Introduction
The value of comprehensive intensive atherosclerotic risk factor control in patients with coronary artery disease (CAD) is well-established. In 2007, the COURAGE trial demonstrated that among patients with stable coronary disease, intensive management of vascular risk factors was as good as endovascular intervention plus intensive medical management for preventing cardiac ischemic events1. Yet, despite the fact that atherosclerotic risk factor control in patients with stroke or TIA is recommended by guidelines2, a multimodal approach to prevention has not previously been tested in patients with atherosclerotic stroke. Older atherosclerotic-stroke prevention trials comparing carotid revascularization to medical therapy, such as NASCET3 and ACAS4, were performed in an era before statins and angiotensin converting enzyme (ACE) inhibitors became standard of care, and therefore risk factor control was not adequate by today’s standards. Even recent trials comparing carotid revascularization procedures5, 6 had little emphasis on risk factor control in their design and therefore had little impact on blood pressure and cholesterol measures at 1 year7, 8. Among stroke prevention trials in patients with heterogeneous causes of stroke, several trials have studied the effects of specific risk factor medications9–11 or of intensive control of a particular risk factor, such as blood pressure12, but no stroke prevention trials have used a muti-modal aggressive risk factor approach.
Among patients with intracranial atherosclerosis, which may be the most common cause of stroke world-wide13, risk factor control is also believed to be important for stroke prevention. The Warfarin Aspirin Symptomatic Intracranial Disease (WASID) trial, in which patients with symptomatic intracranial stenosis were managed with either warfarin or aspirin and usual risk factor management14, showed that patients with poorly controlled blood pressure and elevated cholesterol during follow-up had significantly higher rates of recurrent stroke and other vascular events15, suggesting that aggressive management of vascular risk factors may benefit these patients. However, the impact of multi-modal aggressive risk factor control as a primary treatment strategy in patients with intracranial stenosis had not been assessed. Endovascular treatment of intracranial stenosis was also an emerging, yet unproven therapy. Therefore, in an effort to study these two treatment strategies, the Stenting and Aggressive Medical Management for Prevention of Recurrent stroke in Intracranial Stenosis (SAMMPRIS) Trial was designed to compare aggressive medical management alone versus aggressive medical management plus percutaneous transluminal angioplasty and stenting (PTAS). Thus, the SAMMPRIS trial became the first multicenter stroke prevention trial to encorporate multi-modal, protocol-driven risk factor control in the design. This paper focuses on the rationale, design, and implementation of the intensive risk factor management protocols in SAMMPRIS and describes challenges implementing these protocols during the trial and how the challenges have been managed.
Overall SAMMPRIS Design
The overall design of SAMMPRIS has been described previously16. In brief, SAMMPRIS is an ongoing investigator-initiated and designed Phase III randomized multicenter trial funded by the National Institute of Neurological Disorders and Stroke (NINDS) in which eligible patients were randomized at 50 sites to: aggressive medical therapy alone or percutaneous transluminal angioplasty and stenting (PTAS) using the Wingspan stent system plus aggressive medical therapy. The main eligibility criteria were TIA or non-disabling stroke within 30 days prior to enrollment caused by 70% – 99% stenosis of a major intracranial artery (MCA, carotid, vertebral, or basilar). The primary outcome is stroke or death within 30 days after enrollment or after a revascularization procedure for the qualifying lesion performed during the follow-up period or stroke in the territory of the qualifying artery beyond 30 days. Aggressive medical therapy includes aspirin 325 mg/day during the entire follow-up period (estimated mean follow-up of approximately 32 months), clopidogrel 75mg per day for 90 days after enrollment, and aggressive risk factor management primarily targeting systolic blood pressure (SBP) < 140 mm Hg (< 130 if diabetic) and low-density lipoprotein cholesterol (LDLc) < 70 mg/dl. Risk factor management of both primary and secondary targets (see Table 1) is performed by the study neurologist and coordinator at each site, assisted by an innovative, evidence-based, educational, lifestyle modification program (INTERxVENT) that is administered at regularly scheduled times to all patients throughout the study.
Table 1.
SAMMPRIS Risk Factor Targets and their measurement
| RISK FACTOR | GOAL | MEASUREMENT |
|---|---|---|
|
| ||
| Primary Risk Factors | ||
|
| ||
| LDLc | < 70 mg/dL | Central lab- Direct LDL measurement* |
|
| ||
| Systolic Blood pressure | < 140 mm Hg (< 130 if diabetic) | Using standardized device provided to site |
|
| ||
| Secondary Risk Factors | ||
|
| ||
| Non-HDLc | < 100 mg/dL | Central lab |
|
| ||
| HbA1c | < 7.0% | Local lab |
|
| ||
| Smoking | Cessation | Self reported (PACE score**) |
|
| ||
| Weight Management | For initial BMI of 25 – 27 kg/m2: target BMI < 25 kg/m2 For initial BMI > 27 kg/m2: target 10% weight loss |
Weight at each visit Height at baseline |
|
| ||
| Physical Activity | ≥ 30 min of moderate exercise 3 or more times per week | Self reported (PACE score**) |
measured by homogeneous enzymatic method (www.sekisuidiagnostics.com).
Physician-based Assessment and Counseling for Exercise (PACE) questionaire is performed for smoking and exercise.
The target sample size in SAMMPRIS was 764 patients, but enrollment was stopped early by NINDS based on a recommendation by the independent Data Safety Monitoring Board on April 5, 2011 due primarily to safety concerns regarding the periprocedural stroke and death risk in the PTAS group17. At the time enrollment was stopped, 451 patients had been enrolled and the primary event rate in the medical arm was substantially lower than anticipated17. Follow-up with aggressive medical management of all enrolled patients will continue until March, 2013, after which the final outcome and risk factor data will be available for analysis.
Rationale for Incorporating Aggressive Risk Factor Management
Aggressive management of vascular risk factors was incorporated into the SAMMPRIS design for several reasons: 1. In the WASID study14 (which included patients with stroke or TIA within the previous 90 days that was due to 50–99% intracranial stenosis) risk factors were managed by the study neurologist in association with the patient’s primary care physician. National guidelines for treatment of risk factors were provided to the neurologist at each site, but no specific protocols to address risk factors were followed. Table 2 (reproduced from Chaturvedi S, et al.15) shows how often risk factors exceeded pre-specified target goals at baseline and year 1 (study period: 1999–2003) in WASID. Some progress was made in treating cholesterol and smoking within the first year, but not in lowering blood pressure. Many patients still had uncontrolled vascular risk factors, suggesting that simply providing guidelines for risk factor control was not sufficient to achieve desired targets; 2. Failure to achieve risk factor targets in WASID appeared to have important clinical consequences, since good control of blood pressure and cholesterol levels during follow-up was associated with reductions in the risk of stroke, MI and vascular death15; 3. Other secondary prevention stroke trials of patients with heterogeneous causes of stroke had shown that treatment of elevated LDLc11 and blood pressure10 reduced the risk of recurrent stroke; 4. Among patients with stable coronary artery disease (CAD), intensive risk factor management alone was shown to be as good as endovascular intervention plus either usual medical management or intensive medical management in preventing cardiac ischemic events1, 18; and 5. If usual medical management was compared to stenting plus usual medical management in SAMMPRIS and the stenting arm was found to be superior, stenting would become the standard of care for these patients. It would then be very difficult to perform a subsequent trial to determine if aggressive medical management alone could obviate the need for stenting. Thus, it was critical to determine within SAMMPRIS whether aggressive medical therapy obviated the need for stenting.
Table 2.
Control of Vascular Risk Factors in WASID
| Risk Factor | Baseline | Year 1 visit |
|---|---|---|
| SBP ≥ 140 mm Hg | 51% | 49.9% |
| Cholesterol ≥ 200 mg/dL | 50% | 37%* |
| LDLc ≥ 100 mg/dL | 71% | 58%* |
| LDLc ≥ 70 mg/dL | 94% | 88%** |
| HDLc < 40 mg/dL | 40% | 33%** |
| Triglyceride ≥ 200 mg/dL | 26% | 31% |
| Hemoglobin A1C > 7% † | 64% | 56% |
| Smoking | 21% | 17%*** |
| No alcohol | 55% | 68%* |
SBP indicates systolic blood pressure; LDLc, low-density lipoprotein cholesterol; HDLc, high-density lipoprotein cholesterol
p-values comparing percentage at baseline and 1 year.
P<0.001
P<0.05
P<0.01
Diabetics only
Reproduced with permission from Chatuvedi S. et al, Neurology15
Rationale for Specific Risk Factor Targets
Systolic Blood Pressure
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends a target SBP of less than 140 mm Hg (< 130 mm Hg in diabetics)19 and the American Heart Association/American Stroke Association also endorses this blood pressure target for stroke patients2. In WASID during a mean follow-up of 1.8 years, 23% of patients with mean SBP ≥ 140 mm Hg has a recurrent ischemic stroke compared with 15% of patients with mean SBP < 140 mm Hg (HR 1.63, 95% CI: 1.11–2.40, p=0.0120)15, 20, supporting the use of the JNC7 target in SAMMPRIS.
Low-density Lipoprotein cholesterol
At the time of WASID, the Second Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) recommended targeting LDLc < 100 mg/dL21. In WASID during a mean follow-up of 1.8 years, 19% of patients with a mean LDLc ≥ 100 mg/dL had a recurrent ischemic stroke compared with 11% of patients with a mean LDLc < 100 mg/dL (HR 1.72, 95% CI: 1.04–2.85, p=0.0326)15, 20. Since the completion of WASID, the NCEP guidelines were updated to include a target LDLc of < 70 mg/dL for very high risk patients22, 23. In WASID, only 10% of patients achieved a mean LDLc < 70 mg/dL by the end of Year 2, but the data was strongly suggestive that achieving an LDLc < 70 mg/dL was highly beneficial, as 23% of patients with a mean LDLc ≥ 70 mg/dL during follow-up had a primary endpoint (stroke, vascular death, or MI) compared to 7% of patients with an LDLc < 70 mg/dL (p=0.09)15, supporting the use of the NCEP target for very high risk patients in SAMMPRIS.
Non-High-density Lipoprotein cholesterol
Non-HDLc was selected as a secondary target in SAMMPRIS because the NCEP ATP III guidelines considered it an important secondary target and recommended achieving a target of < 100 mg/dL in high-risk patients23, particularly in patients with triglycerides > 200 mg/dL. In WASID, there was an association between elevated non-HDLc ≥ 130 mg/dL (the NCEP ATP II recommended target at the time of WASID follow-up) and a higher risk of recurrent stroke (HR 1.94, 95% CI: 1.15–3.27, p=0.01)15, 20. Evidence at the time of the SAMMPRIS design did not support specific target levels for other cholesterol sub-fractions, such as HDLc.
Hemoglobin A1c
Hemoglobin A1c (HbA1c) < 7.0% was selected as a secondary target for diabetic patients in SAMMPRIS because the American Diabetes Association24 and the ASA/AHA Stroke Council25 guidelines endorsed this target. In WASID, 26% of patients with a mean HbA1c ≥ 7.0% had a recurrent stroke compared to 15% patients with mean HbA1c < 7.0% (HR= 1.7, 95% CI:0.81–3.58, p=0.15)20.
Smoking Cessation
Smoking cessation was chosen as a risk factor target because the US Department of Health and Human Services Surgeon General’s most recent report on the effects of smoking concluded there was sufficient evidence to establish a causal relationship between smoking and stroke26, and because the AHA/ASA Stroke Council recommended strongly advising every patient with stroke or TIA who has smoked in the last year to quit25.
Weight Management
Weight Management was included because the 2006 Update of the AHA Scientific Statement on Obesity and Heart Disease From the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism27 listed obesity as an independent modifiable risk factor for stroke; and recommended a combination of diet, physical activity, and behavior therapy to achieve a BMI of < 25 kg/m2 in patients with an initial BMI of 25.0– 27 kg/m2 and 10% weight loss for those with an initial BMI > 27 kg/m2.
Physical Activity
The physical activity target was included based on the AHA/ASA Stroke Prevention Guidelines statement recommendation that patients with ischemic stroke or TIA who are capable of engaging in physical activity participate in at least 30 minutes of moderate-intensity physical exercise most days25.
Rationale for Incorporating a Lifestyle Modification Program
Several studies have shown a large gap between recommended target levels for risk factors and those achieved in clinical practice15, 28–30. While cardiac rehabilitation programs designed to address this risk factor treatment gap exist at most large hospitals, not all healthcare facilities offer this service. Commercially available lifestyle modification programs have emerged to help patients and employees of large corporations achieve risk factor targets. These programs have improved the achievement of risk factor targets in a variety of populations, including patients who have previously suffered a stroke or TIA and those with multiple risk factors31–33.
Design and Implementation of the Risk Factor Protocols in SAMMPRIS
The design of risk factor management in SAMMPRIS included the following strategies to help control risk factors: providing study medications to subjects, providing medication titration algorithms for the primary risk factors to study investigators, standardizing the measurement of primary risk factor levels, providing an innovative lifestyle management program to subjects, and central oversight of risk factor performance.
The Risk Factor Team includes the patient’s study neurologist, the patient’s study coordinator, and the patient’s INTERxVENT lifestyle coach. The neurologist and coordinator follow protocols for the primary risk factors and collaborate with the patient’s outside physicians to achieve secondary risk factor targets using national guidelines, which include general recommendations for lifestyle modification and medication use. The lifestyle coach recommends and reinforces specific healthy lifestyle behaviors.
Study Medications
Medications necessary to achieve the primary risk factor targets (LDLc and SBP) were provided to subjects free of charge to enhance compliance. In order to achieve the LDLc target of < 70 mg/dL, rosuvastatin was selected as the lipid-lowering agent for several reasons. The NCEP III22 and ASA/AHA Stroke Council guidelines25 recommended statins for the treatment of hypercholesterolemia, and there was evidence that statins offered pleiotropic benefits such as stabilizing atherosclerotic plaques, enhancing endothelial function, decreasing oxidative stress and inflammation, inhibiting thrombosis, and possibly having neuroprotective effects 34, 35. Rosuvastatin was used in SAMMPRIS because it is more effective for LDLc reduction on a milligram-to-milligram basis than other statins36, 37, FDA post-marketing surveillance of rosuvastatin showed the safety profile to be consistent with other statins38, and the manufacturer agreed to donate rosuvastatin for use in SAMMPRIS patients. SAMMPRIS subjects were encouraged to start or switch to rosuvastatin at enrollment because of the early benefits of statins within 30 days of a vascular event35 and for lowering the risk of stroke, MI, and death within 30 days after revascularization procedures (e.g., carotid stenting39).
The antihypertensive medications provided to SAMMPRIS patients included one drug from almost every class of antihypertensive medications: diuretic (chlorthalidone), ACE inhibitor (lisinopril), potassium-sparing diuretic (spironolactone), angiotensin receptor blocker (candesartan), beta-blocker (atenolol), vasodilator (hydralazine), central alpha agonist (clonidine), and long-acting calcium channel antagonist (felodipine). These medications were selected because most were available in generic form, they were commonly used in practice, and their efficacy in stroke patients had been established in the Secondary Prevention of Small Subcortical Strokes (SPS3) study12, on which the SAMMPRIS blood pressure protocol was based. These medications were paid for by the study and provided at no cost to SAMMPRIS patients, but SAMMPRIS patients could use other non-study medications.
Medication titration algorithms
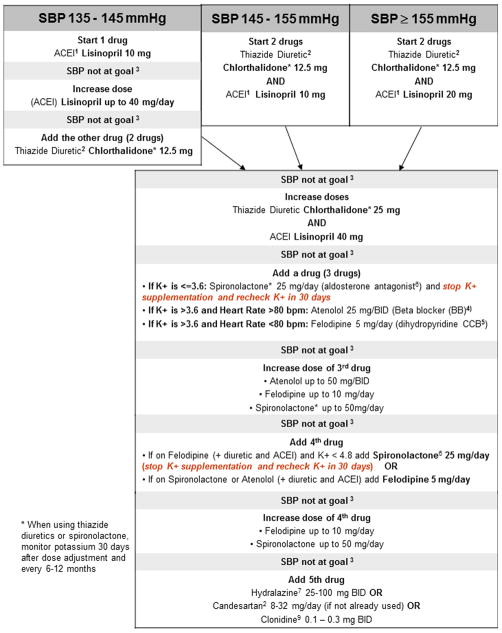
Given the relative inexperience of neurologists compared with internists and cardiologists in managing risk factors, specific step-by-step medication titration algorithms were designed to achieve the primary risk factor targets. These algorithms are provided in Figures 1 and 2. In summary, for LDLc control, each subject was to begin or switch (if on another statin) to rosuvastatin 20 mg per day at the baseline visit, followed by an increase to 40 mg at the 30-day visit if the LDLc was higher than 70 mg/dL. For SBP control, the SAMMPRIS blood pressure algorithm was based on the SPS3 blood pressure algorithm with some modifications. If the subject’s baseline blood pressure was not below target level, the patient was started on antihypertensive medications (ACE inhibitor or diuretic or both were the initial recommendations). Study subjects return every 30 days until their blood pressure is in target. If the study neurologist is unable to lower the participant’s LDLc or SBP to achieve target by following the algorithms, further assistance was provided by the central risk factor management team and conveyed to the sites by email or teleconference.
Figure 1.
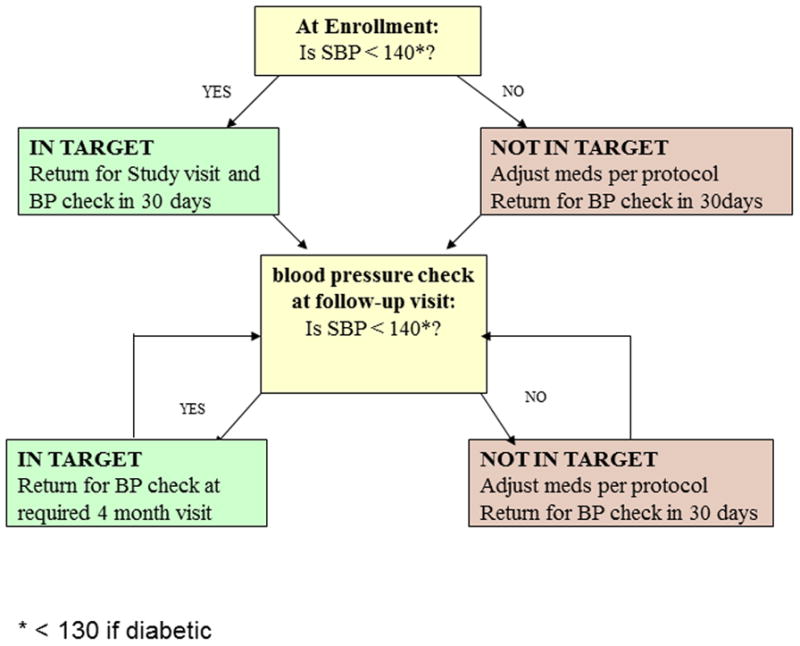
a. Blood Pressure Measurement Algorithm; b. Hypertension Treatment Algorithm
Figure 2.
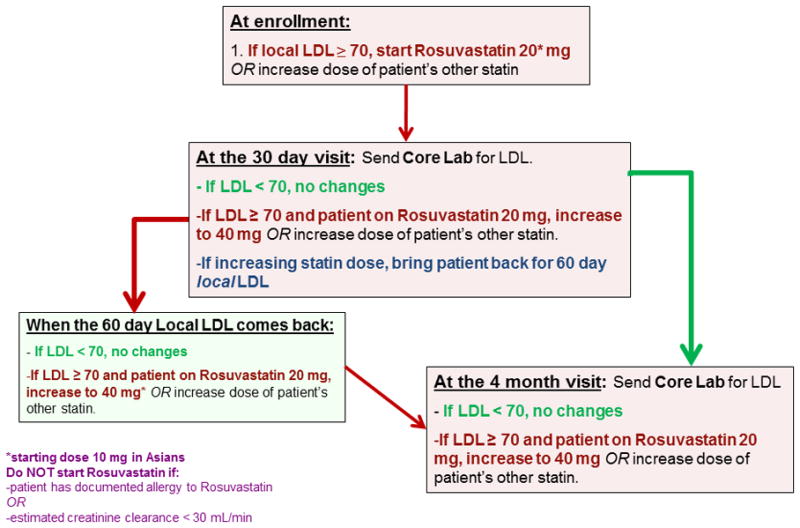
LDLc Management Algorithm
Medication Dispensing
Another unique challenge for successfully addressing risk factor management in a clinical trial is to provide conveniently available risk factor medications to patients. Initially, medications were dispensed through a centralized Pharmacy Coordinating Center (PCC), which handled the procurement, labeling, distribution, inventory management, and monitoring of all drugs used in the trial. SAMMPRIS sites (either the coordinators or site investigational pharmacies) received study medications from the PCC and tracked dispensing to study patients with the use of a web-based inventory system. However, due to unpredictability in study medication use, inventory issues (such as expired medications and running out of study medications) at the individual sites became a challenge.
Since SAMMPRIS study medications are FDA-approved, commercially available medications, they do not require the same amount of oversight as with investigational medications. Therefore, medication dispensing was switched to a national commercial pharmacy chain that was available near all SAMMPRIS sites. The SAMMPRIS trial billing information and the list of SAMMPRIS-provided medications were entered into the commercial pharmacy’s Pharmacy Benefit System, which typically routes charges to pharmaceutical insurance providers, allowing for SAMMPRIS to be billed directly for study medications and requiring no out-of-pocket cost to study patients. This novel system of dispensing medications, besides more closely mimicking clinical practice, resulted in decreased overhead costs since it did not require payment for shipping and there was no wasting of expired medications.
Standardizing the Measurement of Primary Risk Factor Levels
Providing accurate measurements of the primary risk factor levels in SAMMPRIS is important so that LDLc and SBP levels can be reliably correlated with outcomes at the end of the trial. LDLc and SBP can have significant variability between clinical sites. The standard LDLc values reported at most medical laboratories are calculated using the Friedwald equation40. These calculated LDLc values are impacted by levels of other blood lipids, making them more variable, particularly in the postprandial period, than a direct LDLc measurement41. Similarly, blood pressure monitoring devices also have significant variability in accuracy, with varying grades of performance42. Therefore, in SAMMPRIS, the accuracy of the primary risk factor measures was optimized and standardized by using a central lipid laboratory that performs direct LDLc measurement and by providing a highly-rated blood pressure monitoring device for use at all sites.
Secondary risk factor measures were assessed using local site laboratory tests (hemoglobin A1c), central lipid laboratory values (non-HDLc), local site scales (weight), and patient-completed scales (PACE Current Physical Activity Status Score43 and PACE Smoking Score44).
Lifestyle Modification Program
The lifestyle modification program INTERxVENT R, was selected because it is nationally available, is delivered with 1-on-1 counseling via the telephone throughout the United States, and has been demonstrated to be clinically effective in a variety of populations, including patients with prior history of stroke or TIA, and patients with multiple risk factors31–33. The use of a nationally available service provides an additional level of standardization of risk factor education and counseling across all SAMMPRIS sites.
INTERxVENT is provided to SAMMPRIS patients at no cost for the duration of the trial at scheduled intervals (every 2 weeks for first 12 weeks, then monthly thereafter) and includes: 1. Baseline evaluation of health and readiness to change; 2. Computer-generated risk factor goals incorporating SAMMPRIS targets; 3. Computer-generated treatment plans, with a focus on lifestyle intervention (including physical activity, nutrition, weight management, and tobacco cessation); 4. Treatment plan implementation, with brief (15 – 30 minute), behaviorally-oriented counseling sessions by coaches (non-physician healthcare professionals under the direction of a medical director) to help each patient acquire the skills, motivation, and support needed to implement and adhere to their individualized treatment plan (including medication compliance); 5. Follow-up and ongoing revision of treatment plan; 6. Computerized compliance tracking; and 7. Outcomes assessment.
Communication between INTERxVENT and the patient’s healthcare providers presented another challenge in the SAMMPRIS trial. In clinical practice, INTERxVENT sends recommendations for risk factor control to the physician who orders the service (typically a primary care doctor) via mail or fax. However, in SAMMPRIS, the study neurologist and coordinator are the recipients of INTERxVENT recommendations. Since many of the study sites are at large academic centers with complex mail delivery systems, there could have been considerable delay in sites receiving the recommendations. Therefore, the SAMMPRIS electronic data capture system (iDataFax) was used to provide reliable and timely delivery of INTERxVENT recommendations to the sites. INTERxVENT faxes their recommendations into iDataFax, which stores them and automatically notifies the site coordinator that the recommendations are available for retrieval. This novel delivery method also allows the SAMMPRIS Clinical Coordinating Center (CCC) to monitor the interaction between INTERxVENT and the sites.
Central Monitoring of Risk Factor Performance
Another strategy to maximize risk factor control in SAMMPRIS is to combat physician treatment inertia by providing sites feedback on their risk factor performance. Community physician audit and feedback programs have been shown in several studies to improve hypertension control in clinical practice45, 46. The SAMMPRIS CCC adopted a similar strategy for risk factor oversight, providing both auditing and feedback of site performance and patient-specific recommendations.
Overall site risk factor performance is monitored by generating weekly reports of each site’s achievement of risk factor targets (i.e. the percentage of the site’s patients who are in-target for each risk factor). Poorly performing sites are identified and reports on individual patients at those sites are reviewed. Systematic problems in site protocol compliance are identified and additional site coordinator and neurologist training is implemented, if needed. On a monthly basis, sites receive a report detailing their risk factor performance relative to the overall performance in the trial as well as the performance at each of the other SAMMPRIS sites, in order to promote site-to-site comparisons. Other tools used to monitor risk factor performance include automated weekly reports with lists of patients who: 1. have LDLc values ≥ 70 mg/dL; 2. are overdue for repeat LDLc or SBP measurement; and 3. are overdue for safety labs (e.g. liver function tests, serum creatinine, and serum potassium).
Individual patient-specific risk factor recommendations are also provided to sites, typically for patients with particularly challenging risk factors either at the site’s request or if a patient is noted to be consistently out-of-target. These recommendations are generated by the Risk Factor Management Committee Members who have specific expertise in managing key risk factors. Recommendations are conveyed to the sites by email or teleconference. All patient-specific communication between the CCC and SAMMPRIS sites are recorded centrally to ensure consistency and follow-up of prior recommendations.
In addition to this regular oversight of risk factor control at each site by the CCC, the Risk Factor Management Committee meets annually to evaluate the overall success of risk factor management in the trial and to recommend updates in the risk factor guidelines and protocols if necessary.
Discussion
The SAMMPRIS trial employs a multi-modal approach to risk factor control that has not been used in previous stroke prevention trials. Although follow-up of SAMMPRIS-enrolled patients continues, early analyses of risk factor measures show substantial improvement using the aggressive medical management strategies described above17. Within the first 30 days, mean SBP decreased by over 5 mm Hg and mean LDLc decreased by over 20 mg/dL47, with both of these primary risk factor measures continuing to improve at year 117. Improvements in secondary risk factor targets were also seen, with significantly better control of non-HDL cholesterol and HbA1c, weight loss, improved exercise, and smoking cessation compared to baseline47.
These improvements in risk factor control may have contributed to better-than-expected outcomes at this point in the medical management arm of SAMMPRIS. Among WASID patients who met the SAMMPRIS entry criteria (70–99% intracranial stenosis and qualifying event of stroke or TIA within 30 days) and were treated with usual management of risk factors and aspirin or warfarin, the 30-day stroke and death rate was 10.7%, whereas the 30-day rate of stroke and death in the aggressive medical management arm of SAMMPRIS was 5.8%. While the use of dual antiplatelet therapy in SAMMPRIS likely contributed to the early benefit of aggressive medical management, it is possible that intensive risk factor control also played a role in lowering the 30-day event rate compared to WASID. Such early pleiotropic effects of risk factor medications, such as high dose statins, have been shown to reduce 30-day cardiovascular risk in patients with acute coronary syndromes35. Based on currently available follow-up data in SAMMPRIS, aggressive medical management appeared, as well, to lower the 1-year rates of events. In WASID, patients who met SAMMPRIS entry criteria had a primary endpoint rate of 25% at year 1, but SAMMPRIS patients receiving aggressive medical management alone had a primary endpoint rate of 12.2% at year 117. Dual-antiplatelet therapy was only given for 90 days after enrollment, followed by aspirin monotherapy, yet the primary endpoint rate continued to remain lower than expected at year 1, suggesting intensive risk factor control is an important contributor to the better than expected 1-year outcome in the SAMMPRIS patients who were treated with medical management alone. Although historical comparisons between WASID and SAMMPRIS patients do not prove that the SAMMPRIS aggressive medical management strategy improved outcomes, analyses to determine the impact of risk factor control on outcomes (e.g. comparison between the event rates of patients who did achieve risk factor targets versus those who did not achieve targets in the medical arm of SAMMPRIS) will be performed at the end of follow-up to help address this question.
Another important issue is the practicality and ability to generalize the use of the SAMMPRIS risk factor management strategy in clinical practice. Most of the strategies used in SAMMPRIS to control vascular risk factors are available and used outside of clinical trials. For example, cardiac rehabilitation clinics are available at many medical centers and are recommended by the American Heart Association National Guidelines for the Management and Prevention of Coronary Heart Disease48. Monitoring of physician performance for achievement of prevention measures also occurs in clinical practice. In programs known as “pay for performance”, which are common in the U.S. and now worldwide49, healthcare reimbursement is linked to achievement of quality measures. Similarly, performance feedback programs, with ongoing education and training, are also being used to improve control of risk factors in high-risk populations45, 46. In addition, most of the risk factor management in SAMMPRIS is coordinated by the study coordinators (typically nurses) using medication titration algorithms with neurologist oversight, similar to the increasing number of Lipid-Management Clinics that utilize physician-extenders commonly seen in clinical practice. Finally, although some risk factor medications were provided at no cost to the study participants, the method of distributing the medication (via a commercial pharmacy) largely duplicates the methods used in clinical practice. Therefore, aspects of the SAMMPRIS aggressive medical management strategy can be implemented in ‘real-life’ patients. In fact, a recent single-center study of the feasibility of implementing a SAMMPRIS-like intensive medical management protocol showed that blood pressure and LDLc targets could be achieved in clinical practice and were associated with good outcomes50.
While the early SAMMPRIS results suggest that the SAMMPRIS aggressive medical management strategy can be and should be implemented in these high-risk patients, the next challenge is to ensure that aggressive medical management will be implemented in practice. The resources provided to SAMMPRIS study patients (e.g. free risk factor medications, close follow-up by study investigators, and individualized lifestyle management) are not currently available to many patients in the U.S. health-care system. In addition to variability of resources, additional challenges to implementation of the SAMMPRIS aggressive medical management strategy include patient language and cultural barriers, variability in healthcare provider training and education, and variability in healthcare system access and policies. Despite these challenges, the strategies used for risk factor management in SAMMPRIS provide a framework for implementing aggressive risk factor management in clinical practice. Additionally, the success in achieving multiple risk factor targets in SAMMPRIS sets the bar for future stroke prevention and implementation trials.
In summary, the SAMMPRIS aggressive medical management strategy employed a multi-modal approach to risk factor control that has been very effective in achieving risk factor targets in study patients and likely contributed to the lower than expected rate of stroke in the medical arm. Intensive management of vascular risk factors should be incorporated into the management of patients at high risk of stroke in clinical practice and in future secondary prevention trials.
Supplementary Material
Acknowledgments
The PACE self-assessment forms for smoking cessation and physical activity were provided by the San Diego Center for Health Interventions, LLC. Vendors: INTERxVENT provides the lifestyle modification program to the study at a discounted rate. The Veterans Affairs Cooperative Studies Program Clinical Research Pharmacy Coordinating Center (Albuquerque, NM) handled the procurement, labeling, distribution, and inventory management of the study devices and rosuvastatin. Walgreens pharmacies provide study medications except rosuvastatin to study patients at a discounted price (paid for by the study).
Funding Sources
The SAMMPRIS trial was funded by a research grant (U01 NS058728) from the United States Public Health Service National Institute of Neurological Disorders and Stroke (NINDS). In addition, Dr. Tanya Turan’s NIH-funded K23 grant award (1 K23 NS069668-01A1), provided support for her time and effort as the Director of Risk Factor Management. Corporate Support: Stryker Neurovascular (formerly Boston Scientific Neurovascular) provided study devices and supplemental funding for third party device distribution, site monitoring and study auditing. This research is also supported by the Investigator-Sponsored Study Program of AstraZeneca that donates rosuvastatin (Crestor) to study patients.
Biographies
Tanya N. Turan, MD MS is the current recipient of a K23 grant from NIH/NINDS (K23 NS069668) for research related to intracranial stenosis. She currently serves on blinded Neurological Events Adjudication Committees for an industry funded diabetes drug trial (Boehringer Ingelheim), a NINDS-funded observational study of vertebrobasilar stenosis (VERiTAS), and an industry funded patent foramen ovale closure trial (W.L Gore and Associates) and is compensated for those activities. She has also served as an expert witness in medical legal cases.
Michael J. Lynn, MS receives grant support from the National Eye Institute. He is the principal investigator of the Coordinating Center for Infant Aphakia Treatment Study (EY013287) and a co-investigator on the Core Grant for Vision Research (EY006360).
Bethany F. Lane, RN MSN has received consulting fees from Microvention Terumo.
Brent M. Egan, MD has received research support from Medtronic, Takeda and Novartis. He has been a consultant for Medtronic and Blue Cross.
Ngoc-Anh Le, PhD has received research support for the NIH-funded study, BIOSIS: Biomarkers of Ischemic Outcomes in Systemic Intracranial Stroke.
Kathie L. Hermayer, MD MS has received research support from the American Diabetes Association (ADA), Sanofi-Aventis, Eli Lilly, Novo Nordisk, and the NIH (DCCT/EDIC trial). Dr. Hermayer has received speakers’ bureau appointment payments from Sanofi-Aventis, Eli Lilly, Amylin, and Boehringer Ingelheim.
William Virgil Brown, MD has received research support from Astra Zeneca, speakers’ bureau appointment payments from Abbott, Merck, Liposcience and Kowa, and honoraria from Merck, Liposcience and Pfizer. Dr. Brown has acted as a consultant to Amgen, Genzyme, Anthera, Cerenis, Pfizer, and Bristol-Myers Squibb. He has also served as an expert witness in a legal case involving Merck.
Colin Derdeyn, MD receives grant support from the NINDS (P50 55977; R01 NS051631). He is also on the Scientific Advisory Board for W.L Gore and Associates and is the Chair of the Scientific Advisory Board for Pulse Therapeutics.
David Fiorella, MD PhD has received institutional research support from Siemens Medical Imaging and Microvention, consulting fees from Codman/Johnson and Johnson, NFocus, W.L. Gore and Associates, and EV3/Covidien, and royalties from Codman/Johnson and Johnson. He has received honoraria from Scientia and has ownership interest in CVSL and Vascular Simulations.
Scott Janis, PhD is a program director at the National Institute of Neurological Disorders and Stroke.
Marc Chimowitz, MBChB has received research grants from NINDS to fund the WASID trial (1 R01 NS36643) and to fund other research on intracranial stenosis (1 K24 NS050307 and 1 R01 NS051688). He currently serves on the stroke adjudication committee of an industry funded osteoporosis drug trial (Merck and Co., Inc.) and on the DSMB of another industry funded patent foramen ovale closure trial (W.L Gore and Associates) and is compensated for those activities. He has also served as an expert witness in medical legal cases.
Footnotes
Disclosures
Drs. Turan, Derdeyn, Fiorella, Janis, and Chimowitz, and Michael Lynn MS, and Bethany Lane RN, MSN serve on the Executive Committee of the SAMMPRIS trial, which is funded by the National Institute of Neurological Disorders and Stroke (NINDS) (grant number: U01 NS058728). All except Dr. Janis have received salary support from the SAMMPRIS grant. Dr. Turan is currently supported by a NINDS K23 award (K23 NS069668). Dr. Janis is the NINDS Program Officer for the trial. Drs. Egan, Le, Lopes-Virella, Hermayer, Benavente, White, and Brown serve on the SAMMPRIS Risk Factor Committee and were reimbursed from the SAMMPRIS grant for these efforts. Azhar Nizam MS serves at the Statistical Coordinating Center and receives salary support from the SAMMPRIS grant. Michelle Caskey, Meghan Steiner, Nicole Vilardo and Andrew Stufflebean served in support roles for the Risk Factor Committee and received salary support from the SAMMPRIS grant for these efforts. None of the authors have any financial relationship with the INTERxVENT Lifestyle Program. The following investigators report additional support:
References
- 1.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS. Optimal medical therapy with or without pci for stable coronary disease. The New England Journal of Medicine. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 2.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D on behalf of the American Heart Association Stroke Council, Council on Cardiovascular Nursing Council on Clinical Cardiology, Interdisciplinary Council on Quality of Care, Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 3.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. New England Journal of Medicine. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 4.Walker MD, Marler JR, Goldstein M, et al. Endarterectomy for asymptomatic carotid artery stenosis. Executive committee for the asymptomatic carotid atherosclerosis study. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 5.EVA-3S Investigators. Endarterectomy vs. Angioplasty in patients with symptomatic severe carotid stenosis (EVA-3S) trial. Cerebrovascular Diseases. 2004;18:62–65. doi: 10.1159/000078751. [DOI] [PubMed] [Google Scholar]
- 6.Sheffet AJ, Roubin G, Howard G, Howard V, Moore W, Meschia JF, Hobson RW, Brott TG. Design of the carotid revascularization endarterectomy vs. Stenting trial (CREST) International Journal of Stroke. 2010;5:40–46. doi: 10.1111/j.1747-4949.2009.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF. Stenting versus endarterectomy for treatment of carotid-artery stenosis. The New England Journal of Medicine. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mas JL, Trinquart L, Leys D, Albucher JF, Rousseau H, Viguier A, Bossavy JP, Denis B, Piquet P, Garnier P, Viader F, Touze E, Julia P, Giroud M, Krause D, Hosseini H, Becquemin JP, Hinzelin G, Houdart E, Henon H, Neau JP, Bracard S, Onnient Y, Padovani R, Chatellier G. Endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis (EVA-3S) trial: Results up to 4 years from a randomised, multicentre trial. Lancet Neurology. 2008;7:885–892. doi: 10.1016/S1474-4422(08)70195-9. [DOI] [PubMed] [Google Scholar]
- 9.Diener HC, Sacco R, Yusuf S. Rationale, design and baseline data of a randomized, double-blind, controlled trial comparing two antithrombotic regimens (a fixed-dose combination of extended-release dipyridamole plus asa with clopidogrel) and telmisartan versus placebo in patients with strokes: The prevention regimen for effectively avoiding second strokes trial (PROFESS) Cerebrovascular Diseases. 2007;23:368–380. doi: 10.1159/000100105. [DOI] [PubMed] [Google Scholar]
- 10.PROGRESS Collaborative Group. Randomised trial of perindopril-based blood-pressure lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 11.Amarenco P, Bogousslavsky J, Callahan A, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators. High-dose atorvastatin after stroke or transient ischemic attack. New England Journal of Medicine. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 12.Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, Coffey C, McClure LA, Szychowski JM, Conwit R, Heberling PA, Howard G, Bazan C, Vidal-Pergola G, Talbert R, Hart RG SPS3 Investigators. The secondary prevention of small subcortical strokes (SPS3) study. International Journal of Stroke. 2011;6:164–175. doi: 10.1111/j.1747-4949.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorelick P, Wong K, Bae H, Pandey D. Large artery intracranial occlusive disease, a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–2399. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 14.The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Trial Investigators. Design, progress and challenges of a double-blind trial of warfarin versus aspirin for symptomatic intracranial arterial stenosis. Neuroepidemiology. 2003;22:106–117. doi: 10.1159/000068744. [DOI] [PubMed] [Google Scholar]
- 15.Chaturvedi S, Turan TN, Lynn MJ, Kasner SE, Romano J, Cotsonis G, Frankel M, Chimowitz MI. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology. 2007;69:2063–2068. doi: 10.1212/01.wnl.0000279338.18776.26. [DOI] [PubMed] [Google Scholar]
- 16.Chimowitz MI, Lynn MJ, Turan TN, Fiorella D, Lane BF, Janis S, Derdeyn CP. Design of the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis trial. Journal of Stroke and Cerebrovascular Diseases. 2011;20:357–368. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters MF, Hoh BL, Hourihane JM, Levy EI, Alexandrov AV, Harrigan MR, Chiu D, Klucznik RP, Clark JM, McDougall CG, Johnson MD, Pride GL, Jr, Torbey MT, Zaidat OO, Rumboldt Z, Cloft HJ for SAMMPRIS Investigators. Stenting versus aggressive medical therapy for intracranial arterial stenosis. New England Journal of Medicine. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitt B, Waters D, Brown WV, van Boven AJ, Schwartz L, Title LM, Eisenberg D, Shurzinske L, McCormick LS. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus revascularization treatment investigators. The New England Journal of Medicine. 1999;341:70–76. doi: 10.1056/NEJM199907083410202. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart Lung Blood Institute, Joint National Committee on Prevention Detection Evaluation &Treatment of High Blood Pressure, National High Blood Pressure Education Program Coordinating Center. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 20.Turan TN, Lynn MJ, Cotsonis G, Chaturvedi S, Chimowitz MI. Risk factors associated with recurrent ischemic stroke in patients with symptomatic intracranial arterial stenosis (abstract) International Journal of Stroke. 2006;1:S99. [Google Scholar]
- 21.Grundy SM, Bilheimer D, Chait A, et al. Summary of the second report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel II) JAMA. 1993;269:3015–3023. [PubMed] [Google Scholar]
- 22.Expert Panel on Detection Evaluation Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 23.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association. Standards of medical care in diabetes--2006. Diabetes Care. 2006;29 (Suppl 1):S4–42. [PubMed] [Google Scholar]
- 25.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the american heart association/american stroke association council on stroke: Cosponsored by the council on cardiovascular radiology and intervention. Circulation. 2006;113:e409–449. [PubMed] [Google Scholar]
- 26.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Jaen CR, Lando HA, Mecklenburg RE, Mullen PD, Nett LM, Robinson L, Stitzer ML, Tommasello AC, Wewers ME, Kottke TE. Treating tobacco use and dependence. Quick reference guide for clinicians. 2000 Oct; [Google Scholar]
- 27.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 american heart association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 28.Pearson TA, Peters TD. The treatment gap in coronary artery disease and heart failure: Community standards and the post-discharge patient. American Journal Cardiology. 1997;80:45H–52H. doi: 10.1016/s0002-9149(97)00820-5. [DOI] [PubMed] [Google Scholar]
- 29.Marcelino JJ, Feingold KR. Inadequate treatment with hmg-coa reductase inhibitors by health care providers. American Journal Medicine. 1996;100:605–610. doi: 10.1016/s0002-9343(96)00011-3. [DOI] [PubMed] [Google Scholar]
- 30.Muls E, de Bbacker G, de Bacquer D, Brohet M, Heller F. Lipi-watch, a belgian/luxembourg survey on achievement of european atherosclerosis society lipid goals. Clinical Drug Investigation. 2000;19:219–229. [Google Scholar]
- 31.Lafranchise EF, Widener WG, Franklin BA, Salmon RD, Leighton RF, English CD, Gordon NF. Need for and clinical effectiveness of a neurologist supervised, nurse case managed stroke risk reduction program (abstract) Stroke. 2000;32:367-c. [Google Scholar]
- 32.Gordon NF, English CD, Contractor AS, Salmon RD, Leighton RF, Franklin BA, Haskell WL. Effectiveness of three models for comprehensive cardiovascular disease risk reduction. The American Journal of Cardiology. 2002;89:1263–1268. doi: 10.1016/s0002-9149(02)02323-8. [DOI] [PubMed] [Google Scholar]
- 33.Gordon NF, Salmon RD, Franklin BA, Sperling LS, Hall L, Leighton RF, Haskell WL. Effectiveness of therapeutic lifestyle changes in patients with hypertension, hyperlipidemia, and/or hyperglycemia. The American Journal of Cardiology. 2004;94:1558–1561. doi: 10.1016/j.amjcard.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 34.Liao JK, Laufs U. Pleiotropic effects of statins. Annual Review of Pharmacology and Toxicology. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ray KK, Cannon CP, McCabe CH, Cairns R, Tonkin AM, Sacks FM, Jackson G, Braunwald E. Early and late benefits of high-dose atorvastatin in patients with acute coronary syndromes: Results from the prove it-timi 22 trial. Journal American College Cardiology. 2005;46:1405–1410. doi: 10.1016/j.jacc.2005.03.077. [DOI] [PubMed] [Google Scholar]
- 36.Brown WV, Bays HE, Hassman DR, McKenney J, Chitra R, Hutchinson H, Miller E. Efficacy and safety of rosuvastatin compared with pravastatin and simvastatin in patients with hypercholesterolemia: A randomized, double-blind, 52-week trial. American Heart Journal. 2002;144:1036–1043. doi: 10.1067/mhj.2002.129312. [DOI] [PubMed] [Google Scholar]
- 37.Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, Cain VA, Blasetto JW. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* trial) The American Journal of Cardiology. 2003;92:152–160. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 38.Davidson MH. Rosuvastatin safety: Lessons from the FDA review and post-approval surveillance. Expert Opinion on Drug Safety. 2004;3:547–557. doi: 10.1517/14740338.3.6.547. [DOI] [PubMed] [Google Scholar]
- 39.Groschel K, Ernemann U, Schulz JB, Nagele T, Terborg C, Kastrup A. Statin therapy at carotid angioplasty and stent placement: Effect on procedure-related stroke, myocardial infarction, and death. Radiology. 2006;240:145–151. doi: 10.1148/radiol.2401050603. [DOI] [PubMed] [Google Scholar]
- 40.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 41.Lindsey CC, Graham MR, Johnston TP, Kiroff CG, Freshley A. A clinical comparison of calculated versus direct measurement of low-density lipoprotein cholesterol level. Pharmacotherapy. 2004;24:167–172. doi: 10.1592/phco.24.2.167.33142. [DOI] [PubMed] [Google Scholar]
- 42.O’Brien E, Petrie J, Littler W, de Swiet M, Padfield PL, Altmanu DG, Bland M, Coats A, Atkins N. The british hypertension society protocol for the evaluation of blood pressure measuring devices. Journal of Hypertension. 1993;11:S43–S62. doi: 10.1097/00004872-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 43.Calfas KJ, Long BJ, Sallis JF, Wooten WJ, Pratt M, Patrick K. A controlled trial of physician counseling to promote the adoption of physical activity. Preventive Medicine. 1996;25:225–233. doi: 10.1006/pmed.1996.0050. [DOI] [PubMed] [Google Scholar]
- 44.Maron DJ, Boden WE, O’Rourke RA, Hartigan PM, Calfas KJ, Mancini GB, Spertus JA, Dada M, Kostuk WJ, Knudtson M, Harris CL, Sedlis SP, Zoble RG, Title LM, Gosselin G, Nawaz S, Gau GT, Blaustein AS, Bates ER, Shaw LJ, Berman DS, Chaitman BR, Weintraub WS, Teo KK. Intensive multifactorial intervention for stable coronary artery disease: Optimal medical therapy in the COURAGE (clinical outcomes utilizing revascularization and aggressive drug evaluation) trial. Journal American College Cardiology. 2010;55:1348–1358. doi: 10.1016/j.jacc.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 45.Egan BM, Laken MA, Shaun Wagner C, Mack SS, Seymour-Edwards K, Dodson J, Zhao Y, Lackland DT. Impacting population cardiovascular health through a community-based practice network: Update on an ash-supported collaborative. Journal of Clinical Hypertension. 2011;13:543–550. doi: 10.1111/j.1751-7176.2011.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Townsend RR, Shulkin DJ, Bernard D. Improved outpatient hypertension control with disease management guidelines. American Journal of Hypertension. 1999;12:88. [Google Scholar]
- 47.Turan TN, Nizram Azhar, Lynn Michael J, Derdeyn Colin P, Fiorella David, Lane Bethany F, Steiner Megan, Janis LS, Chimowitz Marc I for the SAMMPRIS Investigators. Impact of an aggressive medical management protocol on early risk factor measures in the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis (SAMMPRIS) trial (abstract) Stroke. 2012;43:A141. [Google Scholar]
- 48.Thomas RJ, King M, Lui K, Oldridge N, Pina IL, Spertus J American Association of Cardiovascular Pulmonary Rehabilitation/American College of Cardiology/American Heart Association Cardiac Rehabilitation/Secondary Prevention Performance Measures Writing Committee. AACVPR/ACC/AHA 2007 performance measures on cardiac rehabilitation for referral to and delivery of cardiac rehabilitation/secondary prevention services. Circulation. 2007;116:1611–1642. doi: 10.1161/CIRCULATIONAHA.107.185734. [DOI] [PubMed] [Google Scholar]
- 49.Eijkenaar F. Pay for performance in health care: An international overview of initiatives. Medical Care Research and Review. 2012 Jun;69(3):251–76. doi: 10.1177/1077558711432891. [DOI] [PubMed] [Google Scholar]
- 50.Nahab F, Kingston C, Frankel MR, Dion JE, Cawley CM, Mitchell B, Hammonds LP, Ayala L, Tong FC. Early aggressive medical management for patients with symptomatic intracranial stenosis. Journal of stroke and cerebrovascular diseases. 2011 Jul 25; doi: 10.1016/j.jstrokecerebrovasdis.2011.06.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.