Abstract
Aims
Increasing energy storage capacity by elevating creatine and phosphocreatine (PCr) levels to increase ATP availability is an attractive concept for protecting against ischaemia and heart failure. However, testing this hypothesis has not been possible since oral creatine supplementation is ineffectual at elevating myocardial creatine levels. We therefore used mice overexpressing creatine transporter in the heart (CrT-OE) to test for the first time whether elevated creatine is beneficial in clinically relevant disease models of heart failure and ischaemia/reperfusion (I/R) injury.
Methods and results
CrT-OE mice were selected for left ventricular (LV) creatine 20–100% above wild-type values and subjected to acute and chronic coronary artery ligation. Increasing myocardial creatine up to 100% was not detrimental even in ageing CrT-OE. In chronic heart failure, creatine elevation was neither beneficial nor detrimental, with no effect on survival, LV remodelling or dysfunction. However, CrT-OE hearts were protected against I/R injury in vivo in a dose-dependent manner (average 27% less myocardial necrosis) and exhibited greatly improved functional recovery following ex vivo I/R (59% of baseline vs. 29%). Mechanisms contributing to ischaemic protection in CrT-OE hearts include elevated PCr and glycogen levels and improved energy reserve. Furthermore, creatine loading in HL-1 cells did not alter antioxidant defences, but delayed mitochondrial permeability transition pore opening in response to oxidative stress, suggesting an additional mechanism to prevent reperfusion injury.
Conclusion
Elevation of myocardial creatine by 20–100% reduced myocardial stunning and I/R injury via pleiotropic mechanisms, suggesting CrT activation as a novel, potentially translatable target for cardiac protection from ischaemia.
Keywords: Cardiac energetics, Metabolism, Creatine kinase, Ischaemia, Reperfusion injury, Myocardial stunning
1. Introduction
Directly increasing energy stores in the heart is an attractive strategy for the treatment of ischaemic heart disease, but this is not currently targeted by any available medication. One approach is to increase intracellular creatine levels. Creatine kinase (CK) catalyses the reversible transfer of a high-energy phosphoryl group from ATP onto creatine to form phosphocreatine (PCr), which acts as an energy storage molecule, rapidly regenerating ATP at times of increased energy demand.1 Since impairment of this system is detrimental to functional recovery following myocardial ischaemia,2,3 we hypothesized that augmenting it, by increasing intracellular creatine, would be beneficial.
There is a wealth of evidence that many components of the CK system are impaired in chronic heart failure (CHF), regardless of aetiology or species,4–6 and there exists a close relationship between a low PCr/ATP ratio and mortality in human heart failure.7 A strategy of reversing this energetic deficit is strongly supported by recent findings that M-CK overexpression protects against heart failure in a mouse pressure-overload model.8 We, therefore, also sought to test whether elevated creatine would be beneficial in CHF.
Increasing creatine levels in the heart to test these two hypotheses is not straightforward. Cardiomyocytes cannot synthesize creatine, which enters the cell via a specific creatine transporter (CrT). Simply increasing dietary creatine does not result in elevated tissue creatine in the mature heart due to subsequent down-regulation of the CrT.9 To circumvent this feedback-loop, we previously created mice that constitutively overexpress the CrT in the heart (CrT-OE mice). These mice accumulated creatine to very high levels (four-fold above wild-type), which was associated with left ventricular (LV) hypertrophy and dysfunction.10,11
To determine whether the theoretical benefits of elevating creatine can be realized (free of adverse effects), we have used CrT-OE mice with more modest increases in myocardial creatine (∼20–100% above normal values). We establish that this range is not deleterious to the heart, even within the setting of CHF, and find that elevated creatine is cardio-protective in models of ischaemia/reperfusion via pleiotropic mechanisms. This work establishes elevating creatine by augmenting CrT function as a potentially translatable target to protect against myocardial ischaemia.
2. Methods
2.1. Mouse strain used
Transgenic mice overexpressing the creatine transporter (CrT-OE) using a cardiomyocyte-specific promoter (MLC2v) have previously been described in our laboratory.10 Two strains were created exhibiting a wide range of myocardial creatine from normal to >four-fold above wild-type values. Creatine variability was related to differences in transgene mRNA expression rather transgene copy number or number of integration sites. These mice were back-crossed to C57BL/6J for >10 generations with wild-type littermates used as controls. For the current study, mice were selected for moderate elevation of creatine of between 20 and 100% above WT either in vivo by 1H-MRS or on post-mortem tissue by HPLC. Mice from the Tg46 strain were used preferentially as the frequency distribution is closer to this target range. Mice were kept in specific pathogen-free cages, 12-h light–dark cycle, controlled temperature and humidity, and fed standard chow (naturally creatine-free) and water ad libitum. This investigation was approved by the institutional ethical review committee and conforms to Directive 2010/63/EU of the European Parliament.
2.2. Left ventricular haemodynamics
In vivo LV haemodynamics were performed in closed-chest freely breathing mice under isoflurane general anaesthesia (1–1.5% in medical oxygen) as previously described.10 An adequate anaesthetic depth was determined by the absence of the pedal reflex. The jugular vein was cannulated for the administration of dobutamine at 16 ng/g body weight/min for maximal inotropic stimulation. Measurements were made in n = 31 WT and n = 31 CrT-OE mice (17 males and 14 females in each) at 18 months of age (79 ± 1 weeks). There was no difference between sexes, therefore males and females were analysed together. Mice were euthanized by cervical dislocation and LV tissue snap frozen in liquid nitrogen for the determination of creatine levels by HPLC.
2.3. Chronic myocardial infarction protocol
A total of 67 female mice aged 4–8 months had 1H-MRS examination so that CrT-OE mice could be selected for total creatine in the range 88–140 nmol/mg protein. Mice meeting these criteria received a cine-MRI examination 1 week prior to surgery to ligate the left anterior descending coronary artery. An echocardiogram was performed at 4 weeks to exclude mice with infarct sizes <25%. Cine-MRI was repeated 6 weeks after surgery followed by LV haemodynamics (as described above). Six mice were excluded in order to retrospectively match infarct sizes between groups.
2.4. 1H-MRS and cine-MRI
All MR experiments were carried out on a 9.4T (400 MHz) MR system (Agilent Technologies) using a quadrature-driven birdcage resonator (Rapid biomedical). Mice were anaesthetized with isoflurane and maintained at 1.5–2% in oxygen. Monitoring of ECG and the respiration rate ensured the stable depth of anaesthesia. Cardiac metabolite signals from a 2 µL voxel, placed in the interventricular septum, were acquired using a double-gated, double spin-echo sequence as reported previously.12 Analysis and quantification of these spectra are described in the Supplementary material online, Methods. Global cardiac functional parameters were measured from multi-frame short-axis gradient-echo images covering the heart from the base to the apex. The infarct size (in % of total LV) was measured using ImageJ (version 1.44).
2.5. Coronary artery ligation surgery
Permanent ligation of the left anterior descending (LAD) coronary artery as previously described.5 Briefly, general anaesthesia was induced in female adult mice with 4% isoflurane in medical oxygen followed by oropharyngeal intubation for mechanical ventilation with 2% isoflurane at 250 µL stroke volume and 150 b.p.m. An adequate anaesthetic depth was confirmed by the absence of the pedal reflex. Buprenorphine analgesia was provided (1 mg/kg subcutaneous) prior to surgery. A thoracotomy was performed between the 4th and 5th ribs, the pericardium removed, and the LAD occluded using a 6–0 polyethylene suture (infarct confirmed by blanching of the myocardium). The chest wall was closed and 1 mL subcutaneous saline administered prior to recovery. Mice were provided with softened chow and supplementary heat until fully recovered.
2.6. Ischaemia/reperfusion injury in vivo
For permanent occlusion, surgery was as above, but with the reperfusion of the coronary artery after 45 min of ischaemia, confirmed by direct visualization of blood returning to infarcted myocardium. A total of n = 53 CrT-OE and WT mice aged 4–5 months and n = 6 C57BL/6J mice as a positive control group treated with ischaemic postconditioning (IPC) consisting of three cycles of 10 s reperfusion and 10 s ischaemia immediately after the main period of ischaemia. A further n = 14 C57Bl/6J mice served as non-IPC controls and were combined with the WT control group for analysis purposes. At 24 h, mice were killed by the excision of the heart under deep isoflurane anaesthesia. Area-of-necrosis and area-at-risk were then assessed histologically using tetrazolium staining as previously described.13 The right ventricle was snap frozen in liquid nitrogen for retrospective quantification of creatine by HPLC.
2.7. Ischaemia/reperfusion recovery ex vivo
A total of n = 9 CrT-OE mice and WT littermates (four females and five males of each) were heparinized (5000 U/kg body weight) and anaesthetized (pentobarbitone 140 mg/kg ip). Hearts were excised, cannulated and perfused in the Langendorff mode inside a 10-mm NMR tube at 80 mmHg and 37°C with Krebs–Henseleit buffer gassed with 95% O2/5% CO2. The LV function was assessed using a fluid-filled intraventricular balloon connected to a pressure transducer (ADInstruments Ltd). The end-diastolic pressure was set to ∼10 mmHg.31P-NMR spectra of perfused mouse hearts were acquired on a Bruker Avance 500 spectrometer equipped with an 11.7T magnet and a 20 mm 1H-/31 P-crosscage resonator.31P spectra were acquired using a pulse-and-collect sequence with a repetition time (TR) of 2 s and a flip angle of 60° with 150 averages each (5 min per spectrum) and were corrected for partial saturation. Pre-ischaemic ATP levels were normalized to previously published baseline values,10 with absolute PCr and inorganic phosphate (Pi) calculated from the measured PCr/ATP and Pi/ATP ratios. Intracellular volume was assumed to be 0.5 mL/g wet weight. Intracellular pH was determined from the chemical shift δ between the Pi and PCr peaks using the equation pH = 6.72 − log(δ − 5.72)/(3.17 − δ). The free-energy change of ATP hydrolysis (▵G; in kJ/mol) was calculated from the CK equilibrium assumption as previously described.4 After a 10 min period of control perfusion, hearts were subjected to 20 min of global normothermic no-flow ischaemia, followed by a 30 min reperfusion period. Rate pressure product (RPP) was calculated as the product of heart rate and LV developed pressure (systolic – diastolic pressure).
2.8. Biochemical analysis
Frozen crushed ventricular tissue or cells were prepared for the measurement of CK activity and/or the quantification of creatine by HPLC, and then normalized to protein content using the Lowry method as previously described.14 The myocardial glycogen content was determined spectrophotometrically as glucose equivalents after digestion of tissue with 30% KOH, ethanol precipitation, and acid hydrolysis of glycogen as described in the Supplementary material online, Methods. Immunoblotting for phosphorylated PKC delta and cleaved Caspase-3 used total protein extracted from LV tissue following ex vivo ischaemia/reperfusion as described above. Expression levels were detected with the ECL Advance chemiluminescence kit (GE Healthcare, Amersham, UK) and a FluorChem 8800 imager. For quantification, blots were stripped and re-probed for β-tubulin as described in Supplementary material online, Methods.
2.9. Oxidative stress protection assay
Oxidation of 2′,7′dichlorodihydroflourescein diacetate (H2DCF-DA) by H2O2 in vitro was adapted from Young et al.15 A cell line stably expressing the rabbit creatine transporter (3T3-CrT-HA) and atrial tumour-derived mouse HL-1 cells were used for this purpose. Cells were plated onto 96-well plates at a density of 0.2 × 105 cells/well and creatine monohydrate added to culture media at 250, 500 µM, 5 and 10 mM for 24 h. Cultures were then washed with phosphate-buffered saline and loaded with 10 µM H2DCF-DA (Molecular Probes, Life Technologies, Paisley, UK) in Hanks' balanced salt solution for 30 min at 37°C (95% air, 5% CO2). Cells were then exposed to 100 μM H2O2 with or without Trolox (12 nmol; BDH Prolabo VWR, UK) as a positive antioxidant control. Oxidation of intracellular H2DCF-DA was determined by increase in fluorescence at excitation and emission wavelengths of 485 and 520 nm at 37°C with a microtitre plate reader (POLARstar Omega, BMG Labtech, Aylesbury, UK). Data were recorded every 5 min and were collected in three independent experiments, having n = 6 replicates per treatment group.
2.10. mPTP opening assay
The effect of intracellular creatine on opening of the mitochondrial permeability transition pore (mPTP) was assessed in vitro in HL-1 cells loaded with tetramethylrhodamine methyl ester (TMRM), as previously described.16 HL-1 cells were seeded onto gelatin/fibronectin-coated 35-mm confocal dishes at a density of 1–2 × 105 cells/dish, and incubated for 24 h in either standard Claycomb medium (Sigma, F2442), or Claycomb medium supplemented with 5 or 10 mM creatine. Cells were loaded with 3 µM TMRM (Sigma, T5428) in Hank's buffered salt solution (HBSS with Ca2+, Sigma, H8264) for 15 min at 37°C. As a positive control, some samples were supplemented with 0.5 µM cyclosporin A (CsA) (Merck, 239835). Photoactivation of TMRM was induced using the 1.2 mW 543 nm He–Ne laser of a Zeiss LSM510 confocal microscope. Laser power (5%), detector gain, and optical settings were identical for all experiments. The time to a 50%-maximal increase in TMRM fluorescence was taken as a measure of mPTP opening. For statistical analysis, the results from four independent experiments were normalized to their respective control values and the data averaged for between groups comparison.
2.11. Data analysis
All measurements were analysed blind to the genotype and creatine levels. Data are expressed as mean ± SEM. Unless otherwise stated, statistical comparison between two groups at a single time-point was by Student's t-test, and between three groups by one-way ANOVA. Comparison between groups at multiple time-points was analysed by two-way repeated measures (mixed model) ANOVA with a Bonferroni correction for multiple comparisons. A log-rank (Mantel-Cox) test was used to determine whether survival curves were statistically different. Differences were considered significant when P < 0.05.
3. Results
3.1. Long-term moderate elevation of myocardial creatine is safe
To determine a safe level of creatine elevation, in vivo LV haemodynamic measurements were performed in CrT-OE and WT littermate controls at 18 months (n = 31 of each). Mean myocardial [Cr] was 74 ± 6 in WT and 119 ± 16 nmol/mg protein in CrT-OE mice (maximum 138). No significant differences were observed for any functional parameters (Supplementary material online, Figure S1A–F). Mean values for LV/body weight were 2.79 ± 0.43 WT vs. 2.97 ± 0.43 mg/g CrT-OE (P = ns), and there was no correlation with LV creatine levels (Supplementary material online, Figure S1G). In contrast, n = 10 mice excluded due to high creatine (mean 152 ± 10 nmol/mg protein; range 141–167) had significant LV hypertrophy with LV/body weight 3.21 ± 0.35 mg/g (P = 0.007 vs. WT). Therefore, mice with total myocardial creatine >140 nmol/mg protein were excluded from all subsequent experiments.
3.2. Effect of creatine on LV remodelling and function in chronic heart failure
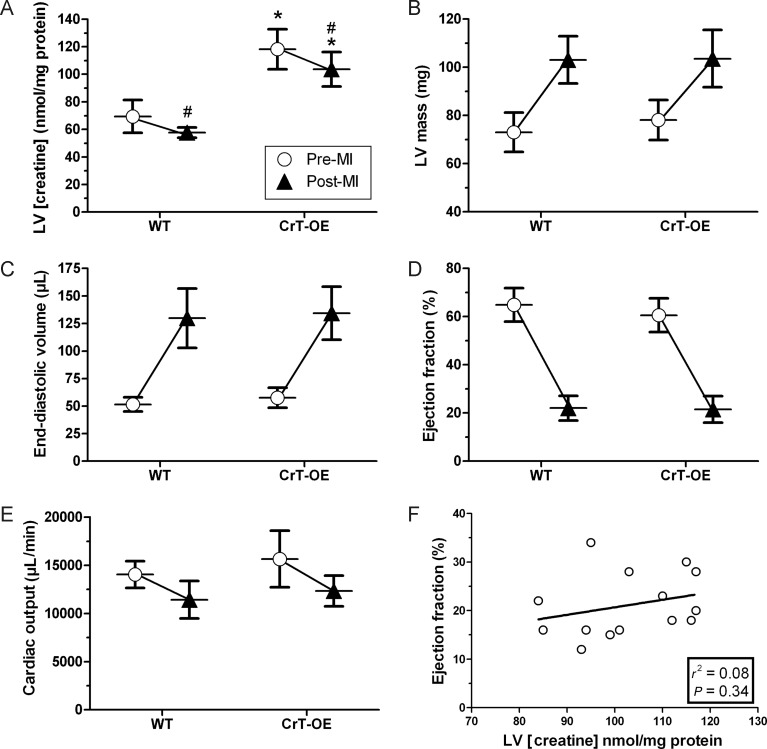
CrT-OE mice were pre-selected for myocardial creatine in the range 88–140 nmol/mg protein by 1H-MRS and subjected to permanent ligation of a coronary artery to induce myocardial infarction (MI). There was no significant difference in all-cause mortality over 6 weeks between WT and CrT-OE mice (31 vs. 33%; P = 0.78). For all further analysis, WT and CrT-OE mice were retrospectively matched for the infarct size in order to study the effect of creatine on heart failure independently of any effect on myocardial injury: WT mean 36%, range 29–50; CrT-OE mean 37%, range 30–50 (n = 10 in each). Total LV creatine levels were higher at baseline in CrT-OE mice compared with WT and remained significantly higher despite a fall in creatine in both groups characteristic of CHF (Figure 1A).
Figure 1.
Elevated creatine is neither beneficial nor detrimental in chronic heart failure. Experimental groups were matched for the infarct size (n = 10 in each). (A) Myocardial creatine measured pre-MI (open circle) by 1H-MRS and post-MI (filled triangle) by HPLC. CrT-OE mice had elevated creatine throughout the experiment (*P < 2 × 10–7 vs. WT at same time-point; #P < 0.05 pre-MI vs. post-MI). (B–E) MRI parameters measured before and after MI. There was no effect of the genotype by 2-way ANOVA, while MI resulted in major changes in all parameters in both groups (P < 0.0001). (F) There was no correlation between ejection fraction and final myocardial creatine levels.
There were no significant differences in baseline structural or functional parameters by cine-MRI between WT and CrT-OE mice (open circles in Figure 1B–E). Six-week post-MI, all animals had highly significant LV hypertrophy, dilatation, and dysfunction compared with the baseline, consistent with the development of CHF, but there were no significant differences between WT and CrT-OE mice, and no relationship between LV creatine and ejection fraction (triangles Figure 1B–F). In vivo LV haemodynamics confirmed these findings (Supplementary material online, Table S1).
3.3. Elevated creatine reduces myocardial injury following ischaemia/reperfusion in vivo
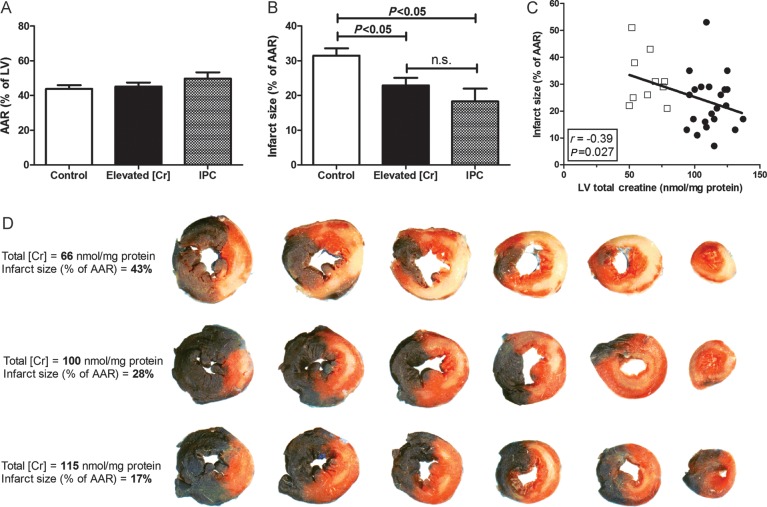
Male mice had 45 min of regional ischaemia followed by 24 h reperfusion. The mean myocardial [Cr] was 75% higher in CrT-OE mice (Supplementary material online, Table S2). Area-at-risk was comparable between groups at 45–46% (Figure 2A), whereas mice with elevated creatine had 27% less myocardial necrosis, P < 0.05 (Figure 2B). There was an inverse correlation between the infarct size and LV creatine levels, with higher creatine being associated with smaller infarct sizes (Figure 2C with examples in Figure 2D). Mice with IPC were included to compare the size of effect: infarct sizes were 31, 23, and 18% for WT, elevated creatine, and IPC groups, respectively.
Figure 2.
Elevated myocardial creatine protects against in vivo I/R injury (45 min ischaemia, 24 h reperfusion). (A) There was no difference in area-at-risk (AAR) between groups. (B) Mice with elevated LV total creatine ([Cr] = 94–137 nmol/mg protein; n = 22) had significantly less myocardial necrosis compared with control mice (n = 25), to a level indistinguishable from ischaemic postconditioning (IPC, n = 6; mean ± SEM). (C) There was a significant inverse correlation between infarct size and LV creatine. Open squares represent WT mice and black circles CrT-OE mice. (D) Representative LV histological sections arranged from the base to the apex. Unaffected myocardium is stained blue, necrotic areas are white, and area-at-risk is red + white.
3.4. Elevated creatine improves functional recovery following I/R ex vivo
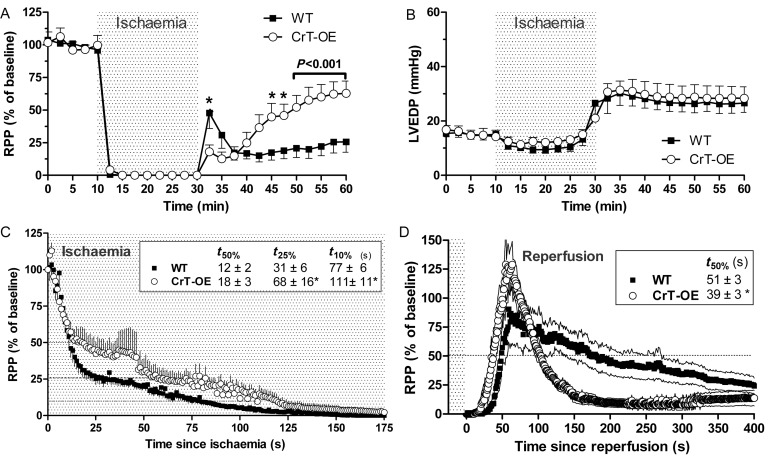
Langendorff-perfused WT and CrT-OE hearts were subject to 20 min global ischaemia and 30 min reperfusion. At baseline there were no significant differences in any functional parameters (Supplementary material online, Table S3). Upon reperfusion CrT-OE hearts had significantly higher LV systolic pressure and RPP during the last 20 min of ischaemia (Supplementary material online, Table S3 and Figure 3A). Diastolic pressure during reperfusion increased to the same extent in both groups (Figure 3B). During the onset of ischaemia functional data were analysed at high temporal resolution (1 s). It took twice as long for RPP to decline to 25% of baseline in CrT-OE hearts (Figure 3C). A similar effect was observed on return to function at reperfusion, with 50% of baseline RPP achieved 12 s quicker in the CrT-OE hearts (P < 0.05; Figure 3D). Both groups had a period of hyper-contractility on initial reperfusion, but in CrT-OE hearts this phase was transient, whereas in WT this phase was markedly prolonged.
Figure 3.
Function in isolated-perfused hearts during 20 min global no-flow ischaemia (grey) and 30 min reperfusion. (A) CrT-OE hearts had better functional recovery during reperfusion. RPP is rate pressure product (developed pressure × heart rate) expressed as % of baseline with data points at 2.5 min intervals (B) Left ventricular end-diastolic pressure (LVEDP) rises during reperfusion to the same extent in both groups. (C) Initial onset of ischaemia at 1 s temporal resolution showing contractile function is maintained for longer in CrT-OE hearts. (D) Detailed analysis of data shown in (A) focusing on early reperfusion with 1 s temporal resolution showing a different pattern of hypercontractility upon reperfusion, i.e. onset is slower but response is prolonged in the WT hearts. *P < 0.05.
3.5. Cardiac energetics during ischaemia/reperfusion
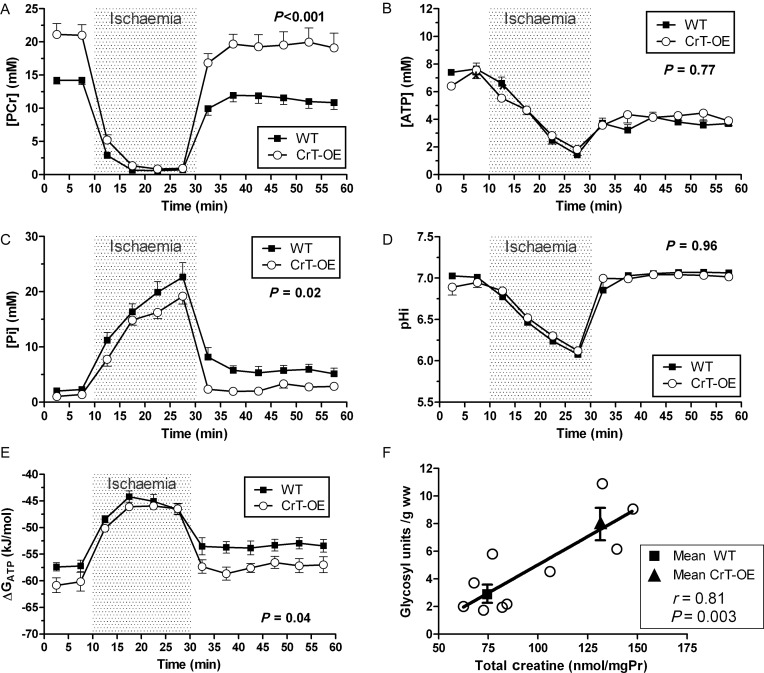
Using 31P-MRS, baseline myocardial PCr levels were 49% higher in CrT-OE hearts compared with WT littermates (21.1 ± 1.6 vs. 14.2 ± 0.6 mM, respectively, P < 0.01; Figure 4A), with no significant difference in Pi or pH (Figure 4C and D). During ischaemia, the relative breakdown of PCr and ATP and the consequent increase in Pi was indistinguishable between groups within the available temporal resolution of 5 min (Figure 4A–C). Intracellular pH fell to 6.08 and 6.12 in control and CrT-OE hearts, respectively, and quickly recovered during reperfusion. PCr rapidly recovered to near baseline in both groups, but returned to higher values in CrT-OE hearts. ATP concentrations recovered only partially and to the same extent in both groups. Pi levels rapidly declined, in line with the rapid re-phosphorylation of Cr, but remained higher in the control hearts than in the CrT-OE hearts (Figure 4C; P = 0.02). There was a trend for lower ADP levels in CrT-OE hearts, e.g. during the reperfusion phase WT 133±35 vs. CrT-OE 77 ± 16 µM (P = 0.16). However, Gibbs free energy change of ATP hydrolysis was greater in CrT-OE hearts, both at baseline and during reperfusion (Figure 4E; P = 0.04), suggesting that more energy was available per molecule of ATP. Under normal baseline conditions the myocardial glycogen content was significantly higher in CrT-OE compared with WT (P = 0.003), and there was a positive correlation with total creatine levels (Figure 4F).
Figure 4.
Ex vivo 31P-MRS during 20 min global ischaemia and 30 min reperfusion in CrT-OE and wild-type hearts. (A) Phosphocreatine, PCr; (B) ATP; (C) inorganic phosphate, Pi; (D) intracellular pH, pHi; (E) free energy change of ATP hydrolysis, ΔGATP; (F) the myocardial glycogen content at baseline is 2.8-fold higher in CrT-OE mice (t-test, P = 0.003) and positively correlates with creatine levels. Data are mean ± SEM and P-value is for the effect of the genotype by two-way repeated measures ANOVA.
To determine whether improved functional recovery was related to reduced cell injury in this acute model, we measured protein expression of cleaved caspase-3 and phosphorylated-PKCδ as early markers of commitment to apoptosis, and no differences were observed between groups (Supplementary material online, Figure S2).
3.6. Creatine does not provide protection via direct antioxidant activity
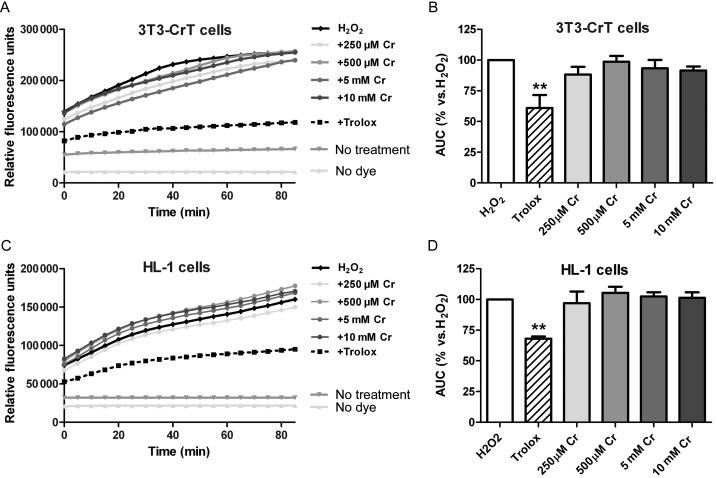
To determine whether creatine has antioxidant effects that could contribute to the cardio-protective mechanisms, we used a direct measure of antioxidant activity in a cell-based assay system. Cells loaded with the dye H2DCF-DA which fluoresces only in the oxidized form, produced a robust increase in fluorescence upon challenge with H2O2. Pre-incubation with a known antioxidant (Trolox) attenuated this response (Figure 5). Fibroblasts constitutively overexpressing the CrT were incubated for 24 h with creatine, resulting in elevated intracellular creatine levels (Table 1). Creatine did not attenuate the response to H2O2 (Figure 5A and B). Identical results were obtained when the experiment was repeated in the cardiomyocyte-derived HL-1 cell line (Figure 5C and D). Creatine levels in HL-1 cells after 24 h exposure show an increase similar in magnitude to the in vivo CrT-OE mouse heart (Table 1).
Figure 5.
Creatine does not have direct anti-oxidant activity. Cells were loaded with the dye H2DCF-DA and exposed to 100 μM H2O2 resulting in oxidation of dye, measured as an increase in fluorescence. Pre-incubation with varying concentrations of creatine (Cr) for 24 h increased intracellular creatine levels, but did not alter the fluorescent signal. In contrast, the anti-oxidant trolox (12 nmol) significantly attenuated oxidation of dye by H2O2 (**P < 0.01). An identical pattern was observed in two cell types: 3T3 fibroblasts overexpressing the creatine transporter (CrT), and in cardiomyocyte-derived HL-1 cells. Example traces from a single experiment are shown in (A) and (C), with mean data in (B) and (D).
Table 1.
Creatine levels and creatine kinase activity in cells cultured with and without creatine
| 3T3-CrT cells |
HL-1 cells |
|||
|---|---|---|---|---|
| Control | +5 mM Cr | Control | +5 mM Cr | |
| Creatine (nmol/mg protein) | 33.8 ± 0.5 | 54.7 ± 0.5 | 20.0 ± 0.4 | 52.0 ± 0.8 |
| Phosphocreatine (nmol/mg Pr) | 2.4 ± 0.1 | 1.8 ± 0.1 | 5.2 ± 0.2 | 16.5 ± 0.4 |
| Total CK activity (IU CK/mg Pr) | ND | ND | 0.32 ± 0.01 | 0.31 ± 0.01 |
| Mito-CK | ND | ND | 0.21 ± 0.01 | 0.19 ± 0.00 |
| MM-CK | ND | ND | 0.12 ± 0.02 | 0.07 ± 0.01 |
| MB-CK | ND | ND | ND | 0.02 ± 0.00 |
| BB-CK | ND | ND | ND | 0.04 ± 0.00 |
Data are mean ± SD. CK, creatine kinase; ND denotes below limits of detection. Cells incubated in normal culture media (control) or supplemented with 5 mM creatine monohydrate for 24 h.
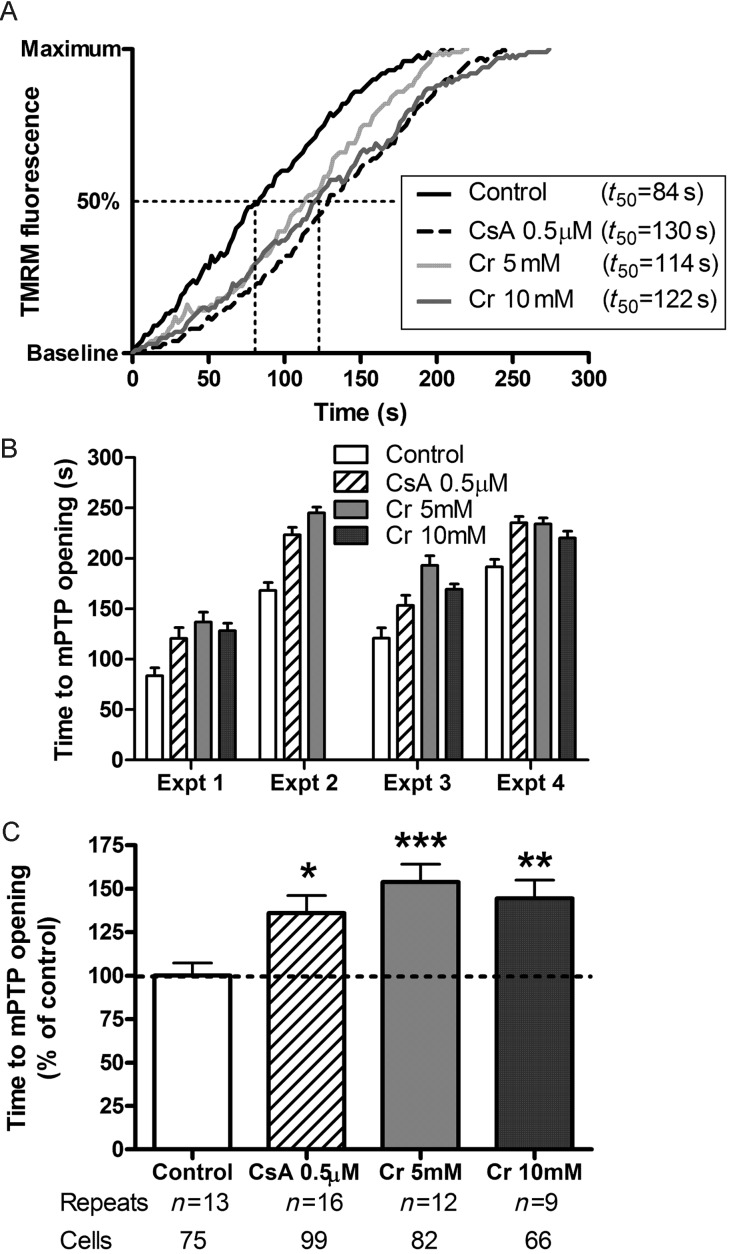
3.7. Creatine attenuates mitochondrial permeability transition pore opening
Opening of the mPTP is a seminal event determining the extent of reperfusion injury, therefore the ability of intracellular creatine levels to modulate mPTP opening was assayed in HL-1 cells loaded with the mitochondrial permeable dye, TMRM. Illumination with laser light generates reactive oxygen species that provide stimulus for pore opening, observed as a gain in fluorescence signal as dye released into the cytosol becomes dequenched. Using CsA as a positive control, we observed a prolongation in time to 50% of maximal response, representing protection from mPTP opening (Figure 6). Incubation of cells for 24 h with creatine resulted in elevated creatine and PCr levels and induced expression of the B-CK isoform (Table 1). Both 5 mM and 10 nM creatine concentrations were as effective as CsA at delaying mPTP opening. This is illustrated in the example experimental trace shown in Figure 6A. The same pattern of protection was observed in all four independent experiments (Figure 6B), which were then normalized to control values and averaged as shown in Figure 6C.
Figure 6.
The effect of creatine on mitochondrial permeability transition pore (mPTP) opening in vitro. (A) Example traces from representative HL-1 cells loaded with TMRM and illuminated with laser light to generate reactive oxygen species. mPTP opening is observed as redistribution of TMRM from mitochondria to the cytosol and quantitated as time to 50% of maximum fluorescence (t50). Treatment groups were cyclosporin A (CsA) as a positive control; and creatine incubation for 24 h at 5 mM and 10 mM (Cr). The experiment was repeated on four independent occasions with the same pattern observed every time (B) n.b. there was no 10 mM creatine group in experiment 2 due to a technical failure. Since absolute time to mPTP opening varied between experiments, data were therefore normalized to the mean value of control samples for each day (C). *P < 0.05; **P < 0.01; ***P < 0.001 vs. control.
4. Discussion
Our study establishes the elevation of creatine by augmenting CrT function as a therapeutic principle in ischaemia/reperfusion injury. This circumvents the difficulty of increasing intracellular creatine in the adult cardiomyocyte and has allowed us to test the concept of elevating myocardial creatine as a therapeutic strategy for the first time.
Our first step was to establish a safe range for creatine elevation in the heart. Using data from our earlier studies,10,11 a cut-off of 140 nmol/mg protein was selected. We clearly demonstrate that prolonged elevation of myocardial creatine up to this level, which is ∼100% above normal values, is safe and does not result in LV hypertrophy even in mice up to 18 months of age. This provided a therapeutic window to investigate our hypotheses.
We did not observe a beneficial effect of elevated creatine in CHF. One limitation is that mice have relatively low starting creatine compared with larger species and the fall in levels during heart failure is less pronounced.5 This might negatively impact on our ability to detect a protective effect in the mouse, especially since the mechanisms underpinning such species difference are unclear. Nevertheless, it is an important finding that creatine is safe within this setting. More refined approaches involving creatine may yet hold promise in CHF. For example, in silico modelling has predicted benefits from the elevation of creatine in combination with total adenine nucleotides and total exchangeable phosphate pools.17 More recently, overexpression of M-CK in the mouse heart was shown to protect against LV dysfunction in a model of heart failure,8 and there is a clear rationale in simultaneously increasing both CK activity and creatine, i.e. substrate and enzyme together.
The in vivo cardio-protective effect of creatine was comparable in scale to IPC, an intervention that has been shown to strongly attenuate I/R injury in mice.18 However, we cannot rule out that chronic overexpression of CrT could have resulted in adaptations that contributed to this effect and unfortunately our attempts to create an inducible model of CrT-OE to address this question were unsuccessful. Improved functional recovery was demonstrated ex vivo, with simultaneous 31P-MRS data suggesting several potential protective mechanisms.
4.1. PCr as energy store
Myocardial PCr levels were 49% higher in CrT-OE mice, which could act like a back-up battery, effectively reducing ischaemic time. Normal metabolic demand for the contracting heart is ∼1 mM of ATP per second,4 so this effect will not be prolonged and was not reflected in PCr or ATP depletion rates, likely due to insufficient temporal resolution (5 min). However, CrT-OE hearts maintained contractile function for longer, which returned more rapidly upon reperfusion. Although small, this effect may be physiologically relevant, since small changes in ischaemic time are known to have a disproportionate effect on the myocardial injury size.19,20 Furthermore, a larger effect of PCr on basal metabolism during ischaemia cannot be discounted.21
4.2. PCr maintains favourable ATP/ADP ratio and therefore ΔG of ATP hydrolysis
The PCr/CK system also functions to keep local free ADP concentrations low, which is important for maintaining high ΔGATP, i.e. the energy liberated from ATP hydrolysis and available to perform work.22 The value for ΔGATP in the normal rodent heart is −55 to −60 kJ/mol,4 and many key ATPases cease to function properly when ΔGATP falls below a certain threshold value, e.g. Na+–K+–ATPase at −50 kJ/mol23 and sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) at −52 kJ/mol. The difference between |ΔGATP| and the threshold value is termed ‘energy reserve’,4 and represents the chemical potential driving ATPase activity. It is notable that ΔGATP started higher in the CrT-OE mice and returned to normal values within minutes of reperfusion. In contrast, WT hearts did not return above −53 kJ/mol, very close to the threshold for SERCA pump function.
Both experimental groups had a hyper-contractile phase peaking 60 s into reperfusion, the inotropic consequence of intracellular calcium accumulation during ischaemia.24,25 That this returns to normal more rapidly in CrT-OE represents indirect evidence for improved ion homoeostasis and suggests the effect of low energy reserve is more than just theoretical. This suggests that WT experienced a relatively longer period of calcium overload, which is known to be detrimental, for example, the impairment of SERCA function increases calcium accumulation and results in greater I/R injury,26 as does knockout of CK.2
4.3. Effect on glycogen storage
During ischaemia, generation of ATP is dependent on glycogen as the only substrate available for glycolysis.27 We observed 2.8-fold higher levels of glycogen in hearts from CrT-OE mice compared with WT, which in rabbit hearts have been shown to reduce ischaemic damage and improve recovery.28
4.4. Oxidative stress and mPTP opening
Other studies have shown direct antioxidant effects of (phospho-) creatine,29,30 however, we were unable to detect protection from H2O2 challenge in cells with intracellular levels of creatine that correspond with our in vivo ‘therapeutic range’. In contrast, we clearly demonstrate that elevated creatine was as effective as CsA in prolonging time to mPTP opening in an assay that models the burst of reactive oxygen species associated with reperfusion. This assay does not induce ischaemia, therefore the energetic mechanisms described above are unlikely to contribute, and we have excluded a direct antioxidant effect of creatine. The precise mechanism of action remains to be elucidated, however, overexpression of mitochondrial-CK in the liver can reduce mPTP opening, with the presence of creatine obligate for this effect.31 This is a potential mechanism here, since we demonstrate that HL-1 cells express enzymatically active CK (including mitochondrial-CK), although we cannot rule out a direct effect of creatine itself to influence pore opening.
4.5. Limitations and potential clinical relevance
We cannot rule out that chronic overexpression of CrT may have resulted in adaptations that contribute to our cardioprotective effect. Our previous attempts to create an inducible model of CrT-OE were unsuccessful and there are no pharmacological modulators currently available to test this. Translation of our own findings will therefore require the development of the necessary pharmacological tools targeting the CrT. While genetic overexpression led to variation in the creatine content due to variability in transgene mRNA expression,32 a drug-based approach mimicking post-translational regulation should provide more reproducible creatine levels. Furthermore, the kinetics of creatine accumulation and elimination are favourable, and can be monitored non-invasively using 1H-MRS. For example, the CrT is highly inducible, capable of increasing activity up to seven-fold above baseline.32 Loss of tissue creatine is a slow process via non-enzymatic cyclization to creatinine (∼1.6% of creatine pool per day),33 suggesting that a single boost in creatine levels will have prolonged effects. Clinical applications of a putative CrT activator might include patients with unstable angina at risk of an imminent MI; patients undergoing cardiac surgery with cardioplegic arrest; and patients with stable angina, where a shift in the ischaemia threshold on exercise would improve symptoms.
Summary and conclusions
We have demonstrated that elevation of creatine by up to 100% above normal values by increasing CrT activity is not detrimental to the heart even over prolonged periods, and importantly, is well tolerated even in the presence of CHF. Although creatine does not improve function in the failing heart, it does protect against ischaemia/reperfusion injury and associated myocardial stunning. The mechanisms in the acute setting are attributed to a combined effect on cardiac energetics by increasing PCr buffer capacity, increasing myocardial glycogen, and improving ‘energy reserve’. Protection from ischaemia/reperfusion injury is likely to be a combination of downstream benefits of improved functional recovery and an effect of creatine to reduce probability of mPTP opening. CrT activation may become a clinically translatable target for anti-ischaemic therapy.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by British Heart Foundation grants (RG/10/002/28187, BS/06/001); the BHF Centre of Research Excellence, Oxford; the Else Kröner-Fresenius-Stiftung, the German Cardiac Society; and Wellcome Trust Core Award (090532/Z/09/Z).
Supplementary Material
Acknowledgements
Dr Derek Hausenloy and Dr Uma Mukherjee from The Hatter Cardiovascular Institute, University College London, provided expert advice and training on conducting the mPTP experiments. We thank Ms Hannah Barnes and Mrs Lee-Anne Stork for technical assistance during the magnetic resonance experiments and Mr Tanmoy Ray for assistance with cell culture.
Conflict of interest: A patent application has been filed by C.A.L. and S.N. to cover therapeutic modulation of myocardial creatine levels, including a method for screening compounds that modulate the creatine transporter.
References
- 1.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. doi:10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 2.Spindler M, Meyer K, Stromer H, Leupold A, Boehm E, Wagner H, et al. Creatine kinase-deficient hearts exhibit increased susceptibility to ischemia-reperfusion injury and impaired calcium homeostasis. Am J Physiol Heart Circ Physiol. 2004;287:H1039–1045. doi: 10.1152/ajpheart.01016.2003. doi:10.1152/ajpheart.01016.2003. [DOI] [PubMed] [Google Scholar]
- 3.ten Hove M, Lygate CA, Fischer A, Schneider JE, Sang AE, Hulbert K, et al. Reduced inotropic reserve and increased susceptibility to cardiac ischemia/reperfusion injury in phosphocreatine-deficient guanidinoacetate-N-methyltransferase-knockout mice. Circulation. 2005;111:2477–2485. doi: 10.1161/01.CIR.0000165147.99592.01. doi:10.1161/01.CIR.0000165147.99592.01. [DOI] [PubMed] [Google Scholar]
- 4.Ingwall JS, Shen W. The chemistry of ATP in the failing heart—the fundamentals. Heart Fail Rev. 1999;4:221–228. doi:10.1023/A:1009857906567. [Google Scholar]
- 5.Lygate CA, Fischer A, Sebag-Montefiore L, Wallis J, ten Hove M, Neubauer S. The creatine kinase energy transport system in the failing mouse heart. J Mol Cell Cardiol. 2007;42:1129–1136. doi: 10.1016/j.yjmcc.2007.03.899. doi:10.1016/j.yjmcc.2007.03.899. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer S. The failing heart—an engine out of fuel. New Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. doi:10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 7.Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, et al. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. doi:10.1161/01.CIR.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A, Akki A, Wang Y, Leppo MK, Chacko VP, Foster DB, et al. Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J Clin Invest. 2012;122:291–302. doi: 10.1172/JCI57426. doi:10.1172/JCI57426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehm E, Chan S, Monfared M, Wallimann T, Clarke K, Neubauer S. Creatine transporter activity and content in the rat heart supplemented by and depleted of creatine. Am J Physiol Endocrinol Metabol. 2003;284:E399–406. doi: 10.1152/ajpendo.00259.2002. [DOI] [PubMed] [Google Scholar]
- 10.Wallis J, Lygate CA, Fischer A, ten Hove M, Schneider JE, Sebag-Montefiore L, et al. Supranormal myocardial creatine and phosphocreatine concentrations lead to cardiac hypertrophy and heart failure: insights from creatine transporter-overexpressing transgenic mice. Circulation. 2005;112:3131–3139. doi: 10.1161/CIRCULATIONAHA.105.572990. doi:10.1161/CIRCULATIONAHA.105.572990. [DOI] [PubMed] [Google Scholar]
- 11.Phillips D, ten Hove M, Schneider JE, Wu CO, Sebag-Montefiore L, Aponte AM, et al. Mice over-expressing the myocardial creatine transporter develop progressive heart failure and show decreased glycolytic capacity. J Mol Cell Cardiol. 2010;48:582–590. doi: 10.1016/j.yjmcc.2009.10.033. doi:10.1016/j.yjmcc.2009.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider JE, Tyler DJ, ten Hove M, Sang AE, Cassidy PJ, Fischer A, et al. In vivo cardiac 1H-MRS in the mouse. Magn Reson Med. 2004;52:1029–1035. doi: 10.1002/mrm.20257. doi:10.1002/mrm.20257. [DOI] [PubMed] [Google Scholar]
- 13.Bohl S, Medway DJ, Schulz-Menger J, Schneider JE, Neubauer S, Lygate CA. Refined approach for quantification of in vivo ischemia-reperfusion injury in the mouse heart. Am J Physiol Heart Circ Physiol. 2009;297:H2054–2058. doi: 10.1152/ajpheart.00836.2009. doi:10.1152/ajpheart.00836.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neubauer S, Horn M, Naumann A, Tian R, Hu K, Laser M, et al. Impairment of energy metabolism in intact residual myocardium of rat hearts with chronic myocardial infarction. J Clin Invest. 1995;95:1092–1100. doi: 10.1172/JCI117756. doi:10.1172/JCI117756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young J, Larsen L, Malmendal A, Nielsen N, Straadt I, Oksbjerg N, et al. Creatine-induced activation of antioxidative defence in myotube cultures revealed by explorative NMR-based metabonomics and proteomics. J Int Soc Sports Nutr. 2010;7:9. doi: 10.1186/1550-2783-7-9. doi:10.1186/1550-2783-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. doi:10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 17.Wu F, Zhang J, Beard DA. Experimentally observed phenomena on cardiac energetics in heart failure emerge from simulations of cardiac metabolism. Proc Natl Acad Sci USA. 2009;106:7143–7148. doi: 10.1073/pnas.0812768106. doi:10.1073/pnas.0812768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skyschally A, van Caster P, Iliodromitis EK, Schulz R, Kremastinos DT, Heusch G. Ischemic postconditioning: experimental models and protocol algorithms. Basic Res Cardiol. 2009;104:469–483. doi: 10.1007/s00395-009-0040-4. doi:10.1007/s00395-009-0040-4. [DOI] [PubMed] [Google Scholar]
- 19.Redel A, Jazbutyte V, Smul TM, Lange M, Eckle T, Eltzschig H, et al. Impact of ischemia and reperfusion times on myocardial infarct size in mice in vivo. Exp Biol Med (Maywood) 2008;233:84–93. doi: 10.3181/0612-RM-308. doi:10.3181/0612-RM-308. [DOI] [PubMed] [Google Scholar]
- 20.Fisher SG, Marber MS. An in vivo model of ischaemia-reperfusion injury and ischaemic preconditioning in the mouse heart. J Pharmacol Toxicol Methods. 2002;48:161–169. doi: 10.1016/S1056-8719(03)00046-7. doi:10.1016/S1056-8719(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 21.Gibbs CL, Loiselle DS. Cardiac basal metabolism. Jpn J Physiol. 2001;51:399–426. doi: 10.2170/jjphysiol.51.399. doi:10.2170/jjphysiol.51.399. [DOI] [PubMed] [Google Scholar]
- 22.ten Hove M, Neubauer S. MR spectroscopy in heart failure–clinical and experimental findings. Heart Fail Rev. 2007;12:48–57. doi: 10.1007/s10741-007-9003-8. doi:10.1007/s10741-007-9003-8. [DOI] [PubMed] [Google Scholar]
- 23.Jansen MA, Shen H, Zhang L, Wolkowicz PE, Balschi JA. Energy requirements for the Na+ gradient in the oxygenated isolated heart: effect of changing the free energy of ATP hydrolysis. Am J Physiol Heart Circ Physiol. 2003;285:H2437–2445. doi: 10.1152/ajpheart.00534.2003. [DOI] [PubMed] [Google Scholar]
- 24.Rynning SE, Birkeland S, Hexeberg E, Grong K. Changes in myocardial contraction pattern during initial reperfusion. Am J Physiol Heart Circ Physiol. 1994;266:H980–H986. doi: 10.1152/ajpheart.1994.266.3.H980. [DOI] [PubMed] [Google Scholar]
- 25.Aksnes G, Kirkeboen KA, Christensen G, Ilebekk A. Characteristics and development of myocardial stunning in the pig. Am J Physiol Heart Circ Physiol. 1992;263:H544–H551. doi: 10.1152/ajpheart.1992.263.2.H544. [DOI] [PubMed] [Google Scholar]
- 26.Talukder MAH, Kalyanasundaram A, Zuo L, Velayutham M, Nishijima Y, Periasamy M, et al. Is reduced SERCA2a expression detrimental or beneficial to postischemic cardiac function and injury? Evidence from heterozygous SERCA2a knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H1426–H1434. doi: 10.1152/ajpheart.01016.2007. doi:10.1152/ajpheart.01016.2007. [DOI] [PubMed] [Google Scholar]
- 27.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Cardiovasc Res. 1997;33:243–257. doi: 10.1016/s0008-6363(96)00245-3. doi:10.1016/S0008-6363(96)00245-3. [DOI] [PubMed] [Google Scholar]
- 28.Vanoverschelde JL, Janier MF, Bakke JE, Marshall DR, Bergmann SR. Rate of glycolysis during ischemia determines extent of ischemic injury and functional recovery after reperfusion. Am J Physiol Heart Circ Physiol. 1994;267:H1785–H1794. doi: 10.1152/ajpheart.1994.267.5.H1785. [DOI] [PubMed] [Google Scholar]
- 29.Lawler JM, Barnes WS, Wu G, Song W, Demaree S. Direct antioxidant properties of creatine. Biochem Biophys Res Commun. 2002;290:47–52. doi: 10.1006/bbrc.2001.6164. doi:10.1006/bbrc.2001.6164. [DOI] [PubMed] [Google Scholar]
- 30.Zucchi R, Poddighe R, Limbruno U, Mariani M, Ronca-Testoni S, Ronca G. Protection of isolated rat heart from oxidative stress by exogenous creatine phosphate. J Mol Cell Cardiol. 1989;21:67–73. doi: 10.1016/0022-2828(89)91494-6. doi:10.1016/0022-2828(89)91494-6. [DOI] [PubMed] [Google Scholar]
- 31.Dolder M, Walzel B, Speer O, Schlattner U, Wallimann T. Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J Biol Chem. 2003;278:17760–17766. doi: 10.1074/jbc.M208705200. [DOI] [PubMed] [Google Scholar]
- 32.ten Hove M, Makinen K, Sebag-Montefiore L, Hunyor I, Fischer A, Wallis J, et al. Creatine uptake in mouse hearts with genetically altered creatine levels. J Mol Cell Cardiol. 2008;45:453–459. doi: 10.1016/j.yjmcc.2008.05.023. doi:10.1016/j.yjmcc.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloch K, Schoenheimer R, Rittenberg D. Rate of formation and disappearance of body creatine in normal animals. J Biol Chem. 1941;138:155–166. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.