Abstract
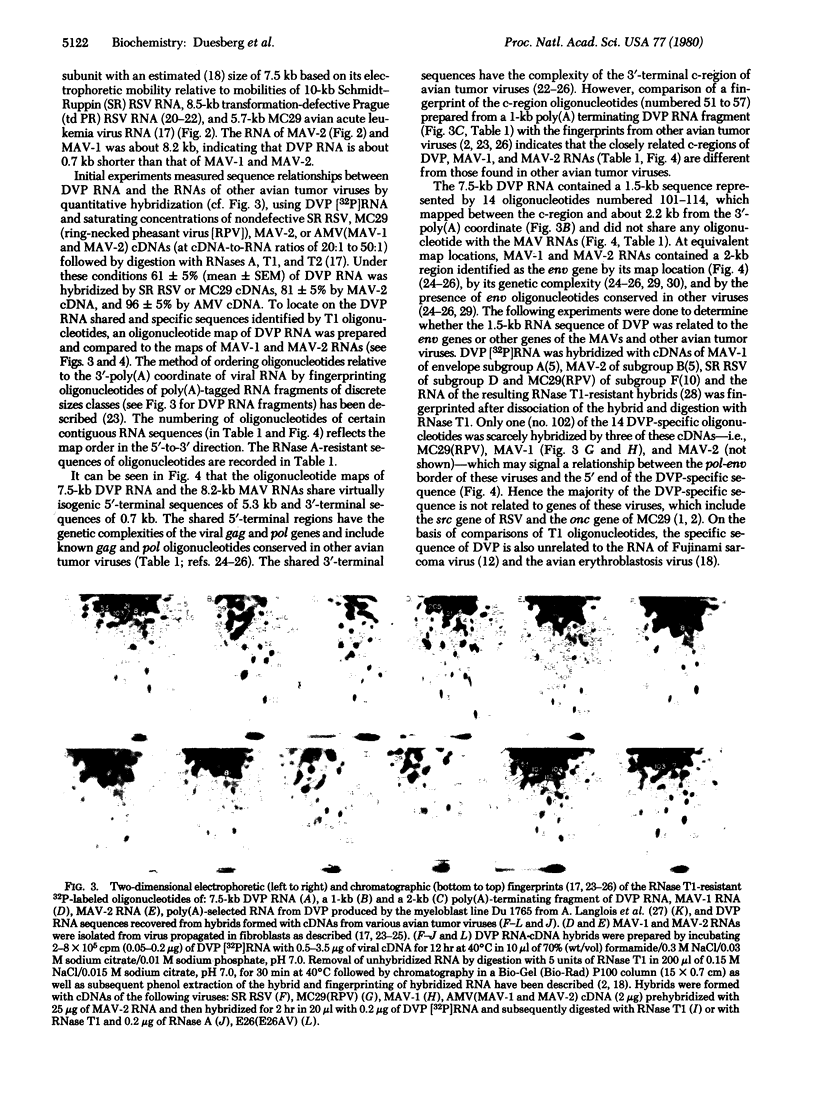
Chicken myeloblasts transformed by avian myeloblastosis virus (AMV) in the absence of nondefective helper virus (termed nonproducer cells) were found to release a defective virus particle (DVP) that contains avian tumor viral gag proteins but lacks envelope glycoprotein and a DNA polymerase. Nonproducer cells contain a Pr76 gag precursor protein and also a protein that is indistinguishable from the Pr180 gag-pol protein of nondefective viruses. The RNA of the DVP is 7.5 kilobases (kb) long and is 0.7 kb shorter than the 8.2-kb RNAs of the helper viruses of AMV, MAV-1 and MAV-2. Comparisons based on RNA·cDNA hybridization and mapping of RNase T1-resistant oligonucleotides indicated that DVP RNA shares with MAV RNAs nearly isogenic 5′-terminal gag and pol-related sequences of 5.3 kb and a 3′-terminal c-region of 0.7 kb that is different from that found in other avian tumor viruses. Adjacent to the c-region, DVP RNA contains a contiguous specific sequence of 1.5 kb defined by 14 specific oligonucleotides. Except for two of these oligonucleotides that map at its 5′ end, this sequence is unrelated to any sequences of nondefective avian tumor viruses of four different envelope subgroups as well as to the specific sequences of fibroblast-transforming avian acute leukemia and sarcoma viruses of four different RNA subgroups. The specific sequence of the DVP RNA is present in infectious stocks of AMV from this and other laboratories in an AMV-transformed myeloblast line from another laboratory, and it is about 70% related to nucleotide sequences of E26 virus, an independent isolate of an AMV-like virus. Preliminary experiments show DVP to be leukemogenic if fused into susceptible cells in the presence of helper virus. We conclude that DVP RNA is the leukemogenic component of infectious AMV and that its specific sequence, termed AMV, may carry genetic information for oncogenicity. Thus we have found here a transformation-specific RNA sequence, unrelated to helper virus, in a highly oncogenic virus that does not transform fibroblasts.
Keywords: RNA and protein electrophoresis, oligonucleotide mapping, RNA·cDNA hybridization, unique c-region, specific
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Genetic structure of avian acute leukemia viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):801–822. doi: 10.1101/sqb.1980.044.01.086. [DOI] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Structure and specific sequences of avian erythroblastosis virus RNA: evidence for multiple classes of transforming genes among avian tumor viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5023–5027. doi: 10.1073/pnas.76.10.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bister K., Löliger H. C., Duesberg P. H. Oligoribonucleotide map and protein of CMII: detection of conserved and nonconserved genetic elements in avian acute leukemia viruses CMII, MC29, and MH2. J Virol. 1979 Oct;32(1):208–219. doi: 10.1128/jvi.32.1.208-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Moscovici M. G., Moscovici C. Isolation of complementary DNA unique to the genome of avian myeloblastosis virus (AMV). Virology. 1980 May;103(1):112–122. doi: 10.1016/0042-6822(80)90130-0. [DOI] [PubMed] [Google Scholar]
- Dougherty R. M., Di Stefano H. S. Virus particles associated with "nonproducer" Rous sarcoma cells. Virology. 1965 Nov;27(3):351–359. doi: 10.1016/0042-6822(65)90115-7. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Vogt P. K. The RNA of avian acute leukemia virus MC29. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4320–4324. doi: 10.1073/pnas.74.10.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Martin G. S., Vogt P. K. Glycoprotein components of avian and murine RNA tumor viruses. Virology. 1970 Aug;41(4):631–646. doi: 10.1016/0042-6822(70)90428-9. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson H. L., Robinson W. S., Huebner R. J., Turner H. C. Proteins of Rous sarcoma virus. Virology. 1968 Sep;36(1):73–86. doi: 10.1016/0042-6822(68)90118-9. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Transforming genes of retroviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):13–29. doi: 10.1101/sqb.1980.044.01.005. [DOI] [PubMed] [Google Scholar]
- Duesberg P., Vogt P. K., Beemon K., Lai M. Avian RNA tumor viruses: mechanism of recombination and complexity of the genome. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):847–857. doi: 10.1101/sqb.1974.039.01.099. [DOI] [PubMed] [Google Scholar]
- Evans L. H., Duesberg P. H., Troxler D. H., Scolnick E. M. Spleen focus-forming Friend virus: identification of genomic RNA and its relationship to helper virus RNA. J Virol. 1979 Jul;31(1):133–146. doi: 10.1128/jvi.31.1.133-146.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Oker-Blom N., Todorov T. G., Beug H. Transforming capacities and defectiveness of avian leukemia viruses OK10 and E 26. Virology. 1979 Dec;99(2):431–436. doi: 10.1016/0042-6822(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Hu S., Knight C. A., Davidson N. Heteroduplex analysis of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1977 Feb;74(2):477–481. doi: 10.1073/pnas.74.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois A. J., Ishizaki R., Beaudreau G. S., Kummer J. F., Beard J. W., Bolognesi D. P. Virus-infected avian cell lines established in vitro. Cancer Res. 1976 Nov;36(11 Pt 1):3894–3904. [PubMed] [Google Scholar]
- Lee W. H., Bister K., Pawson A., Robins T., Moscovici C., Duesberg P. H. Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2018–2022. doi: 10.1073/pnas.77.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel W. F., Delius H., Duesberg P. H. Structure and molecular weight of the 60-70S RNA and the 30-40S RNA of the Rous sarcoma virus. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4541–4545. doi: 10.1073/pnas.71.11.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Mellon P., Pawson A., Bister K., Martin G. S., Duesberg P. H. Specific RNA sequences and gene products of MC29 avian acute leukemia virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5874–5878. doi: 10.1073/pnas.75.12.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C. Leukemic transformation with avian myeloblastosis virus: present status. Curr Top Microbiol Immunol. 1975;71:79–101. doi: 10.1007/978-3-642-66193-8_2. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Zanetti M. Studies on single foci of hematopoietic cells transformed by avian myeloblastosis virus. Virology. 1970 Sep;42(1):61–67. doi: 10.1016/0042-6822(70)90238-2. [DOI] [PubMed] [Google Scholar]
- Pawson T., Mellon P., Duesberg P. H., Martin G. S. env Gene of Rous sarcoma virus: identification of the gene product by cell-free translation. J Virol. 1980 Mar;33(3):993–1003. doi: 10.1128/jvi.33.3.993-1003.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L., Bauer H., Bolognesi D. P. Group-specific antigenic determinants of the large envelope glycoprotein of avian oncornaviruses. Virology. 1975 Sep;67(1):234–241. doi: 10.1016/0042-6822(75)90420-1. [DOI] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Komaromy M. C., Baluda M. A. Identification of a proviral genome associated with avian myeloblastic leukemia. Proc Natl Acad Sci U S A. 1980 May;77(5):3004–3008. doi: 10.1073/pnas.77.5.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Kawai S., Hanafusa H. Location of envelope-specific and sarcoma-specific oligonucleotides on RNA of Schmidt-Ruppin Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):447–451. doi: 10.1073/pnas.73.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Mellon P., Vogt P. K. Distribution of envelope-specific and sarcoma-specific nucleotide sequences from different parents in the RNAs of avian tumor virus recombinants. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1073–1077. doi: 10.1073/pnas.73.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H. The gene order of avian RNA tumor viruses derived from biochemical analyses of deletion mutants and viral recombinants. Annu Rev Microbiol. 1978;32:561–592. doi: 10.1146/annurev.mi.32.100178.003021. [DOI] [PubMed] [Google Scholar]
- Wang L., Galehouse D., Mellon P., Duesberg P., Mason W. S., Vogt P. K. Mapping oligonucleotides of Rous sarcoma virus RNA that segregate with polymerase and group-specific antigen markers in recombinants. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3952–3956. doi: 10.1073/pnas.73.11.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]




