Abstract
Objectives
The extent to which highly active antiretroviral therapy (HAART) affects HPV acquisition and clearance in HIV-infected women is not well-understood. We sought to describe high risk HPV detection and clearance rates over time since HAART initiation, based on time-varying HIV viral load (VL) and CD4+ T-cell count (CD4) using novel statistical methods.
Methods
We conducted retrospective analysis of data from completed AIDS Clinical Trials Group (ACTG) A5029 study using multi-state Markov models. Two sets of high risk HPV types from 2003 and 2009 publications were considered.
Results
There was some evidence that VL>400 copies/mL was marginally associated with higher rate of HPV detection (p=0.068, hazard ratio [HR]=4.67), using the older set of high risk HPV types. Such association was not identified using the latest set of HPV types (p=0.343, HR=2.64). CD4>350 cells/mm3 was significantly associated with more rapid HPV clearance with both sets of HPV types (p=0.001, HR=3.93; p=0.018, HR=2.65). There was no evidence that HPV affects VL or CD4 in all analyses.
Conclusions
High risk HPV types vary in studies, and they can affect analysis results. Use of HAART to improve CD4 may have an impact in the control of HPV infection, and the decrease in VL to a lesser degree.
Keywords: HIV, human papillomavirus, HAART, cervical cancer, Markov models
Introduction
Immuno-suppression is associated with prevalence and persistence of human papillomavirus (HPV), but the extent to which highly active antiretroviral therapy (HAART) affects HPV acquisition and clearance in HIV-infected women is not well-understood. A number of studies have shown that HPV infection and cervical dysplasia risks are elevated among women infected with HIV and immuno-suppressed (1–5), but there are mixed results on the effect of HAART on progression of HPV (1, 2, 6). Classification of high risk HPV types associated with cervical cancer vary in studies, as knowledge has evolved over time, and this may contribute to mixed results in literature. Also, data from HPV studies can be difficult to analyze due to: infrequent observations on HPV detection (every 6–12 months), small number of visits (over 3–5 years) and unknown rate and duration of transient HPV infections (7, 8). For instance, HPV detections at two study visits 12 months apart may indicate a persistent infection or an infection that cleared and recurred between the visits.
To address the data limitations, a statistical approach using multi-state models was applied to describe HPV detection and clearance events that may be recurrent. We conducted a retrospective analysis on AIDS Clinical Trials Group (ACTG) A5029 data to describe and compare HPV detection and clearance rates with time-varying HIV viral load and CD4 count in HIV-infected women initiating HAART, when the exact times of HPV status changes are unavailable. Two sets of high risk HPV types from 2003 and 2009 publications were considered to evaluate how the analysis can be sensitive to evolving HPV types thought to be oncogenic.
Methods
ACTG A5029 Study
A5029 was an observational, prospective study to estimate prevalence of HPV DNA in treatment-naïve women initiating HAART and to explore association of HPV with CD4+ T-cell count (CD4) and HIV viral load (VL) (9). The study enrolled 147 women from 35 sites in the US and Puerto Rico between January 2001 and May 2003, who provided informed consent according to the ACTG procedures and each site’s Institutional Review Board. Scheduled evaluations were infrequent: baseline (within 2 weeks of initiating HAART), weeks 24, 48 and 96. HAART was defined as a regimen of 3 or more drugs containing at least one protease inhibitor or non-nucleoside reverse transcriptase inhibitor or a triple nucleoside regimen containing abacavir. CD4+ T-cell count and plasma HIV-1 VL were conducted in laboratories at the ACTG sites using standardized techniques. Roche polymerase chain reaction/reverse blot strip assay was used to detect specific HPV types in the cervical swab specimens. HPV types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73 and 82 were considered high-risk HPV types for cancer based on a 2003 publication (10) in A5029 (Set I). In addition to this set, we considered Set II based on the review of human carcinogens by the International Agency for Research on Cancer in 2009 (11). Types 26, 53, 66, 68, 73 and 82 were considered to have “limited evidence in humans for cervical cancer” and were placed in a separate category from types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59 which had “sufficient evidence for cervical cancer” and were used as Set II.
Statistical Models
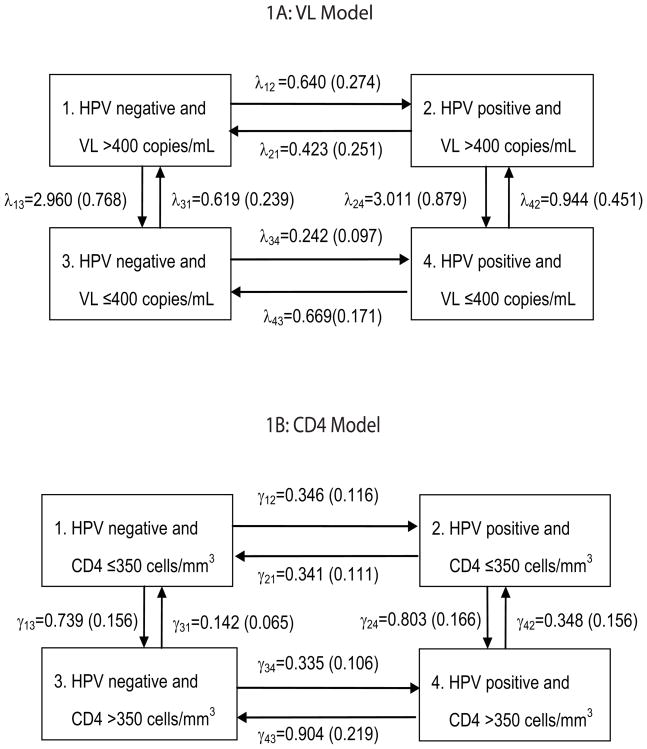
Continuous-time, multi-state Markov models were applied to the status data on HPV detection, VL and CD4. The following four states were defined for VL Model (Figure 1A) to describe the high risk HPV detection and clearance rates with time-varying VL:
Figure 1.
Models to describe HPV detection and clearance with varying HIV-related statuses. Estimated cause-specific hazard rates and standard errors from Set II are presented.
= none of the high risk HPV types (HPV negative) and HIV viral load (VL) >400 copies/mL,
= at least one of the high risk HPV detected (HPV positive) and VL>400,
= HPV negative and VL ≤ 400, and
= HPV positive and VL ≤ 400.
A multi-state model describes a process where an individual is in one of the specified states at any time. An individual’s status at any time can be categorized as one of the four states above, and changes in the state can be followed. The choice of 400 copies was based on the lower limit of quantitation of the Roche assay at the time of A5029. To illustrate the assumed state structure, suppose a woman begins in State 1. She may acquire HPV without improvement of VL (transition to State 2), or she may remain HPV negative and improve on VL (transition to State 3). State 4 may be reached from State 1 via State 2 (change in HPV status first) or State 3 (change in VL first), but not directly from State 1; that is, we assume that simultaneous changes in HPV status and VL do not occur biologically. (However, State 4 may be observed after State 1 in the previous visit.) From State 2, she may clear HPV and return to State 1, or she may retain HPV and transition to State 4 with decreased VL. From State 3, she may acquire HPV while maintaining VL status (transition to State 4), or her VL may increase while remaining HPV negative (transition to State 1). From State 4, she may clear HPV and transition to State 3, or remain HPV positive while her VL increases (transition to State 2). She may also remain in any of the states for the remainder of the study. Analysis methods by Kalbfleisch and Lawless (12) were applied to account for the lack of exact times of HPV detection and clearance times. The methods also account for different visit times, numbers of visits and varying initial states from different study participants.
The transition rates, or cause-specific hazard rates, are denoted by λ’s in Figure 1. For instance, λ12 represents the hazard rate for acquiring HPV when VL remains >400 and λ34 represents the rate when VL remains ≤ 400. For HPV clearance rates, λ21 represents the rate for clearing HPV when VL remains >400, and λ43 when VL remains ≤ 400. To describe the changes in HIV-related status, λ13 represents the rate from VL>400 to ≤ 400 without HPV, and λ24 with HPV. Conversely, λ31 represents the rate from VL ≤ 400 to >400 without HPV, and λ42 with HPV. Inferences on the effect of VL on HPV detection and clearance, and the effect of HPV on VL, can be made by comparing these hazard rates. Hypotheses are tested by fitting unrestricted and restricted models and using likelihood ratio tests. Mathematical details are in the Appendix.
A similar model was used to assess HPV detection and clearance rates with varying CD4 (CD4 Model, Figure 1B). The cause-specific hazard rates are represented by γ’s. The four states were defined as follows:
= HPV negative and CD4 ≤ 350 cells/mm3,
= HPV positive and CD4 ≤ 350,
= HPV negative and CD4>350, and
= HPV positive and CD4>350.
Threshold of 350 cells was chosen to be consistent with guidelines on when to start antiretroviral therapy at the time of A5029. The parameter estimates (and standard errors) using Set II are shown in Figure 1, and model fits are presented in the Appendix. The times to final visit were compared between groups with different baseline characteristics (HPV, VL, CD4 statuses) using log-rank tests. Analyses were conducted using SAS 9.1 and R 1.8.1.
Results
HPV and HIV RNA Viral Load
Of the 147 study subjects in the analysis, 143 had both HPV and HIV RNA results at baseline, 119 at week 24, 103 at week 48 and 85 at week 96. The data from times outside the scheduled visits for some subjects were also included in the analysis. Seventy subjects had four visits; 41 had three, 18 had two, and 18 had only one visit. Of the 143 subjects with both HPV and HIV RNA results at baseline, 120 subjects (84%) had VL>400 and 80 subjects (56%) had HPV. There was a trend of earlier discontinuation for subjects starting with HPV than without HPV, but the time to final visit was not statistically different (p=0.13). The final visit times for the subjects starting with VL>400 and ≤ 400 were not significantly different.
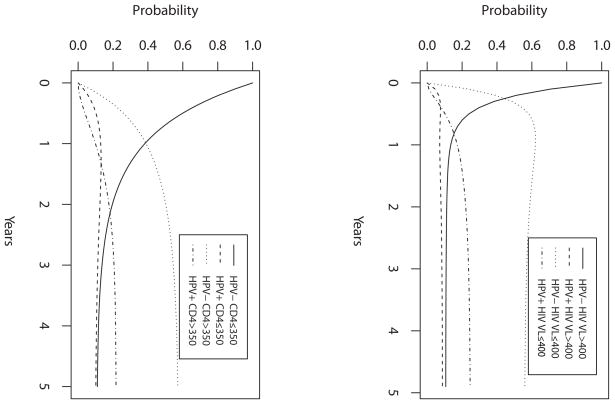
In VL Model (Figure 1A), comparison between λ12 and λ34 marginally suggested that a woman with current VL>400 was more likely to acquire HPV than with VL ≤ 400 using Set I (hazard ratio λ12/λ34 = 4.67, p=0.068). However, no such association was suggested using Set II (λ12/λ34 = 2.64, p=0.34). Other comparison results were similar with both HPV sets. There was no indication of a significant difference between subjects with VL>400 and VL ≤ 400 in clearance of HPV (λ21/λ43 = 0.632, p=0.55) and between HPV positive and HPV negative subjects in VL increase (λ31/λ42 = 0.656, p=0.69) and in VL decrease (λ13/λ24 = 0.983, p=0.98) using both sets of HPV (Set II results shown). Figure 2A presents model-based probability curves over 5 years for a woman initiating HAART, starting HPV negative and VL>400 (State 1), based on estimates using Set II. In half-year, the probability of remaining in State 1 is 0.20. The chances of being in the other states as HPV status changes and/or VL decreases are: 0.08 for HPV positive and VL>400 (State 2), 0.60 for HPV negative and VL<400 (State 3) and 0.12 for HPV positive and VL<400 (State 4). The probabilities stabilize in about 2.5 years to around 0.11, 0.08, 0.58 and 0.23 for the four states, respectively. They suggest that whereas the probability of being HPV positive and HPV negative are similar when VL>400 (at around 0.10), the probability of being HPV positive is about 0.4 times the probability of being HPV negative when VL ≤ 400.
Figure 2.
State probability curves over 5 years from (A) VL Model and (B) CD4 Model, based on the model estimates using Set II. The initial state is State 1: high risk HPV negative and (A) VL>400, or (B) CD4 350.
HPV and CD4+ T-Cell Count
There were 145 subjects in the analysis, because two of the subjects did not provide CD4. Of the 140 subjects with both HPV and CD4 results at baseline, 107 subjects (76%) started with CD4<350, and HPV was detected in 86 subjects (61%). Data availability trends over time were similar to those for the VL model above.
In CD4 Model (Figure 1B), similar conclusions were drawn from the two HPV sets (Set II results shown). Comparison between γ21 and γ43 suggested that a woman with current CD4>350 was more likely to clear HPV than with CD4 ≤ 350 (hazard ratio γ43/γ21 = 2.65, p=0.018). The statistical tests on other comparisons were not significant. There was no evidence to conclude that HPV detection rates differed between subjects with CD4 ≤ 350 and >350 (γ12/γ34 = 1.03, p=0.94), and HPV status did not seem to affect CD4 state transition rates (γ13/γ24 = .920, p=0.78; γ31/γ42 = 0.408, p=0.18). Figure 2B presents the model-based probability curves over 5 years for a HAART-initiating woman, starting HPV negative and CD4 ≤ 350 (State 1). The probabilities stabilize in about 3.5 years to around 0.12 in State 1, 0.11 in State 2 (HPV positive and CD4 ≤ 350), 0.56 in State 3 (HPV negative and CD4>350) and 0.21 in State 4 (HPV positive and CD4>350). The probability of being HPV positive is about 0.4 times the probability of being HPV negative when CD4>350.
Discussion
Studies have shown varying effects of HAART on HPV infection and HPV-related cervical dysplasia. Several studies have shown higher HPV prevalence in women with lower CD4 and clearance of HPV with improving CD4 (9, 13, 14). Research on the association between HIV VL and HPV detection has been limited. One study showed that HIV VL and CD4 in combination appeared to be associated with HPV detection, with varying effects of HIV RNA level on HPV prevalence and incident detection depending on the CD4 stratum (4). Higher HIV VL may be associated with HPV detection due to a possible viral-viral interaction (15), or inflammatory responses induced by HIV may interfere with a woman’s ability to mount an effective immune response to HPV infection (16). In vitro studies have shown an increased expression of HPV E1 and L1 viral genes with the presence of HIV tat proteins (17). Our analysis using an older set of high risk HPV types suggested that higher VL may be associated with HPV detection and hinted at the role of HIV viral load in HPV acquisition. However, such association was not suggested using the latest set of high risk HPV types. Hence, our research shows the importance of considering the HPV types used when reviewing literature. It is postulated that immune reconstitution associated with HAART may lead to clearance of HPV, as has been the case with other viral or nonviral opportunistic infections, and this was consistent with our analyses where higher CD4 was associated with HPV clearance.
There are a couple of limitations to our analysis. There was a trend of earlier discontinuation for subjects starting with HPV, suggesting possible informative censoring, and the small sample size did not allow models that adjust for covariates such as age, cigarette smoking, HPV type and sexual activity. There are several advantages of the statistical methods that we used. The multi-state modeling approach accommodates multiple and recurrent events using intermittent data. The hazard rates are estimated simultaneously in the model, eliminating the need to subset the data to estimate HPV detection rate among the HPV negatives and separately to estimate clearance among the positives. And while other HPV studies have used the midpoint between the visit times as the event time (e.g. time of HPV detection or clearance) or the time of the visit, the methods we used are appropriate when the exact event times are unknown. The approach utilized the incomplete data efficiently and provided a more comprehensive description of the HPV detection and clearance process.
Acknowledgments
We thank the clinicians, study coordinators and study subjects at A5029 sites for their participation and the A5029 study team, headed by Ken Fife, for sharing the data. We also thank Stephen Lagakos, Janet Andersen and Michael Hughes for their thoughtful comments on the analysis. The authors are supported by the AIDS Clinical Trials Group and K24 Mid-Career Research Mentoring Award funded by the National Institute of Allergy and Infectious Diseases (Grants 1U01AI068636-01, 1U01AI068634-01 and K24AI066884).
A5029 participating sites and funding: Univ. of Southern California CTU Grant AI27673; Duke Univ. Med Ctr. CTU Grant AI69684; Beth Israel Deaconess Med. Ctr., Brigham and Women’s Hospital CTU Grant AI69472; AI60354, NYU/NYC HHC at Bellevue CTU Grant AI69532, GCRC Grant RR00096; Northwestern Univ., Cook County CORECenter CTU Grant AI69471; Univ. of Alabama at Birmingham CTU Grant AI69452-01, GCRC Grant RR00032, CFAR Grant AI027767; Indiana Univ. CTU Grant AI25859, GCRC Grant RR000750; Univ. of Rochester CTU Grant AI69411, GCRC Grant RR00044; Case Western Reserve Univ. CTU Grant AI69501; Univ. of Miami CTU Grant AI69477; Univ. of Pittsburgh CTU Grant AI69494; The Miriam Hospital CTU Grant AI46381, AI069472; Univ. of Maryland, Inst. of Human Virology CTU Grant AI69447; San Juan City Hospital NICHD Contract HD33345; SUNY at Stony Brook School of Med., Div. of Ped. Infect. Dis. NICHD Contract HD33345; Univ. of Puerto Rico, Childrens Hospital; Columbia Collaborative HIV/AIDS CTU Grant AI69470; North Broward Hosp. District, Children’s Diagnostic & Treatment Ctr., Inc. Contract HD33345, Washington Univ. St. Louis CTU Grant AI69495; Beth Israel Med. Ctr. CTU Grant AI46370; Vanderbilt Univ. CTU Grant AI69439; Univ. of Hawaii at Manoa CTU Grant AI34853; Univ. of Maryland Med Ctr, Div. of Ped Immunology & Rheumatology; Mt. Sinai Hospital Med Ctr, Womens & Childrens HIV Program, Los Angeles County/Univ. of Southern California PACTU/Maternal-Child-Adolescent HIV Center NICHD Contract HD33345, Westat Subcontract Grant 7735-S042, GCRC Grant RR000043; Univ. of Washington CTU Grant AI27664, AI69434; UNC Chapel Hill CTU Grant AI69423-01, CFAR Grant AI50410, GCRC Grant RR00046; Univ. of Florida/Jacksonville NIHCD Contract HD33345
Appendix
Let pjk(s,t) represent the probability that an individual in state j at time s is in state k at time t, where j,k=1,2,3,4 and s ≤ t. Since the process is assumed to be time-homogeneous, pjk(s,t)= pjk(0,t − s). The intensity function for transition from state j to state k, or cause-specific hazard at time t, is denoted by λij and defined as,
Let P(s,t) and Λ denote the 4×4 matrices of transition probabilities and intensities, respectively, where the jth diagonal element (λjj) is the negative of the rate of leaving state j:
The relationship between the transition probabilities and the transition rates is given by,
The time that the process stays in a state before making a transition to a different state is exponentially distributed, with the mean sojourn time in state j given by −1/λjj.
The transition intensity matrix for the model in Figure 1 is given by,
where λ12=θ1, λ13=θ2, λ21=θ3, λ24=θ4, λ31=θ5, λ34=θ6, λ42=θ7 and λ43=θ8. The likelihood function for θ = (θ1, …,θ8)′ is given by,
where the Markov process is observed intermittently at times ti,0 < ti,1< · · · < ti,mi−1 < ti,mi, i =1, …, n individuals, each with mi observations. Kalbfleisch and Lawless provide a scoring procedure to obtain the maximum likelihood estimate (MLE) for θ and an estimate of its asymptotic covariance matrix.
Hypotheses to compare hazard rates can be tested by fitting unrestricted and restricted models and using likelihood ratio tests (LRT). For example, we can test H0: θ1=θ6 by fitted the unrestricted model given in [1] and fitting the restricted model where the third row is replaced with (θ5, 0, −(θ5+θ1), θ1). The likelihood ratio statistic, 2[log L(θ)unrestricted − log L(θ)restricted] has an approximately distribution if H0 is true.
The observed transition frequencies and the corresponding expected numbers from the models (in parentheses) are listed below to examine the model fits. As an example, consider the 47 subjects who started in State 1: HPV negative and HIV VL>400 copies/mL (HIV VL Model). Of these, 11 subjects remained in State 1, two transitioned to State 2, 28 to State 3 and six to State 4 by Week 28. The model-estimations for these frequencies are 11.23, 3.57, 27.36 and 4.84, respectively. Overall, the comparisons of the observed and expected frequencies indicate adequate model fits.
Table A1.
The HIV viral load (VL) model
| 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|
|
|
|||||
| 1 | 11 (11.23) | 2 (3.57) | 28 (27.36) | 6 (4.84) | 47 |
| 2 | 2 (2.85) | 9 (14.39) | 9 (7.97) | 31 (25.79) | 51 |
| 3 | 1 (1.21) | 1 (0.28) | 6 (7.67) | 2 (0.85) | 10 |
| 4 | 0 (0.25) | 2 (1.12) | 2 (1.53) | 3 (4.10) | 7 |
|
|
|||||
| (t0=0,t1=24) | |||||
| 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|
|
|
|||||
| 1 | 3 (2.87) | 1 (0.91) | 7 (6.99) | 1 (1.23) | 12 |
| 2 | 1 (0.61) | 7 (3.10) | 1 (1.72) | 2 (5.56) | 11 |
| 3 | 3 (4.94) | 4 (1.14) | 31 (31.45) | 3 (3.47) | 41 |
| 4 | 0 (1.05) | 1 (4.79) | 8 (6.57) | 21 (17.59) | 30 |
|
|
|||||
| (t0=24,t1=48) | |||||
| 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|
|
|
|||||
| 1 | 3 (0.68) | 0 (0.36) | 1 (3.10) | 1 (0.86) | 5 |
| 2 | 2 (0.72) | 4 (1.86) | 4 (3.38) | 1 (5.04) | 11 |
| 3 | 2 (4.91) | 2 (2.02) | 31 (26.58) | 4 (5.50) | 39 |
| 4 | 3 (1.16) | 1 (2.65) | 4 (6.15) | 10 (8.04) | 18 |
|
|
|||||
| (t0=48,t1=96) | |||||
Table A2.
The CD4 model
| 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|
|
|
|||||
| 1 | 21 (21.55) | 4 (3.69) | 10 (9.34) | 1 (1.42) | 36 |
| 2 | 4 (5.05) | 30 (29.63) | 3 (3.89) | 13 (11.42) | 50 |
| 3 | 2 (0.95) | 0 (0.25) | 12 (15.66) | 5 (2.15) | 19 |
| 4 | 1 (0.13) | 0 (0.69) | 4 (2.13) | 2 (4.04) | 7 |
|
|
|||||
| (t0=0,t1 =24) | |||||
| 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|
|
|
|||||
| 1 | 13 (14.36) | 3 (2.46) | 6 (6.23) | 2 (0.95) | 24 |
| 2 | 4 (2.83) | 14 (16.60) | 1 (2.18) | 9 (6.40) | 28 |
| 3 | 2 (1.44) | 0 (0.38) | 23 (23.90) | 4 (3.27) | 29 |
| 4 | 1 (0.21) | 2 (1.09) | 3 (3.35) | 5 (6.35) | 11 |
|
|
|||||
| (t0=24,t1=48) | |||||
| 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|
|
|
|||||
| 1 | 7 (6.12) | 3 (2.07) | 5 (6.23) | 1 (1.59) | 16 |
| 2 | 2 (1.54) | 6 (4.62) | 1 (2.47) | 3 (3.36) | 12 |
| 3 | 1 (2.15) | 0 (1.01) | 25 (21.10) | 3 (4.73) | 29 |
| 4 | 0 (0.75) | 3 (1.95) | 10 (7.03) | 3 (6.26) | 16 |
|
|
|||||
| (t0=48,t1=96) | |||||
References
- 1.Ahdieh L, Klein RS, Burk R, et al. Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative women. J Infect Dis. 2001;184:682–90. doi: 10.1086/323081. [DOI] [PubMed] [Google Scholar]
- 2.Schuman P, Ohmit SE, Klein RS, et al. Longitudinal study of cervical squamous intraepithelial lesions in human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women. J Infect Dis. 2003;188:128–36. doi: 10.1086/375783. [DOI] [PubMed] [Google Scholar]
- 3.Massad LS, Ahdieh L, Benning L, et al. Evolution of cervical abnormalities among women with HIV-1: evidence from surveillance cytology in the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2001;27:432–42. doi: 10.1097/00126334-200108150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–86. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 5.Ellerbrock TV, Chiasson MA, Bush TJ, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA. 2000;283:1031–7. doi: 10.1001/jama.283.8.1031. [DOI] [PubMed] [Google Scholar]
- 6.Minkoff H, Ahdieh L, Massad LS, et al. The effect of highly active antiretroviral therapy on cervical cytologic changes associated with oncogenic HPV among HIV-infected women. Aids. 2001;15:2157–64. doi: 10.1097/00002030-200111090-00011. [DOI] [PubMed] [Google Scholar]
- 7.Kang M, Lagakos SW. Statistical methods for panel data from a semi-Markov process, with application to HPV. Biostatistics. 2007;8:252–64. doi: 10.1093/biostatistics/kxl006. [DOI] [PubMed] [Google Scholar]
- 8.Wacholder S. Chapter 18: Statistical issues in the design and analysis of studies of human papillomavirus and cervical neoplasia. J Natl Cancer Inst Monogr. 2003:125–30. doi: 10.1093/oxfordjournals.jncimonographs.a003474. [DOI] [PubMed] [Google Scholar]
- 9.Fife KH, Wu JW, Squires KE, Watts DH, Andersen JW, Brown DR. Prevalence and persistence of cervical human papillomavirus infection in HIV-positive women initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;51:274–82. doi: 10.1097/QAI.0b013e3181a97be5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 11.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. The Lancet Oncology. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 12.Kalbfleisch J, Lawless J. The analysis of panel data under a Markov assumption. Journal of the American Statistical Association. 1985;80:863–71. [Google Scholar]
- 13.Heard I, Palefsky JM, Kazatchkine MD. The impact of HIV antiviral therapy on human papillomavirus (HPV) infections and HPV-related diseases. Antiviral therapy. 2004;9:13–22. [PubMed] [Google Scholar]
- 14.Paramsothy P, Jamieson DJ, Heilig CM, et al. The effect of highly active antiretroviral therapy on human papillomavirus clearance and cervical cytology. Obstetrics and gynecology. 2009;113:26–31. doi: 10.1097/AOG.0b013e31819225cb. [DOI] [PubMed] [Google Scholar]
- 15.Clarke B, Chetty R. Postmodern cancer: the role of human immunodeficiency virus in uterine cervical cancer. Mol Pathol. 2002;55:19–24. doi: 10.1136/mp.55.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vuyst H, Lillo F, Broutet N, Smith JS. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur J Cancer Prev. 2008;17:545–54. doi: 10.1097/CEJ.0b013e3282f75ea1. [DOI] [PubMed] [Google Scholar]
- 17.Dolei A, Curreli S, Marongiu P, et al. Human immunodeficiency virus infection in vitro activates naturally integrated human papillomavirus type 18 and induces synthesis of the L1 capsid protein. The Journal of general virology. 1999;80 ( Pt 11):2937–44. doi: 10.1099/0022-1317-80-11-2937. [DOI] [PubMed] [Google Scholar]