Abstract
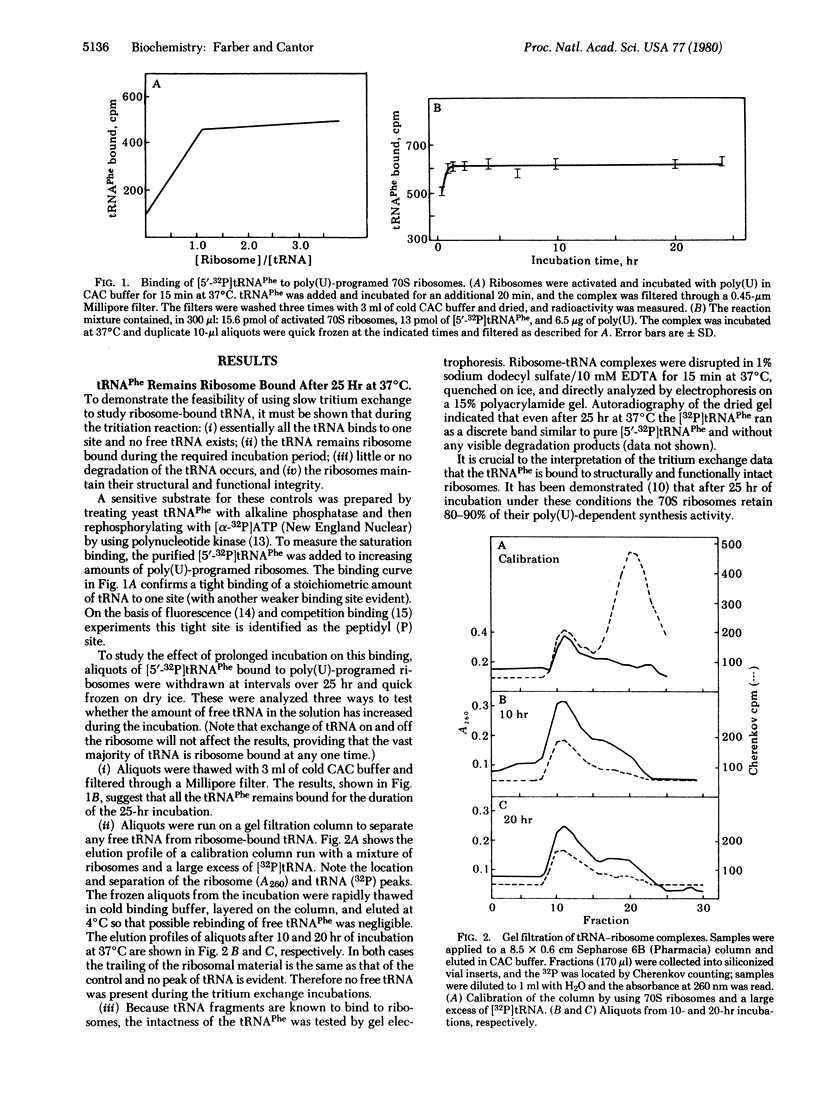
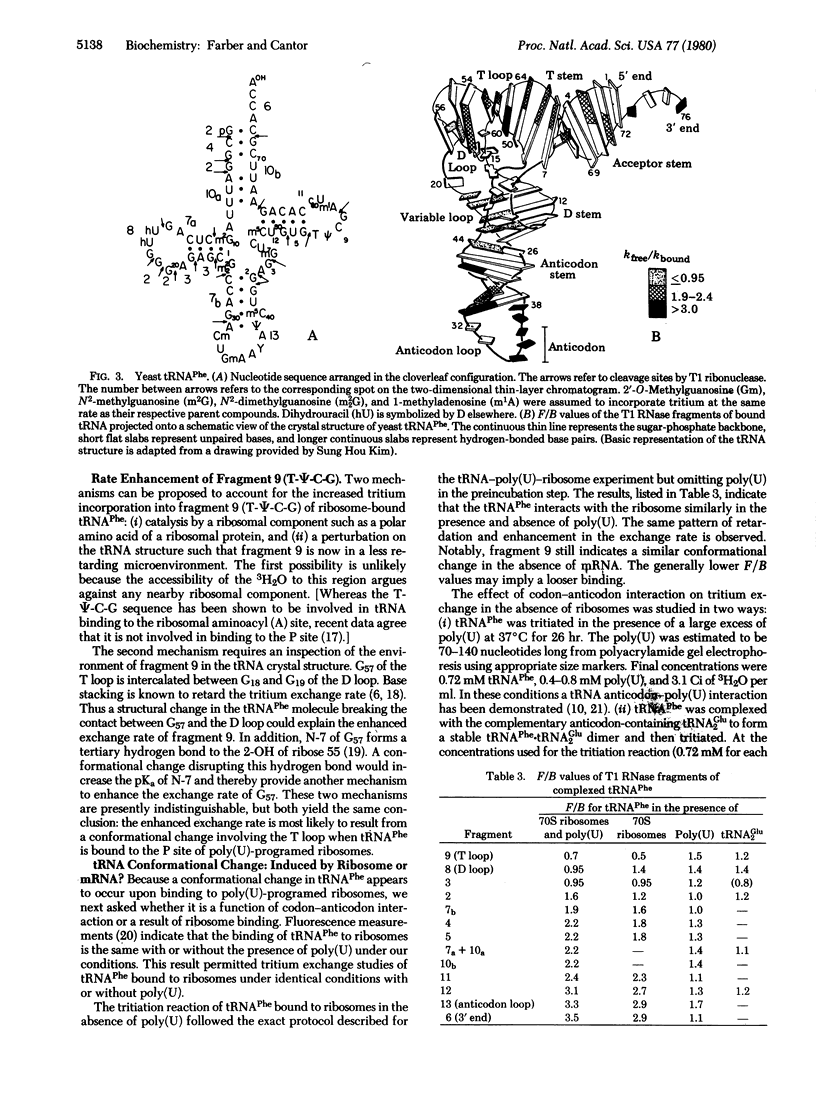
The rate of incorporation of tritium from the solvent into the C-8 position of purines in RNA is markedly sensitive to the microenvironment. This slow tritium exchange reaction has been used to study the structure and interactions of yeast tRNAPhe bound to poly(U)-programed tight-couple 70S ribosomes of Escherichia coli. The tritium incorporation into specific sites of the tRNA was determined by enzymatic digestion and measurement of the specific activity of each of the isolated radioactive fragments. Ribosome binding leads to marked suppression in the exchange rate of a number of fragments. This delineates extensive regions of tRNA-ribosome contact. No change in exchange rates is seen for fragments from the corner of the molecule, indicating that this region of bound tRNA is readily accessible to the solvent. Ribosome binding results in an enhanced exchange rate at the T loop. This appears to be the result of a conformational change that is most likely an unfolding of the T and D loops. Additional tritium exchange reactions suggest this conformational change is induced by ribosomes and not by messenger.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davanloo P., Sprinzl M., Cramer F. Proton nuclear magnetic resonance of minor nucleosides in yeast phenylalanine transfer ribonucleic acid. Conformational changes as a consequence of aminoacylation, removal of the Y base, and codon--anticodon interaction. Biochemistry. 1979 Jul 24;18(15):3189–3199. doi: 10.1021/bi00582a001. [DOI] [PubMed] [Google Scholar]
- De Groot N., Panet A., Lapidot Y. The binding of purified Phe-tRNA and peptidyl-tRNA Phe to Escherichia coli ribosomes. Eur J Biochem. 1971 Dec 10;23(3):523–527. doi: 10.1111/j.1432-1033.1971.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger J., Feuer B., Yamane T. Luminescence and binding studies on tRNA-Phe. Proc Natl Acad Sci U S A. 1970 Mar;65(3):638–644. doi: 10.1073/pnas.65.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander S. W., Crowe D. Rapid microdialysis and hydrogen exchange. Anal Biochem. 1965 Sep;12(3):579–584. doi: 10.1016/0003-2697(65)90225-3. [DOI] [PubMed] [Google Scholar]
- Fairclough R. H., Cantor C. R. The distance between the anticodon loops of two tRNAs bound to the 70 S Escherichia coli ribosome. J Mol Biol. 1979 Aug 25;132(4):575–586. doi: 10.1016/0022-2836(79)90375-9. [DOI] [PubMed] [Google Scholar]
- Fairclough R. H., Cantor C. R., Wintermeyer W., Zachau H. G. Fluorescence studies of the binding of a yeast tRNAPhe derivative to Escherichia coli ribosomes. J Mol Biol. 1979 Aug 25;132(4):557–573. doi: 10.1016/0022-2836(79)90374-7. [DOI] [PubMed] [Google Scholar]
- Gamble R. C., Schoemaker J. P. Rate of tritium labeling of specific purines in relation to nucleic acid and particularly transfer RNA conformation. Biochemistry. 1976 Jun 29;15(13):2791–2799. doi: 10.1021/bi00658a014. [DOI] [PubMed] [Google Scholar]
- Geerdes H. A., van Boom J. H., Hilbers C. W. Codon--anticodon interaction in yeast tRNAPhe: an 1H NMR study. FEBS Lett. 1978 Apr 1;88(1):27–32. doi: 10.1016/0014-5793(78)80599-7. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Söll D. G., Crothers D. M. Studies of the complex between transfer RNAs with complementary anticodons. I. Origins of enhanced affinity between complementary triplets. J Mol Biol. 1976 May 25;103(3):499–519. doi: 10.1016/0022-2836(76)90214-x. [DOI] [PubMed] [Google Scholar]
- Holbrook S. R., Sussman J. L., Warrant R. W., Kim S. H. Crystal structure of yeast phenylalanine transfer RNA. II. Structural features and functional implications. J Mol Biol. 1978 Aug 25;123(4):631–660. doi: 10.1016/0022-2836(78)90210-3. [DOI] [PubMed] [Google Scholar]
- Kuechler E., Bauer K., Rich A. Protein synthesis with ribonuclease digested ribosomes. Biochim Biophys Acta. 1972 Sep 14;277(3):615–627. doi: 10.1016/0005-2787(72)90106-2. [DOI] [PubMed] [Google Scholar]
- Lake J. A. Aminoacyl-tRNA binding at the recognition site is the first step of the elongation cycle of protein synthesis. Proc Natl Acad Sci U S A. 1977 May;74(5):1903–1907. doi: 10.1073/pnas.74.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley G. J., Rich A. Structural domains of transfer RNA molecules. Science. 1976 Nov 19;194(4267):796–806. doi: 10.1126/science.790568. [DOI] [PubMed] [Google Scholar]
- RajBhandary U. L., Chang S. H. Studies on polynucleotides. LXXXII. Yeast phenylalanine transfer ribonucleic acid: partial digestion with ribonuclease T-1 and derivation of the total primary structure. J Biol Chem. 1968 Feb 10;243(3):598–608. [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Robertson J. M., Kahan M., Wintermeyer W., Zachau H. G. Interactions of yeast tRNAPhe with ribosomes from yeast and Escherichia coli. A fluorescence spectroscopic study. Eur J Biochem. 1977 Jan 3;72(1):117–125. doi: 10.1111/j.1432-1033.1977.tb11231.x. [DOI] [PubMed] [Google Scholar]
- Schimmel P. R., Schoemaker H. J. Mapping the structure of specific protein-transfer RNA complexes by a tritium labeling method. Methods Enzymol. 1979;59:332–350. doi: 10.1016/0076-6879(79)59095-8. [DOI] [PubMed] [Google Scholar]
- Schoemaker H. J., Gamble R. C. Comparison of isotope labeling patterns of purines in three specific transfer RNAs. Biochemistry. 1976 Jun 29;15(13):2800–2803. doi: 10.1021/bi00658a015. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Gassen H. G. Codon-dependent rearrangement of the tertiary structure of tRNAPhe from yeast. FEBS Lett. 1977 Jun 15;78(2):267–270. doi: 10.1016/0014-5793(77)80320-7. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Menzel H. M., Gassen H. G. Codon-dependent rearrangement of the three-dimensional structure of phenylalanine tRNA, exposing the T-psi-C-G sequence for binding to the 50S ribosomal subunit. Biochemistry. 1976 Jun 1;15(11):2484–2490. doi: 10.1021/bi00656a035. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Wagner T., Lorenz S., Erdmann V. A. Regions of tRNA important for binding to the ribosomal A and P sites. Biochemistry. 1976 Jul 13;15(14):3031–3039. doi: 10.1021/bi00659a015. [DOI] [PubMed] [Google Scholar]
- Wagner R., Garrett R. A. Chemical evidence for a codon-induced change of tRNA conformation. FEBS Lett. 1978 Jan 15;85(2):291–295. doi: 10.1016/0014-5793(78)80476-1. [DOI] [PubMed] [Google Scholar]
- Wishnia A., Boussert A., Graffe M., Dessen P. H., Grunberg-Manago M. Kinetics of the reversible association of ribosomal subunits: stopped-flow studies of the rate law and of the effect of Mg2+. J Mol Biol. 1975 Apr 25;93(4):499–415. doi: 10.1016/0022-2836(75)90242-9. [DOI] [PubMed] [Google Scholar]
- Woese C. Molecular mechanics of translation: a reciprocating ratchet mechanism. Nature. 1970 May 30;226(5248):817–820. doi: 10.1038/226817a0. [DOI] [PubMed] [Google Scholar]
- Zamir A., Miskin R., Vogel Z., Elson D. The inactivation and reactivation of Escherichia coli ribosomes. Methods Enzymol. 1974;30:406–426. doi: 10.1016/0076-6879(74)30042-0. [DOI] [PubMed] [Google Scholar]



