Abstract
Aims
Existing evidence suggests that amyloid-β precursor protein (APP) causes endothelial dysfunction and contributes to pathogenesis of atherosclerosis. In the present study, experiments were designed to: (1) determine the mechanisms underlying endothelial dysfunction and (2) define the effects of peroxisome proliferator-activated receptor delta (PPARδ) ligand on endothelial function in transgenic Tg2576 mice overexpressing mutated human APP.
Methods and results
Confocal microscopy and western blot analyses of wild-type mice aortas provided evidence that APP protein is mainly present in endothelial cells. Overexpression of APP significantly impaired endothelium-dependent relaxations to acetylcholine and phosphorylation of endothelial nitric oxide synthase at Ser1177 in aortas. HPLC analysis revealed that tetrahydrobiopterin (BH4) levels were reduced in Tg2576 mice aortas. This was caused by increased oxidation of BH4 and reduced expression and activity of GTP-cyclohydrolase I. Furthermore, gp91phox protein expression and superoxide anion production were increased in aortas of Tg2576 mice. This augmented superoxide formation was completely prevented by the NADPH oxidase inhibitor VAS2870. Expression of copper-/zinc-superoxide dismutase (Cu/ZnSOD) and extracellular SOD was downregulated. Treatment with PPARδ ligand GW501516 (2 mg/kg/day) for 14 days significantly increased BH4 bioavailability and improved endothelium-dependent relaxations in Tg2576 mice aortas. GW501516 also normalized protein expression of gp91phox and SODs, thereby reducing production of superoxide anion in the aortas.
Conclusion
Our results suggest that in APP transgenic mice loss of nitric oxide and increased oxidative stress are the major causes of endothelial dysfunction. The vascular protective effects of GW501516 in Tg2576 mice appear to be critically dependent on prevention of superoxide anion production.
Keywords: Amyloid-β precursor protein, Endothelial function, Superoxide anion, Tetrahydrobiopterin, Atherosclerosis
1. Introduction
The amyloid-β precursor protein (APP) is an integral membrane protein expressed abundantly in the brain. Consecutive proteolysis of APP by β- and γ-secretase generates amyloid-β (Aβ) peptides, which are major culprits in pathogenesis of Alzheimer's disease.1 Recent evidence suggests that APP is also present in vascular cells including endothelium and that it may play a role in pathogenesis of atherosclerosis.2,3 Indeed, several studies have demonstrated that APP and Aβ are present in advanced human carotid plaques and in human and apoE-deficient mice atherosclerotic aortas.4,5 The development of non-dietary induced early atherosclerotic lesions has been observed in transgenic Tg2576 mice overexpressing double Swedish mutated human APP (K670N/M671L).6 Moreover, APP overexpression accelerated aortic atherosclerotic development in apolipoprotein E-deficient mice.7 Conversely, genetic deletion of APP attenuated atherogenesis in apolipoprotein E-deficient mice.8
Nitric oxide (NO) plays a key role in the control of the cardiovascular system.9 In the blood vessel wall, NO is mainly formed in endothelial cells from l-arginine by enzymatic activity of endothelial NO synthase (eNOS).10,11 It is well established that tetrahydrobiopterin (BH4) is an essential cofactor for allosteric and redox activation of eNOS enzymatic activity.12 Exposure of vascular endothelial cells to exogenous Aβ peptide results in cell damage and toxicity via oxidative injury.13,14 Likewise, previous studies showed that overexpression of APP is toxic to bovine aortic endothelial cells but not to bovine aortic smooth muscle cells, suggesting that endothelial cells are specifically susceptible to intracellular overexpression of APP and subsequent free radical generation.15 Furthermore, endothelial dysfunction is present in conduit arteries of Tg2576 mice.16 However, the exact mechanisms by which APP causes endothelial dysfunction in APP transgenic Tg2576 mice have not been defined. The central hypothesis of the present study is that overexpression of APP impairs metabolism of BH4 and antioxidant capacity of the arterial wall, thereby causing endothelial dysfunction.
Peroxisome proliferator-activated receptor-delta (PPARδ), together with the other two isoforms, PPARα and PPARγ, constitute the PPAR subfamily of the nuclear receptor superfamily.17 While there are extensive studies on the vascular protective effects of PPARα and PPARγ, i.e. activation of PPARα or PPARγ increases eNOS expression and NO release in endothelial cells,18,19 there are only a few studies with regard to the vascular function of PPARδ. Animal studies have demonstrated that PPARδ activation exerts many favourable effects, including reduction of weight gain, increase in skeletal muscle metabolic rate, and improvement of insulin sensitivity.20 In addition, PPARδ is also expressed in endothelial cells although its function remains poorly understood.21 Recently, treatment with PPARδ agonist was shown to suppress vascular inflammation and to prevent development of atherosclerosis in apoE-deficient mice.22 Because of these effects of PPARδ on vascular homeostasis, we also hypothesized that PPARδ activation might protect endothelial function in APP transgenic Tg2576 mice.
2. Methods
2.1. Experimental animals
Transgenic Tg2576 mice overexpressing double Swedish mutated human APP (K670N/M671L) and non-transgenic littermates were provided by Dr Steven Younkin (Mayo Clinic, Jacksonville, FL, USA).23 Mice were maintained on standard chow with free access to drinking water. Housing facilities and all experimental protocols were approved by the Institutional Animal Care and Use Committee of the Mayo Clinic and complied with the National Institute of Health Guide for the Care and Use of Laboratory Animals. At 16–20 weeks of age, female T2576 transgenic and wild-type littermates were treated daily by gavages for 14 days with either vehicle (DMSO 2%) or PPARδ ligand GW501516 (2 mg/kg of BW, Alexis Biochemical) suspended in 5% carboxymethylcellulose. The dose was selected on the basis of optimal serum GW501516 levels demonstrating selectivity for PPARδ without any cross-reactivity with other PPAR isoforms in mice.22,24,25 After treatments, mice were anaesthetized with pentobarbital (200–300 mg/kg BW, i.p.) followed by exsanguination via cardiac puncture for blood collection. The adequacy of anaesthesia was monitored by lack of twitch after foot pinch. Aortas were carefully removed and dissected free from connective tissue in cold (4°C) modified Krebs–Ringer solution.26
2.2. Glucose and plasma lipid profile
Blood samples were transferred to a tube containing EDTA. Glucose levels were measured in whole blood with Accu Check® (Roche Diagnostics, Indianapolis, IN, USA). After centrifugation at 600 g (4°C, 10 min) supernatants were stored at −80°C until assayed. Plasma cholesterol and HDL levels were measured with a Hitachi 912 chemistry analyzer using Cholesterol CHOD-PAP and direct HDL-C plus reagents, respectively (Roche Diagnostics, Indianapolis, IN, USA).
2.3. Plasma levels of Aβ peptides
ELISA kits (Invitrogen) were used to perform the measurements of plasma Aβ-40 and Aβ-42 levels.
2.4. Protein expression
Western blot analyses were performed in aortas as described.26 Primary antibodies against APP (Invitrogen), ADAM 10, nicastrin (Millipore), BACE (Cell Signaling), Cu/ZnSOD, MnSOD, EcSOD (Enzo Live Sciences), catalase (Sigma), glutathione peroxidase I (GPx; AbFrontier), eNOS, Ser1177-phosphorylated eNOS, nNOS, iNOS, cyclooxygenase-2 (COX-2), gp91phox (BD Biosciences), prostacyclin synthase (PGIS), cyclooxygenase-1 (COX-1; Cayman), and GTPCH I were used. As a loading control, blots were rehybridized with β-actin (Sigma).
2.5. Immunofluorescent imaging
Aortic rings were fixed in a 4% paraformaldehyde solution and embedded in paraffin blocks. Cross-sections of 5 µm were permeabilized using 0.1% Triton X-100 in 10% normal goat serum. Sections were incubated with rabbit anti-APP and anti-VE-Cadherin antibodies overnight at 4°C. Sections were incubated with Cy5-conjugated anti-rabbit secondary antibody and Alexa-Fluor 488 conjugated anti-mouse secondary antibody (Jackson Immuno Research) for 2 h. Sections were visualized using a Zeiss LSM 510 laser scanning confocal microscope.
2.6. Vasomotor reactivity studies
Isometric force of aortic rings was studied in organ bath as described.9 The data were acquired using PowerLab data acquisition system and LabVIEW 7.1 software. Concentration-dependent response curves to acetylcholine (10−9–10−5mol/L) and diethylammonium (Z)-1-(N,N-diethylamino)diazen-1-ium-1,2-diolate (DEA-NONOate; 10−10–10−5mol/L) were cumulatively obtained during submaximal contractions to phenylephrine. Concentrations of phenylephrine (1–3 × 10−7mol/L) were selected in order to obtain the same submaximal contraction in aortic rings from both wild-type and Tg2576 mice.
2.7. Biopterin metabolism
Biopterin levels and GTPCH I activity were measured in aortas by HPLC.26
2.8. Detection of superoxide anion
An HPLC method was used to analyze intracellular superoxide levels in intact aortas in the absence or presence of polyethylene-glycolated superoxide dismutase (PEG-SOD, 10 U/mg) for 30 min, NADPH oxidase inhibitor VAS2870 (10 μm, Enzo Live Sciences) for 30 min, or NOS inhibitor Nω-nitro-l-arginine methyl ester (L-NAME, 30 µm) for 15 min.26
2.9. Calculations and statistical analysis
All results are expressed as means ± SEM and ‘n’ indicates the number of animals from which tissues were harvested. Relaxations (expressed as percentage of contraction) were determined for each individual concentration–response curve by non-linear regression analysis. The concentration–response curves of the different groups were compared by ANOVA for repeated measurements followed by Bonferroni's correction. Wild-type and Tg2576 mice treated with or without GW501516 were compared by ANOVA with Bonferroni's. For simple comparisons, an unpaired Student's t-test was used. A value of P < 0.05 was considered significant.
3. Results
3.1. Characteristics of Tg2576 mice
Plasma levels of HDL were significantly reduced in Tg2576 mice (P < 0.05; Table 1). In contrast, plasma levels of cholesterol, blood glucose, and body weight were not different between wild-type and Tg2575 mice (Table 1). Two weeks treatment with PPARδ ligand GW501516 normalized HDL levels in Tg2576 (Table 1) while it had no effect on other parameters in wild-type and Tg2575 mice (Table 1).
Table 1.
Characteristics of wild-type and Tg2576 mice
| Parameters | Wild-type | Wild-type + GW501516 | Tg2576 | Tg2576 + GW501516 |
|---|---|---|---|---|
| Body weight (g) | 26 ± 2 | 27 ± 1 | 25 ± 1 | 27 ± 1 |
| Glucose (mg/mL) | 138 ± 10 | 154 ± 19 | 122 ± 8 | 134 ± 11 |
| Cholesterol (mg/dL) | 54 ± 4 | 52 ± 3 | 48 ± 2 | 57 ± 3 |
| HDL (mg/dL) | 38 ± 3 | 40 ± 2 | 31 ± 1* | 40 ± 2** |
Data are means ± SEM (n = 5–10).
*P < 0.05 vs. wild-type mice.
**P < 0.05 vs. transgenic T2576 mice without GW501516 (ANOVA with Bonferroni's).
3.2. Expression of APP
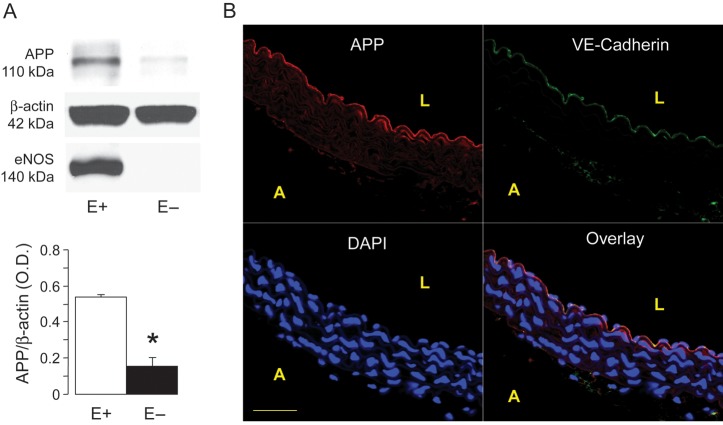
Western blot analyses showed that APP protein expression is present in normal wild-type mice aortas (Figure 1A). In order to evaluate the cellular localization of APP protein, we determined APP levels in mouse aortas with and without endothelium. We found that about 72% of total APP protein is present in endothelial cells of wild-type mice (Figure 1A). Confocal microscopic analysis of mouse aorta slices demonstrated that APP staining was mainly localized to endothelial cells (Figure 1B).
Figure 1.
(A) Effect of endothelial removal on APP expression in the aortas of wild-type mice. Please note that removal of the endothelium (E−) significantly reduced APP protein expression. eNOS expression is shown to control the experiments. Results are mean ± SEM (n = 4). *P < 0.05 vs. aortas with endothelium (E+). (B) Representative confocal images showing staining of thoracic aorta of wild-type mouse aorta with anti-APP (red) and anti-VE-cadherin (green) demonstrates colocalization of the two proteins mainly in vascular endothelium (yellow). 4′,6′-diamiddino-2-phenylindole dilactate (DAPI) was used to visualize nuclei. Data are representative of at least three independent experiments. L, lumen; A, adventitia. Bar denotes 50 µm.
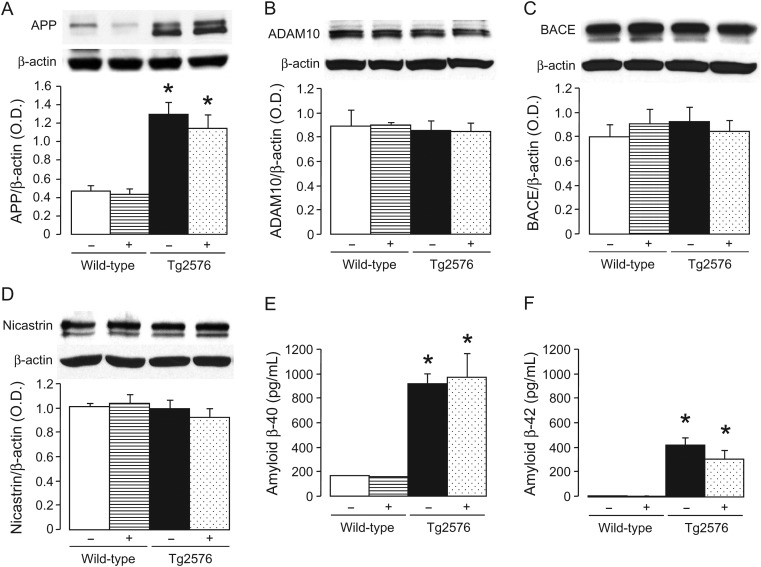
In Tg2576 mice, protein expression of APP was elevated in the aorta (P < 0.05; Figure 2A). Treatment with GW501516 did not affect APP expression in wild-type and Tg2576 mice aortas (Figure 2A). Protein expression of non-amyloidogenic and amyloidogenic enzymes α-secretase ADAM10 as well as β-secretase BACE and γ-secretase nicastrin were not different between wild-type and Tg2576 mice and those treated without or with GW501516 (Figure 2B–D).
Figure 2.
Protein expression of APP (A), α-secretase ADAM10 (B), β-secretase BACE (C), and γ-secretase nacistrin (D) in aortas of wild-type and Tg2576 mice treated without (−) or with (+) GW501516. The bar graphs indicated the results of the relative densitometry compared with β-actin protein. Plasma levels of Aβ-40 and Aβ-42 are shown in (E) and (F), respectively. Results are mean ± SEM (n = 6). *P < 0.05 vs. wild-type mice (ANOVA with Bonferroni's).
3.3. Plasma levels of Aβ peptides
Transgenic Tg2576 mice exhibit elevated circulating levels of Aβ-40 and Aβ-42, which were unaffected by PPAPδ ligand GW501516 (Figure 2E and F, respectively).
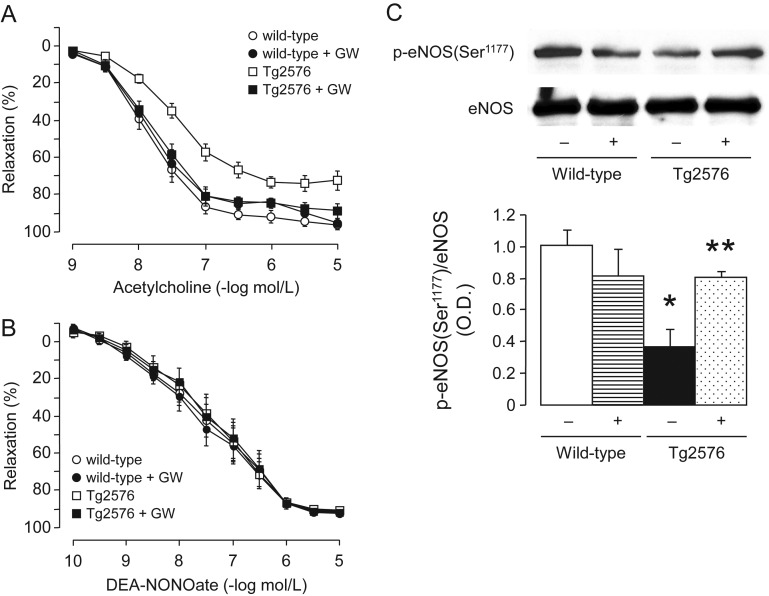
3.4. Endothelial function
Endothelium-dependent relaxations to acetylcholine were significantly diminished in the aorta of Tg2576 mice when compared with wild-type littermates (Figure 3A). This impairment of endothelium-dependent relaxation was completely prevented by treatment with PPAPδ ligand GW501516 (Figure 3A). In contrast, endothelium-independent relaxations to NO-donor DEA-NONOate were unchanged in wild-type and Tg2576 mice treated without or with GW501516 (Figure 3B).
Figure 3.
(A) Endothelium-dependent relaxations to acetylcholine and (B) endothelium-independent relaxations to NO-donor DEA-NONOate in aortas of wild-type littermates and Tg2576 mice. All data are shown as mean ± SEM (n = 6–7) and expressed as percent relaxation of the sub-maximal contraction to phenylephrine (1–3 × 10−7 mol/L). (C) Representative western blot analyses of phosphorylation of eNOS at Ser1177 in aortas of wild-type and Tg2576 mice without (−) or with (+) GW501516 treatment. Bar graph indicates the relative densitometry compared with total eNOS proteins. Results are mean ± SEM (n = 5–6). *P < 0.05 vs. wild-type mice; **P < 0.05 vs. transgenic T2576 mice without GW501516 (ANOVA with Bonferroni's).
3.5. Protein expression of enzymes involved in the production of prostanoids
Protein expression of COX-1, COX-2, and PGIS was unaltered in aortas of Tg2576 mice when compared with wild-type mice and those treated with GW501516 (see Supplementary material online, Figure S1).
3.6. Phosphorylation of eNOS
Western blot analysis showed a significant decrease in protein expression of phosphorylated eNOS at Ser1177 in the aorta of Tg2576 mice when compared with wild-type mice (Figure 3C). Treatment with PPARδ agonist normalized eNOS phosphorylation in Tg2576 (Figure 3C). In contrast, total eNOS proteins did not differ between wild-type littermates and Tg2576 mice and those treated with or without GW501516 (Figure 3C). Protein expression of nNOS was present in mice aortas (see Supplementary material online, Figure S2). However, this was not significantly altered in Tg2576 mice or by GW501516 treatment (see Supplementary material online, Figure S2). Protein expression of iNOS was almost undetectable in all groups of mice (see Supplementary material online, Figure S2).
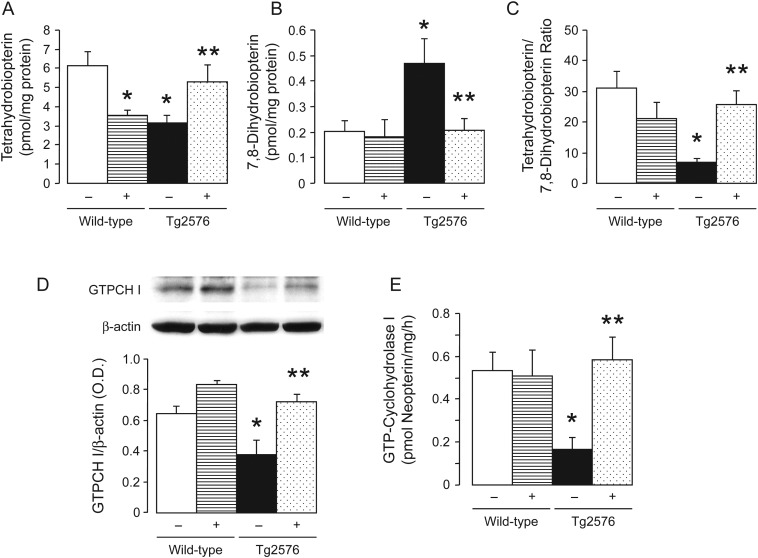
3.7. BH4 metabolism
Measurements of biopterins indicated that BH4 levels were significantly reduced in the aortas of Tg2576 mice (Figure 4A) while oxidative products of BH4, 7,8-dihydrobiopterin (7,8-BH2), were increased in Tg2576 mice (Figure 4B). Consequently, the BH4 to 7,8-BH2 ratio was significantly reduced in Tg2576 mice (Figure 4C). Two weeks treatment with the PPARδ ligand normalized levels of BH4 and 7,8-BH2, and BH4 to 7,8-BH2 ratio in Tg2576 mice (Figure 4A–C). However, treatment with GW501516 significantly decreased BH4 levels in the aorta of non-transgenic mice (Figure 4A).
Figure 4.
Tetrahydrobiopterin (BH4) metabolism and GTP-cyclohydrolase I (GTPCH I) in aortas of wild-type and Tg2576 mice without (−) or with (+) GW501516 treatment. Bar graphs showing levels of BH4 (A), levels of 7,8-dihydrobiopterin (7,8-BH2) (B), and BH4 to 7,8-BH2 ratio (C). Representative western blot analysis showed GTPCH I protein expression of aortas, and bar graph indicated the results of the relative densitometry when compared with β-actin protein (D). Quantitative analysis of enzymatic activity of GTPCH I (E). Data are means ± SEM (6–10). *P < 0.05 vs. wild-type mice; **P < 0.05 vs. transgenic T2576 mice without GW501516 treatment (ANOVA with Bonferroni's).
In order to evaluate further the mechanisms of decreased BH4 levels in Tg2576 mice, the vascular GTPCH I, the rate limiting enzyme for de novo biosynthesis of BH4, was determined. Interestingly, GTPCH I expression and activity were significantly reduced in Tg2576 mice aortas when compared with wild-type littermates (Figure 4D and E). GW501516 treatment normalized expression and activity of GTPCH I in Tg2576 aortas (Figure 4D and E). In addition, GW501516 treatment tended to increase GTPCH I protein expression in wild-type littermates (Figure 4D).
3.8. Production of superoxide anion
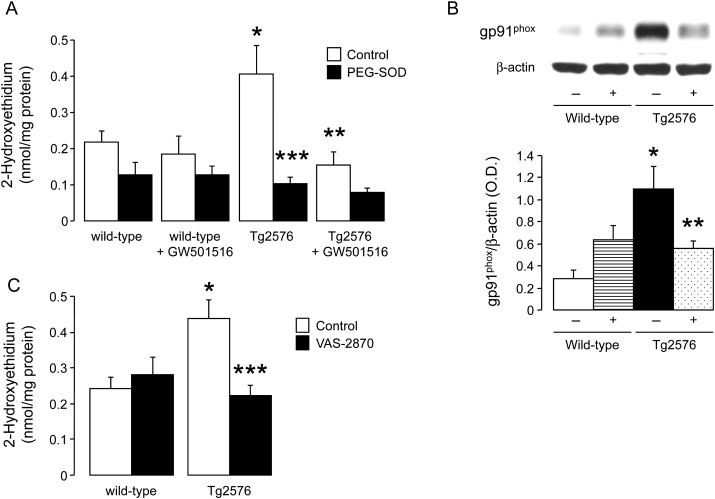
HPLC analysis revealed that PEG-SOD inhabitable production of superoxide anion was increased in the aorta of Tg2576 mice (P < 0.05; Figure 5A). In vitro incubation with L-NAME had no significant effect on superoxide anion levels in Tg2576 mice aortas (0.48 ± 0.08 nmol/mg vs. 0.58 ± 0.09 nmol/mg for Tg2576 aortas without L-NAME; n = 5–6; P > 0.05). Interestingly, protein expression of NADPH oxidase subunit gp91phox was increased in Tg2576 aortas (P < 0.05; Figure 5B). Treatment with GW501516 significantly decreased gp91phox protein expression and superoxide production in Tg2576 mice (P < 0.05; Figure 5). Incubation of aortic rings with NADPH oxidase inhibitor VAS2870 prevented increased superoxide anion production in APP Tg2576 mice while it had no effect in wild-type mice (Figure 5C).
Figure 5.
Quantitative analysis of intracellular superoxide anion (A) and western blot analysis of gp91phox protein expression (B) in aortas of wild-type littermates and Tg2576 mice without (−) or with (+) GW501516 treatment. Results are means ± SEM (n = 6–9 for superoxide anion and n = 4–5 for gp91phox). Effect of NADPH oxidase inhibition with VAS-2870 on superoxide anion levels in wild-type and Tg2576 mice aortas (C). *P < 0.05 vs. wild-type mice; **P < 0.05 vs. transgenic T2576 mice; ***P < 0.05 vs. control aortas (two-way ANOVA).
3.9. Protein expression of antioxidants
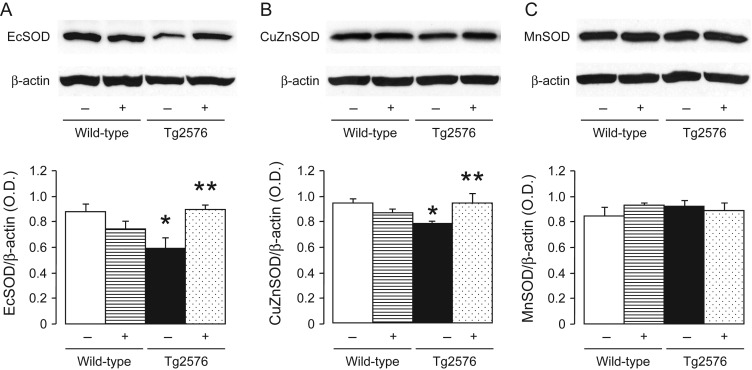
In order to investigate further the mechanism responsible for increased oxidative stress, we performed western blot analyses of antioxidant profile. Protein expression of EcSOD and Cu/ZnSOD were significantly attenuated in the aortas of Tg2576 mice when compared with non-transgenic mice (Figure 6A and B). Treatment with PPARδ ligand normalized EcSOD and Cu/ZnSOD protein expression in Tg2576 mice (Figure 6A and B) while it had no effect in wild-type mice. MnSOD was unaffected in all groups of treated mice (Figure 6C).
Figure 6.
Representative western blot analysis of EC-SOD (A), CuZnSOD (B), and MnSOD (C) protein expression in aortas of wild-type and Tg2576 mice without (−) or with (+) treatment with GW501516. Bar graphs indicate the results of the relative densitometry when compared with β-actin protein. Results are mean ± SEM (n = 6–8). *P < 0.05 vs. wild-type mice; **P < 0.05 vs. transgenic T2576 mice without GW501516 (ANOVA with Bonferroni's).
Protein expression of catalase and GPx were not different between wild-type and Tg2576 mice and those treated with GW501516 (see Supplementary material online, Figure S3).
4. Discussion
There are several new findings in the present study. First, endothelium-dependent relaxations and phosphorylation of eNOS at Ser1177 were significantly impaired in aortas of transgenic Tg2576 mice overexpressing APP. Secondly, BH4 levels and its rate-limiting enzyme GTPCH I were significantly reduced in Tg2576 mice aortas while 7,8-BH2 levels were increased. Thirdly, APP-overexpression significantly increased gp91phox expression and production of superoxide anion in the aorta while protein expression of Cu/ZnSOD and EcSOD were decreased. Fourthly, 2 weeks treatment with the PPARδ ligand GW501516 normalized Ser1177-eNOS phosphorylation, BH4 biosynthesis, and significantly improved endothelial function in the aorta of Tg2576 mice. Fifthly, GW501516 normalized protein expression of gp91phox, Cu/ZnSOD, and EcSOD and significantly reduced superoxide anion production in APP transgenic mice. Our results demonstrate that endothelial cells and NO production are specifically susceptible to oxidative stress induced by overexpression of APP. Treatment with PPARδ ligand reduced oxidative stress thereby improving endothelial function in Tg2576 mice.
Reduced NO bioavailability is a major mechanism responsible for initiation and progression of endothelial dysfunction in cardiovascular diseases such as hypercholesterolaemia, diabetes, ageing, and hypertension. All of these conditions are considered risks factors for development of atherosclerosis.9 Furthermore, both atherosclerosis and Alzheimer's disease share many common pathogenetic mechanisms, such as inflammation and the generation of free radicals.4,14,27 It is well established that transgenic Tg2576 mice overexpressing double Swedish mutated human APP (K670N/M671L) are normotensive but exhibit several pathological features of Alzheimer's disease.16,23,28 Relevant to our study, these mice develop spontaneous atherosclerosis in the aorta demonstrating that the detrimental vascular effects of overexpressed APP are not limited to the brain.6 Moreover, recent study has reported that APP is expressed in the adipocyte tissue and is upregulated in obesity.29 In the present study, we observed that circulating levels of HDL were decreased in APP transgenic mice. The association between low levels of HDL and an increased risk for cardiovascular disease has been well established by epidemiological and clinical studies.30
Overexpression of APP caused an impairment of endothelium-dependent relaxations to acetylcholine in aortas of Tg2576 mice. In contrast, endothelium-independent relaxations to NO-donor DEA-NONOate were unaltered in Tg2576 mice demonstrating that the effects of overexpressed APP are endothelium specific. In addition, phosphorylation of eNOS at Ser1177 was significantly impaired in the aorta of Tg2576 mice suggesting that enzymatic activity of eNOS is diminished. This observation may help to explain endothelial dysfunction detected in Tg2576 mice. Although we did not detect any alteration of the APP processing enzymes α-secretase, β-secretase, as well as γ-secretase in Tg2576 mice aortas, circulating levels of Aβ peptides were significantly increased in APP transgenic mice consistent with the fact that this mutation causes increased secretion of Aβ peptides.23 It is very likely that elevated concentration of Aβ causes impairment of endothelium-dependent relaxations since several experimental studies have demonstrated that Aβ can directly induce endothelial dysfunction of both cerebral and peripheral blood vessels.13,14,31
We have previously shown that under physiological conditions, the vascular endothelium of wild-type mouse aorta has a high rate of BH4 production while vascular smooth muscle cells do not contain detectable quantities of BH4.32 Our study is the first to show that overexpression of APP reduced BH4 levels and increased oxidation of BH4 to 7,8-BH2 in the aorta of transgenic mice. We also provided evidence that GTPCH I activity was decreased in Tg2576 mice aortas and that reduced activity of BH4-synthetizing enzyme was caused by reduced protein expression of GTPCH I. Decrease in BH4 to 7,8-BH2 ratio is considered an important index of reduced enzymatic activity of eNOS. Since both BH4 and 7,8-BH2 has been shown to bind eNOS with similar affinity, increased availability of 7,8-BH2 can efficiently replace BH4 thereby inhibiting eNOS enzymatic activity.33
Evidence from experimental and human studies suggests that oxidative stress is an important mechanism of endothelial dysfunction and vascular injury as the local concentrations of NO in arterial wall are not only dependent on enzymatic activity of eNOS but are also determined by concentrations of superoxide anions.9 APP overexpression reduced protein expression of EcSOD and Cu/ZnSOD and increased PEG-SOD sensitive production of superoxide anion in the aorta of Tg2576 mice. This is consistent with reported study showing that endothelial dysfunction in cerebral arteries of Tg2576 mice was prevented by overexpression of Cu/ZnSOD.34 However, it is important to note that superoxide anion levels and endothelium-dependent relaxations are comparable in young heterozygous Cu/ZnSOD and wild-type mice suggesting that partial reduction in SOD is not sufficient to impair endothelial function.35
We and others have shown that phosphorylated eNOS at Ser1177 is an important source for superoxide anion generation during BH4 deficiency.26,36 However, in Tg2576 mice, non-selective NOS inhibitor L-NAME did not affect increased superoxide anion production most likely because eNOS phosphorylation at Ser1177 was significantly reduced thereby minimizing production of superoxide anion by eNOS. In contrast, we demonstrated that protein expression of gp91phox was upregulated and that inhibition of NADPH oxidase completely prevented increased production of superoxide anion thereby suggesting that gp91phox-containing NADPH oxidase complex mainly contributed to the increased generation of superoxide anion in the aorta of Tg2576 mice. This observation is in line with the results of prior studies in peripheral and cerebral arteries.37,38
On the basis of the results obtained in human and animal studies it appears that APP and/or Aβ play a role in the development of atherosclerosis.4–7 Recently, PPARδ agonist has been shown to reduce vascular inflammation and development of atherosclerosis suggesting that activation of PPARδ exerts vascular protective effects.22 In the present study, we detected several beneficial effects of PPARδ agonist GW501516. We observed similar elevation of HDL levels by PPARδ activation as reported by previous study.22 Remarkably, 2 weeks treatment with GW501516 normalized eNOS phosphorylation and improved endothelium-dependent relaxations to acetylcholine in aortas of Tg2576 mice without affecting Aβ levels thus indicating that vascular protective effects of PPARδ ligand are independent of APP processing. Moreover, the improvement in endothelial function of Tg2576 mice by GW501516 was associated with increased BH4 bioavailability, a critical cofactor required for enzymatic activity of eNOS.12 Indeed, BH4 levels were significantly improved in aortas of Tg2576 mice treated with PPARδ ligand. Increase in BH4 levels were caused by increased de novo biosynthesis of BH4 via GTPCH I, as protein expression and enzymatic activity of GTPCH I were augmented by GW501516. Our findings are consistent with reported study showing that the beneficial effects of GW501516 on re-endothelialization after vascular injury are dependent on increased production of BH4.39
Unexpectedly, BH4 levels were reduced in aortas of GW501516 treated wild-type mice. The decrease in BH4 levels by PPARδ ligand was not caused by the decreased de novo biosynthesis of BH4 via GTPCH I or by the downregulation of GTPCH I protein expression. Furthermore, the decrease in BH4 levels were not caused by the increased oxidative stress as the production of superoxide anion was not elevated. We did not observe any increase in oxidation of BH4 to 7,8- BH2 that would compete with BH4 to bind eNOS and thus inhibit its enzymatic activity. Moreover, endothelium-dependent relaxations to acetylcholine were normal in GW501516 treated wild-type mice despite decreased BH4 levels. This finding suggests that reduced BH4 levels are not sufficient to cause impairment of endothelial function.33 The exact mechanism of selective reduction in BH4 levels by GW501516 remains to be determined.
We cannot rule out the possibility that endothelium-dependent relaxations in aortas of GW501516 treated mice are normal because of compensatory increase in production of other relaxing factors such as prostacyclin or hydrogen peroxide. However, we did not observe any effects of PPARδ ligand on prostacyclin generating enzymes in transgenic Tg2576 mice and their wild-type littermates. On the other hand, our study also showed that expression and activity of eNOS was unchanged in GW501516 treated wild-type mice. It is thus unlikely that hydrogen peroxide is involved because hydrogen peroxide has been shown to increase expression and phosphorylation of eNOS.40
Interestingly, treatment with PPARδ agonist decreased protein expression of gp91phox, normalized protein expression of both vascular Cu/ZnSOD and EcSOD isoforms, and prevented increased production of superoxide anion in the aorta of APP transgenic mice. The putative binding site for PPAR transcription factors has been found in the promoter region of Cu/ZnSOD gene suggesting that Cu/ZnSOD is regulated by PPARs.41,42 Indeed, PPARδ deficiency attenuated cardiac expression of Cu/ZnSOD in the adult mouse heart, leading to increased oxidative damage.43 Conversely, activation of PPARδ enhances expression of Cu/ZnSOD in endothelial cells and increases endothelial cell resistance against oxidative stress.21,44 We did not investigate whether the vascular protective effects of PPARδ agonist is blood pressure dependent. However, previous studies have demonstrated that in vivo treatment with GW501516 did not affect systolic blood pressure in healthy volunteers.45 Moreover, treatment with the other PPARδ ligand had no effect on blood pressure in angiotensin II-infused mice.46
In conclusion, presented findings support the concept that APP causes endothelial dysfunction by increasing oxidative stress and reducing availability of NO. Activation of PPARδ in Tg2576 mice exerts a number of vascular protective effects thereby leading to normalization of NO production and prevention of endothelial dysfunction.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by National Institutes of Health grants HL-91867 and HL111062, by the American Heart Association Scientist Development grants #07–30133N (to L.V.d.) and #08-35436N (to A.V.R.S.), and by the Mayo Foundation.
Conflict of interest: none declared.
Supplementary Material
References
- 1.De Strooper B, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol. 2010;6:99–107. doi: 10.1038/nrneurol.2009.218. doi:10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldgaber D, Harris HW, Hla T, Maciag T, Donnelly RJ, Jacobsen JS, et al. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci USA. 1989;86:7606–7610. doi: 10.1073/pnas.86.19.7606. doi:10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forloni G, Demicheli F, Giorgi S, Bendotti C, Angeretti N. Expression of amyloid precursor protein mRNAs in endothelial, neuronal and glial cells: modulation by interleukin-1. Brain Res Mol Brain Res. 1992;16:128–134. doi: 10.1016/0169-328x(92)90202-m. doi:10.1016/0169-328X(92)90202-M. [DOI] [PubMed] [Google Scholar]
- 4.De Meyer GR, De Cleen DM, Cooper S, Knaapen MW, Jans DM, Martinet W, et al. Platelet phagocytosis and processing of beta-amyloid precursor protein as a mechanism of macrophage activation in atherosclerosis. Circ Res. 2002;90:1197–1204. doi: 10.1161/01.res.0000020017.84398.61. doi:10.1161/01.RES.0000020017.84398.61. [DOI] [PubMed] [Google Scholar]
- 5.Austin SA, Sens MA, Combs CK. Amyloid precursor protein mediates a tyrosine kinase-dependent activation response in endothelial cells. J Neurosci. 2009;29:14451–14462. doi: 10.1523/JNEUROSCI.3107-09.2009. doi:10.1523/JNEUROSCI.3107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Cao D, Garber DW, Kim H, Fukuchi K. Association of aortic atherosclerosis with cerebral beta-amyloidosis and learning deficits in a mouse model of Alzheimer's disease. Am J Pathol. 2003;163:2155–2164. doi: 10.1016/s0002-9440(10)63572-9. doi:10.1016/S0002-9440(10)63572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tibolla G, Norata GD, Meda C, Arnaboldi L, Uboldi P, Piazza F, et al. Increased atherosclerosis and vascular inflammation in APP transgenic mice with apolipoprotein E deficiency. Atherosclerosis. 2010;210:78–87. doi: 10.1016/j.atherosclerosis.2009.10.040. doi:10.1016/j.atherosclerosis.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Van De Parre TJ, Guns PJ, Fransen P, Martinet W, Bult H, Herman AG, et al. Attenuated atherogenesis in apolipoprotein E-deficient mice lacking amyloid precursor protein. Atherosclerosis. 2011;216:54–58. doi: 10.1016/j.atherosclerosis.2011.01.032. doi:10.1016/j.atherosclerosis.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 9.d'Uscio LV, Baker TA, Mantilla CB, Smith L, Weiler D, Sieck GC, et al. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1017–1022. doi: 10.1161/01.atv.21.6.1017. doi:10.1161/01.ATV.21.6.1017. [DOI] [PubMed] [Google Scholar]
- 10.Palmer RMJ, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. doi:10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 11.Pollock JS, Förstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, et al. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci USA. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. doi:10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raman CS, Li H, Martasek P, Kral V, Masters BS, Poulos TL. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–950. doi: 10.1016/s0092-8674(00)81718-3. doi:10.1016/S0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- 13.Suo Z, Fang C, Crawford F, Mullan M. Superoxide free radical and intracellular calcium mediate A beta(1–42) induced endothelial toxicity. Brain Res. 1997;762:144–152. doi: 10.1016/s0006-8993(97)00383-1. doi:10.1016/S0006-8993(97)00383-1. [DOI] [PubMed] [Google Scholar]
- 14.Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–171. doi: 10.1038/380168a0. doi:10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- 15.Jahroudi N, Kitney J, Greenberger JS, Bowser R. Endothelial cell dysfunction in response to intracellular overexpression of amyloid precursor protein. J Neurosci Res. 1998;54:828–839. doi: 10.1002/(SICI)1097-4547(19981215)54:6<828::AID-JNR11>3.0.CO;2-M. doi:10.1002/(SICI)1097-4547(19981215)54:6<828::AID-JNR11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Elesber AA, Bonetti PO, Woodrum JE, Zhu XY, Lerman LO, Younkin SG, et al. Bosentan preserves endothelial function in mice overexpressing APP. Neurobiol Aging. 2006;27:446–450. doi: 10.1016/j.neurobiolaging.2005.02.012. doi:10.1016/j.neurobiolaging.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Marx N, Duez H, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors and atherogenesis: regulators of gene expression in vascular cells. Circ Res. 2004;94:1168–1178. doi: 10.1161/01.RES.0000127122.22685.0A. doi:10.1161/01.RES.0000127122.22685.0A. [DOI] [PubMed] [Google Scholar]
- 18.Goya K, Sumitani S, Xu X, Kitamura T, Yamamoto H, Kurebayashi S, et al. Peroxisome proliferator-activated receptor alpha agonists increase nitric oxide synthase expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:658–663. doi: 10.1161/01.ATV.0000118682.58708.78. doi:10.1161/01.ATV.0000118682.58708.78. [DOI] [PubMed] [Google Scholar]
- 19.Polikandriotis JA, Mazzella LJ, Rupnow HL, Hart CM. Peroxisome proliferator-activated receptor gamma ligands stimulate endothelial nitric oxide production through distinct peroxisome proliferator-activated receptor gamma-dependent mechanisms. Arterioscler Thromb Vasc Biol. 2005;25:1810–1816. doi: 10.1161/01.ATV.0000177805.65864.d4. doi:10.1161/01.ATV.0000177805.65864.d4. [DOI] [PubMed] [Google Scholar]
- 20.Reilly SM, Lee CH. PPAR delta as a therapeutic target in metabolic disease. FEBS Lett. 2008;582:26–31. doi: 10.1016/j.febslet.2007.11.040. doi:10.1016/j.febslet.2007.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan Y, Wang Y, Tang Z, Zhang H, Qin X, Zhu Y, et al. Suppression of pro-inflammatory adhesion molecules by PPAR-delta in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:315–321. doi: 10.1161/ATVBAHA.107.149815. doi:10.1161/ATVBAHA.107.149815. [DOI] [PubMed] [Google Scholar]
- 22.Barish GD, Atkins AR, Downes M, Olson P, Chong LW, Nelson M, et al. PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc Natl Acad Sci USA. 2008;105:4271–4276. doi: 10.1073/pnas.0711875105. doi:10.1073/pnas.0711875105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliver WR, Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, et al. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci USA. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. doi:10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sznaidman ML, Haffner CD, Maloney PR, Fivush A, Chao E, Goreham D, et al. Novel selective small molecule agonists for peroxisome proliferator-activated receptor delta (PPARdelta)–synthesis and biological activity. Bioorg Med Chem Lett. 2003;13:1517–1521. doi: 10.1016/s0960-894x(03)00207-5. doi:10.1016/S0960-894X(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 26.d'Uscio LV, Smith LA, Katusic ZS. Differential effects of eNOS uncoupling on conduit and small arteries in GTP-cyclohydrolase I-deficient hph-1 mice. Am J Physiol Heart Circ Physiol. 2011;301:H2227–2234. doi: 10.1152/ajpheart.00588.2011. doi:10.1152/ajpheart.00588.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosendorff C, Beeri MS, Silverman JM. Cardiovascular risk factors for Alzheimer's disease. Am J Geriatr Cardiol. 2007;16:143–149. doi: 10.1111/j.1076-7460.2007.06696.x. doi:10.1111/j.1076-7460.2007.06696.x. [DOI] [PubMed] [Google Scholar]
- 28.Howlett DR, Richardson JC. The pathology of APP transgenic mice: a model of Alzheimer's disease or simply overexpression of APP? Histol Histopathol. 2009;24:83–100. doi: 10.14670/HH-24.83. [DOI] [PubMed] [Google Scholar]
- 29.Lee YH, Tharp WG, Maple RL, Nair S, Permana PA, Pratley RE. Amyloid precursor protein expression is upregulated in adipocytes in obesity. Obesity (Silver Spring) 2008;16:1493–1500. doi: 10.1038/oby.2008.267. doi:10.1038/oby.2008.267. [DOI] [PubMed] [Google Scholar]
- 30.Assmann G, Gotto AM., Jr HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109:III8–14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 31.Price JM, Chi X, Hellermann G, Sutton ET. Physiological levels of beta-amyloid induce cerebral vessel dysfunction and reduce endothelial nitric oxide production. Neurol Res. 2001;23:506–512. doi: 10.1179/016164101101198758. doi:10.1179/016164101101198758. [DOI] [PubMed] [Google Scholar]
- 32.d'Uscio LV, Katusic ZS. Increased vascular biosynthesis of tetrahydrobiopterin in apolipoprotein E-deficient mice. Am J Physiol Heart Circ Physiol. 2006;290:H2466–H2471. doi: 10.1152/ajpheart.00366.2005. doi:10.1152/ajpheart.00366.2005. [DOI] [PubMed] [Google Scholar]
- 33.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol. 2008;294:H1530–1540. doi: 10.1152/ajpheart.00823.2007. doi:10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. doi:10.1038/5715. [DOI] [PubMed] [Google Scholar]
- 35.Didion SP, Kinzenbaw DA, Schrader LI, Faraci FM. Heterozygous CuZn superoxide dismutase deficiency produces a vascular phenotype with aging. Hypertension. 2006;48:1072–1079. doi: 10.1161/01.HYP.0000247302.20559.3a. doi:10.1161/01.HYP.0000247302.20559.3a. [DOI] [PubMed] [Google Scholar]
- 36.Chen CA, Druhan LJ, Varadharaj S, Chen YR, Zweier JL. Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J Biol Chem. 2008;283:27038–27047. doi: 10.1074/jbc.M802269200. doi:10.1074/jbc.M802269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Görlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. doi:10.1161/01.RES.87.1.26. [DOI] [PubMed] [Google Scholar]
- 38.Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, et al. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. doi:10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He T, Smith LA, Lu T, Joyner MJ, Katusic ZS. Activation of peroxisome proliferator-activated receptor-{delta} enhances regenerative capacity of human endothelial progenitor cells by stimulating biosynthesis of tetrahydrobiopterin. Hypertension. 2011;58:287–294. doi: 10.1161/HYPERTENSIONAHA.111.172189. doi:10.1161/HYPERTENSIONAHA.111.172189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. doi:10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Yoo HY, Chang MS, Rho HM. Induction of the rat Cu/Zn superoxide dismutase gene through the peroxisome proliferator-responsive element by arachidonic acid. Gene. 1999;234:87–91. doi: 10.1016/s0378-1119(99)00176-6. doi:10.1016/S0378-1119(99)00176-6. [DOI] [PubMed] [Google Scholar]
- 42.Chang MS, Yoo HY, Rho HM. Transcriptional regulation and environmental induction of gene encoding copper- and zinc-containing superoxide dismutase. Methods Enzymol. 2002;349:293–305. doi: 10.1016/s0076-6879(02)49344-5. doi:10.1016/S0076-6879(02)49344-5. [DOI] [PubMed] [Google Scholar]
- 43.Wang P, Liu J, Li Y, Wu S, Luo J, Yang H, et al. Peroxisome proliferator-activated receptor {delta} is an essential transcriptional regulator for mitochondrial protection and biogenesis in adult heart. Circ Res. 2010;106:911–919. doi: 10.1161/CIRCRESAHA.109.206185. doi:10.1161/CIRCRESAHA.109.206185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali F, Ali NS, Bauer A, Boyle JJ, Hamdulay SS, Haskard DO, et al. PPARdelta and PGC1alpha act cooperatively to induce haem oxygenase-1 and enhance vascular endothelial cell resistance to stress. Cardiovasc Res. 2010;85:701–710. doi: 10.1093/cvr/cvp365. doi:10.1093/cvr/cvp365. [DOI] [PubMed] [Google Scholar]
- 45.Sprecher DL, Massien C, Pearce G, Billin AN, Perlstein I, Willson TM, et al. Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor delta agonist. Arterioscler Thromb Vasc Biol. 2007;27:359–365. doi: 10.1161/01.ATV.0000252790.70572.0c. doi:10.1161/01.ATV.0000252790.70572.0c. [DOI] [PubMed] [Google Scholar]
- 46.Takata Y, Liu J, Yin F, Collins AR, Lyon CJ, Lee CH, et al. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc Natl Acad Sci USA. 2008;105:4277–4282. doi: 10.1073/pnas.0708647105. doi:10.1073/pnas.0708647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.