Abstract
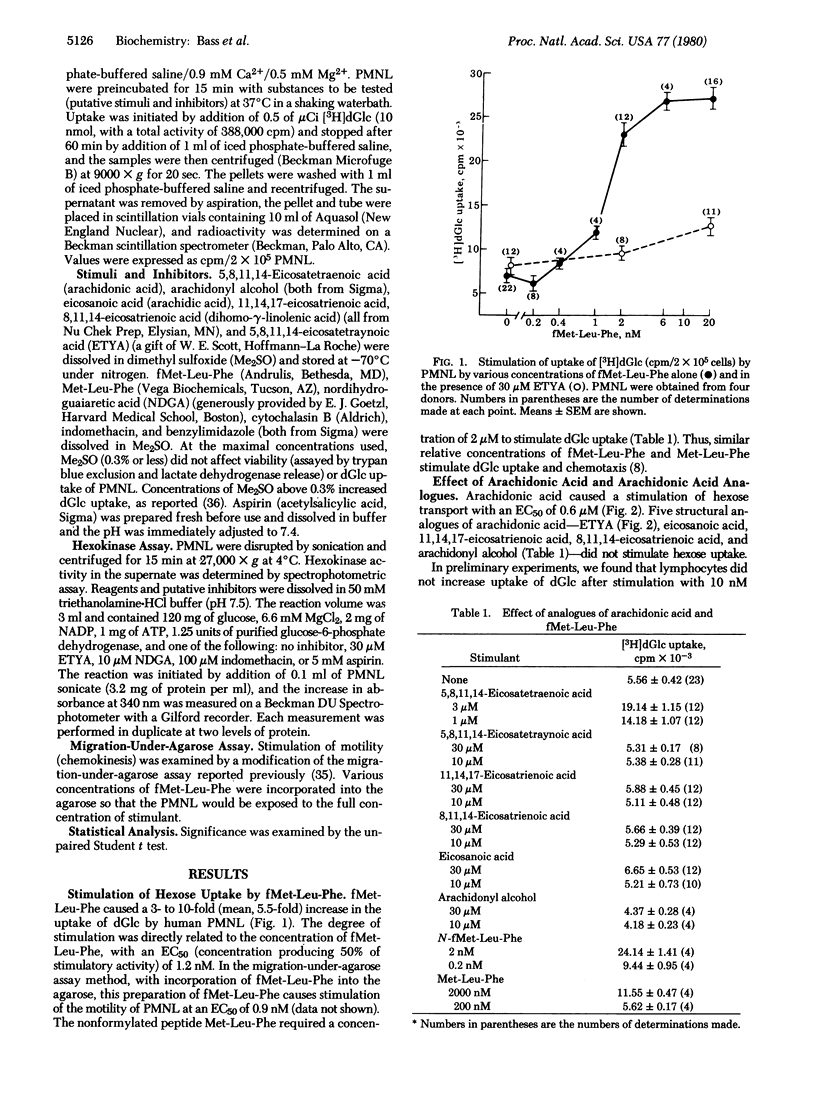
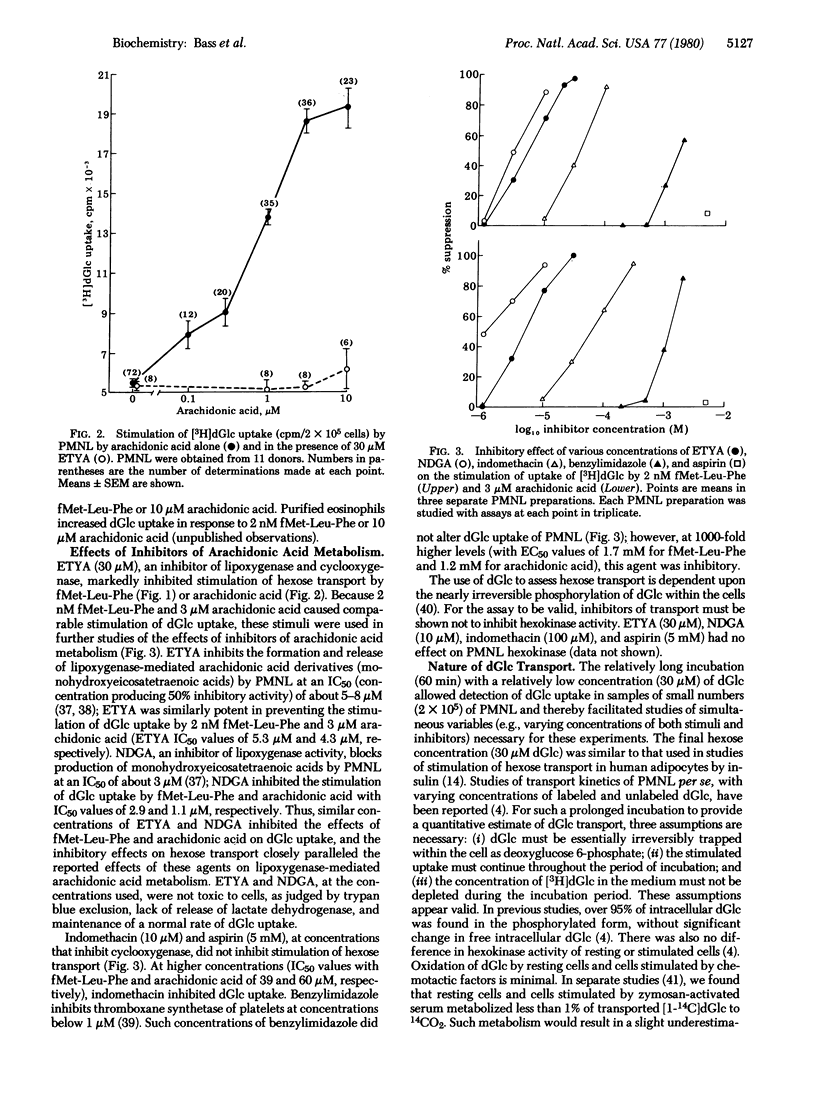
Whereas insulin does not stimulate hexose transport in polymorphonuclear leukocytes, we recently reported that C5a causes the leukocytes to take up 2-[3H]deoxyglucose. We now find that fMet-Leu-Phe, in a concentration-related manner with an EC50 (concentration producing 50% of stimulatory activity) of 1.2 nM, causes a 5.5-fold stimulation of deoxyglucose uptake. Moreover, arachidonic acid (5,8,11,14-eicosatetraenoic acid) similarly stimulated deoxyglucose uptake with an EC50 of 0.6 μM. Stimulation by arachidonic acid exhibited structural specificity; five structural analogues of arachidonic acid, including arachidonyl alcohol, 8,11,14-eicosatrienoic acid, 11,14,17-eicosatrienoic acid, 5,8,11,14-eicosatetraynoic acid, and arachidic acid, did not stimulate deoxyglucose uptake. Release and metabolism of arachidonic acid may also be involved in the stimulation of deoxyglucose uptake by fMet-Leu-Phe. Inhibitors of arachidonic acid metabolism (5,8,11,14-eicosatetraynoic acid, nordihydroguaiaretic acid, indomethacin, aspirin, and benzylimidazole) caused parallel changes in the responses to both arachidonic acid and fMet-Leu-Phe. Stimulation of deoxyglucose uptake of polymorphonuclear leukocytes by chemotactic factors or arachidonic acid had the characteristics of carrier-facilitated hexose transport. The response was saturable with increasing concentrations of stimulant or substrate (deoxyglucose). It was stereospecific (inhibited by D-glucose but not by L-glucose) and was inhibited in resting and stimulated cells by 5 μg of cytochalasin B per ml. It was separable from the stimulation of oxidative metabolism; it occurred normally in polymorphonuclear leukocytes from a patient with chronic granulomatous disease (these are incapable of an oxidative metabolic response to membrane stimuli). Thus, stimulation of polymorphonuclear leukocytes is associated with enhanced hexose transport. Moreover, carrier-facilitated hexose transport and arachidonic acid metabolism may be linked, at least in these leukocytes: arachidonic acid mimies the stimulatory effects of chemotactic factors, and blockade of arachidonic acid metabolism inhibits the stimulation of hexose transport by these agents.
Keywords: chemotactic factors, leukocyte metabolism
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswanikumar S., Corcoran B., Schiffmann E., Day A. R., Freer R. J., Showell H. J., Becker E. L. Demonstration of a receptor on rabbit neutrophils for chemotactic peptides. Biochem Biophys Res Commun. 1977 Jan 24;74(2):810–817. doi: 10.1016/0006-291x(77)90375-8. [DOI] [PubMed] [Google Scholar]
- Bass D. A., Dechatelet L. R., McCall C. E. Independent stimulation of motility and the oxidative metabolic burst of human polymorphonuclear leukocytes. J Immunol. 1978 Jul;121(1):172–178. [PubMed] [Google Scholar]
- Becker E. L., Sigman M., Oliver J. M. Superoxide production induced in rabbit polymorphonuclear leukocytes by synthetic chemotactic peptides and A23187. Am J Pathol. 1979 Apr;95(1):81–97. [PMC free article] [PubMed] [Google Scholar]
- Blackwell G. J., Duncombe W. G., Flower R. J., Parsons M. F., Vane J. R. The distribution and metabolism of arachidonic acid in rabbit platelets during aggregation and its modification by drugs. Br J Pharmacol. 1977 Feb;59(2):353–366. doi: 10.1111/j.1476-5381.1977.tb07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecher M. Effects of insulin and phospholipase A on glucose transport across the plasma membrane of free adipose cells. Biochim Biophys Acta. 1967 Jun 6;137(3):557–571. doi: 10.1016/0005-2760(67)90137-3. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Reed P. W. Inhibition of the neutrophil oxidative response to a chemotactic peptide by inhibitors of arachidonic acid oxygenation. Biochem Biophys Res Commun. 1979 Sep 27;90(2):481–487. doi: 10.1016/0006-291x(79)91260-9. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Hamberg M., Samuelsson B. Transformation of arachidonic acid and homo-gamma-linolenic acid by rabbit polymorphonuclear leukocytes. Monohydroxy acids from novel lipoxygenases. J Biol Chem. 1976 Dec 25;251(24):7816–7820. [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: effects of ionophore A23187. Proc Natl Acad Sci U S A. 1979 May;76(5):2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Transformation of arachidonic acid by rabbit polymorphonuclear leukocytes. Formation of a novel dihydroxyeicosatetraenoic acid. J Biol Chem. 1979 Apr 25;254(8):2643–2646. [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignarelli M., Giovine A., Di Reda L., Giorgino R. On the mechanism of action on insulin on the adipose tissue: role of endogenous prostaglandins. Boll Soc Ital Biol Sper. 1977 Aug 30;53(16):1373–1377. [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietze G., Wicklmayr M., Böttger I., Mayer L. Insulin action on glucose uptake into skeletal muscle: inhibition of endogenous biosynthesis of prostaglandins. FEBS Lett. 1978 Aug 15;92(2):294–298. doi: 10.1016/0014-5793(78)80773-x. [DOI] [PubMed] [Google Scholar]
- Fehr J., Jacob H. S. In vitro granulocyte adherence and in vivo margination: two associated complement-dependent functions. Studies based on the acute neutropenia of filtration leukophoresis. J Exp Med. 1977 Sep 1;146(3):641–652. doi: 10.1084/jem.146.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- Franson R., Waite M. Relation between calcium requirement, substrate charge, and rabbit polymorphonuclear leukocyte phospholipase A2 activity. Biochemistry. 1978 Sep 19;17(19):4029–4033. doi: 10.1021/bi00612a024. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. Stimulation of human neutrophil leukocyte aerobic glucose metabolism by purified chemotactic factors. J Clin Invest. 1974 Feb;53(2):591–599. doi: 10.1172/JCI107594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Valone F. H., Reinhold V. N., Gorman R. R. Specific inhibition of the polymorphonuclear leukocyte chemotactic response to hydroxy-fatty acid metabolites of arachidonic acid by methyl ester derivatives. J Clin Invest. 1979 Jun;63(6):1181–1186. doi: 10.1172/JCI109412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Woods J. M., Gorman R. R. Stimulation of human eosinophil and neutrophil polymorphonuclear leukocyte chemotaxis and random migration by 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid. J Clin Invest. 1977 Jan;59(1):179–183. doi: 10.1172/JCI108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Malmsten C. L., Kindahl H., Kaplan H. B., Rådmark O., Samuelsson B., Weissmann G. Thromboxane generation by human peripheral blood polymorphonuclear leukocytes. J Exp Med. 1978 Sep 1;148(3):787–792. doi: 10.1084/jem.148.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M. On the formation of thromboxane B2 and 12l-hydroxy-5,8,10,14-eicosatetraenoic acid (12 ho-20:4) in tissues from the guinea pig. Biochim Biophys Acta. 1976 Jun 22;431(3):651–654. doi: 10.1016/0005-2760(76)90232-0. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S., Falardeau P. Resolution of prostaglandin endoperoxide synthase and thromboxane synthase of human platelets. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3691–3695. doi: 10.1073/pnas.74.9.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S. Selective inhibition of platelet n-8 lipoxygenase by 5,8,11-eicosatriynoic acid. Biochim Biophys Acta. 1977 Jun 22;487(3):517–519. doi: 10.1016/0005-2760(77)90221-1. [DOI] [PubMed] [Google Scholar]
- Hirata F., Corcoran B. A., Venkatasubramanian K., Schiffmann E., Axelrod J. Chemoattractants stimulate degradation of methylated phospholipids and release of arachidonic acid in rabbit leukocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2640–2643. doi: 10.1073/pnas.76.6.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor H. S., Hampton M. Indomethacin in submicromolar concentrations inhibits cyclic AMP-dependent protein kinase. Nature. 1978 Dec 21;276(5690):841–842. doi: 10.1038/276841a0. [DOI] [PubMed] [Google Scholar]
- Kaplan L., Weiss J., Elsbach P. Low concentrations of indomethacin inhibit phospholipase A2 of rabbit polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2955–2958. doi: 10.1073/pnas.75.6.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J. 2-Deoxyglucose transport by intestinal epithelial cells isolated from the chick. J Membr Biol. 1976 Jun 30;27(4):363–379. doi: 10.1007/BF01869146. [DOI] [PubMed] [Google Scholar]
- Knapp H. R., Oelz O., Roberts L. J., Sweetman B. J., Oates J. A., Reed P. W. Ionophores stimulate prostaglandin and thromboxane biosynthesis. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4251–4255. doi: 10.1073/pnas.74.10.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall C. E., Bass D. A., Cousart S., DeChatelet L. R. Enhancement of hexose uptake in human polymorphonuclear leukocytes by activated complement component C5a. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5896–5900. doi: 10.1073/pnas.76.11.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Arachidonic acid induced degranulation of rabbit peritoneal neutrophils. Biochem Biophys Res Commun. 1979 Mar 15;87(1):292–299. doi: 10.1016/0006-291x(79)91678-4. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Arachidonic acid induced degranulation of rabbit peritoneal neutrophils. Biochem Biophys Res Commun. 1979 Mar 15;87(1):292–299. doi: 10.1016/0006-291x(79)91678-4. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Changes in ionic movements across rabbit polymorphonuclear leukocyte membranes during lysosomal enzyme release. Possible ionic basis for lysosomal enzyme release. J Cell Biol. 1977 Dec;75(3):635–649. doi: 10.1083/jcb.75.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Volpi M., Showell H. J., Becker E. L., Sha'afi R. I. Chemotactic factor-induced release of membrane calcium in rabbit neutrophils. Science. 1979 Feb 2;203(4379):461–463. doi: 10.1126/science.760200. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Chemotactic factor influences on the aggregation, swelling, and foreign surface adhesiveness of human leukocytes. Am J Pathol. 1978 Mar;90(3):537–550. [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Neutrophil aggregation and swelling induced by chemotactic agents. J Immunol. 1977 Jul;119(1):232–239. [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Becker E. L., Ward P. A. Neutrophil aggregation and degranulation. Effect of arachidonic acid. Am J Pathol. 1979 May;95(2):433–444. [PMC free article] [PubMed] [Google Scholar]
- Parker C. W., Stenson W. F., Huber M. G., Kelly J. P. Formation of thromboxane B2 and hydroxyarachidonic acids in purified human lymphocytes in the presence and absence of PHA. J Immunol. 1979 Apr;122(4):1572–1577. [PubMed] [Google Scholar]
- Pickett W. C., Jesse R. L., Cohen P. Initiation of phospholipase A2 activity in human platelets by the calcium ion ionophore A23187. Biochim Biophys Acta. 1976 Jan 18;486(1):209–213. doi: 10.1016/0005-2760(77)90086-8. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M. I., McConnell R. T., Cuatrecasas P. Aspirin-like drugs interfere with arachidonate metabolism by inhibition of the 12-hydroperoxy-5,8,10,14-eicosatetraenoic acid peroxidase activity of the lipoxygenase pathway. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3774–3778. doi: 10.1073/pnas.76.8.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid, a chemotactic fatty acid, is incorporated into neutrophil phospholipids and triglyceride. Prostaglandins. 1979 Aug;18(2):285–292. doi: 10.1016/0090-6980(79)90115-1. [DOI] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Metabolism of arachidonic acid in ionophore-stimulated neutrophils. Esterification of a hydroxylated metabolite into phospholipids. J Clin Invest. 1979 Nov;64(5):1457–1465. doi: 10.1172/JCI109604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Monohydroxyeicosatetraenoic acids (HETEs) induce degranulation of human neutrophils. J Immunol. 1980 May;124(5):2100–2104. [PubMed] [Google Scholar]
- Tai H. H., Yuan B. On the inhibitory potency of imidazole and its derivatives on thromboxane synthetase. Biochem Biophys Res Commun. 1978 Jan 13;80(1):236–242. doi: 10.1016/0006-291x(78)91128-2. [DOI] [PubMed] [Google Scholar]
- Turner S. R., Tainer J. A., Lynn W. S. Biogenesis of chemotactic molecules by the arachidonate lipoxygenase system of platelets. Nature. 1975 Oct 23;257(5528):680–681. doi: 10.1038/257680a0. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis A. L., Kuhn D. C., Weiss H. J. Acetylenic analog of arachidonate that acts like aspirin on platelets. Science. 1974 Jan 25;183(4122):327–330. doi: 10.1126/science.183.4122.327. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Cytochalasin B: inhibition of D-2-deoxyglucose transport into leukocytes and fibroblasts. Science. 1972 Jun 30;176(4042):1432–1434. doi: 10.1126/science.176.4042.1432. [DOI] [PubMed] [Google Scholar]


