Abstract
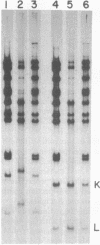
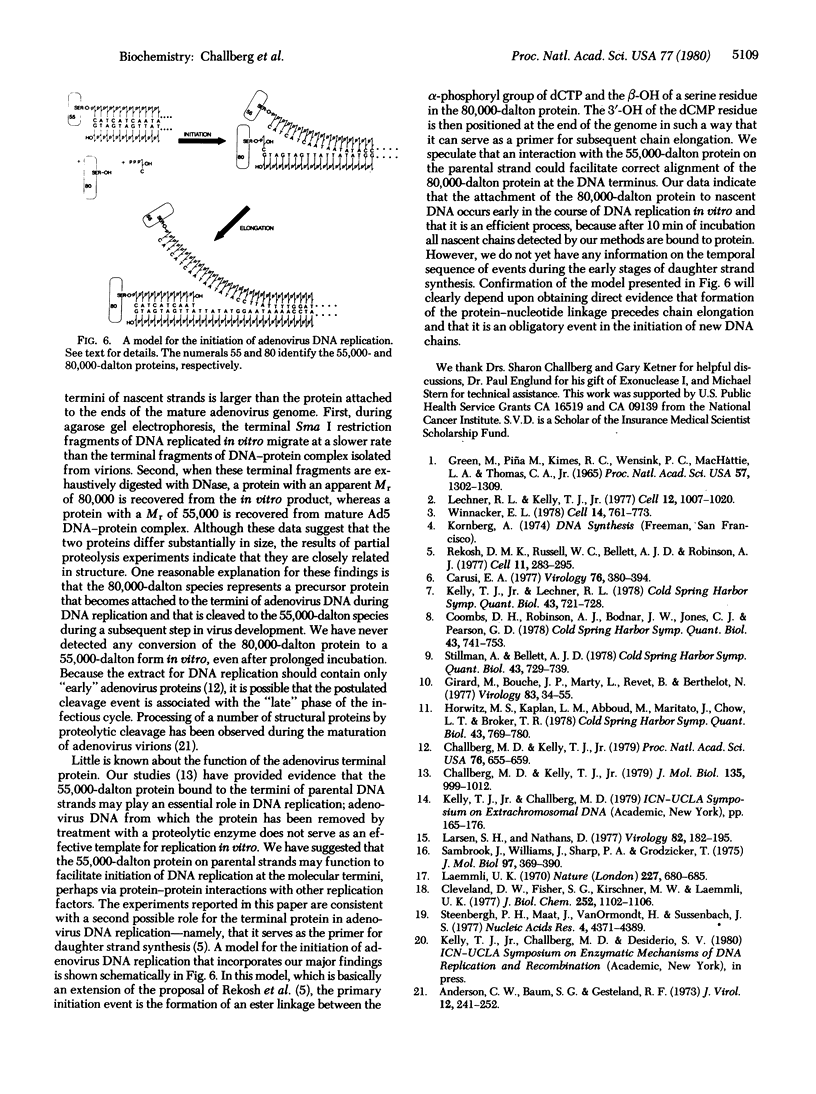
The 5' terminus of each nascent daughter strand of adenovirus DNA replicated in vitro is covalently linked to a protein with an apparent Mr of 80,000. This protein may represent a precursor to the 55,000-dalton protein known to be linked to the 5' ends of mature adenovirus DNA strands. Partial proteolysis experiments indicate that the 80,000-dalton and 55,000-dalton proteins are structurally related. Furthermore, both proteins are attached to DNA by the same linkage: a phosphodiester bond between the beta-OH of a serine residue in the protein and the 5'-OH of the terminal deoxycytidine residue of the DNA. The role of the 80,000-dalton protein in adenovirus DNA replication is not yet clear, although one reasonable possibility is that it serves as the primer for daughter strand synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carusi E. A. Evidence for blocked 5'-termini in human adenovirus DNA. Virology. 1977 Jan;76(1):380–394. doi: 10.1016/0042-6822(77)90310-5. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro: origin and direction of daughter strand synthesis. J Mol Biol. 1979 Dec 25;135(4):999–1012. doi: 10.1016/0022-2836(79)90524-2. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Coombs D. H., Robinson A. J., Bodnar J. W., Jones C. J., Pearson G. D. Detection of covalent DNA-protein complexes: the adenovirus DNA-terminal protein complex and HeLa DNA-protein complexes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):741–753. doi: 10.1101/sqb.1979.043.01.082. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Kaplan L. M., Abboud M., Maritato J., Chow L. T., Broker T. R. Adenovirus DNA replication in soluble extracts of infected cell nuclei. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):769–780. doi: 10.1101/sqb.1979.043.01.084. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lechner R. L. The structure of replicating adenovirus DNA molecules: characterization of DNA-protein complexes from infected cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):721–728. doi: 10.1101/sqb.1979.043.01.080. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen S. H., Nathans D. Mouse adenovirus: growth of plaque-purified FL virus in cell lines and characterization of viral DNA. Virology. 1977 Oct 1;82(1):182–195. doi: 10.1016/0042-6822(77)90041-1. [DOI] [PubMed] [Google Scholar]
- Lechner R. L., Kelly T. J., Jr The structure of replicating adenovirus 2 DNA molecules. Cell. 1977 Dec;12(4):1007–1020. doi: 10.1016/0092-8674(77)90165-9. [DOI] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Williams J., Sharp P. A., Grodzicker T. Physical mapping of temperature-sensitive mutations of adenoviruses. J Mol Biol. 1975 Sep 25;97(3):369–390. doi: 10.1016/s0022-2836(75)80046-5. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Maat J., van Ormondt H., Sussenbach J. S. The nucleotide sequence at the termini of adenovirus type 5 DNA. Nucleic Acids Res. 1977 Dec;4(12):4371–4389. doi: 10.1093/nar/4.12.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Bellett A. J. Replication of DNA in adenovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):729–739. doi: 10.1101/sqb.1979.043.01.081. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L. Adenovirus DNA: structure and function of a novel replicon. Cell. 1978 Aug;14(4):761–773. doi: 10.1016/0092-8674(78)90332-x. [DOI] [PubMed] [Google Scholar]