Abstract
This study evaluated a novel test strip designed to assess thiol levels as they relate to gingival/periodontal health in dogs. The simple to use strip (similar in form to a pH test strip) provides a colorimetric signal which estimates the level of thiols dissolved in oral fluid. Among several oral sites tested (left and right lingual vestibules, lower buccal vestibule, and upper buccal gingival margin), fluid from the maxillary gingival margin gave results with the best dynamic range, and its thiol levels correlated well with several oral health parameters (Pearson coefficients between 0.55 and 0.84; P < 0.001), especially those relating directly to the gingiva. The strip, which can be used on animals which are awake, may be useful as a quick, objective assessment of periodontal health, potentially enhancing compliance for thorough examinations, and promoting earlier and better-sustained treatment programs.
Résumé
Évaluation pilote d’un nouveau bâtonnet diagnostique pour l’évaluation des taux de thiol dissous comme indicateur de la santé gingivale canine et de la situation parodontale. Cette étude a évalué un nouveau bâtonnet diagnostique conçu pour évaluer les taux de thiol tels qu’ils se rapportent à la santé gingivale et parodontale des chiens. Le bâtonnet facile à utiliser (de forme semblable à un bâtonnet diagnostique de pH) fournit un signal colorimétrique qui estime le taux de thiols dissous dans le liquide buccal. Parmi plusieurs sites buccaux testés (vestibules linguaux gauche et droit, vestibule buccal inférieur et bord marginal de la gencive buccale supérieure), le liquide du bord marginal de la gencive maxillaire a donné les résultats avec le meilleur écart dynamique et ses taux de thiol correspondaient bien à plusieurs paramètres de la santé buccale (coefficients de Pearson entre 0,55 et 0,84; P < 0,001), particulièrement ceux se rapportant directement à la gencive. Le bâtonnet, qui peut être utilisé sur les animaux lorsqu’ils sont éveillés, pourra être utile comme évaluation objective rapide de la santé parodontale, ce qui rehaussera potentiellement l’observance pour des examens complets et fera la promotion de programmes de traitement mieux soutenus qui sont administrés plus tôt.
(Traduit par Isabelle Vallières)
Introduction
As dogs mature and age the prevalence of periodontal disease increases, with clinical attachment loss being observed in > 80% of individuals over 3 y old in certain breeds (1). In order to perform thorough assessments of canine periodontal health, anesthesia is needed for dental radiographs (2). In the patient that is awake, the pet owner must rely on the veterinarian’s visual examination, which would not involve dental probing and radiography. Complete dental examinations can be costly and time consuming, and pet owners may decide against them, due in part perhaps to reservations about sedation/anesthesia. Additional objective evidence about the patient that is awake could be beneficial, since it could help prompt more appropriate decisions relating to subsequent assessment and treatment.
As evidence continues to mount linking dental health and systemic health (3) in dogs and humans, the need for diagnostic techniques which can quickly and easily provide results correlated to oral health parameters is becoming more critical. The purposes of this study were to determine the most appropriate sample collection site, and then (using that site) examine correlations between the test results (thiol levels) and a number of clinical parameters germane to canine periodontal health.
Materials and methods
Seventy-one dogs were included in the study. Forty-two of the dogs received thorough periodontal examinations, including radiographs, and measurements of gingival index, probing depth, calculus score, periodontal disease staging, furcation stage, and tooth mobility. Twenty-nine of 32 dogs that were presumed to be healthy did not receive the thorough assessment. All dental examinations were performed by members of the College of Veterinary Medicine at the University of Illinois at Urbana-Champaign. All testing using test strips was done by one examiner (SM), while others at the College performed the periodontal health assessments. All examiners were trained in these assessment techniques by SM.
Initial information collected for each subject included age, gender, weight, and breed. Upon presentation, all subjects underwent visual dental examinations (i.e., not involving dental probing or radiography) and had oral fluid samples collected using the prototype test strip. The examinations included an initial impression of overall, whole-mouth gingival health, and a “gingival inflammation score” was assigned (description follows).
Prototype test strips and color (“comparator”) charts were provided by PDx BioTech, Lexington, Kentucky. Strips were composed of a dry porous pad (pretreated with a proprietary mixture containing a thiol detection reagent) attached to a plastic backing (4). Each comparator chart consisted of a numbered set of 10 colors, showing progressively more intense color chips (white to deep yellow), on a white background. More intense colors are generated by higher levels of dissolved thiols.
Samples were collected from several sites within the oral cavity: left lingual vestibule (LV-left); right lingual vestibule (LV-right); the entire lower buccal vestibule; and the entire maxillary gingival margin. Test strips were wetted with oral fluid samples by passive absorption (buccal and lingual vestibule samples) or by gently contacting and then gliding the absorbent pad of the test strip across the maxillary gingival margin (Figure 1). The test result is manifested as a change in the color of the pad surface (from off-white to various intensities of yellow). This result color was compared to the colors on the comparator chart, which correspond to those generated using a model thiol system, across a range of concentrations (0 to 2000 μM), 10 seconds after removing the strip from the animal’s mouth. The number corresponding to the color on the chart closest in intensity to the test strip color was recorded for each sampled site in each subject. The examiner also recorded whether the test strip could be used appropriately without sedation/anesthesia.
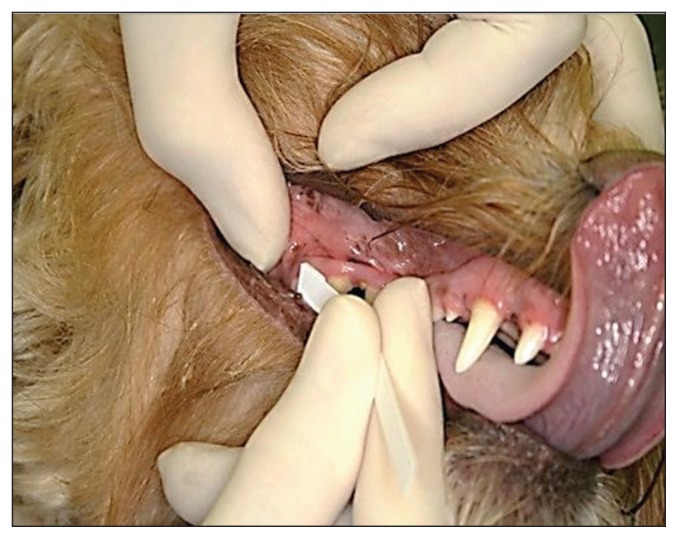
Figure 1.
Clinical examiner using test strip on the maxillary buccal gingival margin.
An initial assessment of gingival inflammation was made for all dogs in the study, and scores were assigned as follows: 0) — no apparent inflammation (healthy gingiva), 1) — mild inflammation, and 2) — moderate to severe inflammation. For dogs receiving thorough examinations, a gingival index (GI) score was recorded as well, again based on an overall assessment of the level of inflammation, but after the examination: 0) — no inflammation, 1) — mild inflammation, 2) — moderate inflammation, and 3) — severe inflammation.
Periodontal disease classification was determined according to the American Veterinary Dental College (AVDC) staging guidelines (www.avdc.org/nomenclature), and based on the worst affected tooth, and was scored as follows:
0) Normal (PD 0) — clinically normal — no gingival inflammation or periodontitis clinically evident. 1) Stage 1 (PD 1) — gingivitis only without attachment loss. The height and architecture of the alveolar margin are normal. 2) Stage 2 (PD 2) — early periodontitis; less than 25% of attachment loss or at most, there is a stage 1 furcation involvement in multirooted teeth. There are early radiologic signs of periodontitis. The loss of periodontal attachment is less than 25% as measured either by probing of the clinical attachment level, or radiographic determination of the distance of the alveolar margin from the cemento-enamel junction relative to the length of the root. 3) Stage 3 (PD 3) — moderate periodontitis; 25% to 50% of attachment loss as measured either by probing of the clinical attachment level, radiographic determination of the distance of the alveolar margin from the cemento-enamel junction relative to the length of the root, or there is a stage 2 furcation involvement in multirooted teeth. 4) Stage 4 (PD 4) — advanced periodontitis; more than 50% of attachment loss as measured either by probing of the clinical attachment level, or radiographic determination of the distance of the alveolar margin from the cemento-enamel junction relative to the length of the root, or there is a stage 3 furcation involvement in multirooted teeth.
Probing depths were done on all teeth and pocket depths > 3 mm were recorded. Furcation was scored as follows (per AVDC nomenclature):
0) no furcation exposure; 1) Stage 1 (F1, furcation involvement) exists when a periodontal probe extends less than half way under the crown in any direction of a multirooted tooth with attachment loss; 2) Stage 2 (F2, furcation involvement) exists when a periodontal probe extends greater than half way under the crown of a multirooted tooth with attachment loss but not through and through; 3) Stage 3 (F3, furcation exposure) exists when a periodontal probe extends under the crown of a multirooted tooth, through and through from one side of the furcation out the other.
Tooth mobility was scored as follows (per AVDC nomenclature):
0) Stage 0 (M0) — physiologic mobility up to 0.2 mm; 1) Stage 1 (M1) — the mobility is increased in any direction other than axial over a distance of more than 0.2 mm and up to 0.5 mm. 2) Stage 2 (M2) — the mobility is increased in any direction other than axial over a distance of more than 0.5 mm and up to 1.0 mm; 3) Stage 3 (M3) — the mobility is increased in any direction than axial over a distance exceeding 1.0 mm or any axial movement.
Calculus was scored as follows:
0) No calculus seen; 1) Mild — small amount along gingival margin; 2) Moderate — moderate amount, but not bridging along the adjacent teeth; 3) Heavy — large amount of thick calculus, with bridging between teeth
Tabulated data (for all subjects, or for the ‘full-exam’ subgroup) were analyzed using “statistiXL” (an add-on statistical software designed to function within Microsoft Excel, available from www.statistixl.com), the output for which provides descriptive statistics for each variable considered, a matrix of Pearson correlation coefficients (r-values), and corresponding matrix showing correlation significance (P-values).
Results
Of the 71 dogs enrolled in this study, 40 had been scheduled for dental cleaning or treatment and 31 were “presumed healthy” subjects based on presentation. The average age of the healthy dogs was 1.1 y, while the average age of the dogs scheduled for dental treatment or cleaning was 7.4 y. All subjects were grouped and analyzed by extent of gingival inflammation seen on initial visual examination. Forty-two dogs underwent thorough dental examinations with sedation/anesthesia, including 3 dogs that were presumed healthy. Twenty-nine dogs received a visual dental examination only. The test strip was used without sedation/anesthesia in most cases. Five of 62 dogs (~8%) required sedation/anesthesia for proper sample collection, due to biting concerns. The remaining 9 dogs were anesthetized for their procedure by another practitioner before the examiner was available to use the test strip.
Evaluation of several sampling sites (on the initial 64 of 71 subjects) indicated the maxillary buccal gingival margin provided the clearest distinction and best dynamic range for thiol concentrations among the sites (Table 1). Subsequent statistical analyses were conducted using data from this site.
Table 1.
Test strip results for the various sites examined
| Dogs receiving visual examination only (n = 29) | Dogs receiving full examination (n = 42)a | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Site sampled | Range | Average | Median | Range | Average | Median |
| LV-left | 1–2 | 1.6 | 2 | 1–4 | 2.0 | 2 |
| LV-right | 1–3 | 1.8 | 2 | 1–3 | 2.1 | 2 |
| Lower buccal | 1–4 | 2.0 | 2 | 1–5 | 2.9 | 3 |
| Maxillary | 1–5 | 2.3 | 2 | 2–9 | 4.9 | 5 |
Only 35 of the 42 dogs receiving the full examination were sampled at all 4 sites. All 42, however, were sampled at the maxillary site.
LV — lingual vestibule; Lower buccal refers to the lower buccal vestibule.
Thiol levels, as “test strip scores” (i.e., color values, per the numeric scale of the comparator chart) appeared to correlate with a number of measured parameters [Pearson correlation (r) values between 0.55 and 0.84, P < 0.001]. These included the initial assessment of gingival inflammation, the subsequent gingival index, calculus scores, subject age, and periodontal stage (when it was recorded), as shown in Table 2. P-values (all < 0.001) suggested the correlations were statistically significant. Variables not shown in Table 2 had correlation coefficients < 0.50.
Table 2.
Correlation coefficients (r), and statistical significance (P), for variables with r ≥ 0.5 (results relate to maxillary test strip scores)
| Variable | Population | Correlation coefficient (r) | Correlation significance (P) |
|---|---|---|---|
| Gingival inflammation score (initial visual assessment) | All dogs (n = 71) | 0.841 | < 0.001 |
| Full examination (n = 42) | 0.727 | < 0.001 | |
| Gingival index | All dogs (n = 71) | 0.842 | < 0.001 |
| Full examination (n = 42) | 0.739 | < 0.001 | |
| Calculus score | All dogs (n = 71) | 0.789 | < 0.001 |
| Full examination (n = 42) | 0.579 | < 0.001 | |
| Age | All dogs (n = 71) | 0.744 | < 0.001 |
| Full examination (n = 42) | 0.575 | < 0.001 | |
| Periodontal stage | Full examination (n = 42) | 0.549 | < 0.001 |
Two populations are shown since not all animals received the full examination; periodontal stage was determined only for those subjects receiving the full examination.
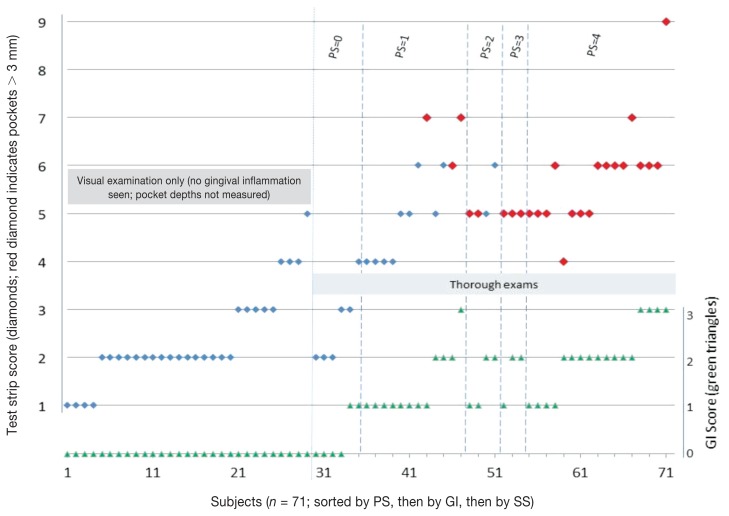
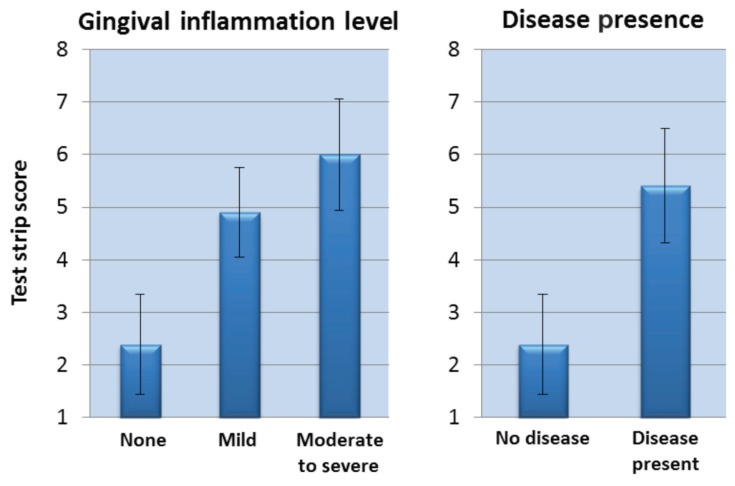
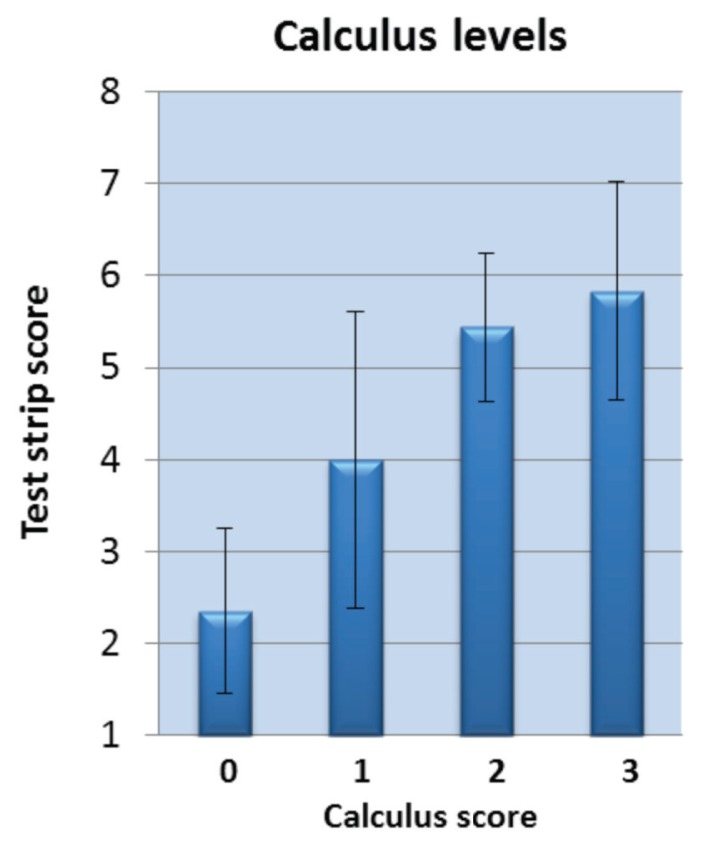
Test strip scores from the maxillary gingival margin were generally lower for healthier dogs, and generally elevated for dogs with visual signs of periodontal disease. Note that there were dogs presumed to be “healthy,” which had strip scores overlapping dogs undergoing thorough examinations defined as having stage 1 periodontal disease (Figure 2). Based on degree of gingival inflammation seen in the initial visual examination (Figure 3, left), test strip scores averaged 2.4, 4.9 and 6.0, respectively (for subjects grouped as: no inflammation, mild inflammation, and moderate/severe inflammation). Combining individuals presenting with any visible evidence of gingival inflammation (“disease”), the Figure 3, left graph condenses to that shown in Figure 3, right. Calculus scores also correlated with average test strip scores (Figure 4), as did periodontal stage (not shown), though not to the same extent in this data set as did the inflammation and calculus scores. Finally, the number of deeper pockets (> 3.0 mm) present correlated with maxillary test strip scores, although the correlation was only moderate (r = 0.440, P = 0.0029).
Figure 2.
Test strip scores and Gingival Index scores for study subjects. Test strip scores (SS, blue or red diamonds; use axis at left) were arranged by sup-population (groups are denoted by labeled gray rectangles), then sorted (left to right) by Periodontal Stage (PS) (for dogs with thorough examinations; labeled along top edge), then by Gingival Index (GI, green triangles; use axis at right), and finally by SS. Strip Scores for dogs with pocket depths of > 3 mm are shown by the red diamond markers. Probing was not done in the “visual examination only” sup-population.
Figure 3.
Maxillary test strip score averages relate to the extent of gingival inflammation (graph at left). Subjects with any gingival inflammation were grouped together, and portrayed as a single bar (graph at right).
Figure 4.
Test strip score averages relate to calculus level.
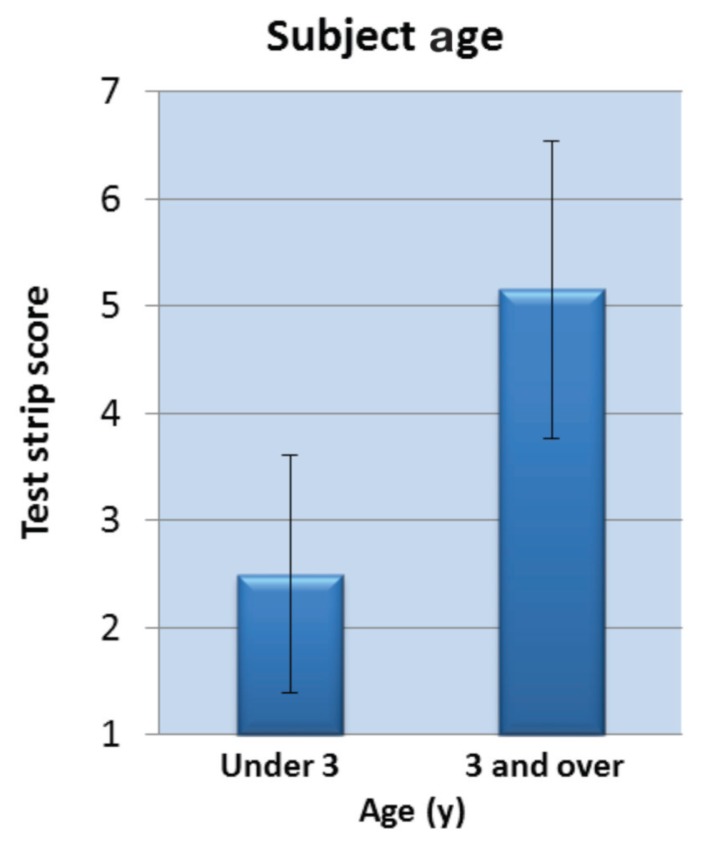
When test strip scores were graphed as a function of subject age, comparing strip score averages for dogs “under 3” and “3 or older,” (Figure 5 and Table 2) suggest that older dogs generally exhibit higher thiol levels along the maxillary gingival margin. Although the dogs in this pilot study were not recruited according to an age criterion, the majority being presented for wellness checks/neutering were younger animals than those being presented for dental cleaning/treatment.
Figure 5.
Test strip score averages relate to subject age.
Discussion
The volatile sulfur compounds hydrogen sulfide (H2S) and methylmercaptan (CH3SH) produced by oral microbes are the primary contributors to oral halitosis in humans (5). The responsible microbes may be present as biofilms on the posterior surface of the tongue, or within periodontal pockets (sites where periodontal tissue destruction and bone loss can occur). In addition, a number of studies suggest that these same compounds, toxic to gingival tissues, appear to be involved in the development and progression of periodontal disease (6–11). Parallel situations exist in dogs, in which malodorous volatile sulfur compounds are associated with periodontitis (12) and decrease following treatment for periodontal disease (13). The sites at which such compounds are produced depend on the location in the oral cavity occupied by the microbes responsible for their production. The times when such levels of thiols would be produced correspond to the times during which the microbes producing them are active (i.e., during an “active” disease process, as opposed to a quiescent phase, or a phase in which thiol-producers are perhaps being “successfully controlled”) (13,14). In this study, we did not control for “time since last meal.” Thiol levels may be affected, for instance, due to fasting prior to anesthesia.
We attempted to determine which one of several sampling sites provided the broadest visual signal range (thiol levels, based on test strip score), and how well semi-quantitative thiol detection at this site correlated with a number of variables associated with periodontal disease. The maxillary gingival margin displayed the largest range of thiol concentrations, and correlated strongly (and significantly) with several variables, even given the limitations and preliminary nature of this pilot study. Average test strip scores for dogs with healthy-appearing gingiva were typically 3 or less, suggesting that lower thiol concentrations in this site’s fluid generally correlate with better periodontal health. We would expect this to be generally true, although this study does not provide for parallel control in the “presumed healthy” group, by requiring these subjects as well to undergo thorough dental examinations. Additional studies in which all subjects are given thorough examinations are currently underway, which will allow us to better explore the relationships between thiol levels and the important clinical variables. In terms of technical manipulation of the strip, it may be important to ensure that the tongue (which can also harbor thiol-producing microbes) does not contact or otherwise contaminate the absorptive pad on the test strip.
Clinical utility of the test strip may also be evident in correlations between certain test strip cut-off values and the presence of pockets with depth > 3 mm. For the dogs receiving thorough examinations, the majority with such pockets show test strip scores of “5” or higher (Figure 2). If we use this cut-off, and calculate the strip’s accuracy at predicting abnormal periodontal pockets (i.e., deeper than 3 mm), the percentage is > 80. In this scenario, the strip is accurate 34 of 42 times (81%).
Not surprisingly, there was good correlation between strip result and the age of the subject (r = 0.744, P < 0.001). Employing an age cut-off of 3 y, and then examining the subjects in the ≥ 3 year group, we found that only 3 of 39 individuals (~8%) had test strip results < 4 (results of 1, 2, or 3 were generally associated with “good oral health;” the corresponding dissolved thiol level for a strip score of “3” was 25 μM). Similarly, in the subjects < 3 y of age (n = 32), only 5 individuals (~16%) had test strip values of ≥ 4. There seemed then to be a concentration cut-off of ~25 μM thiol for periodontally “healthy” individuals, with higher thiol levels suggesting progressively deteriorating oral health.
In response to microbial infection, many compounds can be produced by a host and may be either up- or down-regulated during the infectious process (15); others may simply be microbial toxins or effector molecules, or cellular components originating from microbes themselves. We propose, given these preliminary results, that dissolved oral thiols have sufficient diagnostic potential with regard to periodontal disease assessment in dogs, to merit further study. The relatively low ambient thiol levels evident in dogs with a healthier periodontium may represent those of a pre- or sub-inflammatory state; higher levels seem to accompany proportionately worsening inflammation. We found the liquid coating the maxillary gingival margin exhibited the best dynamic range of thiol concentrations, and was therefore the most appropriate among several samples tested, especially given the dynamic range of the colorimetric test strip. Analyzing this fluid in this fashion allows one to easily estimate dissolved thiol “chemomarkers,” which may, at a certain threshold level, signal the onset of inflammation and generally track with the severity of active periodontal infections.
Employing a screening test as part of an overall veterinary health check could have a positive impact on canine oral health care, by helping to boost compliance rates for home oral care regimens. The test is easy to administer and interpret. The visual result develops within 10 s of sample collection, and can typically be used on awake animals. In this study, only ~8% of the subjects required sedation/anesthesia, due to biting concerns. The test strip can be shown to the pet owner as an additional indication supporting or augmenting an examiner’s visual assessment, thereby enabling joint decisions regarding appropriate treatment and/or home care options.
Studies now in progress (in dogs and cats) should allow us to extend and strengthen these preliminary findings. Testing larger numbers of subjects, and employing thorough examinations and radiographs in all cases will afford more accurate comparisons of healthy and diseased populations. Also, the statistical correlations presented here assume linear relationships between the variables being compared. In future studies, we will consider how well current diagnostic criteria correlate with one another, and also that relationships between or among periodontal variables (including thiol levels) may not be linear, an assumption made for the simple correlations presented in this study. Future studies may also allow us to further elucidate the relationships between thiol levels and periodontal pockets, pocket depths, and periodontal staging. In addition, we hope to examine whether thiol levels may be affected by subject weight, and to follow animals over periods of time, to determine the potential of the test strip as a monitoring tool.
Acknowledgment
This study was made possible through financial support provided by PDx BioTech. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Kortegaard HE, Eriksen T, Baelum V. Periodontal disease in research beagle dogs — An epidemiological study. J Small Anim Pract. 2008;49:610–616. doi: 10.1111/j.1748-5827.2008.00609.x. [DOI] [PubMed] [Google Scholar]
- 2.Tsugawa AJ, Verstraete FJ. How to obtain and interpret periodontal radiographs in dogs. Clin Tech Small Anim Pract. 2000;15:204–210. doi: 10.1053/svms.2000.21042. [DOI] [PubMed] [Google Scholar]
- 3.Rawlinson JE, Goldstein RE, Reiter AM, Attwater DZ, Harvey CE. Association of periodontal disease with systemic health indices in dogs and the systemic response to treatment of periodontal disease. J Am Vet Med Assoc. 2011;238:601–609. doi: 10.2460/javma.238.5.601. [DOI] [PubMed] [Google Scholar]
- 4.Burgess-Cassler A, Leesman M. Rapid method for detecting/estimating dissolved oral thiols. Int Assoc for Dent Res; 87th General Session, abstract #787; 2009. [Last accessed October 16, 2012]. Available from http://iadr.confex.com/iadr/2009miami/webprogram/Paper117509.html. [Google Scholar]
- 5.Van den Velde S, van Steenberghe D, Van Hee P, Quirynen M. Detection of odorous compounds in breath. J Dent Res. 2009;88:285–289. doi: 10.1177/0022034508329741. [DOI] [PubMed] [Google Scholar]
- 6.Ng W, Tonzetich J. Effect of hydrogen sulfide and methyl mercaptan on the permeability of oral mucosa. J Dent Res. 1984;63:994–997. doi: 10.1177/00220345840630071701. [DOI] [PubMed] [Google Scholar]
- 7.Johnson PW, Ng W, Tonzetich J. Modulation of human gingival fibro-blast cell metabolism by methyl mercaptan. J Periodontal Res. 1992;27:476–483. doi: 10.1111/j.1600-0765.1992.tb01820.x. [DOI] [PubMed] [Google Scholar]
- 8.Persson S. Hydrogen sulfide and methyl mercaptan in periodontal pockets. Oral Microbiol Immunol. 1992;7:378–379. doi: 10.1111/j.1399-302x.1992.tb00641.x. [DOI] [PubMed] [Google Scholar]
- 9.Ratcliff PA, Johnson PW. The relationship between oral malodor, gingivitis, and periodontitis. A review. J Periodontol. 1999;70:485–489. doi: 10.1902/jop.1999.70.5.485. [DOI] [PubMed] [Google Scholar]
- 10.Yaegaki K. Oral malodorous compounds are periodontally pathogenic and carcinogenic. Jpn Dent Sci Rev. 2008;44:100–108. [Google Scholar]
- 11.Aoyama I, Calenic B, Imai T, Ii H, Yaegaki K. Oral malodorous compound causes caspase-8 and -9 mediated programmed cell death in osteoblasts. J Periodontal Res. 2012;47:365–373. doi: 10.1111/j.1600-0765.2011.01442.x. [DOI] [PubMed] [Google Scholar]
- 12.Nordhoff M, Rühe B, Kellermeier C, et al. Association of Treponema spp. with canine periodontitis. Vet Microbiol. 2008;127:334–342. doi: 10.1016/j.vetmic.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Rawlings JM, Culham N. Halitosis in dogs and the effect of periodontal therapy. J Nutr. 1998;128:2715S–2716S. doi: 10.1093/jn/128.12.2715S. [DOI] [PubMed] [Google Scholar]
- 14.Langendijk-Genevaux PS, Hanssen JT, van der Hoeven JS. Decrease of sulfate-reducing bacteria after initial periodontal treatment. J Dent Res. 2001;80:1637–42. doi: 10.1177/00220345010800070801. [DOI] [PubMed] [Google Scholar]
- 15.Bostanci N, Heywood W, Mills K, Parkar M, Nibali L, Donos N. Application of label-free absolute quantitative proteomics in human gingival crevicular fluid by LC/MSE (gingival exudatome) J Proteome Res. 2010;9:2191–2199. doi: 10.1021/pr900941z. [DOI] [PubMed] [Google Scholar]