Abstract
AcPhe-tRNAPhe from yeast can be photocross-linked to poly(U) on Escherichia coli ribosomes. The photoreaction occurs at the wybutine base situated next to the 3' side of the anticodon. The kinetics and efficiency of crosslinking of AcPhe-Phe-tRNA are the same at both the acceptor site and the peptidyl site. Therefore, the orientation of wybutine with respect to the mRNA is similar in both the pretranslocational and posttranslocational states. AcPhe-Phe-tRNA crosslinked at the acceptor site can still be translocated to the peptidyl site, demonstrating that tRNA and mRNA are transported together. The experiments support a model of translocation in which the conformation of the anticodon loop of tRNA is similar in both the peptidyl site and the acceptor site.
Full text
PDF

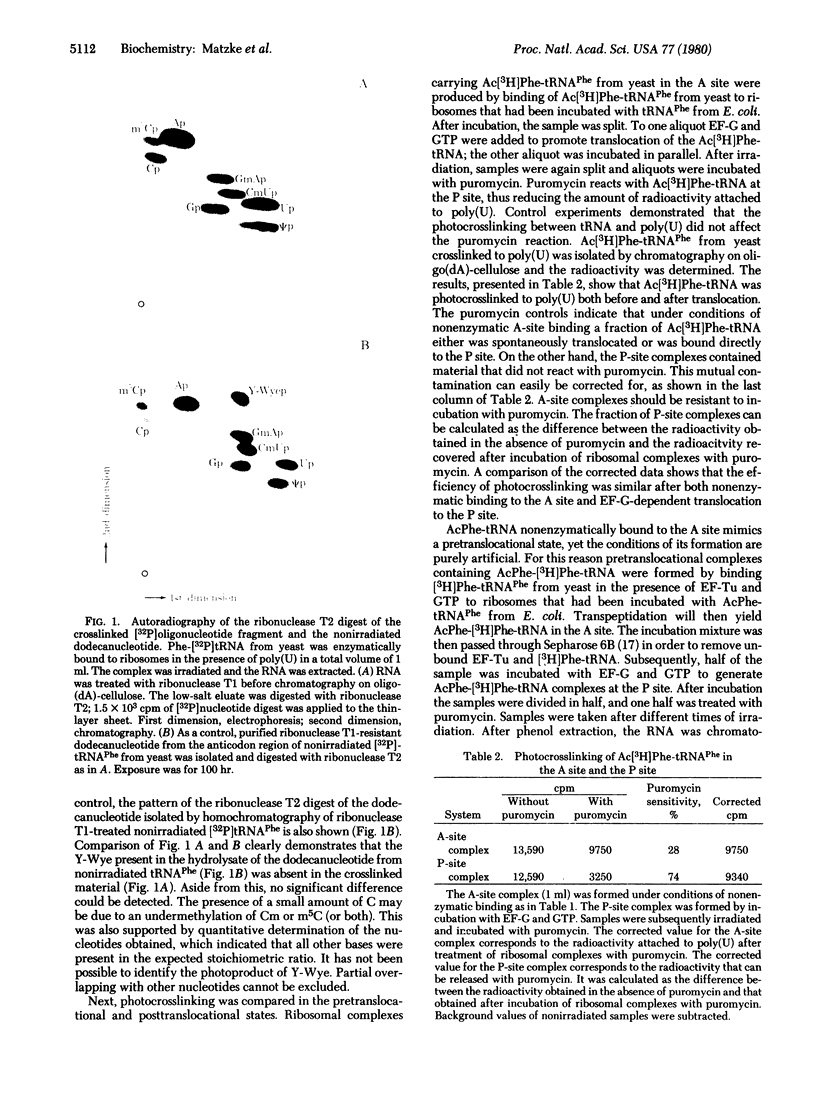
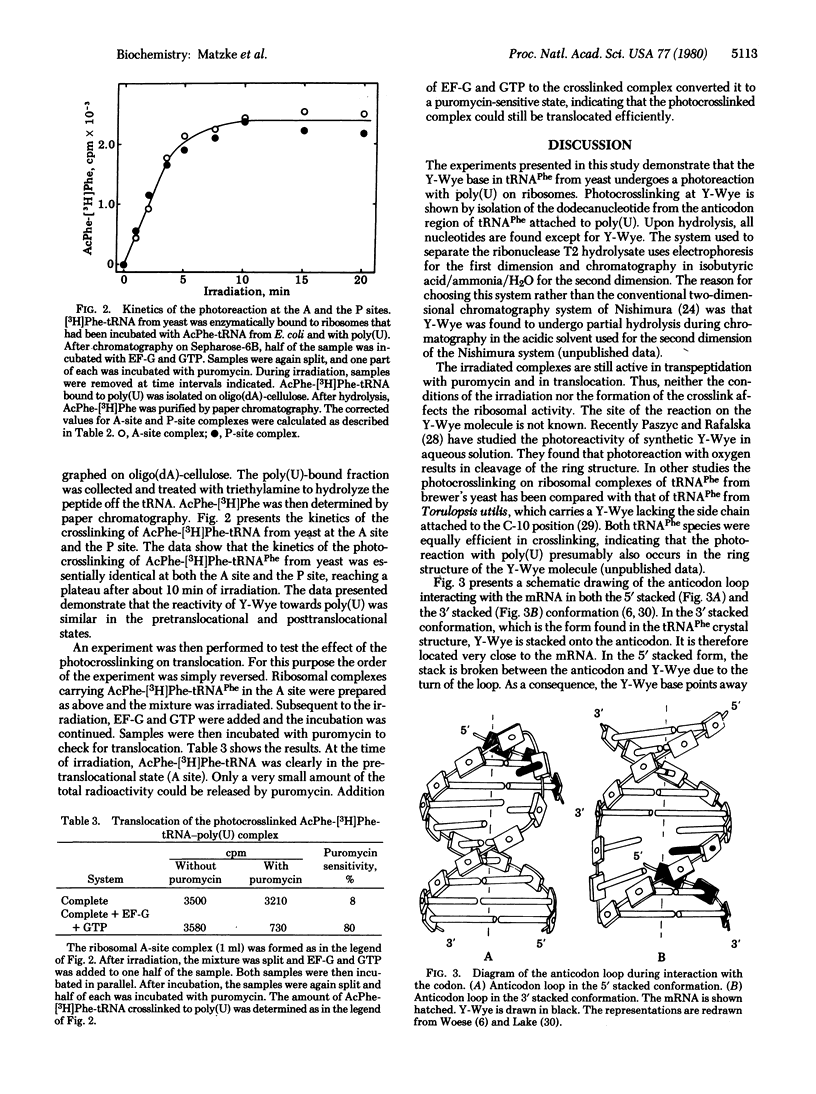

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Kawakita M., Kaziro Y. Studies on polypeptide elongation factors from Escherichia coli. II. Purification of factors Tu-guanosine diphosphate, Ts, and Tu-Ts, and crystallization of Tu-guanosine diphosphate and Tu-Ts. J Biol Chem. 1972 Nov 10;247(21):7029–7037. [PubMed] [Google Scholar]
- Baltzinger M., Fasiolo F., Remy P. Yeast phenylalanyl-tRNA synthetase. Affinity and photoaffinity labelling of the stereospecific binding sites. Eur J Biochem. 1979 Jul;97(2):481–494. doi: 10.1111/j.1432-1033.1979.tb13136.x. [DOI] [PubMed] [Google Scholar]
- Bartmann P., Hanke T., Hammer-Raber B., Holler E. Equilibrium analysis of L-Phe-tRNA Phe complexes with L-phenylalanyl transfer ribonucleic acid synthetase of Escherichia coli K 10. Biochemistry. 1974 Sep 24;13(20):4171–4175. doi: 10.1021/bi00717a016. [DOI] [PubMed] [Google Scholar]
- Crick F. H., Brenner S., Klug A., Pieczenik G. A speculation on the origin of protein synthesis. Orig Life. 1976 Dec;7(4):389–397. doi: 10.1007/BF00927934. [DOI] [PubMed] [Google Scholar]
- De Groot N., Panet A., Lapidot Y. The binding of purified Phe-tRNA and peptidyl-tRNA Phe to Escherichia coli ribosomes. Eur J Biochem. 1971 Dec 10;23(3):523–527. doi: 10.1111/j.1432-1033.1971.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Gavrilova L. P., Kostiashkina O. E., Koteliansky V. E., Rutkevitch N. M., Spirin A. S. Factor-free ("non-enzymic") and factor-dependent systems of translation of polyuridylic acid by Escherichia coli ribosomes. J Mol Biol. 1976 Mar 15;101(4):537–552. doi: 10.1016/0022-2836(76)90243-6. [DOI] [PubMed] [Google Scholar]
- Gavrilova L. P., Spirin A. S. Stimulation of "non-enzymic" translocation in ribosomes by p-chloromercuribenzoate. FEBS Lett. 1971 Oct 1;17(2):324–326. doi: 10.1016/0014-5793(71)80177-1. [DOI] [PubMed] [Google Scholar]
- Girbes T., Vazquez D., Modolell J. Polypeptide-chain elongation promoted by guanyl-5'-yl imidodiphosphate. Eur J Biochem. 1976 Aug 1;67(1):257–265. doi: 10.1111/j.1432-1033.1976.tb10657.x. [DOI] [PubMed] [Google Scholar]
- Lake J. A. Aminoacyl-tRNA binding at the recognition site is the first step of the elongation cycle of protein synthesis. Proc Natl Acad Sci U S A. 1977 May;74(5):1903–1907. doi: 10.1073/pnas.74.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Bursztyn H. Initiation of protein synthesis II. A convenient assay for the ribosome-dependent synthesis of N-formyl-C14-methionylpuromycin. Biochem Biophys Res Commun. 1966 Oct 20;25(2):233–238. doi: 10.1016/0006-291x(66)90586-9. [DOI] [PubMed] [Google Scholar]
- Lührmann R., Eckhardt H., Stöffler G. Codon-anticodon interaction at the ribosomal peptidyl-site. Nature. 1979 Aug 2;280(5721):423–425. doi: 10.1038/280423a0. [DOI] [PubMed] [Google Scholar]
- Mizuno H., Sundaralingam M. Stacking of Crick Wobble pair and Watson-Crick pair: stability rules of G-U pairs at ends of helical stems in tRNAs and the relation to codon-anticodon Wobble interaction. Nucleic Acids Res. 1978 Nov;5(11):4451–4461. doi: 10.1093/nar/5.11.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolell J., Cabrer B., Vázquez D. The stoichiometry of ribosomal translocation. J Biol Chem. 1973 Dec 25;248(24):8356–8360. [PubMed] [Google Scholar]
- Modolell J., Vazquez D. Inhibition by aminoacyl transfer ribonucleic acid of elongation factor G-dependent binding of guanosine nucleotide to ribosomes. J Biol Chem. 1973 Jan 25;248(2):488–493. [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Noll M., Hapke B., Schreier M. H., Noll H. Structural dynamics of bacterial ribosomes. I. Characterization of vacant couples and their relation to complexed ribosomes. J Mol Biol. 1973 Apr 5;75(2):281–294. doi: 10.1016/0022-2836(73)90021-1. [DOI] [PubMed] [Google Scholar]
- Odom O. W., Craig B. B., Hardesty B. A. The conformation of the anticodon loop of yeast tRNAPhe in solution and on ribosomes. Biopolymers. 1978 Dec;17(12):2909–2931. doi: 10.1002/bip.1978.360171212. [DOI] [PubMed] [Google Scholar]
- Paszyc S., Rafalska M. Photochemical properties of Yt base in aqueous solution. Nucleic Acids Res. 1979 Jan;6(1):385–397. doi: 10.1093/nar/6.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. 3. The formation of peptide bonds by ribosomes in the absence of supernatant enzymes. J Biol Chem. 1968 May 25;243(10):2810–2820. [PubMed] [Google Scholar]
- RajBhandary U. L., Chang S. H. Studies on polynucleotides. LXXXII. Yeast phenylalanine transfer ribonucleic acid: partial digestion with ribonuclease T-1 and derivation of the total primary structure. J Biol Chem. 1968 Feb 10;243(3):598–608. [PubMed] [Google Scholar]
- Rappoport S., Lapidot Y. The chemical preparation of acetylaminoacyl-tRNA. Methods Enzymol. 1974;29:685–688. doi: 10.1016/0076-6879(74)29060-8. [DOI] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Riddle D. L., Carbon J. Frameshift suppression: a nucleotide addition in the anticodon of a glycine transfer RNA. Nat New Biol. 1973 Apr 25;242(121):230–234. doi: 10.1038/newbio242230a0. [DOI] [PubMed] [Google Scholar]
- Watanabe S. Interaction of siomycin with the acceptor site of Escherichia coli ribosomes. J Mol Biol. 1972 Jun 28;67(3):443–457. doi: 10.1016/0022-2836(72)90462-7. [DOI] [PubMed] [Google Scholar]
- Wimmer E., Maxwell I. H., Tener G. M. A simple method for isolating highly purified yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1968 Jul;7(7):2623–2628. doi: 10.1021/bi00847a026. [DOI] [PubMed] [Google Scholar]
- Woese C. Molecular mechanics of translation: a reciprocating ratchet mechanism. Nature. 1970 May 30;226(5248):817–820. doi: 10.1038/226817a0. [DOI] [PubMed] [Google Scholar]
- Wrede P., Woo N. H., Rich A. Initiator tRNAs have a unique anticodon loop conformation. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3289–3293. doi: 10.1073/pnas.76.7.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmbach P., Nierhaus K. H. Codon-anticodon interaction at the ribosomal P (peptidyl-tRNA)site. Proc Natl Acad Sci U S A. 1979 May;76(5):2143–2147. doi: 10.1073/pnas.76.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]