Abstract
Light is used to release a drug from a cell impermeable small molecule, uncloaking its cytotoxic effect on cancer cells.
Off-target toxicity plagues conventional cancer chemotherapy. One strategy to enhance selectivity of anti-cancer drugs involves unmasking the cytotoxicity of a molecule in the vicinity of the tumor1. This type of activation can be mediated by enzymes2, 3, changes in pH4, or exogenous factors such as temperature5 or light6–8.
Light is an ideal external stimulus since it provides a broad range of adjustable parameters9 that can be optimized for biological compliance. Several approaches that use light for biomolecular activation have been reported10–15. One established method to enable selectivity of drug action using light is photodynamic therapy (PDT). In PDT, 16, 17 light activation of a photosensitizer generates cytotoxic singlet oxygen killing only illuminated cells. PDT is currently used in several types of malignancies16 including skin, lung, esophageal, bladder, head and neck, and prostate cancer. A related strategy, photochemical internalization18, 19, also uses photosensitizers. In this case, the photosensitizers are used to release macromolecular cytotoxins from endosomes, enabling their entry into the cytosol. However, these approaches suffer from disadvantages20, including unpredictable drug uptake rates, the limited diffusion and lifetime of 1O2, and the requirement for moderate levels of O2 which may not always be available in the tumor environment.
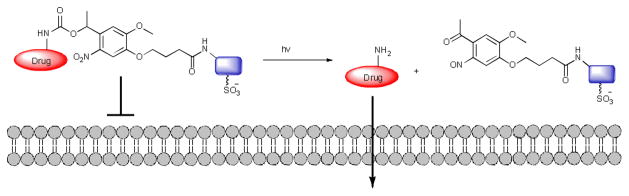
In this communication, we report a new light-targeted drug delivery system, which operates independently of the creation of 1O2. The basis of the system is the attachment of a cell impermeable small molecule to a drug via a linker that can be removed in presence of light, allowing cellular entry (Fig. 1). We call this new strategy photocaged permeability (PCP)21.
Fig. 1.
Photocaged permeability strategy for drug delivery
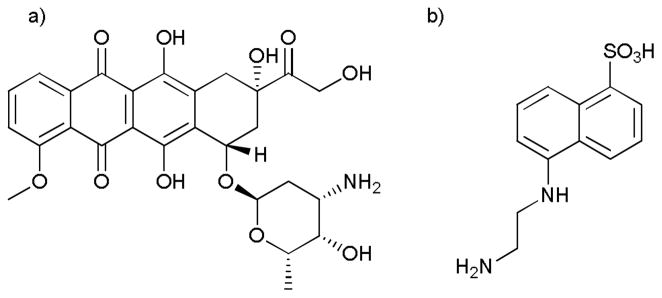
More specifically, we report the controlled release of the anticancer drug, doxorubicin (Dox), upon illumination (Fig. 2). To prevent entry of Dox in the dark we attached Dox to EDANS, a small fluorophore, chosen because it contains a sulfonic acid moiety known to hinder cellular entry22. To connect Dox to EDANS we utilized a light-cleavable nitroveratryl23 linker. The nitroveratryl moiety has been used previously as a photocaging group for a wide variety of biomolecules24.
Fig. 2.
a) The anticancer drug doxorubicin b) Cell impermeable EDANS
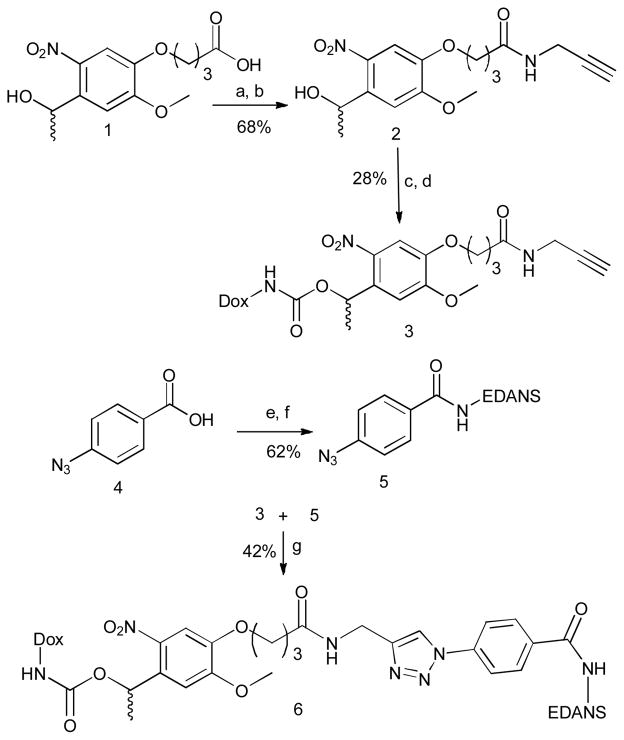
The synthesis (Scheme 1) began with commercially available nitroveratryl carboxylic acid (1). The N-hydroxy succinimide ester was prepared followed by coupling with propargylamine to give amide (2). This compound was converted to the p-nitrophenyl carbonate which was then treated with doxorubicin3 to generate photocaged doxorubicin carbamate (3). EDANS was coupled with azido benzoic acid (5) and was finally attached to the photocage using click chemistry to generate the final Dox-EDANS conjugate (6).
Scheme 1.
Synthesis of photocaged-cell impermeable drug conjugate. Reagents and conditions: (a) NHS, EDC. HCl; (b) propargylamine, Et3N; (c) bis p-nitrophenylcarbonate, Et3N; (d) doxorubicin HCl., Et3N; (e) NHS, EDC. HCl (f) EDANS, Et2N(iPr)2; (g) CuSO4. 5H2O, sodium ascorbate, tris-(benzyltriazolylmethyl)amine, (1:1) (DMSO: water).
The photolytic release of doxorubicin from the drug conjugate was analyzed using HPLC. The drug conjugate was dissolved in PBS buffer and was exposed to UV light at 365 nm (9.0 mW/cm2). Aliquots of the reaction mixture were collected at various times and were analyzed on RP-HPLC. During the course of the reaction, the peaks corresponding to the Dox-EDANS conjugate disappeared with concomitant increase in the intensity of a peak with the same retention time as Dox (See ESI Fig. S3), confirming time-depended drug release in the presence of light.
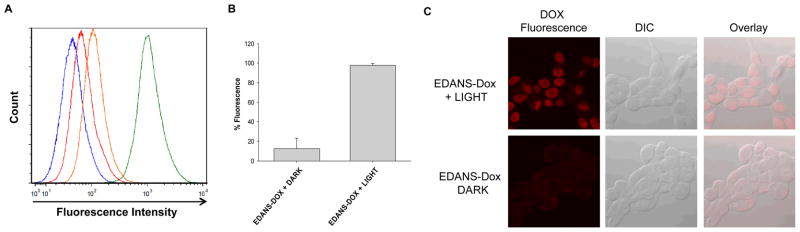
We then investigated whether the attachment of EDANS to Dox via the veratryl linker would enable drug delivery. JH-EsoAd1 cells, a Barrett’s esophagus associated adenocarcinoma cell line25, were incubated with Dox-EDANS in the dark or with illumination. Cell permeability was measured with flow cytometry; upon illumination a significant enhancement of cellular Dox fluorescence was observed (Fig. 3A–B). This enhancement was mirrored in confocal studies with the same cell line (Fig. 3C)—only light-treated cells show significant Dox fluorescence in the nucleus, where it is known to accumulate26.
Fig. 3.
A). The flow cytometry histogram displays the relative fluorescence intensity of untreated controls in the dark (blue) or light (red) or JH-EsoAd1 cells treated with EDANS-Dox in the dark (orange) or light (green). B) Quantification of Dox fluorescence for the indicated treatment conditions. Data are representative of two independent experiments, N=9. C) Representative confocal images of JHEsoAd1 cells treated with EDANS-Dox in the dark or light. The red channel shows Dox fluorescence, the gray channel is a DIC image, and the overlay represents the cellular localization of Dox fluorescence.
With permeability enhancement established, we proceeded to investigate the extent to which the release of Dox with light would lead to enhanced cellular toxicity. Indeed, increased illumination lead to decreased survival as measured by an MTT assay (Fig. 4). Survival of cells treated with EDANS-Dox in the dark was equivalent to controls with no drug added. Moreover, treatment with light alone at the same dose was not cytotoxic (See ESI, Fig. S5).
Fig 4. Light-dependent cytotoxicity of Dox-EDANS.
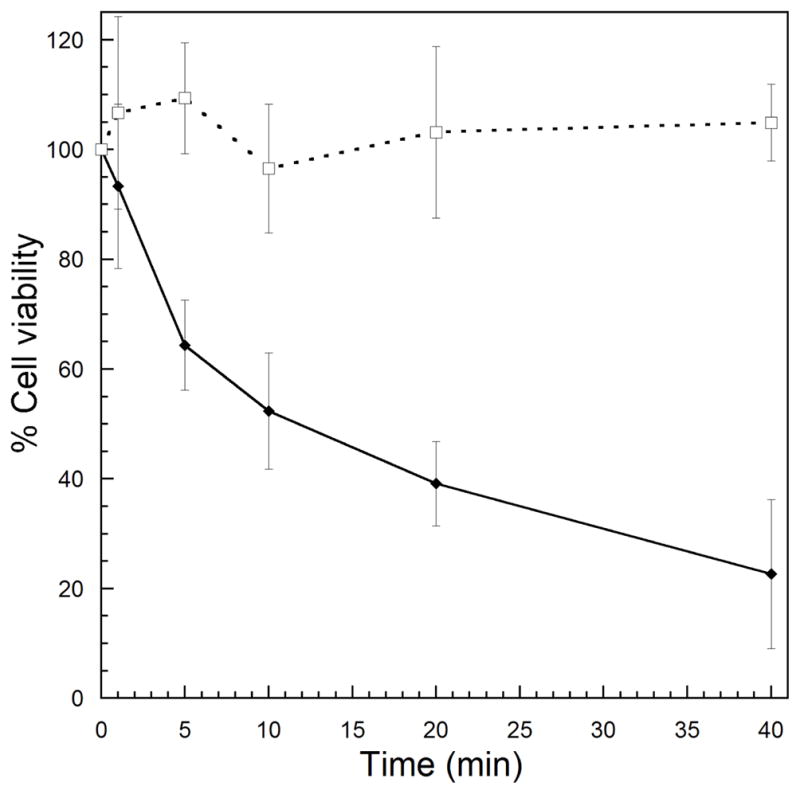
Cells were treated with 10 μM of Dox-EDANS with (solid diamonds) or without (open squares) light for the specified times. After 2 h, the cells were washed with fresh media and allowed to grow for 72 h. The fraction of surviving cells was evaluated using the absorbance of the formazan product of MTT reduction. Error bars denote one standard deviation from the mean.
Finally, we sought to compare the concentration dependence for our method vs. Dox alone. We first measured the IC50 of Dox alone with the JH-EsoAd1 cells and found it to be 1.0 ± 0.4 μM (see ESI Fig S6). As expected by PCP, EDANS-Dox was not toxic to the cells in the dark at the highest value tested (16 μM). However, illumination of EDANS-Dox (365 nm, 9.0 mW/cm2) leads to cytotoxicity with an IC50 of 1.6 ± 1.0 μM (Fig. 5), comparable to that of Dox alone. Based on our FACS study (Fig. 3), we deduce that this enhanced cytotoxicity is caused by efficient light-stimulated release of free Dox from the impermeable EDANS.
Fig. 5. Concentration dependent Light-stimulated cytotoxicity.
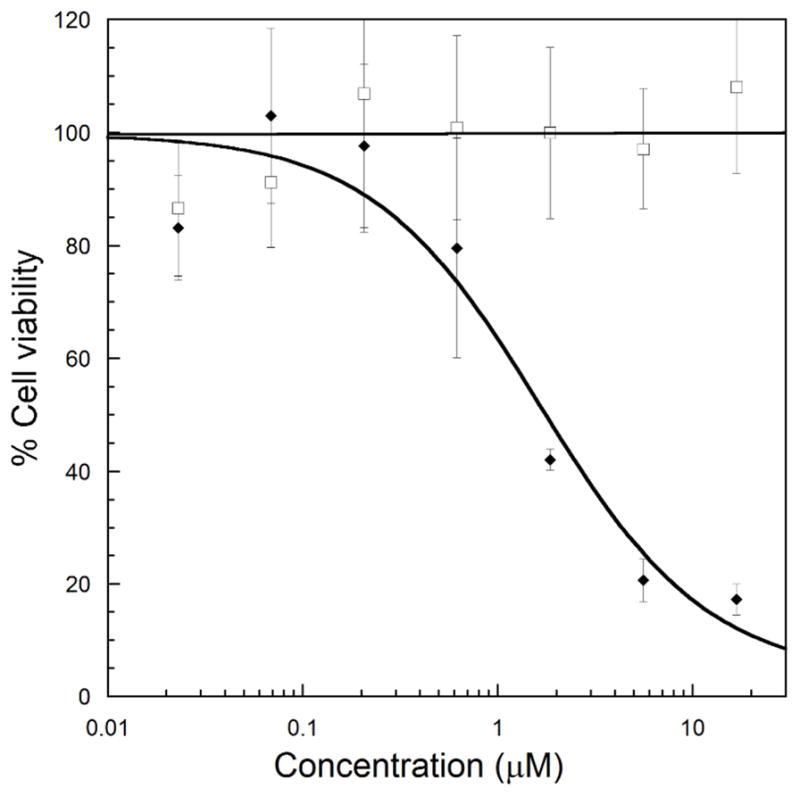
Cells were treated with EDANS-Dox at various concentrations in the dark (open squares) or with UV light for 20 minutes (black diamonds). After 2 h, the cells were washed with fresh media and allowed to grow for 72 h. The fraction of surviving cells relative to no drug controls was evaluated using the absorbance of the formazan product of MTT reduction. Error bars denote one standard deviation from the mean.
In conclusion, we have developed a new and efficient strategy for drug release based on photocaged permeability (PCP). In this first report, we have focused on applying PCP to the light-stimulated delivery of Dox into esophageal adenocarcinoma cells. In principle, the PCP approach could be applied to any small, cell permeable molecule that has a free amine, hydroxyl, or carboxylic acid group for attachment of the veratryl-EDANS molecule. Further experiments will focus on use of other light-scissile linkers that can operate at longer wavelengths that are able to penetrate farther into tissues, as well as the use of other molecules that block permeability.
Supplementary Material
Acknowledgments
The authors acknowledge financial support from the American Cancer Society (IRG-73-001-34) and the Massey Cancer Center. Flow cytometry and confocal microscopy were supported in part by grants P30CA16059 and 5P30NS047463. We thank Dr. M. Bertino (VCU) and M. Foussekis (VCU) for help in measuring light intensity, Dr. J. Eshleman (Johns Hopkins) for the JH-EsoAd1 cells, and Dr. A. Cropp (VCU) for helpful comments.
Footnotes
Electronic Supplementary Information (ESI) available: Details of synthesis and characterization of the photocaged drug conjugate, photolytic drug release studies, cytotoxicity studies, confocal and flow cytometry data.
Notes and References
- 1.Kratz F, Muller IA, Ryppa C, Warnecke A. ChemMedChem. 2008;3:20–53. doi: 10.1002/cmdc.200700159. [DOI] [PubMed] [Google Scholar]
- 2.Fischel-Ghodsian F, Brown L, Mathiowitz E, Brandenburg D, Langer R. Proc Natl Acad Sci U S A. 1988;85:2403–2406. doi: 10.1073/pnas.85.7.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubowchik GM, Firestone RA, Padilla L, Willner D, Hofstead SJ, Mosure K, Knipe JO, Lasch SJ, Trail PA. Bioconjug Chem. 2002;13:855–869. doi: 10.1021/bc025536j. [DOI] [PubMed] [Google Scholar]
- 4.Langer M, Kratz F, Rothen-Rutishauser B, Wunderli-Allenspach H, Beck-Sickinger AG. J Med Chem. 2001;44:1341–1348. doi: 10.1021/jm001065f. [DOI] [PubMed] [Google Scholar]
- 5.Choi SW, Zhang Y, Xia Y. Angew Chem Int Ed Engl. 2010;49:7904–7908. doi: 10.1002/anie.201004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCoy CP, Rooney C, Edwards CR, Jones DS, Gorman SP. J Am Chem Soc. 2007;129:9572–9573. doi: 10.1021/ja073053q. [DOI] [PubMed] [Google Scholar]
- 7.Agasti SS, Chompoosor A, You CC, Ghosh P, Kim CK, Rotello VM. J Am Chem Soc. 2009;131:5728–5729. doi: 10.1021/ja900591t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi SK, Thomas T, Li MH, Kotlyar A, Desai A, Baker JR., Jr Chem Commun (Camb) 2010;46:2632–2634. doi: 10.1039/b927215c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerasimov OV, Boomer JA, Qualls MM, Thompson DH. Adv Drug Deliv Rev. 1999;38:317–338. doi: 10.1016/s0169-409x(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 10.Lee HM, Larson DR, Lawrence DS. ACS Chem Biol. 2009;4:409–427. doi: 10.1021/cb900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deiters A, Groff D, Ryu Y, Xie J, Schultz PG. Angew Chem Int Ed Engl. 2006;45:2728–2731. doi: 10.1002/anie.200600264. [DOI] [PubMed] [Google Scholar]
- 12.Deiters A. Chem Bio Chem. 2010;11:47–53. doi: 10.1002/cbic.200900529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis-Davies GC. Nat Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goguen BN, Hoffman BD, Sellers JR, Schwartz MA, Imperiali B. Angew Chem Int Ed Engl. 2011;50:5667–5670. doi: 10.1002/anie.201100674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riggsbee CW, Deiters A. Trends Biotechnol. 2010;28:468–475. doi: 10.1016/j.tibtech.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolmans DE, Fukumura D, Jain RK. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 18.Berg K, Weyergang A, Prasmickaite L, Bonsted A, Hogset A, Strand MT, Wagner E, Selbo PK. Methods Mol Biol. 2010;635:133–145. doi: 10.1007/978-1-60761-697-9_10. [DOI] [PubMed] [Google Scholar]
- 19.Berg K, Folini M, Prasmickaite L, Selbo PK, Bonsted A, Engesaeter BO, Zaffaroni N, Weyergang A, Dietze A, Maelandsmo GM, Wagner E, Norum OJ, Hogset A. Curr Pharm Biotechnol. 2007;8:362–372. doi: 10.2174/138920107783018354. [DOI] [PubMed] [Google Scholar]
- 20.Verhille M, Couleaud P, Vanderesse R, Brault D, Barberi-Heyob M, Frochot C. Curr Med Chem. 2010;17:3925–3943. doi: 10.2174/092986710793205453. [DOI] [PubMed] [Google Scholar]
- 21.Note: Drug delivery via light-stimulated release of cytotoxins from cell impermeable polymers or nanoparticles has recently been described (e.g see refs 6–8). Our PCP approach is distinct because permeability is blocked by a cell impermeable small molecule.
- 22.Fichert T, Yazdanian M, Proudfoot JR. Bioorg Med Chem Lett. 2003;13:719–722. doi: 10.1016/s0960-894x(02)01035-1. [DOI] [PubMed] [Google Scholar]
- 23.Holmes CP. J Org Chem. 1997;62:2370–2380. doi: 10.1021/jo961602x. [DOI] [PubMed] [Google Scholar]
- 24.Marriott G. Biochemistry. 1994;33:9092–9097. doi: 10.1021/bi00197a010. [DOI] [PubMed] [Google Scholar]; Iwase R, Kitani A, Yamaoka T, Murakami A. Nucleic Acids Res Suppl. 2003;(3):61–62. doi: 10.1093/nass/3.1.61. [DOI] [PubMed] [Google Scholar]; Baumann L, Beck-Sickinger AG. Biopolymers. 2010;94:771–778. doi: 10.1002/bip.21465. [DOI] [PubMed] [Google Scholar]; Berrade L, Kwon Y, Camarero JA. Chembiochem. 2010;11:1368–1372. doi: 10.1002/cbic.201000157. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yang R, Pasunooti KK, Li F, Liu XW, Liu CF. Chem Commun (Camb) 2010;46:7199–7201. doi: 10.1039/c0cc01382j. [DOI] [PubMed] [Google Scholar]; Pellois JP, Hahn ME, Muir TW. J Am Chem Soc. 2004;126:7170–7171. doi: 10.1021/ja0499142. [DOI] [PubMed] [Google Scholar]; Sanchez MI, Vazquez O, Vazquez ME, Mascarenas JL. Chem Commun (Camb) 2011;47:11107–11109. doi: 10.1039/c1cc13355a. [DOI] [PubMed] [Google Scholar]; Cho S, Lee SH, Chung WJ, Kim YK, Lee YS, Kim BG. Electrophoresis. 2004;25:3730–3739. doi: 10.1002/elps.200406103. [DOI] [PubMed] [Google Scholar]; Gu Z, Biswas A, Joo KI, Hu B, Wang P, Tang Y. Chem Commun (Camb) 2010;46:6467–6469. doi: 10.1039/c0cc01439g. [DOI] [PubMed] [Google Scholar]; Luo Y, Eldho NV, Sintim HO, Dayie TK. Nucleic Acids Res. 2011;39:8559–8571. doi: 10.1093/nar/gkr464. [DOI] [PMC free article] [PubMed] [Google Scholar]; Biggins JB, Hashimoto A, Koh JT. Chembiochem. 2007;8:799–803. doi: 10.1002/cbic.200600497. [DOI] [PubMed] [Google Scholar]; Mayer G, Heckel A. Angew Chem Int Ed Engl. 2006;45:4900–4921. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- 25.Hector A, Koorstra JM, Hong S, Boonstra JJ, Dinjens WN, Foratiere AA, Wu T, Mongomery EA, Eshleman JR, Maitra A. Cancer Biol Ther. 2008;7:1753–1755. doi: 10.4161/cbt.7.11.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speelmans G, Staffhorst RW, Steenbergen HG, de Kruijff B. Biochim Biophys Acta. 1996;1284:240–246. doi: 10.1016/s0005-2736(96)00137-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.