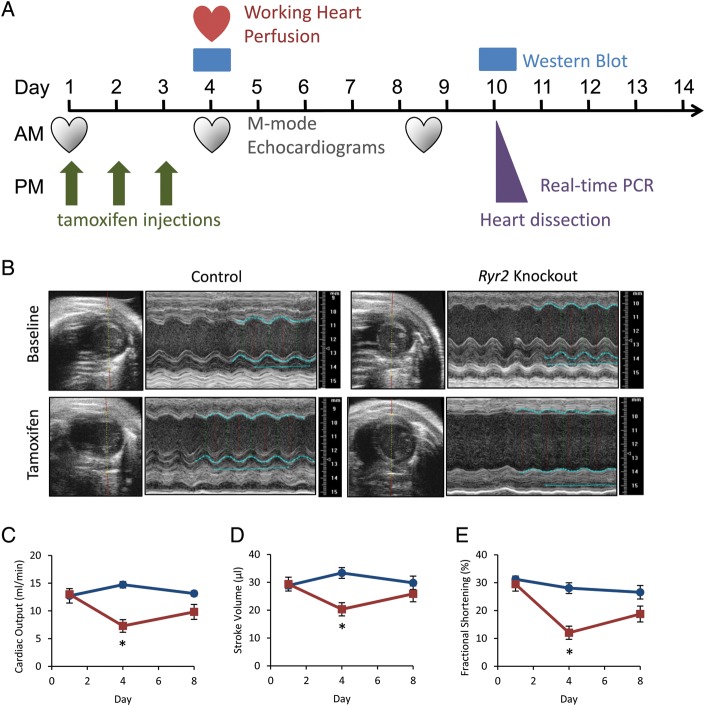
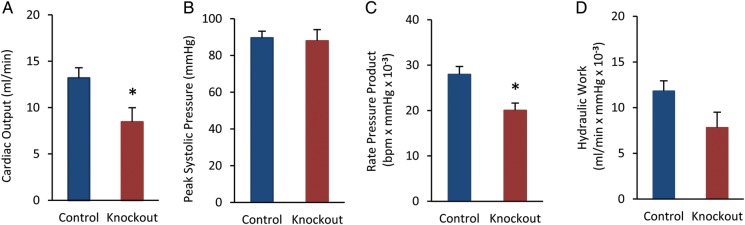
To assess cardiac function, we performed M-mode echocardiography on cRyr2KO mice and littermate controls (Figure 3A and B, Table 1). In these experiments, tamoxifen-injected and non-injected Ryr2flox/flox control mice were similar and therefore pooled. The absence of differences in tamoxifen-injected control mice indicated that tamoxifen exposure played no significant role in the profound cardiac phenotype. Acute Ryr2 deletion resulted in a dramatic and rapid >50% decline in cardiac output after just four days (Figure 3C–E). Interestingly, the contractile parameters of cRyr2KO hearts did not appear to decline further over 10 days (Figure 3C–E), and in some tests trended towards normal as part of a compensatory process. To assess cardiac function further in cRyr2KO hearts, we utilized the isolated working heart model (Figure 4A–D). In these experiments, we observed similar decreases in cardiac output, rate pressure product, and hydraulic work, demonstrating that these defects were intrinsic to the heart. Thus, Ryr2 is required for normal cardiac function, but there appears to be compensatory mechanisms that transiently ameliorate the detrimental effects of Ryr2 deletion.
Figure 3.
Conditional Ryr2 knockout mice rapidly lose cardiac function. (A) Timeline of experimental events. (B) Echocardiograms of control and conditional Ryr2 knockout mice. Images are representative (Control n = 7, cRyr2KO n = 5). (C–E) Cardiac output, stroke volume, and fractional shortening as measured by echocardiography. Blue lines are control, red lines are knockout. (Control n = 7, cRyr2KO n = 5; *P ≤ 0.05).
Table 1.
Echocardiography parameters and cardiac hypertrophy
| Timepoint | Baseline Echo 1 |
Echo 2 |
Final Echo 3 |
|||
|---|---|---|---|---|---|---|
| Mouse | Control | Knockout | Control | Knockout | Control | Knockout |
| Body weight (g) | 25.4 ± 1.4 | 25.8 ± 1.2 | 24.5 ± 1.5 | 23.2 ± 1.0 | 25.4 ± 1.0 | 22.7 ± 1.6 |
| Heart weight/body weight (%) | n/a | n/a | 0.68 ± 0.02a | 0.84 ± 0.03*,a | 0.74 ± 0.06 | 0.98 ± 0.09* |
| Systolic diameter (mm) | 2.4 ± 0.1 | 2.5 ± 0.18 | 2.7 ± 0.1 | 3.7 ± 0.1* | 2.7 ± 0.2 | 3.2 ± 0.2 |
| Diastolic diameter (mm) | 3.4 ± 0.1 | 3.5 ± 0.1 | 3.8 ± 0.1 | 4.2 ± 0.1* | 3.7 ± 0.2 | 4.0 ± 0.1 |
| Systolic volume (μL) | 19.8 ± 2.1 | 24.1 ± 4.3 | 29.3 ± 3.7 | 60.1 ± 5.9* | 29.3 ± 4.9 | 43.5 ± 7.7 |
| Diastolic volume (μL) | 48.7 ± 4.7 | 53.4 ± 5.3 | 62.6 ± 4.4 | 80.4 ± 3.2* | 59.1 ± 6.4 | 69.3 ± 5.7 |
| Stroke volume (μL) | 28.8 ± 2.7 | 29.4 ± 1.4 | 33.4 ± 1.1 | 20.3 ± 3.5* | 29.8 ± 2.2 | 25.9 ± 2.7 |
| Ejection fraction (%) | 59.9 ± 1.7 | 56.9 ± 3.9 | 54.6 ± 3.0 | 25.7 ± 4.8* | 52.2 ± 3.8 | 38.8 ± 5.4 |
| Fractional shortening (%) | 31.2 ± 1.2 | 29.5 ± 2.5 | 28.0 ± 1.9 | 12.0 ± 2.4* | 26.6 ± 2.4 | 18.8 ± 2.9 |
| Cardiac output (mL/min) | 12.7 ± 1.3 | 13.0 ± 0.4 | 14.7 ± 0.6 | 7.3 ± 1.1* | 13.1 ± 0.5 | 9.8 ± 1.3* |
Echocardiography mouse hearts (Control n = 7, cRyr2KO n = 5; *P ≤ 0.05).
aWorking mouse hearts from the same timepoint as Echo #2 (Control n = 5, cRyr2KO n = 9; *P ≤ 0.05).
Figure 4.
Conditional Ryr2 knockout hearts display reduced cardiac function. Measurement of cardiac output (A), peak systolic pressure (B), rate pressure product (C), and hydraulic work (D) in the isolated working heart model (t = 30 min; Control n = 6, cRyr2KO n = 8; *P ≤ 0.05).
3.4. Loss of Ryr2 reduces heart rate and results in severe arrhythmias
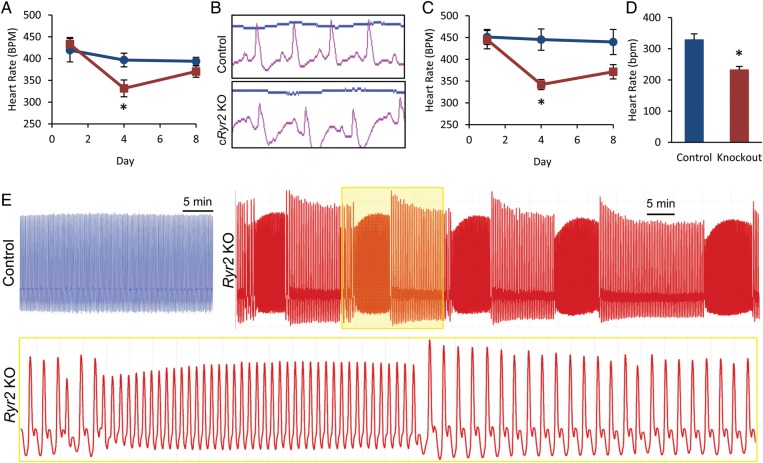
Echocardiography and four-lead ECG were used to assess heart rate in cRyr2KO animals. At times when RYR2 protein levels were ∼50% reduced, cRyr2KO mice exhibited a significantly lower rate of heart contraction by echocardiography relative to tamoxifen injected and non-injected littermate controls (Figure 5A). We also observed a concurrent decrease in heart rate, as defined by ECG, suggesting that this drop in heart rate is linked to a decrease in pacemaking at the organ level (Figure 5C). In addition, we observed altered ECG wave patterns in cRyr2KO mice indicating that this decrease in heart rate is genuine sinus bradycardia (Figure 5B). We also observed instances of mice exhibiting a secondary atrioventricular block. This indicates that RYR2 ablation is sufficient to decrease heart rate at both contractile and signalling levels.
Figure 5.
cRyr2 knockout hearts exhibit bradycardia and arrhythmias. (A) Average heart rate of contraction as measured by echocardiography. Measurements were taken at the minimal isoflurane dose necessary to maintain a light plane of anaesthesia. (Control n = 7, cRyr2KO n = 5; *P ≤ 0.05). (B) Four-lead ECG of control and cRyr2KO mice taken during echocardiogram. ECG leads were affixed to fore and hind paws. Representative plots (Control n = 7, cRyr2KO n = 5). (C) Quantification of heart rate by ECG during echocardiography experiments (Control n = 6, cRyr2KO n = 4; *P ≤ 0.05). (D) Average heart rate of isolated, perfused working hearts (Control n = 5, cRyr2KO n = 5; *P ≤ 0.05). (E) Examples of heart function traces from isolated, perfused working hearts. Trace depicts changes in pressure reflecting fluctuations in cardiac output over time. All cRyr2KO hearts exhibited dramatic periodic arrhythmias that were never observed in control hearts (Control n = 5, cRyr2KO n = 5).
cRyr2KO mice were also assessed with the working heart perfusion model to further characterize the link between heart function and heart rate. We consistently observed bradycardia in cRyr2KO hearts having ∼50% decrease in RYR2 when compared with tamoxifen-treated controls (Figure 5D). A similar decrease in heart rate was observed in all cRyr2KO hearts regardless of their relative cardiomyopathy; hearts in failure showed similar rate decreases to cRyr2KO hearts with relatively normal cardiac function. We observed striking episodes or tachycardic arrhythmia in isolated, working cRyr2KO hearts (Figure 5E). These repetitive arrhythmias were observed in the majority of cRyr2KO hearts throughout the perfusion, although they were more frequently observed early in the perfusion following the stress of isolation and cannulation. Arrhythmias were never observed in tamoxifen-treated control hearts. These working heart experiments suggest that Ryr2 deletion causes an organ-autonomous decrease in heart rate punctuated by brief periods of tachycardic arrhythmia, effects that are independent from systemic factors or neuroregulatory factors in the body.
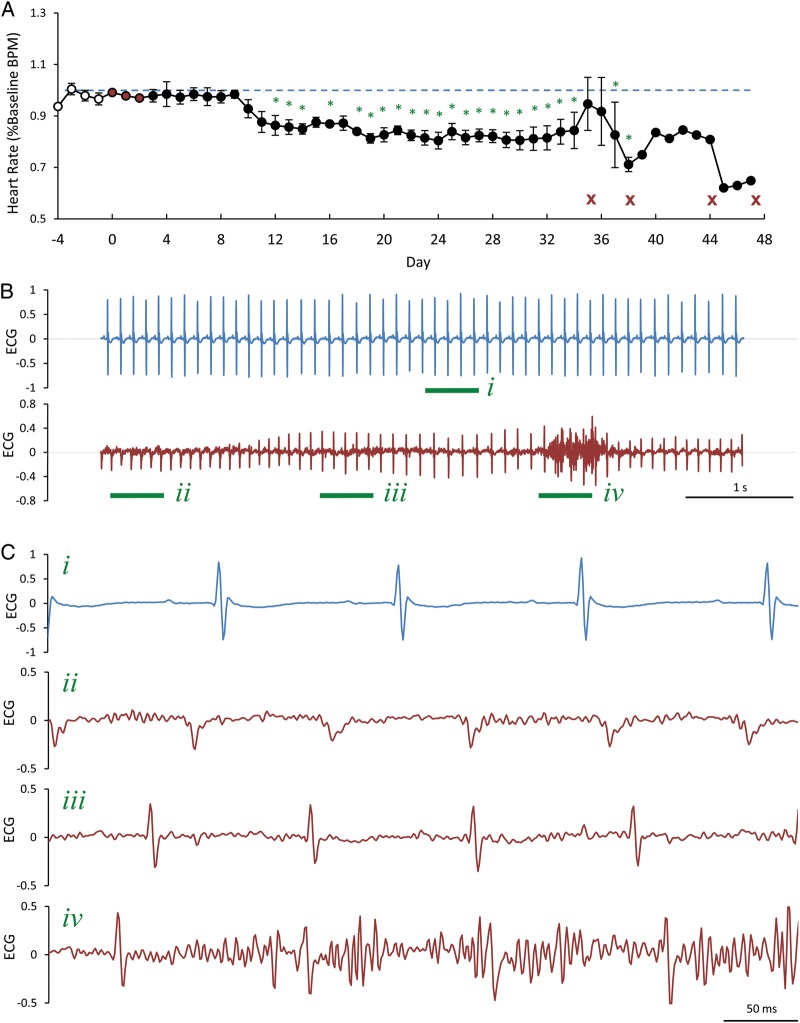
To account for the narrow time frame and to address caveats of echocardiography and working heart perfusion approaches, we studied in vivo heart rate and function in cRyr2KO mice using implantable ECG radio telemetry. Miniaturized two-lead ECG monitors were subcutaneously implanted permitting us to track animal heart rate and activity before and after the induction of gene deletion in cRyr2KO mice. This allowed us to monitor both ECG patterns and heart rate at a very high time resolution in freely moving, unanaesthetized animals continuously for extended periods of time. Indeed, we observed a ∼20% decrease in average daily heart rate following the induction of cardiac Ryr2 gene deletion when compared with the average daily heart rate of naive animals (Figure 6A). This decrease in heart rate was not accompanied by any detectable change in average daily activity and was not observed acutely following tamoxifen treatment, suggesting the decrease in heart rate was caused by RYR2 ablation due to gene deletion. During this experiment we also observed periods of repetitive tachycardic arrhythmias at times near humane endpoints (Figure 6B and C). We observed these arrhythmias following cage changes, a classic stressor of mice, well before humane endpoints and when mice looked healthy by visual inspection. ECG traces showed distinct T-waves and other abnormalities using two ECG leads placed on the left pectoral and right ribs, which is state-of-the-art for in vivo mouse telemetry. This study shows that in vivo Ryr2 deletion is sufficient to cause decreased heart rate and episodes of tachycardic arrhythmias in mice.
Figure 6.
cRyr2 knockout mice exhibit bradycardia and arrhythmias in vivo. (A) Average heart rate collected from freely moving, unanaesthetized cRyr2KO mice using implantable ECG telemetry (n = 4, initially). White points denote pre-knockout timepoints, red points denote the days of tamoxifen injections, and black points denote days following gene knockout. Red X's indicate days when mice reached humane endpoints. Heart rate data is expressed as a percentage of the average heart rate of pre-knockout days, which is depicted as a dashed blue line (*P ≤ 0.05 when compared with average naive heart rate). (B) Representative ECG traces from a single cRyr2KO knockout mouse prior to gene knockout (blue) and during a characteristic arrhythmic event (red) observed 20 days following gene knockout (n = 4). (C) Enlarged regions of ECG traces seen in (B). Green roman numerals and underlines designate enlarged regions.
4. Discussion
In this study, we used inducible, tissue-specific gene ablation to examine the in vivo functions of Ryr2 in the adult heart. Inducible Ryr2 deletion circumvented chronic gene compensation and the embryonic lethality of the global Ryr2 knockout mice. This is the first reported tissue-specific deletion of any RYR isoform in any tissue. This model permitted the first in vivo and ex vivo analysis of specific cardiac Ryr2 loss-of-function in mice. Our in vivo data shed light on controversial roles for this gene in heart rate and rhythmicity, while confirming expected cardiac dysfunction following Ryr2 deletion. Specifically, we found that Ryr2 is required for setting normal heart rate, and that deletion of this gene causes general bradycardia. We further found that Ryr2 is required to maintain normal heart rhythm, and that Ryr2 deletion causes intermittent tachycardic arrhythmias. These results indicate that Ryr2 has a non-redundant role in regulating heart rate. We also found that a reduction in RYR2 protein is sufficient to induce aspects of heart failure in mice. These effects were observed following Ryr2 deletion and in the absence of other stressors. Although tamoxifen, the drug used to initiate Mer-Cre-Mer mediated gene deletion, is known to have mild cardiac effects, our use of tamoxifen-injected control mice excludes the possibility that tamoxifen plays any significant role in the observed phenomena.
Our data support the hypothesis that RYR2 has a non-redundant role in controlling heart rate as we observed a consistent decrease in global heart rate following induction of gene deletion both in vivo and ex vivo. Our results are consistent with previous in vitro work. For example, ryanodine, a pharmacological modulator of RYR2 opening probability, is sufficient to alter the contraction rate of atrial tissue cultures.21 Similarly, model cardiac cells derived from mouse embryonic stem cells lacking Ryr2 show a reduced rate of spontaneous contraction.18 Our results provide dramatic evidence that RYR2 does indeed play this role on an organ level in adult cardiac tissues, in vivo and ex vivo. As such, our results support the ‘calcium clock’ hypothesis, which predicts that the periodicity of Ca2+ transients at the level of the SR/ER, a process governed in part by RYR2, is critical to the electrochemical signalling underlying heart rate.20 It is worth noting that our results do not exclude parallel or sequential involvement of other cardiac ion channels (e.g. HCN) in the regulation of heart rate.
Our data suggest that in vivo deletion of Ryr2 may have altered the rate at which pacemaking cells signalled the initiation of heart contraction or in the manner cardiac action potentials are conducted. However, we cannot yet conclusively say whether the effects are dependent on nodal cells, conduction tissues, or primarily atrial and ventricular cardiomyocytes, or some combination. The model system that we employed relies on the α-MHC promoter to drive Cre recombinase expression and gene deletion. Cre is abundantly expressed in both atrial and ventricular cells in young adult mouse hearts. Unfortunately, it is not technically feasible for us to quantitative assess RYR2 protein levels specifically in isolated sinoatrial nodes of cRyr2KO mice and littermate controls by immunoblot. However, a recent study using the same C57Bl6-mhy6-mer-Cre-mer transgenic mouse strain to drive gene knockout broadly in the heart confirmed robust cre-lox recombination in the sinoatrial node.19 Given that we used the identical Cre-deleter mouse model, it is reasonable to expect that a similar degree of Ryr2 gene deletion occurred in the sinoatrial nodes of our mice.
Our results reveal that ‘decreased’ RYR2 activity can cause tachycardic arrhythmias. RYR2 has a well-documented role in the genesis of arrhythmias as mutations in the human RYR2 gene underlie tachycardic arrythmogenic conditions such as CPVT and ARVD.33 The vast majority of mutations studied are predicted to increase RYR2 open probabilities by increasing the channels sensitivity to cytosolic or SR luminal Ca2+ levels.8,33,34 Thus, these Ryr2 mutants are considered ‘leaky’ as they produce prolonged or inappropriate calcium transients.9,14,16,35 The prevailing model for how Ryr2 mutations cause CPVT suggests that aberrant, increased RYR2 activity during diastole drives NCX currents in reverse (Na+ influx, Ca2+ efflux) potentiating delayed after-depolarizations which can initiate rapid, arrhythmic heart contractions in a feed-forward manner.36 However, this model cannot explain our observation of tachycardic arrhythmias following Ryr2 deletion and reduced RYR2 activity. Therefore, an alternate mechanism must underlie observed ventricular fibrillation in cRyr2KO mice following gene deletion.
Previous studies conducted on L433P and A4860G Ryr2 mutants, known to cause either catecholaminergic idiopathic ventricular fibrillation or sudden cardiac death respectively, found decreased RYR2 open probability and insensitivity to SR luminal Ca2+ levels.16,17 The authors of those papers suggested that these mutations may potentiate arrhythmias via an alternans-dependent mechanism.16,17 Alternans, in this case, refer to alternating strong and weak cellular calcium transients which are associated with heart failure and ventricular fibrillation.37–39 Although a variety of stimuli can cause cellular alternans: such as metabolic alterations, acidosis, and certain electrophysiological stimulations, it is thought that virtually all alternans are caused by alterations in RYR2 behaviour.37–39 One study used tetracaine, an inhibitor of RYR2 action, to induce alternans by decreasing RYR2 activity which functionally uncoupled groups of RYR2 from one another leading to heterogeneous subcellular Ca2+ waves that alternated spatially and temporally.40 It is possible that this phenomenon may lead to prolonged or inappropriate calcium transients, which could potentially lead to delayed after-depolarizations and arrhythmias. While our research does not describe the precise mechanism by which Ryr2 deletion causes arrhythmias, our results are consistent with the model of alternans-associated ventricular fibrillation caused by decreased RYR2 activity.16,17 Overall, our research in combination with other work suggests that appropriate RYR2 function, that is neither elevated nor decreased, is essential for preventing arrhythmias.
It is thought that up to 50% of heart failure associated deaths are caused, in an acute sense, by arrhythmias.41 It is also thought that these arrhythmias are caused by cardiac alternans,37 which are frequently observed during heart failure,42,43 and are known to be a pathology of RYR2 dysfunction.39,40 In addition, decreased RYR2 levels or reduced RYR2 activity have been observed in several models of heart failure, whether induced by pressure overload,25,26 chronic hypertension,23 or diabetes.24 The degree of RYR2 dysfunction correlates with the degree of cardiac dysfunction.44 Whether such decreases in RYR2 function are sufficient to cause heart failure, or are merely consequences of pathological dysfunction remains a somewhat controversial idea because RYR2 loss is not observed in all studies. However, our results provide compelling evidence that decreased RYR2 function can contribute to heart failure in vivo and that Ryr2 deletion alone is sufficient to recapitulate functional characteristics of heart failure: reduced contractility, decreased cardiac output, and ventricular fibrillation. Our results, along with other work,16,17,40 also suggest that the decreased RYR2 function observed in heart failure may be responsible for ventricular fibrillation caused by subcellular Ca2+ alternans. Collectively, our research suggests that RYR2 may be a key player in cardiac dysfunction during heart failure.
Funding
This work was supported by individual Canadian Institutes for Health Research (CIHR) operating grants to J.D.J. and E.M. The generation of the floxed Ryr2 alleles (K.R.B.) was supported by the Intramural Research Program of the NIH, National Institute on Aging. J.D.J. was a CIHR New Investigator and also received salary support from the Canadian Diabetes Association (CDA) and the Michael Smith Foundation for Health Research (MSFHR).
Acknowledgements
We thank Dr A. Lai for reagents and Anca Toma for colony maintenance. We thank Dr Tatyana B. Kalynyak and Farnaz Taghizadeh for technical assistance. We also thank Jessica Fox and other members of the Kieffer laboratory for advice on surgical implantation of telemetry devices.
Conflict of interest: none declared.
References
- 1.Gomez AM, Richard S. Mutant cardiac ryanodine receptors and ventricular arrhythmias: is ‘gain-of-function’ obligatory? Cardiovasc Res. 2004;64:3–5. doi: 10.1016/j.cardiores.2004.07.018. doi:10.1016/j.cardiores.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. doi:10.1161/01.CIR.103.2.196. [DOI] [PubMed] [Google Scholar]
- 3.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. doi:10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, Tateishi H, et al. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res. 2010;106:1413–1424. doi: 10.1161/CIRCRESAHA.109.209312. doi:10.1161/CIRCRESAHA.109.209312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, Villani L, et al. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96:e77–e82. doi: 10.1161/01.RES.0000169067.51055.72. doi:10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 6.Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–2245. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang D, Jones PP, Davis DR, Gow R, Green MS, Birnie DH, et al. Characterization of a novel mutation in the cardiac ryanodine receptor that results in catecholaminergic polymorphic ventricular tachycardia. Channels. 2010;4:302–310. doi: 10.4161/chan.4.4.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, et al. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. doi:10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 9.George CH, Higgs GV, Lai FA. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ Res. 2003;93:531–540. doi: 10.1161/01.RES.0000091335.07574.86. doi:10.1161/01.RES.0000091335.07574.86. [DOI] [PubMed] [Google Scholar]
- 10.Kushnir A, Shan J, Betzenhauser MJ, Reiken S, Marks AR. Role of CaMKIIdelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proc Natl Acad Sci USA. 2010;107:10274–10279. doi: 10.1073/pnas.1005843107. doi:10.1073/pnas.1005843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Oort RJ, Respress JL, Li N, Reynolds C, De Almeida AC, Skapura DG, et al. Accelerated development of pressure overload-induced cardiac hypertrophy and dysfunction in an RyR2-R176Q knockin mouse model. Hypertension. 2010;55:932–938. doi: 10.1161/HYPERTENSIONAHA.109.146449. doi:10.1161/HYPERTENSIONAHA.109.146449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannankeril PJ, Mitchell BM, Goonasekera SA, Chelu MG, Zhang W, Sood S, et al. Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc Natl Acad Sci USA. 2006;103:12179–12184. doi: 10.1073/pnas.0600268103. doi:10.1073/pnas.0600268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, et al. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. doi:10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- 15.Lakatta EG, DiFrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol. 2009;47:157–170. doi: 10.1016/j.yjmcc.2009.03.022. doi:10.1016/j.yjmcc.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas NL, George CH, Lai FA. Functional heterogeneity of ryanodine receptor mutations associated with sudden cardiac death. Cardiovasc Res. 2004;64:52–60. doi: 10.1016/j.cardiores.2004.06.009. doi:10.1016/j.cardiores.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Jiang D, Chen W, Wang R, Zhang L, Chen SR. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc Natl Acad Sci USA. 2007;104:18309–18314. doi: 10.1073/pnas.0706573104. doi:10.1073/pnas.0706573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang HT, Tweedie D, Wang S, Guia A, Vinogradova T, Bogdanov K, et al. The ryanodine receptor modulates the spontaneous beating rate of cardiomyocytes during development. Proc Natl Acad Sci USA. 2002;99:9225–9230. doi: 10.1073/pnas.142651999. doi:10.1073/pnas.142651999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baruscotti M, Bucchi A, Viscomi C, Mandelli G, Consalez G, Gnecchi-Rusconi T, et al. Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene Hcn4. Proc Natl Acad Sci USA. 2011;108:1705–1710. doi: 10.1073/pnas.1010122108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res. 2010;106:659–673. doi: 10.1161/CIRCRESAHA.109.206078. doi:10.1161/CIRCRESAHA.109.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honjo H, Boyett MR, Niwa R, Inada S, Yamamoto M, Mitsui K, et al. Pacing-induced spontaneous activity in myocardial sleeves of pulmonary veins after treatment with ryanodine. Circulation. 2003;107:1937–1943. doi: 10.1161/01.CIR.0000062645.38670.BD. doi:10.1161/01.CIR.0000062645.38670.BD. [DOI] [PubMed] [Google Scholar]
- 22.Honjo H, Inada S, Lancaster MK, Yamamoto M, Niwa R, Jones SA, et al. Sarcoplasmic reticulum Ca2+ release is not a dominating factor in sinoatrial node pacemaker activity. Circ Res. 2003;92:e41–e44. doi: 10.1161/01.res.0000055904.21974.be. doi:10.1161/01.RES.0000055904.21974.BE. [DOI] [PubMed] [Google Scholar]
- 23.Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, et al. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. doi:10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 24.Bidasee KR, Dincer UD, Besch HR., Jr Ryanodine receptor dysfunction in hearts of streptozotocin-induced diabetic rats. Mol Pharmacol. 2001;60:1356–1364. doi: 10.1124/mol.60.6.1356. [DOI] [PubMed] [Google Scholar]
- 25.Naudin V, Oliviero P, Rannou F, Sainte Beuve C, Charlemagne D. The density of ryanodine receptors decreases with pressure overload-induced rat cardiac hypertrophy. FEBS Lett. 1991;285:135–138. doi: 10.1016/0014-5793(91)80743-m. doi:10.1016/0014-5793(91)80743-M. [DOI] [PubMed] [Google Scholar]
- 26.Matsui H, MacLennan DH, Alpert NR, Periasamy M. Sarcoplasmic reticulum gene expression in pressure overload-induced cardiac hypertrophy in rabbit. Am J Physiol. 1995;268:C252–C258. doi: 10.1152/ajpcell.1995.268.1.C252. [DOI] [PubMed] [Google Scholar]
- 27.Assayag P, CHarlemagne D, Marty I, de Leiris J, Lompre AM, Boucher F, et al. Effects of sustained low-flow ischemia on myocardial function and calcium-regulating proteins in adult and senescent rat hearts. Cardiovasc Res. 1998;38:169–180. doi: 10.1016/s0008-6363(97)00283-6. doi:10.1016/S0008-6363(97)00283-6. [DOI] [PubMed] [Google Scholar]
- 28.Tellez J, Maczewski M, Yanni J, Sutyagin P, Mackiewicz U, Atkinson A, et al. Ageing-dependent remodelling of ion channel and Ca2+ clock genes underlyingsinoatrial node pacemaking. Exp Physiol. 2011;96:1163–1178. doi: 10.1113/expphysiol.2011.057752. [DOI] [PubMed] [Google Scholar]
- 29.Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. doi:10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou Y, Liang Y, Gong H, Zhou N, Ma H, Guan A, et al. Ryanodine receptor Type 2 is required for the development of pressure overload-induced cardiac hypertrophy. Hypertension. 2011;58:1099–1110. doi: 10.1161/HYPERTENSIONAHA.111.173500. [DOI] [PubMed] [Google Scholar]
- 31.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. doi:10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 32.Wambolt R, Parsons H, Saeedi R, Allard M. Metabolic and functional phenotype of pathologic cardiac hypertrophy in rodents. FASEB J. 2006;20:A739. [Google Scholar]
- 33.George CH, Jundi H, Thomas NL, Fry DL, Lai FA. Ryanodine receptors and ventricular arrhythmias: emerging trends in mutations, mechanisms and therapies. J Mol Cell Cardiol. 2007;42:34–50. doi: 10.1016/j.yjmcc.2006.08.115. doi:10.1016/j.yjmcc.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 34.Tung CC, Lobo PA, Kimlicka L, Van Petegem F. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature. 2010;468:585–588. doi: 10.1038/nature09471. [DOI] [PubMed] [Google Scholar]
- 35.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci USA. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. doi:10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. doi:10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Surawicz B, Fisch C. Cardiac alternans: diverse mechanisms and clinical manifestations. J Am Coll Cardiol. 1992;20:483–499. doi: 10.1016/0735-1097(92)90122-4. doi:10.1016/0735-1097(92)90122-4. [DOI] [PubMed] [Google Scholar]
- 38.Huser J, Wang YG, Sheehan KA, Cifuentes F, Lipsius SL, Blatter LA. Functional coupling between glycolysis and excitation-contraction coupling underlies alternans in cat heart cells. J Physiol. 2000;524(Pt 3):795–806. doi: 10.1111/j.1469-7793.2000.00795.x. doi:10.1111/j.1469-7793.2000.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picht E, DeSantiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res. 2006;99:740–748. doi: 10.1161/01.RES.0000244002.88813.91. doi:10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- 40.Diaz ME, Eisner DA, O'Neill SC. Depressed ryanodine receptor activity increases variability and duration of the systolic Ca2+ transient in rat ventricular myocytes. Circ Res. 2002;91:585–593. doi: 10.1161/01.res.0000035527.53514.c2. doi:10.1161/01.RES.0000035527.53514.C2. [DOI] [PubMed] [Google Scholar]
- 41.Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the Framingham Study. Am Heart J. 1988;115:869–875. doi: 10.1016/0002-8703(88)90891-5. doi:10.1016/0002-8703(88)90891-5. [DOI] [PubMed] [Google Scholar]
- 42.Kodama M, Kato K, Hirono S, Okura Y, Hanawa H, Ito M, et al. Mechanical alternans in patients with chronic heart failure. J Card Fail. 2001;7:138–145. doi: 10.1054/jcaf.2001.24122. doi:10.1054/jcaf.2001.24122. [DOI] [PubMed] [Google Scholar]
- 43.Euler DE. Cardiac alternans: mechanisms and pathophysiological significance. Cardiovasc Res. 1999;42:583–590. doi: 10.1016/s0008-6363(99)00011-5. doi:10.1016/S0008-6363(99)00011-5. [DOI] [PubMed] [Google Scholar]
- 44.Belevych AE, Terentyev D, Terentyeva R, Nishijima Y, Sridhar A, Hamlin RL, et al. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc Res. 2011;90:493–502. doi: 10.1093/cvr/cvr025. doi:10.1093/cvr/cvr025. [DOI] [PMC free article] [PubMed] [Google Scholar]