Abstract
Patients with hereditary nonpolyposis colorectal cancer syndrome (HNPCC) develop microsatellite-unstable colorectal cancers that tend to be more proximally located and histologically are more likely to show high numbers of tumor-infiltrating lymphocytes, a lack of dirty necrosis, mucinous or poor differentiation, and a Crohn’s-like host response, when compared to microsatellite-stable cancers. However, histologic features that are characteristic of and can perhaps distinguish colorectal adenomas in HNPCC patients from those occurring in the general population have not been previously reported. We compared 16 adenomas endoscopically removed from patients with genetically proven HNPCC to 32 control adenomas, group-matched for patient age and sex, as well as endoscopic size, shape, anatomic location, and presence of high-grade dysplasia. Adenomas from HNPCC patients were more likely to contain high numbers of adenoma-infiltrating lymphocytes (AILs) with 12 of 16 (75%) adenomas having ≥5 AILs per high power field (HPF) as opposed to 4 of 32 (12%) adenomas in the control group (p=0.00003). HNPCC adenomas were also less likely to contain increased numbers of apoptotic bodies: 7 of 16 (44%) contained ≥5 apoptoses per HPF, compared to 27 of 36 (84%) control adenomas (p=0.006). The presence of necrosis or serrated architecture, percent villous component, and numbers of mitotic figures per HPF did not differ significantly between the two groups. Therefore, increased numbers of AILs and decreased numbers of apoptoses in colorectal adenomas are simple and inexpensive markers that raise the possibility of HNPCC.
Keywords: Colorectal adenoma, lymphocytes, apoptosis, HNPCC, microsatellite instability
Introduction
While the histologic features of colorectal carcinomas (CRC) in hereditary nonpolyposis colorectal cancer syndrome (HNPCC) have been extensively investigated and reported, little is known about the precursor adenomas in this syndrome. Invasive carcinomas in HNPCC patients are classically microsatellite-unstable (MSI-H) and have increased numbers of tumor infiltrating lymphocytes (TILs), a Crohn’s-like host response at the advancing edge of the tumor, a circumscribed growth pattern, mucinous or signet ring cell features, histologic heterogeneity, a lack of dirty necrosis, and are well- or poorly-differentiated (1,6,28,29,35).
Several lines of evidence support the conclusion that colorectal adenomas are the precursor lesion of invasive adenocarcinoma in patients with HNPCC, much like sporadic adenomas herald CRC in the general population (16,17,21,33). Furthermore, HNPCC adenomas are thought to be more aggressive clinically, perhaps because they are often flat and difficult to detect endoscopically and because they are thought to progress to invasive carcinoma at much faster rates than sporadic adenomas (10,11,14,15).
Various studies have reported that among adenomas occurring in HNPCC patients 67-86% are MSI-H and 60-86% show loss of expression of mismatch repair (MMR) proteins by immunohistochemistry, suggesting that the second hit in MMR genes occurs in the adenoma stage (5,7,9,12,19,21). Thus, some authors advocate testing selected adenomas (for example, those occurring in patients with strong family history of colon cancer or in patients younger than 40 years old) for MSI-H and/or MMR protein expression loss (4,13,23,30). In fact, the original Bethesda guidelines included colorectal adenomas in patients younger than 40 years old as one of the lesions that should be further subjected to MSI testing, but more recent data suggested that the yield of identifying MSI in these cases was low, causing this category to be dropped from the subsequent revised guidelines (31). Clearly, if such recommendations are to be made, identifying histologic features characteristic of adenomas arising in the setting of HPNCC compared to sporadic adenomas in non-syndromic patients would be desirable, so that pathologists and clinicians can narrow down the subset of adenomas that should be subjected to MSI-H or MMR protein expression testing. We undertook the following study to identify reliable histologic features that might differentiate adenomas arising in HNPCC patients from sporadic adenomas.
Materials and Methods
Case Selection
Patients with genetically proven HNPCC who had undergone screening colonoscopy were retrospectively identified from the University of Michigan Cancer Genetics Registry. Sixteen endoscopically-removed colorectal adenomas, one each from 16 patients, were collected from the Department of Pathology records. Thirty two (twice as many) control adenomas that as a group matched the study cases for patient age and sex, as well as endoscopic size, shape, and anatomic location of the polyp were identified from routine biopsies submitted to the Department of Pathology. Adenomas arising in patients with HNPCC have increased rates of high-grade dysplasia, as reported in the literature, so we preferentially included adenomas with high-grade dysplasia in the control group (9,23). Indications for colonoscopy in control cases included routine screening, anemia, bleeding, and follow-up of prior adenomas. None of the control patients had a personal or family history of colorectal cancer or other HNPCC-associated tumors. Information about clinical and endoscopic features of patients and adenomas was obtained from medical records and endoscopy reports. The study was approved by the institutional review board of the University of Michigan.
Histopathologic Analysis
Routinely processed, hematoxylin and eosin-stained sections obtained for diagnostic purposes in the HNPCC and control adenomas, were blindly and independently reviewed by three gastrointestinal pathologists and evaluated for: presence of high-grade dysplasia, percentage of villous component (0-100%), presence of necrosis, and presence of serrated architecture. Pathologists were instructed to scan the adenoma under low power and identify the area with the highest number of intraepithelial lymphocytes, mitoses, or apoptoses, then evaluate under high power five neighboring fields and record the average of their measurements. A collection of nuclear fragments within a space previously occupied by an epithelial cell, inside the ghost of a cell, or in an area roughly equivalent to a cell, was considered to be a single apoptotic body. Luminal apoptotic fragments were considered to represent necrosis rather than apoptotic bodies. The number of adenoma-infiltrating lymphocytes (AILs), mitotic figures and apoptotic bodies was scored as low (<5/high-power field {HPF; 400x on an Olympus BX40 microscope, total area equal to 0.94 mm2}), intermediate (5-10/HPF), or high (>10/HPF). For the presence of high-grade dysplasia, necrosis, or serrated architecture, the majority opinion among the three gastrointestinal pathologists was used, when they did not unanimously agree. The average of the three pathologists’ scores for percentage of villous component was calculated and distributed among three groups: low (<25%), intermediate (25-50%), and high (>50%). For the number of intra-adenomatous lymphocytes, mitotic figures, and apoptoses (low, intermediate, or high), the consensus opinion was recorded for each case where there was no unanimity.
Statistical Analysis
Binary/categorical clinical variables (patient sex, endoscopic shape and anatomic location of the polyps) and histologic features (presence of high-grade dysplasia, necrosis, or serrated architecture, percentage of villous component, and amount of intra-adenomatous lymphocytes, mitoses, and apoptoses) in adenomas from HNPCC and non-syndromic patients were compared using Fisher’s exact test. The means of continuous variables (patient age and endoscopic size of adenomas) were compared between cases and controls using the two-sample t test. Logistic regression analysis with case/control status as the binary response was conducted. Each predictor was first assessed individually in a single variable logistic regression model. The model was then extended to include both AILs and apoptoses in a multivariate logistic analysis using both unadjusted analysis and adjusting for other potential confounders. Tests of significance for the odds ratios were carried out based on the fitted logistic regression models. A p value <0.05 was considered statistically significant.
Results
Clinical features, including mean patient age, male/female ratio, and mean size, shape (sessile/flat or pedunculated) or anatomic location (proximal vs. distal colon) of the polyps, were not significantly different between HNPCC and control adenomas, given the selection criteria (Table 1). Histologic features such as presence of high-grade dysplasia, necrosis or serrated architecture, percent villous component, and amount of intra-adenomatous mitoses also did not differ significantly between the two groups (Table 2). For the number of intra-adenomatous lymphocytes, mitoses and apoptoses, unanimity among the three gastrointestinal pathologists was present in 26 (54%), 41 (85%), and 32 (67%) cases, respectively. Consensus was present in the remaining cases; there were no cases with one vote for each of the three levels (low, intermediate, high).
Table 1.
Clinical Features of HNPCC Patients with Adenomatous Colorectal Polyps and Group-matched Controls *
| Controls (N = 32) | HNPCC (N = 16) | |
|---|---|---|
|
|
||
| Mean age (y ± SD) (range) | 55.8 ± 11.1 (31-78) | 51.4 ± 11.3 (33-69) |
| Male/female ratio | 26 (81%) / 6 (19%) | 12 (75%) / 4 (25%) |
| Mean size (mm ± SD) (range) | 7.0 ± 3.2 (2-15) | 6.1 ± 3.5 (2-12) |
| Endoscopic shape: | ||
| Sessile or flat | 23 (72%) | 12 (75%) |
| Pedunculated | 9 (28%) | 4 (25%) |
| Anatomic location † | ||
| Proximal colon: | 18 (56%) | 12 (75%) |
| Cecum | 6 (19%) | 1 (6%) |
| Ascending colon | 6 (19%) | 4 (25%) |
| Transverse colon | 6 (19%) | 7 (44%) |
| Distal colon: | 14 (44%) | 4 (25%) |
| Descending colon | 3 (9%) | 0 (0%) |
| Sigmoid colon | 9 (28%) | 3 (19%) |
| Rectum | 2 (6%) | 1 (6%) |
Unless otherwise indicated, values are given as number (percentage).
Ascending colon includes hepatic flexure; transverse colon includes splenic flexure.
SD: standard deviation
Table 2.
Histopathologic Features of HNPCC Adenomas and Group-matched Controls *
| Controls (N = 32) | HNPCC (N = 16) | |
|---|---|---|
|
|
||
| Grade of dysplasia | ||
| Low | 22 (69%) | 13 (81%) |
| High | 10 (31%) | 3 (19%) |
| Presence of necrosis | 6 (19%) | 1 (6%) |
| Presence of serrated architecture | 4 (13%) | 4 (25%) |
| Percent of villous component | ||
| Low (<25%) | 28 (88%) | 16 (100%) |
| Intermediate (25-50%) | 3 (9%) | 0 (0%) |
| High (>50%) | 1 (3%) | 0 (0%) |
| Intra-adenomatous mitotic figures | ||
| Low (<5/HPF) | 24 (75%) | 14 (87%) |
| Intermediate (5-10/HPF) | 8 (25%) | 2 (13%) |
| High (>10/HPF) | 0 (0%) | 0 (0%) |
| Adenoma-infiltrating lymphocytes | ||
| Low (<5/HPF) | 28 (88%) | 4 (25%) |
| Intermediate (5-10/HPF) | 2 (6%) | 5 (31%) |
| High (>10/HPF) | 2 (6%) | 7 (44%) |
| Intra-adenomatous apoptotic bodies | ||
| Low (<5/HPF) | 5 (16%) | 9 (56%) |
| Intermediate (5-10/HPF) | 13 (41%) | 5 (31%) |
| High (>10/HPF) | 14 (43%) | 2 (13%) |
Values are given as number (percentage).
HPF: high power field (400x)
Adenoma-infiltrating lymphocytes (AILs) in HNPCC patients numbered <5 per high power field (HPF) in 4 (25%) adenomas, 5-10/HPF in 5 (31%), and >10/HPF in the remaining 7 (44%). These amounts of AILs in the control adenomas were seen in 28 (88%), 2 (6%) and 2 (6%) of cases, respectively (Table 2). Using a cut-off of 5 AILs/HPF, i.e. combining intermediate and high categories, results in 12 (75%) of HNPCC adenomas having ≥5 AILs/HPF compared to only 4 (12%) of controls, an association found to be highly significant (p=0.00003) (Table 3).
Table 3.
Statistical Results
| Logistic Regression Analysis |
|||||||
|---|---|---|---|---|---|---|---|
| Controls (N = 32) |
HNPCC (N = 16) |
Fisher’s Exact (two-sided) |
Single variable model | Bivariate model | |||
|
|
|||||||
| Lymphocytes: | |||||||
| Low (<5/HPF) | 28 (88%) | 4 (25%) | p=3×10−5 | p=1×10−5 | OR=21.0 (4.5, 98.2) |
p=0.0005 | OR=17.7 (3.5, 89.9) |
| High (≥5/HPF) | 4 (12%) | 12 (75%) | |||||
| Apoptoses: | |||||||
| Low (<5/HPF) | 5 (16%) | 9 (56%) | p=0.006 | p=0.006 | OR=0.14 (0.04, 0.57) |
p=0.058 | OR=0.19 (0.04, 1.06) |
| High (≥5/HPF) | 27 (84%) | 7 (44%) | |||||
Unless otherwise indicated, values are given as number (percentage). HPF: high power field (400x). OR: odds ratio (95% confidence intervals).
In contrast, apoptotic bodies were less numerous in adenomas from HNPCC patients, numbering <5/HPF in 9 (56%), 5-10/HPF in 5 (31%), and >10/HPF in 2 (13%) compared to 5 (16%), 13 (41%), and 14 (44%) respectively, in control adenomas (Table 2). Using the same cut-off of 5 apoptotic bodies per HPF, HNPCC adenomas had ≥5 apoptoses/HPF in 7 (44%) polyps, as opposed to 27 (84%) control adenomas, which was again highly significant (p=0.006) (Table 3).
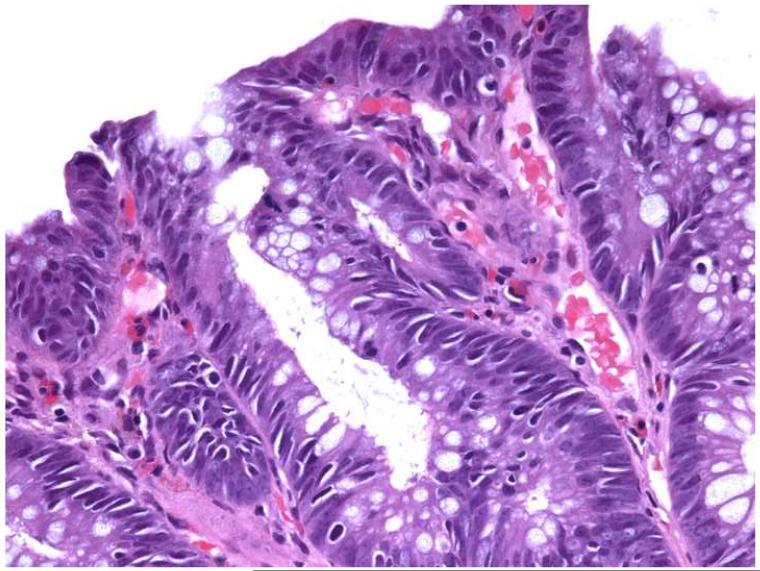
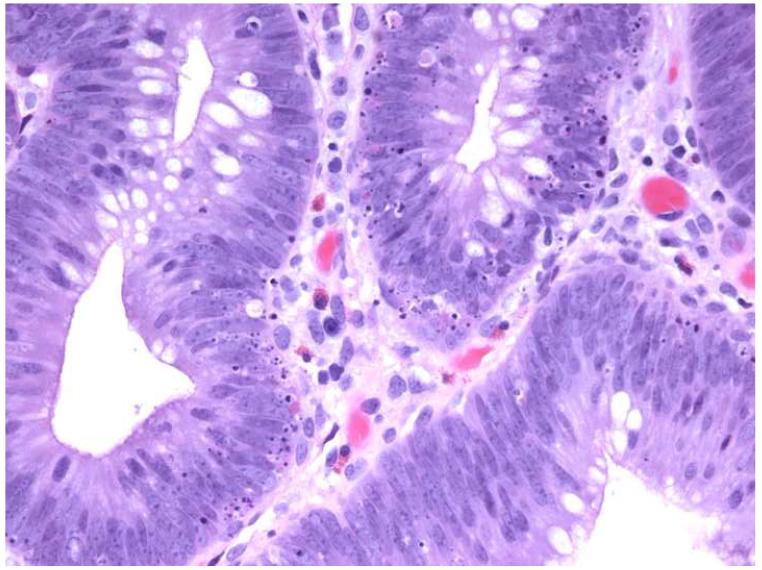
There was no association between grade of dysplasia and numbers of AILs or intra-adenomatous apoptoses. Overall, of 13 HNPCC and control adenomas with high-grade dysplasia, 4 (31%) had ≥5 AILs/HPF and 10 (77%) contained ≥5 apoptoses/HPF, compared to 12/35 (34%) adenomas with low grade dysplasia having ≥5AILs/HPF and 24/35 (69%) with ≥5 apoptoses/HPF. A representative adenoma from an HNPCC patient with >10 AILs/HPF and <5 apoptoses/HPF and a control adenoma with <5 AILs/HPF, but >10 apoptoses/HPF are shown in Figure 1.
Figure 1.
Photomicrographs of hematoxylin and eosin-stained tissue sections from an adenoma in an HNPCC patient (a) showing high numbers of AILs (>10/HPF) and low numbers of intra-adenomatous apoptoses (0-4/HPF) and a control adenoma (b) with low numbers of AILs (0-4/HPF) and high numbers of intra-adenomatous apoptoses (>10/HPF) (original magnification: 400x).
Patient-authorized, PCR-based DNA sequencing identified germline mutations in hMSH2 in 10 (63%), hMLH1 in 5 (31%), and hMSH6 in 1 (6%) of these 16 HNPCC patients. There were no statistically significant differences among the specific mutations in terms of any of the clinical or histologic features evaluated, including the number of intra-adenomatous lymphocytes, apoptoses, or mitoses.
Logistic regression analysis using a single variable model between histopathologic predictors and presence of an HNPCC-defining germline mutation documented strong statistical results for intra-adenomatous lymphocytes and apoptotic bodies, but not for any of the other clinical or histologic variables examined (Table 3). For AILs, a cut-off of ≥5/HPF results in p=0.00001 and odds ratio of 21.0 (95% confidence interval: 4.5-98.2), while for apoptoses, the same cut off gives p=0.006 and odds ratio of 0.14 (95% confidence interval: 0.04-0.57).
Interestingly, because lymphocytes and apoptotic bodies are negatively associated with odds ratio of 0.23 (p=0.03), a bivariate logistic regression model using these two variables modestly changes the statistical significance and point estimates of odds ratios for these two factors. However, the same pattern of association remains true (Table 3): the finding of ≥5 AILs/HPF is still strongly significant (p=0.0005) with odds ratio of 17.7 (95% confidence interval: 3.5-89.9) in HNPCC compared to control adenomas. A result of ≥5 apoptoses/HPF is marginally statistically significant with p=0.058 and odds ratio of 0.19 (95% confidence interval: 0.04-1.06) in control relative to HNPCC adenomas. Adjusted analysis accounting for any remaining variation in patient age and sex, polyp size, shape, and anatomic location, as well as presence of high-grade dysplasia, even after group-matching cases and controls, reveals essentially similar findings for intra-adenomatous lymphocytes and apoptoses.
Discussion
We compared histologic features between colorectal adenomas from patients with HNPCC and group-matched controls. We found that HNPCC adenomas are significantly more likely to contain ≥5 AILs/HPF and less likely to contain ≥5 intra-adenomatous apoptoses/HPF. The only other study, of which we are aware, that directly compared HNPCC adenomas to sporadic adenomas occurring in unrelated individuals, found that HNPCC adenomas were more likely to be smaller (<5 mm), located in the proximal colon (up to and including the transverse colon) and, of those that were larger and more proximal, they were more likely to harbor high-grade dysplasia (21). A separate study comparing adenomas from patients with a known MMR mutation to those occurring in non-carrier relatives found that HNPCC adenomas were more likely to be larger (≥7 mm), proximally located and to have high-grade dysplasia, the latter regardless of size or location (12). Neither study commented on AILs or apoptoses. We purposely chose our control group to control for size, proximal location, and high-grade dysplasia.
Two studies from collaborating groups have examined the presence of intraepithelial lymphocytes in sporadic colorectal adenomas from non-syndromic patients. The first study evaluated 118 flat colorectal adenomas in a Japanese population and found intraepithelial lymphocyte infiltration in 62% of them, even though the authors did not define their cutoff values for presence vs. absence of this infiltration (26). That study found a significant difference in the presence of infiltrating lymphocytes among adenomas with low-grade dysplasia (52%) compared to those with high-grade dysplasia (72%), but their overall rate of adenomas with high-grade dysplasia was remarkably high (51%). In the second study, of 159 adenomatous polyps in a Swedish population, the authors found more than 1 lymphocyte per 200 consecutive epithelial cells in 70% of tubular, 84% of villous, and 81% of serrated adenomas (27). In terms of dysplasia, 68% of adenomas with low-grade dysplasia contained intraepithelial lymphocytes compared to 83% of adenomas with high-grade dysplasia, but the overall rate of high-grade dysplasia in these adenomas was again high (55%). We found no significant association between grade of dysplasia and amount of AILs/HPF in HNPCC or control adenomas, perhaps because of different thresholds for high-grade dysplasia, which in our study we diagnosed in 13/48 (27%) adenomas, or perhaps due to different populations studied.
An interesting finding in our study is that the level of intraepithelial lymphocytes where significant differences between HNPCC and control adenomas occurred (i.e. ≥5AILs/HPF) was more than twice the highest published number (>2 TILs/HPF) that distinguishes invasive CRC with MSI-H from microsatellite-stable tumors, even though we did not specifically investigate a lower cutoff for AILs (6). This result, while at first perhaps contradictory, given the host immune response that mounts against tumor invasion, was also reported in studies of intraepithelial lymphocytes in sporadic adenomas described above, where none of the 50 adenocarcinomas evaluated had levels of lymphocytes similar to those seen in adenomas with high-grade dysplasia (25,27). Additionally, we have observed that in cases of resected MSI-H CRC where an adenoma is present in the vicinity of the invasive component that it presumably gave rise to, the number of AILs is greater than the number of TILs present in the carcinoma (Greenson JK, personal communication). Taken together, these observations suggest that the antigenic stimuli that attract lymphocytes to HNPCC tumors may be present in the premalignant stage and that perhaps invasion is in part due to reduced or failing antitumor immunity.
The second histologic characteristic that we identified in adenomas that develop in patients with HNPCC is a reduced rate of epithelial apoptosis. It is noteworthy that even in the bivariate logistic regression model, apoptoses contribute independently to case/control status and are an important variable even in the presence of lymphocytes. However, due to limited sample size, their effect does not reach statistical significance. The number of intra-adenomatous apoptoses also failed to correlate with grade of dysplasia in our study. Reports in the literature offer conflicting evidence on the association between the number of apoptotic bodies and the grade of dysplasia in sporadic adenomas (2,20,24). Our result may also be contradictory to data that suggest that neoplastic cell apoptosis is increased in MSI-H CRC (3,18). Interestingly however, other studies have shown that pro-apoptotic genes, such as bax, can be mutated with high frequency and that their protein product can be expressed at reduced levels in HNPCC adenomas (22,34). This suggests that apoptosis may be defective in this setting and may explain why we found decreased numbers of intra-adenomatous apoptotic bodies in HNPCC adenomas. Defective apoptosis in HNPCC might also help explain why carcinomas in these patients appear not to respond well to certain chemotherapy agents, such as 5-fluorouracil (32). Finally, defective or reduced rate of apoptosis in adenomas occurring in HNPCC patients may explain their observed rapid proliferation and faster progression to carcinoma (11,17).
The percent distribution of different HNPCC-defining mutations among patients in our study was similar to prior reports in population groups of a comparable geographic area (8). There was no association between particular germline mutations and the histologic features we evaluated, including AILs and intra-adenomatous apoptotic bodies.
This small study has shown that adenomas from HNPCC patients have increased AILs and decreased apoptotic bodies compared to sporadic controls. While these simple histologic findings may be helpful in identifying which adenomas should be tested for MSI and/or MMR protein expression loss, our findings will clearly need to be validated in a large, prospective, population-based study.
References
- 1.Alexander J, Watanabe T, Wu TT, et al. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158:527–535. doi: 10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai T, Kino I. Role of apoptosis in modulation of the growth of human colorectal tubular and villous adenomas. J Pathol. 1995;176:37–44. doi: 10.1002/path.1711760107. [DOI] [PubMed] [Google Scholar]
- 3.Dolcetti R, Viel A, Doglioni C, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154:1805–1813. doi: 10.1016/S0002-9440(10)65436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaglia P, Atkin WS, Whitelaw S, et al. Variables associated with the risk of colorectal denomas in asymptomatic patients with a family history of colorectal cancer. Gut. 1995;36:385–390. doi: 10.1136/gut.36.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuffrè G, Müller A, Brodegger T, et al. Microsatellite analysis of hereditary nonpolyposis colorectal cancer-associated colorectal adenomas by laser-assisted microdissection: correlation with mismatch repair protein expression provides new insights in early steps of tumorigenesis. J Mol Diagn. 2005;7:160–170. doi: 10.1016/S1525-1578(10)60542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenson JK, Bonner JD, Ben-Yzhak O, et al. Phenotype of microsatellite unstable colorectal carcinomas: Well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am J Surg Pathol. 2003;27:563–570. doi: 10.1097/00000478-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Halvarsson B, Lindblom A, Johansson L, et al. Loss of mismatch repair protein immunostaining in colorectal adenomas from patients with hereditary nonpolyposis colorectal cancer. Mod Pathol. 2005;18:1095–1101. doi: 10.1038/modpathol.3800392. [DOI] [PubMed] [Google Scholar]
- 8.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (Hereditary Nonpolyposis Colorectal Cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 9.Iino H, Simms L, Young J, et al. DNA microsatellite instability and mismatch repair protein loss in adenomas presenting in hereditary non-polyposis colorectal cancer. Gut. 2000;47:37–42. doi: 10.1136/gut.47.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 11.Johnson PM, Gallinger S, McLeod RS. Surveillance colonoscopy in individuals at risk for hereditary nonpolyposis colorectal cancer: an evidence-based review. Dis Colon Rectum. 2006;49:80–93. doi: 10.1007/s10350-005-0228-0. [DOI] [PubMed] [Google Scholar]
- 12.de Jong AE, Morreau H, van Puijenbroek M, et al. The role of mismatch repair gene defects in the development of adenomas in patients with HNPCC. Gastroenterology. 2004;126:42–48. doi: 10.1053/j.gastro.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 13.de Jong AE, Morreau H, Nagengast FM, et al. Prevalence of adenomas among young individuals at average risk for colorectal cancer. Am J Gastroenterol. 2005;100:139–143. doi: 10.1111/j.1572-0241.2005.41000.x. [DOI] [PubMed] [Google Scholar]
- 14.Lindgren G, Liljegren A, Jaramillo E, et al. Adenoma prevalence and cancer risk in familial non-polyposis colorectal cancer. Gut. 2002;50:228–234. doi: 10.1136/gut.50.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA. 2006;296:1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 16.Lynch HT, Smyrk T, Jass JR. Hereditary nonpolyposis colorectal cancer and colonic adenomas: aggressive adenomas? Semin Surg Oncol. 1995;11:406–410. doi: 10.1002/ssu.2980110607. [DOI] [PubMed] [Google Scholar]
- 17.Mecklin JP, Aarnio M, Läärä E, et al. Development of colorectal tumors in colonoscopic surveillance in Lynch syndrome. Gastroenterology. 2007;133:1093–1098. doi: 10.1053/j.gastro.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Michael-Robinson JM, Biemer-Hüttmann A, Purdie DM, et al. Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut. 2001;48:360–366. doi: 10.1136/gut.48.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller A, Beckmann C, Westphal G, et al. Prevalence of the mismatch-repair-deficient phenotype in colonic adenomas arising in HNPCC patients: results of a 5-year follow-up study. Int J Colorectal Dis. 2006;21:632–641. doi: 10.1007/s00384-005-0073-6. [DOI] [PubMed] [Google Scholar]
- 20.Nomura M, Watari J, Yokota K, et al. Morphogenesis of nonpolypoid colorectal adenomas and early carcinomas assessed by cell proliferation and apoptosis. Virchows Arch. 2000;437:17–24. doi: 10.1007/s004280000198. [DOI] [PubMed] [Google Scholar]
- 21.Rijcken FE, Hollema H, Kleibeuker JH. Proximal adenomas in hereditary non-polyposis colorectal cancer are prone to rapid malignant transformation. Gut. 2002;50:382–386. doi: 10.1136/gut.50.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rijcken FE, Koornstra JJ, van der Sluis T, et al. Early Carcinogenic Events in HNPCC Adenomas: Differences with Sporadic Adenomas. Dig Dis Sci. 2007 doi: 10.1007/s10620-007-0041-9. Epub Nov 13. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Bigas MA, Boland CR, Hamilton SR, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89:1758–1762. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 24.Rubio CA, Kumagai J, Kanamori T, et al. Apoptosis in flat neoplasias of the colorectal mucosa. In Vivo. 1995;9:173–176. [PubMed] [Google Scholar]
- 25.Rubio CA. Intraepithelial lymphocytes vs. colorectal neoplastic cells: Who is winning the apoptotic battle? Apoptosis. 1997;2:489–493. doi: 10.1023/a:1026430313275. [DOI] [PubMed] [Google Scholar]
- 26.Rubio CA, Kato Y, Hirota T. Intraepithelial lymphocytes in flat colorectal adenomas. In Vivo. 1997;11:393–394. [PubMed] [Google Scholar]
- 27.Rubio CA, Jacobsson B, Castaños-Velez E. Cytotoxic intraepithelial lymphocytes in colorectal polyps and carcinomas. Anticancer Res. 1999;19:3221–3227. [PubMed] [Google Scholar]
- 28.Shia J, Ellis NA, Paty PB, et al. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol. 2003;27:1407–1417. doi: 10.1097/00000478-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Smyrk TC, Watson P, Kaul K, et al. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcioma. Cancer. 2001;91:2417–2422. [PubMed] [Google Scholar]
- 30.Stoffel EM, Syngal S. Adenomas in young patients: what is the optimal evaluation? Am J Gastroenterol. 2005;100:1150–1153. doi: 10.1111/j.1572-0241.2005.41967.x. [DOI] [PubMed] [Google Scholar]
- 31.Velayos FS, Allen BA, Conrad PG, et al. Low rate of microsatellite instability in young patients with adenomas: reassessing the Bethesda guidelines. Am J Gastroenterol. 2005;100:1143–1149. doi: 10.1111/j.1572-0241.2005.40862.x. [DOI] [PubMed] [Google Scholar]
- 32.Warusavitarne J, Schnitzler M. The role of chemotherapy in microsatellite unstable (MSI-H) colorectal cancer. Int J Colorectal Dis. 2007;22:739–748. doi: 10.1007/s00384-006-0228-0. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe T, Muto T, Sawada T, et al. Flat adenoma as a precursor of colorectal carcinoma in hereditary nonpolyposis colorectal carcinoma. Cancer. 1996;77:627–634. doi: 10.1002/(sici)1097-0142(19960215)77:4<627::aid-cncr7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 34.Woerner SM, Kloor M, Mueller A, et al. Microsatellite instability of selective target genes in HNPCC-associated colon adenomas. Oncogene. 2005;24:2525–2535. doi: 10.1038/sj.onc.1208456. [DOI] [PubMed] [Google Scholar]
- 35.Yearsley M, Hampel H, Lehman A, et al. Histologic features distinguish microsatellite-high from microsatellite-low and microsatellite-stable colorectal carcinomas, but do not differentiate germline mutations from methylation of the MLH1 promoter. Hum Pathol. 2006;37:831–838. doi: 10.1016/j.humpath.2006.02.009. [DOI] [PubMed] [Google Scholar]