Abstract
Extranuclear estrogen receptor protein (estrophilin) of MCF-7 human breast cancer cells was purified by passage of the cytosol fraction of a cell homogenate through an affinity column of estradiol linked to Sepharose by a substituted di-n-propyl sulfide bridge in the 17 alpha position. Elution with 50 micro M [3H]estradiol in 10% (vol/vol) dimethyl formamide/0.5 M sodium thiocyanate gave 40% recovery of [3H]estradiol-estrophilin showing 14% of the specific radioactivity expected for the pure complex. Serum from a Lewis rat immunized with this partially purified estradiol-receptor complex contained antiestrophilin antibodies that reacted not only with nuclear and extranuclear estradiol-receptor complexes from MCF-7 cells but also with estrophilin from rat, calf, and monkey uterus, hen oviduct, and human breast cancers. Splenic lymphocytes from the immunized rat were fused with cells of two different mouse myeloma lines (P3-X63-Ag8 and Sp2/0-Ag14) to yield hybridoma cultures, 2% of which produced antibodies to estrophilin. After cloning by limiting dilution, three hybridoma lines secreting antiestrophilin were expanded in suspension culture and as ascites tumors in athymic mice to provide substantial quantities of monoclonal antibodies that recognize mammalian but not avian estrophilin and that show different degrees of reactivity with receptor from nonprimate sources. By growing the clone from Sp2/0 in the presence of [35S]methionine, radiolabeled monoclonal IgG has been prepared. These monoclonal antibodies should prove useful in the study of estrogen receptors of human reproductive tissues, in particular for the radioimmunochemical assay and immunocytochemical localization of receptors in breast cancers.
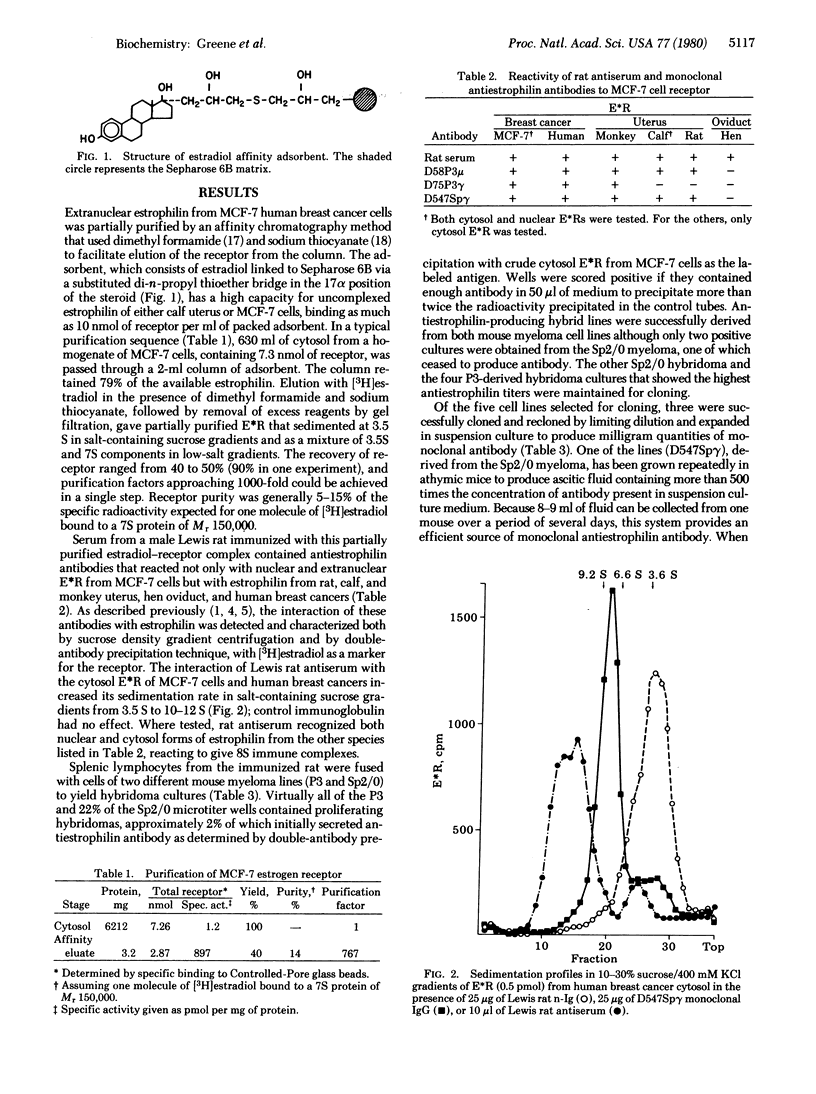
Full text
PDF

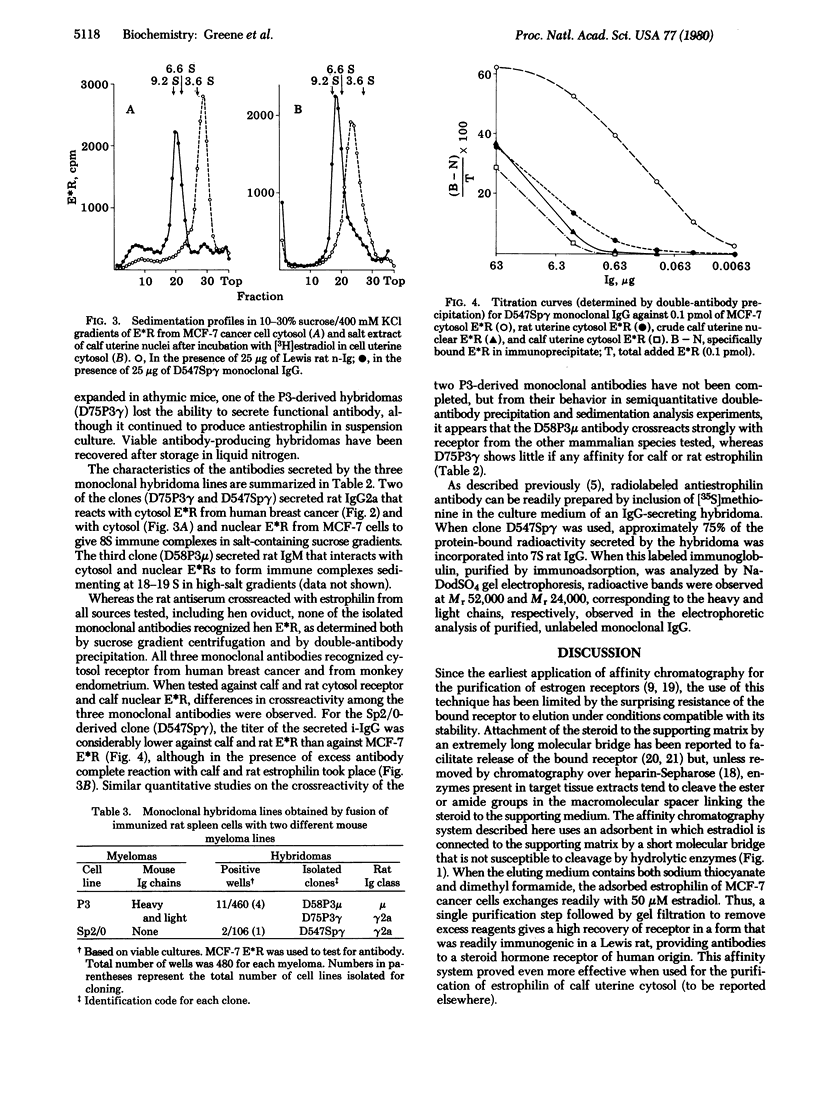

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. K., Veysoglu T. A simple procedure for the epoxidation of acid-sensitive olefinic compounds with m-chloroperbenzoic acid in an alkaline biphasic solvent system. J Org Chem. 1973 Jun 15;38(12):2267–2268. doi: 10.1021/jo00952a046. [DOI] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bazin H., Beckers A., Querinjean P. Three classes and four (sub)classes of rat immunoglobulins: IgM, IgA, IgE and IgG1, IgG2a, IgG2b, IgG2c. Eur J Immunol. 1974 Jan;4(1):44–48. doi: 10.1002/eji.1830040112. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Engers H. D., Macdonald H. R., Brunner T. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):703–717. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSombre E. R., Carbone P. P., Jensen E. V., McGuire W. L., Wells S. A., Jr, Wittliff J. L., Lipsett M. B. Special report. Steriod receptors in breast cancer. N Engl J Med. 1979 Nov 1;301(18):1011–1012. doi: 10.1056/NEJM197911013011826. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Closs L. E., De Sombre E. R., Jensen E. V. Estrophilin: pro and anti. J Steroid Biochem. 1980 Jan;12:159–167. doi: 10.1016/0022-4731(80)90265-4. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Closs L. E., DeSombre E. R., Jensen E. V. Antibodies to estrophilin: comparison between rabbit and goat antisera. J Steroid Biochem. 1979 Jul;11(1A):333–341. doi: 10.1016/0022-4731(79)90316-9. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Closs L. E., Fleming H., DeSombre E. R., Jensen E. V. Antibodies to estrogen receptor: immunochemical similarity of estrophilin from various mammalian species. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3681–3685. doi: 10.1073/pnas.74.9.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene G. L., Fitch F. W., Jensen E. V. Monoclonal antibodies to estrophilin: probes for the study of estrogen receptors. Proc Natl Acad Sci U S A. 1980 Jan;77(1):157–161. doi: 10.1073/pnas.77.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKearn T. J., Fitch F. W., Smilek D. E., Sarmiento M., Stuart F. P. Properties of rat anti-MHC antibodies produced by cloned rat-mouse hybridomas. Immunol Rev. 1979;47:91–115. doi: 10.1111/j.1600-065x.1979.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Musto N. A., Gunsalus G. L., Miljković M., Bardin C. W. A novel affinity column for isolation of androgen binding protein from rat epididymis. Endocr Res Commun. 1977;4(2):147–157. doi: 10.3109/07435807709073919. [DOI] [PubMed] [Google Scholar]
- Radanyi C., Redeuilh G., Eigenmann E., Lebeau M. C., Massol N., Secco C., Baulieu E. E., Richard-Foy H. Production et détection d'anticorps antirécepteur de l'oesstradiol d'utérus de veau. Interaction avec le récepteur d'oviducte de poule. C R Seances Acad Sci D. 1979 Jan 15;288(2):255–258. [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Sica V., Bresciani F. Estrogen-binding proteins of calf uterus. Purification to homogeneity of receptor from cytosol by affinity chromatography. Biochemistry. 1979 May 29;18(11):2369–2378. doi: 10.1021/bi00578a036. [DOI] [PubMed] [Google Scholar]
- Sica V., Parikh I., Nola E., Puca G. A., Cuatrecasas P. Affinity chromatography and the purification of estrogen receptors. J Biol Chem. 1973 Sep 25;248(18):6543–6558. [PubMed] [Google Scholar]
- Truong H., Geynet C., Millet C., Soulignac O., Bucourt R., Vignau M., Torelli V., Baulieu E. E. Purification of estradiol receptor by affinity chromatography. Representative experiments. FEBS Lett. 1973 Sep 15;35(2):289–294. doi: 10.1016/0014-5793(73)80306-0. [DOI] [PubMed] [Google Scholar]
- Vonderhaar B., Mueller G. C. Binding of estrogen receptor to estradiol immobilized on insoluble resins. Biochim Biophys Acta. 1969 Apr 29;176(3):626–631. doi: 10.1016/0005-2760(69)90229-x. [DOI] [PubMed] [Google Scholar]


