Abstract
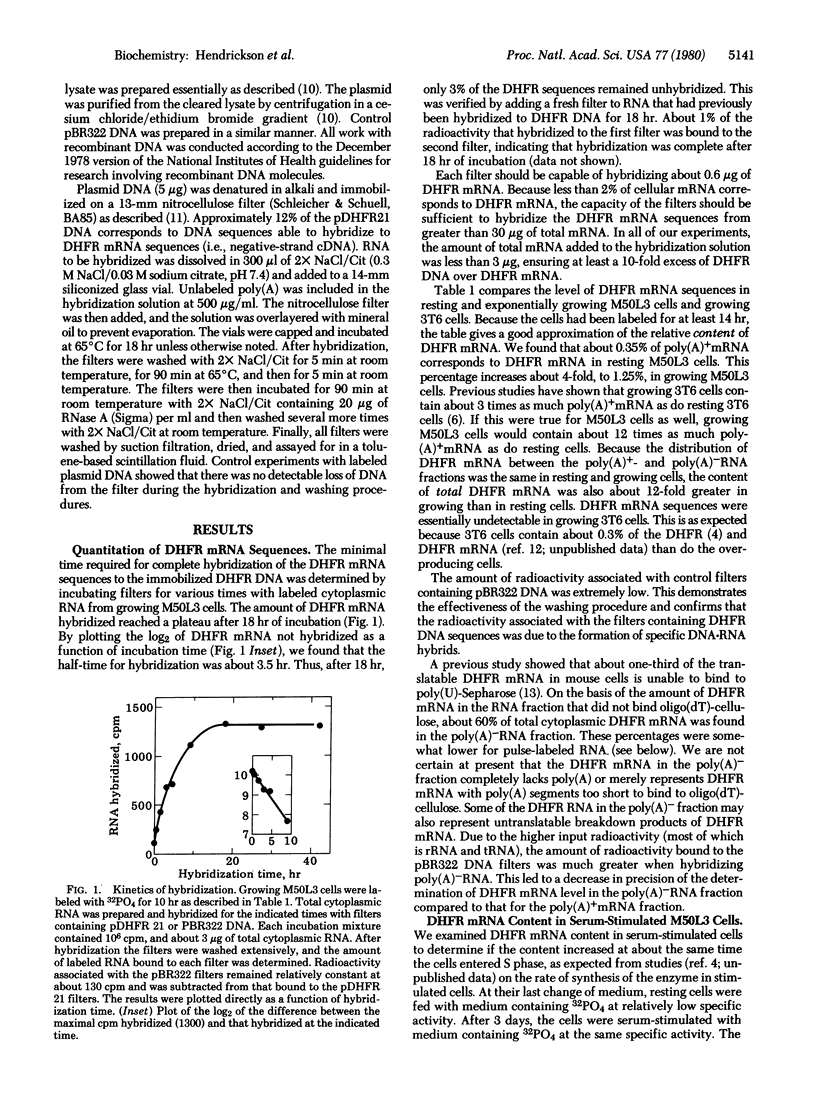
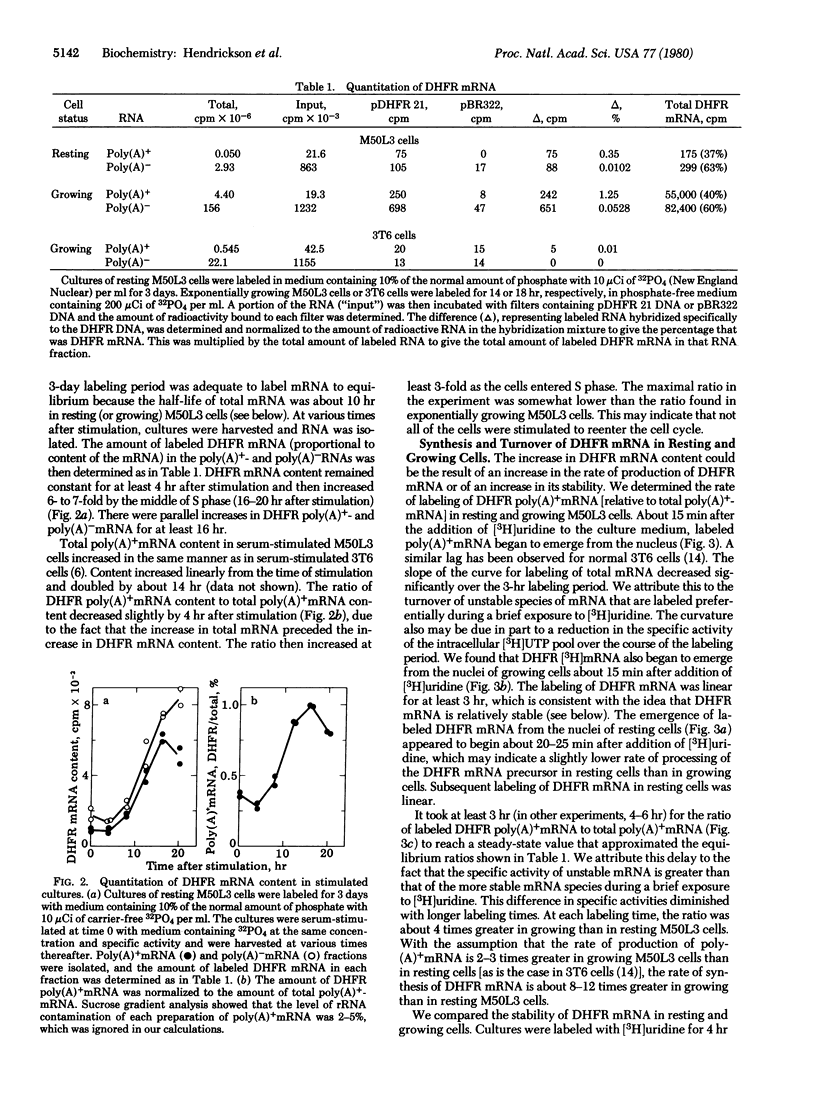
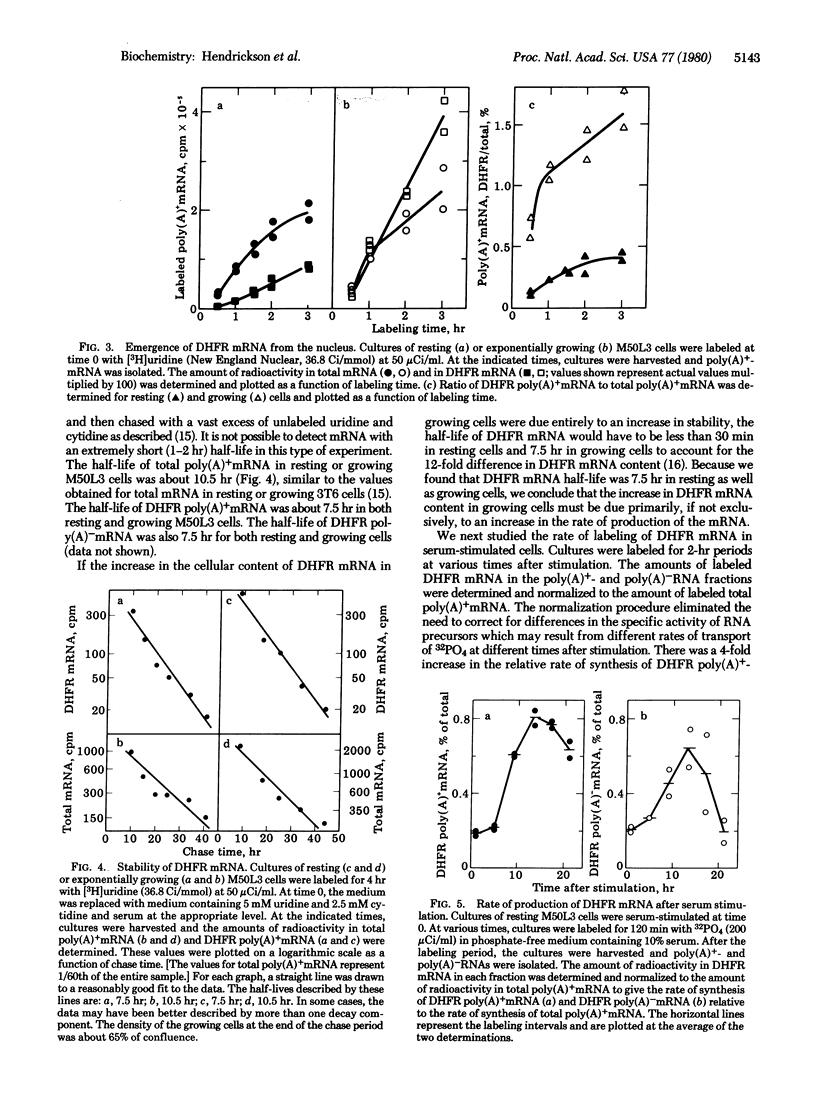
We have used the technique of DNA-excess filter hybridization to measure directly the content and metabolism of the mRNA for dihydrofolate reductase (DHFR; 5,6,7,8-tetrahydrofolate:NADP+ oxidoreductase, EC 1.5.1.3). The studies were conducted with a methotrexate-resistant derivative of mouse 3T6 fibroblasts (M50L3) that overproduces the enzyme and its mRNA by a factor of 300 but regulates the level of the enzyme during the cell cycle in the same manner as normal 3T6 cells. We found that, when resting (G0) M50L3 cells were serum-stimulated to reenter the cell cycle, the 10-fold increase in the rate of synthesis of DHFR that occurs at the beginning of S phase was the result of a corresponding increase in DHFR mRNA content. In pulse-labeling experiments, we found that there was a similar increase in the rate of production of the mRNA just prior to S phase. However, the half-life of the mRNA was the same (7.5 hr) in resting and exponentially growing cells. Therefore, the increase in DHFR mRNA content was due to an increase in the rate of production rather than an increase in the stability of the message. The delay between addition of [3H]-uridine to the culture medium and the emergence of DHFR mRNA from the nucleus was 15-20 min for both resting and growing M50L3 cells. A similar delay was observed for total mRNA. Therefore, the time required for the processing of newly synthesized DHFR heterogeneous nuclear RNA into DHFR mRNA is about the same as that for the average mRNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Chang S. E., Littlefield J. W. Elevated dihydrofolate reductase messenger RNA levels in methotrexate-resistant BHK cells. Cell. 1976 Mar;7(3):391–396. doi: 10.1016/0092-8674(76)90168-9. [DOI] [PubMed] [Google Scholar]
- Colman A., Byers M. J., Primrose S. B., Lyons A. Rapid purification of plasmid DNAs by hydroxyapatite chromatography. Eur J Biochem. 1978 Nov 2;91(1):303–310. doi: 10.1111/j.1432-1033.1978.tb20966.x. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Johnson L. F., Fuhrman C. L., Abelson H. T. Resistance of resting 3T6 mouse fibroblasts to methotrexate cytotoxicity. Cancer Res. 1978 Aug;38(8):2408–2412. [PubMed] [Google Scholar]
- Johnson L. F., Fuhrman C. L., Wiedemann L. M. Regulation of dihydrofolate reductase gene expression in mouse fibroblasts during the transition from the resting to growing state. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):397–306. doi: 10.1002/jcp.1040970314. [DOI] [PubMed] [Google Scholar]
- Johnson L. F., Levis R., Abelson H. T., Green H., Penman S. Changes in RNA in relation to growth of the fibroblast. IV. Alterations in theproduction and processing of mRNA and rRNA in resting and growing cells. J Cell Biol. 1976 Dec;71(3):933–938. doi: 10.1083/jcb.71.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellems R. E., Alt F. W., Schimke R. T. Regulation of folate reductase synthesis in sensitive and methotrexate-resistant sarcoma 180 cells. In vitro translation and characterization of folate reductase mRNA. J Biol Chem. 1976 Nov 25;251(22):6987–6993. [PubMed] [Google Scholar]
- Kellems R. E., Morhenn V. B., Pfendt E. A., Alt F. W., Schimke R. T. Polyoma virus and cyclic AMP-mediated control of dihydrofolate reductase mRNA abundance in methotrexate-resistant mouse fibroblasts. J Biol Chem. 1979 Jan 25;254(2):309–318. [PubMed] [Google Scholar]
- Milcarek C. HeLa cell cytoplasmic mRNA contains three classes of sequences: predominantly poly(A)-free, predominantly poly(A)-containing and bimorphic. Eur J Biochem. 1979 Dec 17;102(2):467–476. doi: 10.1111/j.1432-1033.1979.tb04261.x. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Chang A. C., Cohen S. N., Schimke R. T. Structure and genomic organization of the mouse dihydrofolate reductase gene. Cell. 1980 Feb;19(2):355–364. doi: 10.1016/0092-8674(80)90510-3. [DOI] [PubMed] [Google Scholar]
- Roop D. R., Nordstrom J. L., Tsai S. Y., Tsai M. J., O'Malley B. W. Transcription of structural and intervening sequences in the ovalbumin gene and identification of potential ovalbumin mRNA precursors. Cell. 1978 Oct;15(2):671–685. doi: 10.1016/0092-8674(78)90035-1. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Curtis P. J., Tiemeier D. C., Leder P., Weissmann C. The intervening sequence of a mouse beta-globin gene is transcribed within the 15S beta-globin mRNA precursor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1309–1313. doi: 10.1073/pnas.75.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann L. M., Johnson L. F. Regulation of dihydrofolate reductase synthesis in an overproducing 3T6 cell line during transition from resting to growing state. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2818–2822. doi: 10.1073/pnas.76.6.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]


