Abstract
Introduction:
Hirsutism has a significant impact on the quality of life and serves as a marker of underlying hormonal and systemic conditions. The aim of this study was to study the clinical, biochemical characteristics of these patients and other associations.
Materials and Methods:
Fifty (n=50) consecutive newly diagnosed patients of hirsutism were assessed during a period from August 2009 to July 2010 using modified Ferriman Gallwey (mF-G) score.
Results:
Idiopathic hirsutism (IH) was found in 30 (60%) patients followed by polycystic ovarian syndrome (PCOS) in 19 (38%) patients. Other causes included late-onset classic adrenal hyperplasia in two (4%) and hypothyroidism in four (8%) patients. The mean age at presentation was 23.8±6.657 years. Total (T) and free testosterone (fT), 17-hydroxyprogesterone was significantly higher in PCOS than IH.
Conclusion:
The present data show IH as the commonest cause of hirsutism in our study population. Face, chest, and lower abdomen have a higher impact on the hirsutism score while upper back, abdomen, and lower back are rarely involved.
Keywords: Ferriman–gallwey score, hirsutism, polycystic ovarian syndrome
INTRODUCTION
Hirsutism is defined as the presence of excessive terminal hair in androgen-dependent areas of a woman's body.[1] It is different from hypertrichosis which actually refers to excessive hair in areas that are not predominantly androgen dependent.[2] The degree of hirsutism is the most important determinant of quality of life, second to obesity in patients of polycystic ovarian syndrome (PCOS).[3] A modification of the original method proposed by Ferriman and Gallwey is in general use for visually scoring excess terminal hair for clinical and investigational assessment.[4]
The multifactorial and multiorgan nature of this disorder makes it difficult to identify a single etiologic cause which necessitates a detailed clinical and diagnostic evaluation of the involved organs.
Idiopathic hirsutism (IH) constitutes an important cause of hirsutism. It refers to hirsutism in the presence of normal ovulatory function and normal circulating androgen levels. The underlying pathophysiology is believed to be due to exaggerated activity of 5α reductase, androgen receptor polymorphisms, and altered androgen metabolism.[5,6]
Hyperandrogenemia is one of the common causes of endocrinopathy in women of reproductive age group. The most important of ovarian hyperandrogenic disorder is PCOS. This prototype of chronic hyperandrogenic anovulation provides the hormonal milieu for several conditions. These include the risk of diabetes,[7] hypertension,[8] dyslipidemia,[8] cardiovascular disease,[8] and malignancies.[9] There is no consensus as to the clinical definition of PCOS. No consistent clinical marker or phenotype is unique to PCOS, distinguishing it from other causes of hyperandrogenism. An International Consensus group recently proposed that the syndrome can be diagnosed if at least two of the following are present: oligoovulation or anovulation (usually manifested as oligomenorrhea or amenorrhea), elevated levels of circulating androgens (hyperandrogenemia) or clinical manifestations of androgen excess (hyperandrogenism), and polycystic ovaries as defined by ultrasonography (USG).[10]
Androgen-secreting ovarian tumors such as arrhenoblastoma, hilar cell tumor, microadenoma, leydig cell tumor, granular thecal cell tumor, and luteoma are associated with rapidly progressive hirsutism and virilization.[11] They are relatively rare, affecting between one per 300 to 1,000 hirsute patients.
Between 1 – 8% of hyperandrogenic women suffer from 21-hydroxylase-deficient non-classic adrenal hyperplasia (CAH) which may be asymptomatic until adolescence or adulthood when they present with hirsutism.[12] Cushing's syndrome is caused by a pituitary micro adenoma, ectopic adrenocorticotropic hormone (ACTH) secretion from a non-pituitary neoplasm or from an adrenal neoplasm. Both adenomas and carcinomas may present with isolated virilization in 10% of patients.[13] Hyperprolactinemia is an occasional cause of hirsutism. The exact mechanism by which it causes hirsutism is not exactly known but may involve direct effects on ovarian and adrenal steroidogenesis or decreased sex hormone binding globulin production.[14] Twenty percent of the patients with hypothyroidism have coexistent hyperprolactinemia that may contribute to excessive androgen production and hirsutism.[14]
The non-androgenic causes like excess hair growth seen in acromegalics are rare. In addition, coarsening of the hairs may develop with chronic skin irritation because teleologically hair is designed to protect the skin. Non-androgenic anabolic drugs will cause a generalized growth of many tissues, particularly hair generally leading to vellus hypertrichosis and not hirsutism.
MATERIALS AND METHODS
The study was conducted in the Department of Dermatology, Venereology and Leprosy, Indira Gandhi Medical College, Shimla, over a period of one year from August 1, 2009 to July 31, 2010 and was approved by the Ethical committee. An informed consent was taken from each participant along with a written permission to use the subject photographs for academic purposes.
Selection of patients
Inclusion criteria
All the consecutive newly diagnosed 50 patients of hirsutism in the reproductive age group (15 to 45 years) attending the Out Patient Department with modified Ferriman-Gallwey (mF-G) score of eight or more were included in the study.
Exclusion criteria
Pregnant or Lactating women
Those who received oral contraceptive pills or/and other anti-androgen drugs in previous three months
Those who received drugs known to cause hirsutism or interfere with the hormonal studies
Those patients with modified Ferriman-Gallwey (mF- G) score less than 8.
Study design
A meticulous menstrual history included the age at menarche and the presence of menstrual irregularity. The menstrual patterns were defined as regular cycles if the length of cycle was between 22 and 40 days. The cycle was considered as irregular if the patient had oligomenorrhea (bleeding at interval greater than 40 days), polymenorrhea (bleeding at intervals of less than 22 days), or amenorrhea (absence of menstruation for 12 months or more). A careful family history of hirsutism, infertility, and other metabolic disorders was obtained in every patient. Height, weight, waist circumference (midway between iliac crest and lower margin of ribs), hip circumference (maximum circumference of buttocks), waist/hip ratio, muscle bulk, and blood pressure were recorded in every patient.
Obesity was taken as BMI, i.e., body mass index (weight in kg/height in meters square) ≥ 30 kg/m2 while patients with BMI≥25kg/m2 were labeled as overweight. The patients were examined for the clinical evidence of acne, androgenic alopecia, acanthosis nigricans (AN), striae, clitoromegaly, and increased muscle mass.
The degree of hirsutism was assessed using the modified Ferriman-Gallwey (mF-G) score in the upper lip, chin, areola and chest, upper back, lower back, upper abdomen, lower abdomen, thighs, and upper arms. Hirsutism was classified as mild (score 8-16), moderate (score17-24), and severe (score >24). A complete systemic examination (including thyroid, breast, and pelvic) was performed in these patients.
Hormonal profile was assessed in all patients. Blood sampling was done in the early follicular phase of spontaneous or induced (by Medroxyprogesterone acetate 10 mg/day for 7 days) menstrual cycle (day 3 to 4) after overnight fasting for 10 to 12 hours and was collected around 8-9 am.
Serum Follicle-Stimulating Hormone (FSH), luteinizing hormone (LH), Prolactin was measured using the principle of Sandwich Enzyme-linked immunosorbent Assay (ELISA) method. Serum Testosterone, free (F) and total (T), Dehydroepiandrosterone Sulfate (DHEAS), 17-hydroxyprogesterone (17-OHP), lipid profile, and thyroid profile were assayed using competitive ELISA technique.
Transabdominal USG was done in all patients during the follicular phase of menstrual cycle (Day 5). The USG criteria to define polycystic ovaries were presence of 12 or more follicles in each ovary measuring 2 to 9 mm in diameter, and/or increase in ovarian volume (>10 ml) and only one ovary fitting this definition is sufficient for diagnosis.[10]
The Rotterdam 2003 criteria were used for classification of patients as PCOS, whereas presence of regular ovulation and normal androgen levels were diagnostic of IH.[10]
Statistical analysis was done using Minitab version 14 and Chi square test was used for comparison between categorical variables. Two-sample t-test was used for the comparison of means between two continuous variables. One-way ANOVA (Analysis of variance) was used for comparison of means from two or more sample populations. One-way ANOVA is a technique used to compare means of two or more samples. It is a way to test the equality means at one time by using variances. The one-way ANOVA is useful when we want to compare the effect of multiple levels of one factor and we have multiple observations at each level. P value of <0.05 was considered significant. An attempt to establish a possible etiologic profile and correlation between various parameters was done.
OBSERVATIONS AND RESULTS
Majority (40%) of patients were in the age group of 21 to 25 years. The mean age of the patients enrolled in the study was 23.8 years with a standard deviation of 6.657. There were 40 (80%) unmarried patients and 10 (20%) married patients in the studied population. The commonest cause of mild to moderate hirsutism (mF-G score >8) was IH (50%) followed by PCOS (38%), late onset CAH (4%), and hypothyroidism (8%). None of our patients had androgen-secreting tumors, hyperandrogenic insulin-resistant acanthosis nigricans syndrome (HAIR-AN), or Cushing's syndrome.
There was history of regular menstrual cycle in 36 patients (72%) and irregular menstruation in 14 patients (28%). Of the patients with irregular menstruation, two patients (4% of total) had amenorrhea and 12 (24% of total) had oligomenorrhea. PCOS was diagnosed in 64.2% of the patients with irregular menstrual cycle, of which two patients had amenorrhea and seven patients had oligomenorrhea. Significant association was noted between menstrual irregularity and PCOS (chi square, P value is 0.007).
One patient (2%) gave history of voice changes while three patients (6%) had history of decreased libido. Four patients (8%) presented with a history of malodorous perspiration. These infrequently asked complaints can serve as an important pointer in the diagnosis of hyperandrogenic disorder.
Easy fatigability was seen in seven patients (14%). Of these, PCOS was diagnosed in three (6%) patients, IH in three (6%), and one patient (2%) had hypothyroidism.
Eight patients (8%) of total sample had positive history of hirsutism in the first degree relative. Of the eight patients (16%) with a positive family history, four patients (8%) were diagnosed with PCOS, two (4%) had IH, one (2%) was hypothyroid, and one (2%) patient was diagnosed with late-onset CAH (LoCAH).
The mean weight of the PCOS group was 63.42±10.52 kg and 57.91±10.24 kg in IH while LoCAH had a mean weight of 62±5.66 kg. Comparison of mean weight amongst the different groups with one-way ANOVA revealed no statistical difference (P value - 0.109).
The mean BMI for PCOS patients was 25.48±3.82 kg/ m2 and 23.67±3.8 kg/m2 in IH and 24.95±0.71 kg/m2 in LoCAH but comparison of mean weight amongst the different groups with one-way ANOVA revealed no statistical difference.
Acne (73.68%) and AN (26.32%) was more significant in PCOS (P value 0.049 and 0.033, respectively). Clitoromegaly was present in two (4%) patients both of which belonged to LoCAH. Female pattern hair loss (FPHL) was more commonly seen in PCOS (21.05%) than IH (12%) and was significant (P value 0.030).
Forty (80%) patients had mild hirsutism score while 10 (20%) patients had moderate hirsutism; however, severe hirsutism was not found in any patient. Figure 1 depicts the mean m F-G score of different sites.
Figure 1.
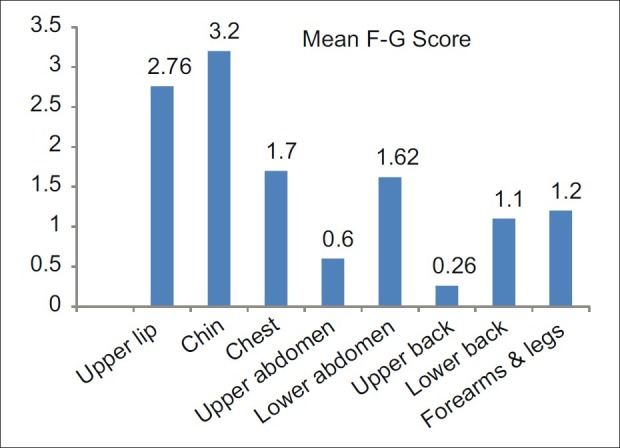
Mean mF-G score of different sites
A two-sample t-test for comparison of mean values of mF-G score for PCOS and IH groups revealed no significant difference (P value, 0.674), although they were higher for LoCAH than PCOS.
Serum levels of LH and FSH were not significantly different between these two groups, whereas LH: FSH ratio (>2) was significantly elevated in PCOS than IH.
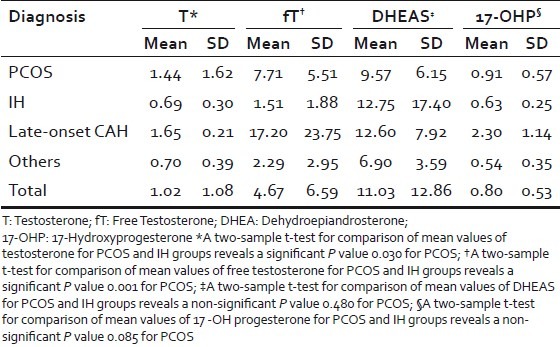
Circulating androgen levels (T, fT, 17-OHP) were significantly raised in PCOS group, compared with IH women (P value <0.05 in all comparisons) [Table 1]. However, there was no statistically significant difference between serum levels of DHEAS between the groups. USG changes consistent with polycystic ovarian morphology were observed in 79% of PCOS patients.
Table 1.
Comparison of androgen profile between major diagnostic groups
DISCUSSION
Hirsutism is no longer a benign cosmetically disfiguring condition, but it can serve as a pointer to underlying hormonal and other systemic conditions. Hence, a rational differential diagnosis of this condition becomes an utmost concern. Most of the patients in our study group (n=50) which comprised of women of reproductive age group (15–45 years) belonged to the age group of 21 to 25 years (40%) with a mean age of 23.8±6.657 years followed by 16 to 20 years (32%). Ansarin et al. reported 16 to 35 years (86.6%) to be the commonest with the mean age range of 20.9 years.[15] Most of the hyperandrogenic disorders begin to clinically manifest in peripubertal age group which can explain the high prevalence in this age group.
Most of the patients attending our hirsutism clinic were unmarried (80%) as also seen in 73.8% of patients in Iranian study.[15] The cosmetic concern and matrimonial prospects can be a possible reason for more unmarried women seeking treatment.
Zargar et al. found menstrual cycles to be irregular in 50.8% of patients complaining of hirsutism.[16] Azziz et al. reported irregular menstrual cycle in 22.8% of patients similar to the rate of 22.9% reported by 101,073 women participating in the Nurses’ Health Study II for cycles 32 days or more.[17,18] This is similar to our finding of menstrual irregularity in 28% of our study population. A significant associ[ation was noted between menstrual history and PCOS (P<0.05) in our study as 64.2% of patients reporting irregular menstrual cycle were positive for the diagnosis. A hospital-based study also pointed that 75-90% of women with oligomenorrhea had PCOS.[19]
Decreased libido was present in three patients (6%) and voice changes in one (2%) patient of PCOS. Malik et al. reported voice changes in 2.7% of their patients.[20] Malodorous perspiration was more commonly seen in PCOS (6%) than IH (2%), although one patient of IH had concomitant diabetes mellitus.
Obesity (BMI>30 kg/m2) was noted in 20% of our study population and 16% were overweight. Ansarin et al. reported obesity in 6.4% of their patients, while 24.8% of hirsute patients were found to be obese (30 kg/m2) by Zargar et al. similar to our study.[15,16] This can be possibly explained by the ethnic similarity between the two North Indian population groups. The mean BMI was slightly higher in PCOS (25.48±3.82) group than in IH (23.67±3.8) but was not significant (P value >0.05), similar to that reported by Carmina.[21]
Acne was the most frequent sign of hyperandrogenism in our study population seen in 60% of patients. Balen et al. and Franks et al. reported the frequency of acne in 35% and 27% of patients, respectively.[19,22] Malik et al. also found it to be the commonest sign of hyperandrogenism in 36% of patients.[20] AN was significantly more in patients of PCOS (73.68%) than IH (44%). FPHL was significantly present in 47.36% of patients with PCOS. Abdominal striae were more common in patients of PCOS (36.84%) than IH which to our best of knowledge has not been reviewed in the literature before in patients of hirsutism without any evidence of Cushing's syndrome.
The hirsutism score between the different groups revealed no significant difference in our study. This finding is also supported by Azziz et al.[17] The mean mF-G scores were highest for chin and upper lip followed by chest and lower abdomen in our study while lower back, upper back, and abdomen were rarely involved. The mean hirsutism score of the entire sample in our study was 12.44±3.64. Similar mean hirsutism score of 13.5±4.6 was reported in a study on Kashmiri women.[16] Similar ethnic and racial background can possibly explain this similarity.
The chin scored the maximum mF-G score followed by upper lip, chest, and lower abdomen. This is similar to the finding of Malik et al. who reported similar sites to be more commonly involved.[20] Knochenhauer et al. concluded that a score of ≥2 in either chin or lower abdomen detected virtually all hirsute patients with sensitivity of 100%, although the authors emphasized this finding to be of low positive predictive value and hence more relevant in population with high frequency of hirsutism.[23] The upper back, abdomen, and lower back were rarely affected in our patients. Hassa et al. reported similar finding in a study of Turkish hirsute women, but the authors found thigh to have highest mean score of 2.26±0.16, whereas we recorded a mean score of 1.2.[24] The authors also found perineum and side burn area to be more importantly involved, although they are not included in routine scoring methods.
Ten of thirty-one patients of PCOS had elevated LH : FSH ratio in a study by Hassa et al.[24] This has been found in our study where PCOS patients were found to have twice elevated LH : FSH ratio (>2) than IH. On the other hand, Carmina concluded that there was no difference in the ratio of LH : FSH between IH and PCOS.[21] The LH : FSH ratio can be found in a broad range due to pulsatile nature of gonadotropins and hence can explain the varied results in different studies.
PCOS patients had higher levels of free testosterone (fT) than IH as seen in our study and supported in the literature.[18] The level of 17-OHP were elevated in 36% of our patients, although they were significantly elevated only in three patients of whom two belonged to LoCAH group (ACTH stimulation test positive).
Among the PCOS patients, ultrasonographic (USG) changes were found in 79% of patients. Ansarin et al. and Hassa et al. had reported similar findings in their respective study population.[15,24] The relatively high yield of positive abdominal USG in the diagnosis of PCOS appeared to make it essential for the diagnostic criteria. More accurate assessment would require the use of vaginal USG, especially for the obese patients which were not available for our patients.
IH (50%) was the commonest cause of hirsutism in our study. It is noteworthy that a history of regular menstrual cycle is not sufficient to exclude anovulation as also observed by Azziz et al.[12] This may be the reason for the greater incidence of IH in our study group. PCOS was found in 38% in our patients which was similar to Zargar et al., although a higher incidence has been reported in literature.[16] The lack of consensus on the criteria used in different studies could be a possible explanation for variable incidence reported in literature. LoCAH was seen in two (4%) patients who had notably increased basal 17-OHP levels and were positive for ACTH stimulation test. The frequency of these patients is less as compared with other similar studies.[25] LoCAH is a relatively rare condition and the frequency ranges from 1.3% to 30% in the literature. This can be explained on the basis of several factors. First, the frequency of the genetic mutation in 21 – hydroxylase may vary among the population. Second, the study design and severity of hirsutism is variable in different studies. Hypothyroidism (8%), as seen in our study population, has also been reported in literature.
CONCLUSION
From this study, it can be concluded that face, chest, and lower abdomen have a high impact on the hirsutism score, whereas areas like upper back, abdomen, and lower back are rarely involved. As severe hirsutism was not found in our study with mF-G score of ≥8, we propose a lower cut off of mF-G score on a larger study sample for arriving at population-specific criteria for defining hirsutism. The major drawback of this study is that the results of smaller sample size used in the study cannot be used to arrive at population-specific criteria. IH is the commonest cause followed by PCOS, whereas LoCAH is a less prevalent cause. Signs and symptoms suggestive of hyperandrogenism are more prevalent in PCOS than IH, but subtle evidence of functional hyperandrogenism highlights a more detailed investigative approach to the idiopathic group. A luteal progesterone level or daily basal body temperature for documentation of ovulation which was not possible in our settings would have confirmed anovulation. A follow up is essential in patients of PCOS for prevention of metabolic and other complications.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Redmond GP, Bergfeld WF. Diagnostic approach to androgen disorders in women: Acne, Hirsutism and Alopecia. Cleve Clin J Med. 1990;57:423–7. doi: 10.3949/ccjm.57.5.423. [DOI] [PubMed] [Google Scholar]
- 2.Wendelin S, Pope D, Mallory S. Hypertrichosis. J Am Acad Dermatol. 2003;48:161–79. doi: 10.1067/mjd.2003.100. [DOI] [PubMed] [Google Scholar]
- 3.Guyatt G, Weaver B, Cronin L, Dooley JA, Azziz R. Health-related quality of life in women with polycystic ovary syndrome, a self administered questionnaire, was validated. J Clin Epidemiol. 2004;57:1279–87. doi: 10.1016/j.jclinepi.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Ferriman D, Galleway JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–7. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 5.Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74–8. [PubMed] [Google Scholar]
- 6.Calvo RM, Asunción M, Sancho J, San Millán JL, Escobar-Morreale HF. The role of the CAG repeat polymorphism in the androgen receptor gene and of skewed X-chromosome inactivation, in the pathogenesis of hirsutism. J Clin Endocrinol Metab. 2000;85:1735–40. doi: 10.1210/jcem.85.4.6561. [DOI] [PubMed] [Google Scholar]
- 7.Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 1998;83:2694–8. doi: 10.1210/jcem.83.8.5054. [DOI] [PubMed] [Google Scholar]
- 8.Dahlgren E, Janson P, Johansson S, Lapidus L, Oden A. Polycystic ovary syndrome and risk for myocardial infarction: Evaluated from a risk factor model based on a prospective population study of women. Acta Obstet Gynecol Scand. 1992;71:599–604. doi: 10.3109/00016349209006227. [DOI] [PubMed] [Google Scholar]
- 9.Dahlgren E, Friberg LG, Johansson S, Lindstrom B, Oden A, Samsioe G, et al. Endometrial carcinoma: Ovarian dysfunction – A risk factor in young women. Eur J Obstet Gynecol Reprod Biol. 1991;41:143–50. doi: 10.1016/0028-2243(91)90092-y. [DOI] [PubMed] [Google Scholar]
- 10.Revised 2003 Consensus on diagnostic criteria and long term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 11.Cole CH. Hirsutism, hypertrichosis, and precocious sexual hair development. In: Hoekelman RS, Friedman SB, Nelson NM, Siedel HM, editors. Primary pediatric care. St. Louis: Mosby Year Book; 1992. p. 976. [Google Scholar]
- 12.Azziz R, Dewailly D, Owerbach D. Non classic adrenal hyperplasia: Current concepts. J Clin Endocrinol Metab. 1994;78:810–5. doi: 10.1210/jcem.78.4.8157702. [DOI] [PubMed] [Google Scholar]
- 13.King DR, Lack EE. Adrenal cortical carcinoma: A clinical and pathologic study of 49 cases. Cancer. 1979;44:239–44. doi: 10.1002/1097-0142(197907)44:1<239::aid-cncr2820440139>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz FL, Flink EB. Hirsutism: Pathophysiology, clinical evaluation, treatment. Postgrad Med. 1985;77:81–93. doi: 10.1080/00325481.1985.11699027. [DOI] [PubMed] [Google Scholar]
- 15.Ansarin H, Azziz-Jalali MH, Rasi Abbas, Arabshahi RS. Clinical presentation and etiologic factors of hirsutism in premenopausal Iranian women. Arch Iranian Med. 2007;10:7–13. [PubMed] [Google Scholar]
- 16.Zargar AH, Wani AI, Masoodi SR, Laway BA, Bashir MI, Salahuddin M. Epidemiologic and etiologic aspects of hirsutism in Kashmiri women in the Indian subcontinent. Fertil Steril. 2002;77:674–8. doi: 10.1016/s0015-0282(01)03241-1. [DOI] [PubMed] [Google Scholar]
- 17.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2003;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 18.Solomon CG, Hu FB, Dunaif A, Rich-Edwards J, Willet WC, Hunter DJ, et al. Prevalence of polycystic ovarian syndrome in women seeking treatment from community electrologists. J Reprod Med. 1999;44:870–4. [PubMed] [Google Scholar]
- 19.Balen AH, Conway GS, Kaltsas G, Techatraisak K, Manning PJ, West C, et al. Polycystic ovary syndrome: The spectrum of the disorder in 1741 patients. Hum Reprod. 1995;10:2107–11. doi: 10.1093/oxfordjournals.humrep.a136243. [DOI] [PubMed] [Google Scholar]
- 20.Malik L, Khursheed K, Haroon T, Malik M. An aetiological study of moderate to severe hirsutism. Pak J Med Sci. 2007;23:167–71. [Google Scholar]
- 21.Carmina E. Prevalence of idiopathic hirsutism. Eur J Endocrinol. 1998;139:421–3. doi: 10.1530/eje.0.1390421. [DOI] [PubMed] [Google Scholar]
- 22.Franks S. Polycystic ovary syndrome: A changing perspective. Clinical Endocrinol. 1989;31:87–120. doi: 10.1111/j.1365-2265.1989.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 23.Knochenhauer E, Hines G, Myers CB, Azziz R. Examination of the chin or lower abdomen only for the prediction of hirsutism. Fertil Steril. 2000;74:980–3. doi: 10.1016/s0015-0282(00)01602-2. [DOI] [PubMed] [Google Scholar]
- 24.Hassa H, Tanir HM, Yildirim A, Senses T, Eskalen M. The hirsutism scoring system should be population specific. Fertil Steril. 2005;84:778–80. doi: 10.1016/j.fertnstert.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 25.Elder-Geva T, Hurwitz A, Vecsei P, Palti Z, Milwidsky A, Rösler A. Secondary biosynthetic defects in women with late- onset congenital adrenal hyperplasia. N Engl J Med. 1990;13:855–63. doi: 10.1056/NEJM199009273231302. [DOI] [PubMed] [Google Scholar]


