Abstract
Background and Purpose
Impairments in CVR have been variably associated with increased risk of ischemic events and may stratify stroke risk in patients with high grade internal carotid artery (ICA) stenosis or occlusion. The purpose of this study is to perform a systematic review and meta-analysis to summarize the association of CVR impairment and stroke risk.
Methods
We performed a literature search evaluating the association of impairments in CVR with future stroke or transient ischemic attack (TIA) in patients with high grade ICA stenosis or occlusion. We included studies with a minimum of one year patient follow up with baseline CVR measures performed via any modality and primary outcome measures of stroke and/or TIA. A meta-analysis with assessment of study heterogeneity and publication bias was performed. Results were presented in a forest plot and summarized using a random-effects model.
Results
Thirteen studies met the inclusion criteria, representing a total of 1061 independent CVR tests in 991 unique patients with a mean follow up of 32.7 months. We found a significant positive relationship between impairment of CVR and development of stroke, with a pooled random effects odds ratio of 3.86 (95% CI, 1.99–7.48). Subset analysis showed that this association between CVR impairment and future risk of stroke/TIA remained significant regardless of ischemic outcome measure, symptomatic or asymptomatic disease, stenosis or occlusion, or CVR testing method.
Conclusions
CVR impairment is strongly associated with increased risk of ischemic events in carotid stenosis or occlusion and may be useful for stroke risk stratification.
Keywords: cerebrovascular reserve, cerebrovascular reactivity, stroke, TIA, risk, systematic review, meta-analysis
Introduction
Atherosclerotic disease occurs frequently at the common carotid artery bifurcation. Such extracranial atherosclerotic disease accounts for 15 to 20% of ischemic strokes1. Traditional imaging-based risk assessment of stroke, focused on defining the degree of arterial narrowing, has not taken into account downstream hemodynamic effects distal to the stenosis and the cerebrovascular reserve (CVR). For example, when carotid stenosis is severe and reduces cerebral perfusion pressure (CPP), autoregulation of the vasculature will maximally dilate the cerebral arterioles to maintain cerebral blood flow (CBF). With further reduction in CPP and maximally dilated arterioles, the CBF will also decrease and potentially increase the risk of stroke.
In symptomatic severe ICA stenosis, carotid endarterectomy (CEA) has been shown to significantly lower the risk of ipsilateral cerebral infarction2. The benefit of CEA is less clear in patients with asymptomatic high grade stenosis. For example, in the Asymptomatic Carotid Surgery Trial, the modest 5.4% reduction in absolute stroke risk at 5 years in patients with asymptomatic carotid stenosis who were treated with CEA requires serious consideration of the risks of surgery, including local surgical expertise in the procedure3. In such a population, integration of cerebral hemodynamics such as CVR or oxygen extraction fraction derived from positron emission tomography (PET) into assessment of stroke risk could potentially help isolate a group of patients who might most benefit from surgical revascularization. On the other end of the spectrum, further risk stratification may improve prognosis and motivation for adherence to medical therapy in patients with symptomatic occlusion, as indications for surgical revascularization in this group also remain unclear with a recent randomized trial4 showing no benefit of surgical revascularization relative to medical therapy.
There have been two main approaches to measuring CVR. One approach attempts direct CBF measurements of the brain tissue with flow sensitive imaging techniques such as positron-emission tomography (PET), nuclear medicine (NM) techniques, CT perfusion, or MR perfusion before and after a vasodilatory stimulus. The second approach involves transcranial Doppler (TCD) measurement of flow velocities (typically in the middle cerebral artery) distal to a lesion both before and after a vasodilatory stimulus, with the increase flow velocity considered a surrogate for CVR. Vasodilatory stimuli include increasing levels of CO2 (such as with breath holding or inhalation of CO2 gas mixtures) and pharmacologic challenge with acetazolamide.
It is difficult to draw reliable conclusions about the role of CVR in predicting stroke based on individual research studies in the literature given their relatively small sample sizes. While there have been attempts to summarize stroke risk based on existing studies evaluating CVR impairment in specific patients with a particular modality5,6 no recent attempt at a systematic review and meta-analysis of the entire literature across all patient populations and modalities has been performed. It is important to improve our understanding of the role of CVR in patients with carotid artery stenosis for determining stroke prevention regimens. The purpose of this study is to perform a systematic review and meta-analysis to summarize the association of CVR impairment and stroke risk.
Methods
We employed the methods described in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement7.
Eligibility Criteria
We identified studies evaluating CVR impairment and association with future stroke or TIA in patients with high grade carotid stenosis (≥70%) or occlusion. Inclusion criteria were 1) published English language manuscripts; 2) original research studies (retrospective or prospective); 3) patients with high grade carotid stenosis (≥70%) or occlusion measured by any imaging modality including ultrasound, CT angiography (CTA), MR angiography (MRA), or digital subtraction angiography (DSA); 4) administration of a physiologic challenge and measurement of CVR after this challenge via any modality; 5) follow up of 1 year or greater assessing development of ipsilateral stroke and/or TIA; and 6) non-surgical management of patients. In the case of duplicated published cohorts, we included the report with the longest follow up and greatest number of patients. If a subset of patients underwent surgical revascularization during follow up, we only included these studies in our meta-analysis if these patients were separately identified and analyzed by the authors so that they could either 1) be excluded from the meta-analysis or 2) be included in the meta-analysis if the authors made clear that such patients were censored after revascularization so that follow-up up to but not after revascularization could be included. In addition, in any study where more than one testing method for CVR was performed on the same set of patients, both methods were included separately in the statistical analysis.
Information Sources and Search
A systematic search was performed by an experienced medical librarian (D.D.) to identify studies according to the inclusion criteria. Potential articles were found by searching the electronic databases Ovid MEDLINE, EMBASE and The Cochrane Library. Relevant subject heading and free text terms were used. Additional records were identified by employing the Related Articles feature in PubMed and the Cited Reference Search in ISI Web of Science. All studies included in each database through September 2011 were searched. A representative primary search conducted through MEDLINE is available in the online supplement (S1).
Study Selection and Data Collection Process
After removal of duplicate manuscripts, all potentially eligible manuscripts were screened by a single reader with all screened manuscripts read in their entirety by three readers. Two data extractors populated a form collecting key qualitative and quantitative data from the studies. Details of study selection and data collection are available in the online supplement (S2).
Assessment of Study Methods
Risk of bias estimates described by PRISMA are most applicable to randomized control trials 7and no such similar published tool exists to evaluate time-to-event or longitudinal studies according to our literature search. Thereby, 2 data extractors assessed each study's methodology (with discrepancies resolved by consensus with a third reader) according to the following guidelines. First, an assessment of reference standard bias was made regarding the blinding of observers to the CVR results when the clinical ischemic outcomes were determined. Heterogeneity between blinded versus non-blinded studies was made using the I2 statistic described below. Second, an assessment of confounding bias was made regarding statistical adjustment of pre-existing vascular risk factors for the ischemic outcomes. Third, we assessed the completeness of follow-up data by recording the number of patients lost to follow-up or censored during the follow up period.
Statistical Analyses
Heterogeneity across studies was examined by both the I2 statistic and Breslow-Day method that measure the proportion of inconsistency in individual studies that cannot be explained by chance. The upper 95% confidence limit of I2 greater than 30% was used as a cutoff for accepting studies that were moderately heterogeneous in which case the odds ratios were pooled using random effects models8. Fixed effects model was chosen if studies were found statistically homogeneous according to the I2 statistic. For the Breslow-Day method, all P values less than 0.05 were considered statistically significant and indicative of significant heterogeneity. Continuity correction was used for sparse tables before pooling the odds ratios. We performed additional subset analysis to assess heterogeneity. Subsets were limited to presence or absence of symptoms, severity of disease, outcome measure used, location of disease, testing modality, and type of vasodilatory challenge. Publication bias was examined by Egger's and Begg's tests. A biostatistician conducted all analyses using StatsDirect version 2.7.8.
Results
Study Selection
After the initial screening review of 2,238 titles and abstracts, 21 potential manuscripts were selected for further detailed review (Figure 1). Manuscripts were excluded for failure to meet all of the inclusion criteria (n=5) and duplicated patient populations (n=3). The remaining 13 studies5,9–20 were included in the qualitative systematic review. Of these studies, 85% (11/13) divided ischemic outcomes into categories amenable to meta-analysis. In one of the two studies where outcome data was originally presented in a fashion not amenable to meta-analysis16, the original data was provided by the study corresponding author in a fashion amenable to meta-analysis, allowing for 92% (12/13) of studies used in the final analysis. In the remaining study14, the data remained unavailable after several attempts to directly contact the corresponding authors.
Figure 1.
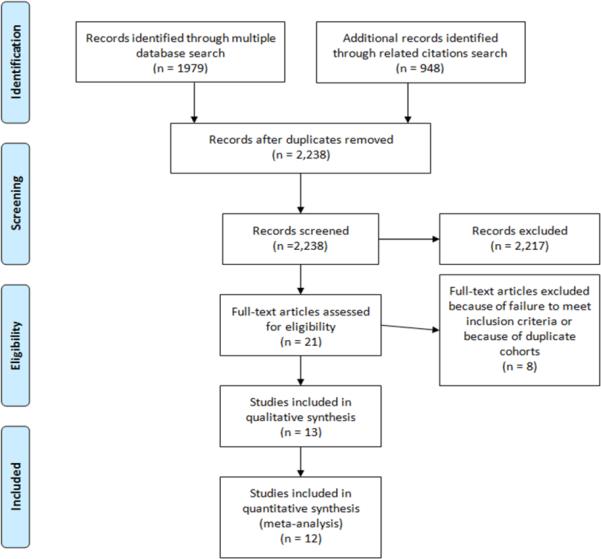
Study selection flow diagram, adapted from the PRISMA group statement
Qualitative Assessment and Study Characteristics
The 13 studies meeting inclusion criteria (Table 1) were all prospective, time-to-event studies, with 4 conducted in Japan10,13,15,20 2 in Israel9,11, 2 in Germany12,16, 2 in Italy17,18, 1 in the United States19, and 1 in multiple countries as a multi-center trial5. A total of 1061 independent CVR tests in 991 unique patients were included with a mean patient follow up of 32.7 months. All study populations had a minimum of 70% stenosis of the ICA, with some studies including patients with carotid occlusion or extension of occlusion from the ICA into the middle cerebral artery. Twelve of the 13 studies included exclusively ipsilateral ischemic outcomes measures and 1 study19included 4 contralateral ischemic outcomes which were excluded in the statistical analysis.
Table 1.
Overview of Studies Evaluating the Association Between Baseline Measures of Cerebrovascular Reserve (CVR) and Risk of Ischemic Outcome.
| Study Number | Author and Year | Number of Patients | Mean Age (SD) | Maie (%) | Disease Site | Disease Severity | Symptomatic versus Asymptomatic | Vasodilatory Stimulus | CVR Testing Modality | Number of Subjects with Normal CVR | Number of Subjects with Impaired CVR | Mean Follow up Duration (Mo) | Ischemic Events in Normal CVR grouP | Ischemic Events in Impaired CVR grouP | Outcome Measure |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Gur9 1996 | 44 | 69 (6.5) | 47.7 | ICA | stenosis | asymptomatic | ACZ | TCD | 23 | 21 | 24 | 0 | 7 | stroke or TIA |
| 2 | Isozaki10 2010 | 30 | 59.6 (9.7) | 86.7 | ICA & MCA | stenosis or occlusion | symptomatic | ACZ | H2O PET | 16 | 14 | 49 | 0 | 0 | stroke or TIA |
| 3 | Kimiagar11 2010 | 35 | 68 (7.5) | 60 | ICA | occlusion | asymptomatic | ACZ | TCD | 14 | 21 | 48 | 1 | 7 | stroke or TIA |
| 4 | King 20115 | 106 | 72.3 (8.1) | 79.2 | ICA | stenosis | asymptomatic | varied (ACZ and inspired CO2 variation) | TCD | 74 | 32 | 22.7 | 2 | 3 | stroke or TIA |
| 5 | Kleiser12 1992 | 85 | Range 43 to 81 | 90.6 | ICA | occlusion | both | inspired CO2 variation | TCD | 48 | 1112 | 38 | 4 | 12 | stroke or TIA |
| 6 | Kuroda13 2001 | 77 | 64 (SD n/a) | 75.3 | ICA & MCA | occlusion | symptomatic | ACZ | Xenon SPECT | 52 | 25 | 42.7 | 7 | 9 | stroke |
| 7 | Markus14 2001 | 107 | n/a | n/a | ICA | stenosis or occlusion | asymptomatic | inspired CO2 variation | TCD | n/a | n/a | 21.7 | n/a | n/a | stroke or TIA |
| 8 | Ogasawara15 2002 | 70 | 57 (n/a) | 75.7 | ICA & MCA | occlusion | symptomatic | ACZ | 133-Xenon SPECT | 47 | 23 | 60 | 5 | 8 | stroke |
| 8* | Ogasawara15 2002 | 70 | 57 (n/a) | 75.7 | ICA & MCA | occlusion | symptomatic | ACZ | 1231-IMP SPECT | 43 | 27 | 60 | 6 | 7 | stroke |
| 9 | Reinhard16 2008 | 161 | 66 (8) | 85.4 | ICA | stenosis or occlusion | both | inspired CO2 variation | TCD | 128 | 33 | 24.5 | 9 | 7 | stroke or TIA |
| 10 | Silvestrini17 2000 | 94 | 71.1 (5) | 78.7 | ICA | stenosis | asymptomatic | inspired CO2 variation | TCD | 54 | 40 | 28.5 | 5 | 11 | stroke or TIA |
| 11 | Vernieri18 1999 | 65 | 67.8 (5.5) | 76.9 | ICA | occlusion | both | inspired CO2 variation | TCD | 29 | 36 | 24 | 1 | 11 | stroke or TIA |
| 12 | Webster19 1995 | 95 | 65.9 (n/a) | 69.5 | ICA | stenosis or occlusion | symptomatic | ACZ | Xenon SPECT | 43 | 52 | 19.6 | 0 | 12 | stroke |
| 13 | Yamamoto20 2006 | 22 | (n/a) | n/a | ICA | stenosis | both | ACZ | 1231-IMP SPECT | 13 | 9 | 23 | 0 | 3 | stroke |
ACZ=acetazolamide; CT=computed tomography; ICA=internal carotid artery; n/a = data not available; MCA=middle cerebral artery; PET=positron emission tomography; SPECT=single photon emission computed tomography; TCD=transcranial Doppler; TIA=transient ischemic attack; IMP=Isopropyl Iodo-Amphetamine;
Ogasawara15 et al performed two methods on the same cohort of patients, both are listed here
Assessment of Study Methods
Thirty-eight percent (5/13) of the studies5,15–18 reported that observers were blinded to the CVR results when assessing ischemic outcomes (I2=0, CI=0 to 61%). In the remaining 8 manuscripts, no blinding method was explicitly described (I2=0, CI=0 to 58.5%). Sixty-nine percent (9/13) of the studies5,13–20explicitly described a statistical correction or adjustment for pre-existing vascular risk factors in the assessment of ischemic outcome likelihood. In the remaining 4 studies, no adjustment was explicitly described. Finally, in the assessment of the completeness of follow-up, in Reinhard et. al16 five patients had CEA after a mean of 23.2 months; these patients were censored in their analysis and were included in the meta-analysis. In Yamamoto et. al20, 18 of 40 patients were surgically managed; these patients were excluded from our meta-analysis as the outcome data from this group was clearly separated from the remaining patients. In the remaining 11 studies, no loss to follow up and no censoring from surgery was described by the authors.
Meta-Analysis Results
In pooling the results of the 13 eligible studies for the meta-analysis, both the I2 statistic and Breslow-Day statistic showed low heterogeneity (I2=0, CI=0 to 48.6% and Breslow-Day=7.31, df=12, p=0.84). Begg's tests did not reveal any publication bias of the meta-analyses (Begg-Mazumdar: Kendall's tau = 0.36, p-value of 0.1). The summarized random effects odds ratio of 3.96 (CI, 2.60–6.04) indicates a significant positive relationship between baseline CVR impairment and future development of TIA and/or stroke (Figure 2). Each study had a positive association between baseline CVR impairment and future development of stroke/TIA, though 38% (5/13) of the studies5,10,11,13,20 did not have statistically significant odds ratios.
Figure 2.
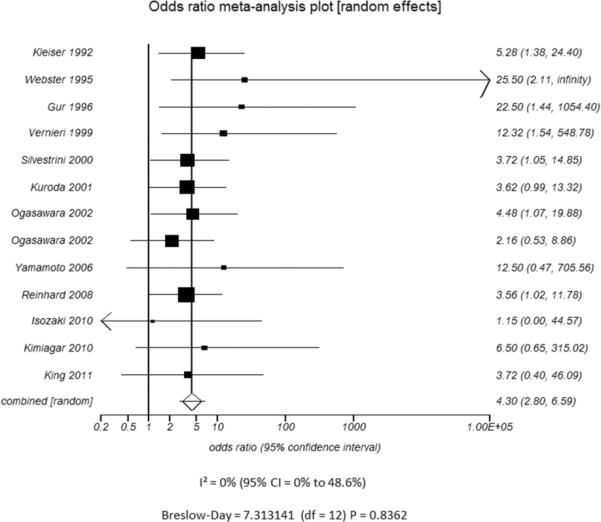
Meta-analysis of the association between cerebrovascular reserve (CVR) and stroke/TIA. Studies are listed by date. Squares indicate point estimates for effect size (OR), with the size proportional to the inverse variance of the estimate. Diamonds indicate pooled estimates. Lines represent 95% CIs. Vertical line indicates the null effect. I2 and Breslow-Day heterogeneity statistic listed below the forest plot.
Subset Analysis
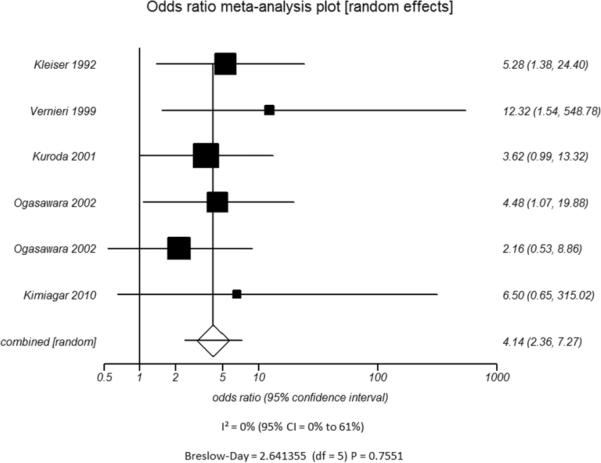
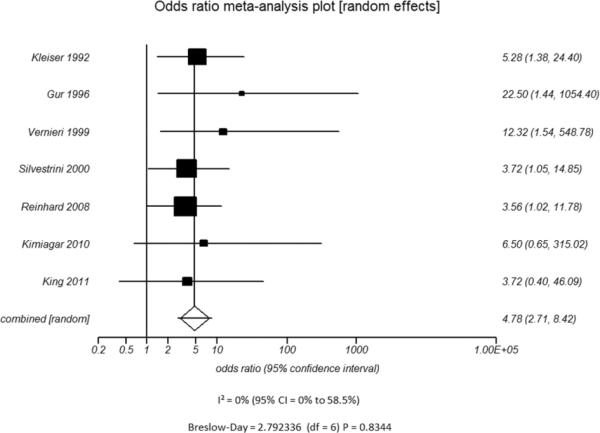
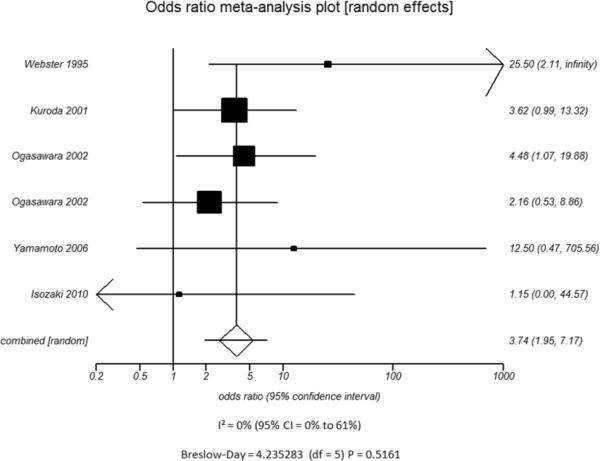
Additional subset analyses with heterogeneity measures were performed according to clinically relevant features using a random effects model based on the criteria described above. A statistically significant random effects odds ratio was preserved in all of the following subset analyses:(1) symptomatic (Figure 3A) versus asymptomatic disease (Figure 3B), (2) outcome measure of only stroke (Figure 3C) versus combination of stroke and/or TIA (Figure 3D), (3) disease severity of high grade stenosis (Figure 4A) versus occlusion (Figure 4B), (4) disease extent involving only the ICA (Figure 4C) versus ICA and MCA disease (Figure 4D), (5) CVR testing modality of TCD (Figure 5A) versus NM-SPECT techniques (Figure 5B), and (6) CVR challenge using acetazolamide (Figure 5C) versus inspired carbon dioxide (Figure 5D).
Figure 3.
Odds ratio and 95% confidence ratio for studies divided by the presence of n patients with only asymptomatic (A) or symptomatic (B) disease and whether the study outcome measure was only stroke (C) or stroke and TIA (D). The symbols and abbreviations are as in Figure 2.
A - Asymptomatic Patients
B - Symptomatic Patients
C - Stroke only as outcome measure
D - Stroke and TIA as outcome measure
A.
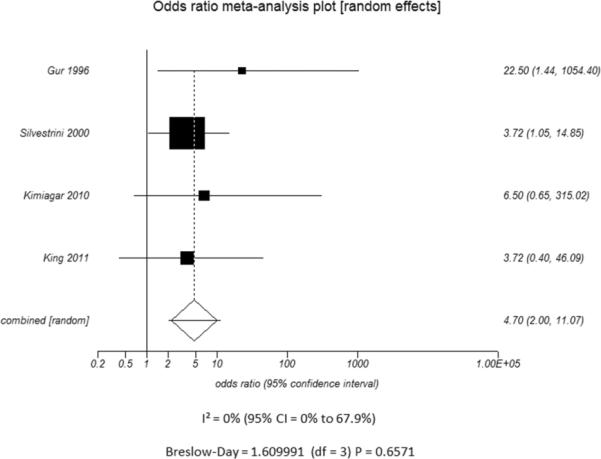
B.
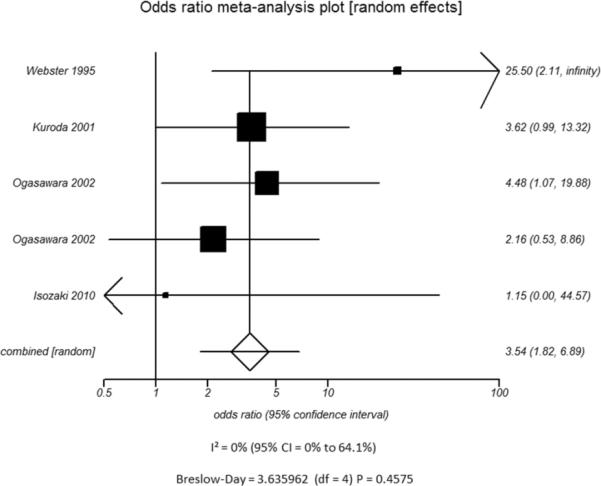
C.
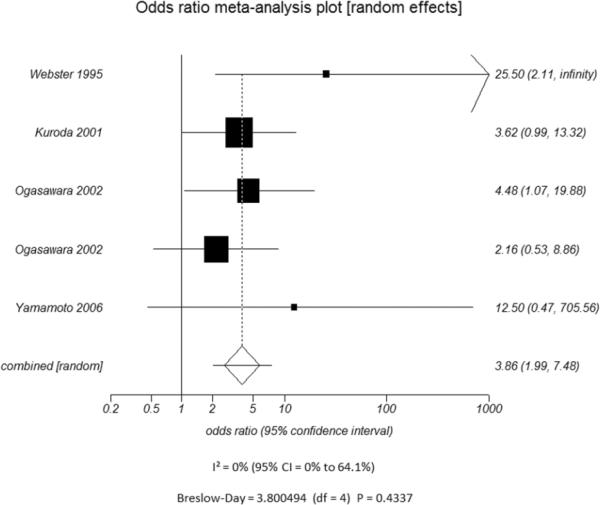
D.
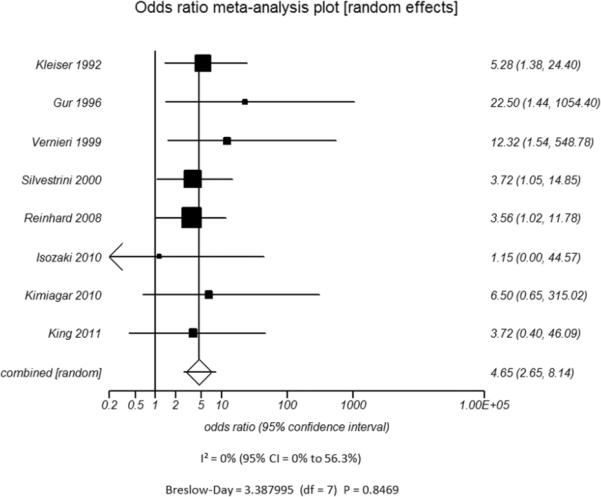
Figure 4.
Odds ratio and 95% confidence ratio for studies divided by patient disease characteristics, including studies of patients with only high grade stenosis (A) or only vessel occlusion (B), and patients with disease only in the ICA only (C) or in both the ICA and MCA (D). The symbols and abbreviations are as in Figure 2.
A - High Grade Stenosis
B - Occlusion
C - ICA disease only
D - ICA and MCA mixed populations
A.
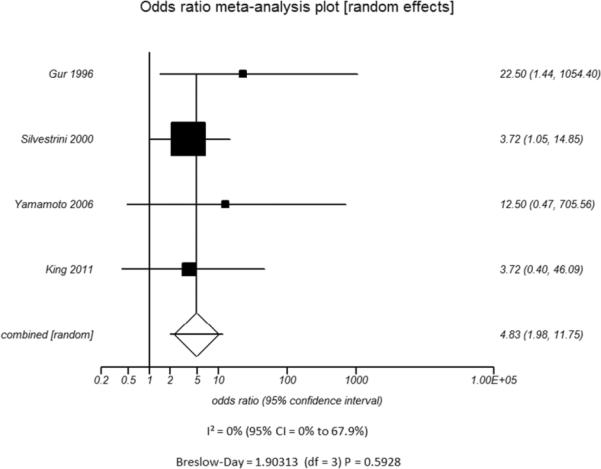
B.
C.
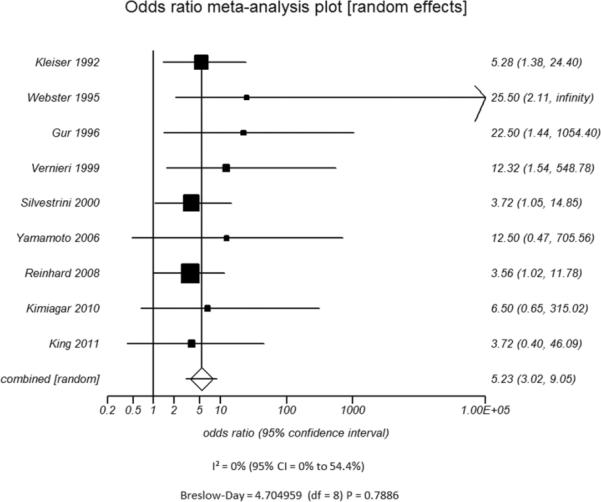
D.
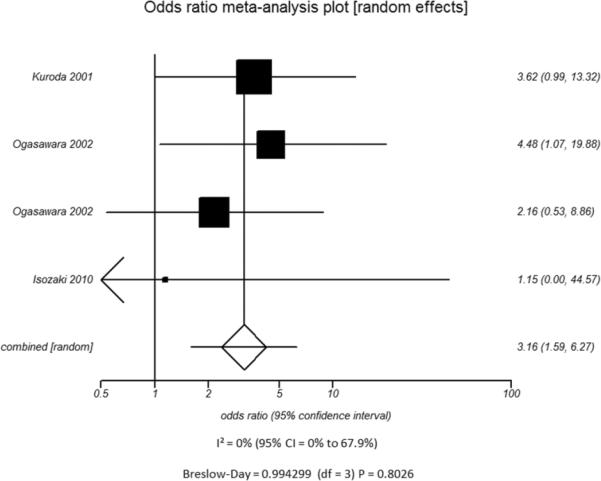
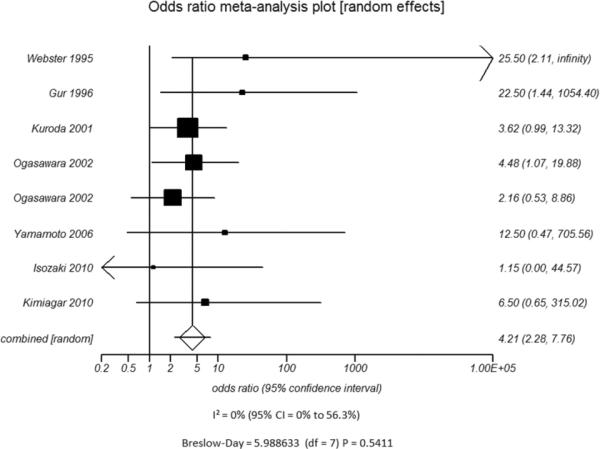
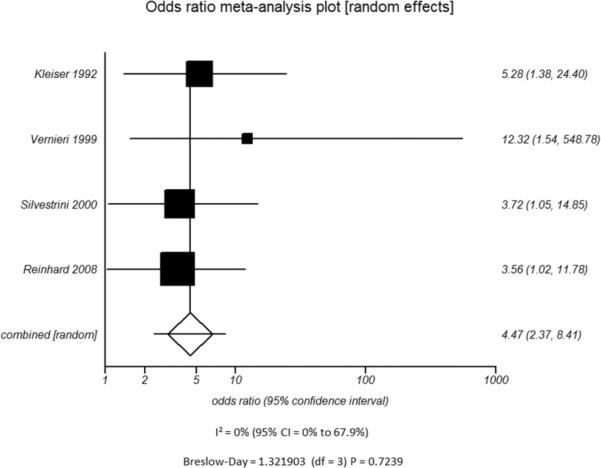
Figure 5.
A - Modality used to Measure CVR - TCD
B - Modality used to Measure CVR - Nuclear Medicine Scintigraphy
C - Type of Vasodilatory Challenge Used - Acetazolamide
D - Type of Vasodilatory Challenge Used - Variation in Inspired Carbon DioxideFigure 5. Odds ratio and 95% confidence ratio for studies divided by distinguishing features of CVR testing methodology used, including whether TCD (A) or nuclear medicine techniques (B) was the modality of choice and whether acetazolamide challenge (C) or variations in inspired carbon dioxide (D) was the vasodilatory stimulus used. The symbols and abbreviations are as in Figure 2.
A.
B.
C.
D.
Discussion
Most imaging-based risk assessments of stroke or TIA rely on the degree of arterial narrowing with the highest incidence of stroke associated with the most severe narrowing. The yearly incidence of stroke varies from approximately 1.2 to 5.9% per year for asymptomatic ICA stenosis5,17 to about 10% per year for symptomatic ICA occlusion21. Though these estimates are integral to current treatment and stroke prevention paradigms, most consensus recommendations do not include assessments of cerebral hemodynamics in their management algorithms22.
In this systematic review and meta-analysis of 1061 independent CVR tests in 991 unique patients with carotid stenosis or occlusion with a mean follow up of 32.7 months, baseline CVR impairment was associated with increased risk of stroke/TIA. Our findings suggest a positive relationship between baseline CVR impairment and future ischemic events, with a pooled odds ratio suggesting that patients with impaired CVR are approximately 4 times more likely to develop stroke or TIA. To our knowledge, though there have been two previous published meta-analyses of the role of CVR in predicting future stroke risk, one was limited in scope as it examined only 3 studies limited to patients with asymptomatic disease5 and another was performed in 1997 before a majority of the current studies in the meta-analysis were published and was focused instead on baseline CBF impairments6. Our literature search found 5 studies limited to asymptomatic patients, and is the first study to evaluate the effect of CVR impairment across different disease characteristics and by combining studies that used different methods to measure CVR. Importantly, our study suggests that CVR impairment is strongly associated with stroke or TIA in both high grade stenosis and occlusion, as well as in asymptomatic and symptomatic patients. These findings suggest that, in combination or in addition to the risk of embolic stroke arising from carotid atheromatous plaque, these patients face stroke risk from hypoperfusion in vascular territories where vasodilatory capacity is maximally exhausted.
The choice of modality for evaluating CVR varies. We found the association between CVR impairment and risk of stroke conserved across testing modality (TCD or NM techniques) as well as the nature of the vasodilatory stimulus (acetazolamide or variation in inspired CO2 levels). TCD is relatively inexpensive and fairly widely available, but does not provide additional information of the brain parenchyma and is technically impossible in some cases due to lack of acoustic windows. Modalities which measure brain tissue perfusion, such as NM techniques, often have limited use in the clinical setting due to expense, availability, and low spatial and temporal resolution. Though there are radiation and cost considerations for newer cross sectional methods such as CT and MRI perfusion techniques, to our knowledge no prospective studies assessing CVR impairment and stroke risk have been performed with these newer modalities, so their utility requires further investigation.
Our study has some limitations that should be considered. Though no studies in the review described any differences in risk factors or treatment that might explain differences between normal and impaired CVR groups, an explicit statistical correction of these risk factors occurred in a majority (9 of 13) but not all of the studies. In addition, no methodology for blinding of investigators to the CVR results was explicitly made in a majority of the studies. Additional limitations inherent to the generalization of data for the purposes of pooled statistical analysis also should be acknowledged. Study endpoints (stroke or TIA) were defined variably by authors with many aggregating these outcomes and preventing distinction between them in our summary meta-analysis, and also preventing a distinction between minor versus disabling stroke. In addition, definitions of normal versus impaired CVR and symptomatic versus asymptomatic disease varied, and though some similarities existed, no one standard definition could be applied across all studies. Similarly, more precise description of the severity of stenosis (percentages) and timing of this measurement relative to CVR determination was reported in a variable fashion and was difficult to generalize. Lastly, due to the nature of the data available for statistical analysis, assessment of risk per unit of time as a hazard ratio could not be performed.
Despite these potential limits, the preservation of association between CVR impairment and risk of stroke/TIA is robust across many patient subsets and methods of CVR assessment suggesting an important potential role in stroke/TIA risk assessment. The feasibility of integrating routine CVR measurements into the care of patients with carotid stenosis or occlusion and validation of newer methods of CVR using cross-sectional imaging techniques requires continued investigation.
Supplementary Material
Acknowledgments
Sources of Funding: The Association of University Radiologists General Electric Radiology Research Academic Fellowship (GERRAF) is acknowledged for supporting a portion of Dr. Gupta's effort. Center for Education and Research in Therapeutics (AHRQ RFA-HS-05-14) and Clinical Translational Science Center (NIH UL1-RR024996) are acknowledged for supporting a portion of Dr. Mazumdar's effort.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Chaturvedi S, Bruno A, Feasby T, Holloway R, Benavente O, Cohen SN, et al. Carotid endarterectomy--an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005;65:794–801. doi: 10.1212/01.wnl.0000176036.07558.82. [DOI] [PubMed] [Google Scholar]
- (2).Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- (3).Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- (4).Powers WJ, Clarke WR, Grubb RL, Jr, Videen TO, Adams HP, Jr, Derdeyn CP, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA. 2011;306:1983–1992. doi: 10.1001/jama.2011.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).King A, Serena J, Bornstein NM, Markus HS, ACES Investigators Does impaired cerebrovascular reactivity predict stroke risk in asymptomatic carotid stenosis? A prospective substudy of the asymptomatic carotid emboli study. Stroke. 2011;42:1550–1555. doi: 10.1161/STROKEAHA.110.609057. [DOI] [PubMed] [Google Scholar]
- (6).Klijn CJ, Kappelle LJ, Tulleken CA, van Gijn J. Symptomatic carotid artery occlusion. A reappraisal of hemodynamic factors. Stroke. 1997;28:2084–2093. doi: 10.1161/01.str.28.10.2084. [DOI] [PubMed] [Google Scholar]
- (7).Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- (9).Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients? Stroke. 1996;27:2188–2190. doi: 10.1161/01.str.27.12.2188. [DOI] [PubMed] [Google Scholar]
- (10).Isozaki M, Arai Y, Kudo T, Kiyono Y, Kobayashi M, Kubota T, et al. Clinical implication and prognosis of normal baseline cerebral blood flow with impaired vascular reserve in patients with major cerebral artery occlusive disease. Ann Nucl Med. 2010;24:371–377. doi: 10.1007/s12149-010-0367-9. [DOI] [PubMed] [Google Scholar]
- (11).Kimiagar I, Bass A, Rabey JM, Bornstein NM, Gur AY. Long-term follow-up of patients with asymptomatic occlusion of the internal carotid artery with good and impaired cerebral vasomotor reactivity. Eur J Neurol. 2010;17:1285–1290. doi: 10.1111/j.1468-1331.2010.03008.x. [DOI] [PubMed] [Google Scholar]
- (12).Kleiser B, Widder B. Course of carotid artery occlusions with impaired cerebrovascular reactivity. Stroke. 1992;23:171–174. doi: 10.1161/01.str.23.2.171. [DOI] [PubMed] [Google Scholar]
- (13).Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke. 2001;32:2110–2116. doi: 10.1161/hs0901.095692. [DOI] [PubMed] [Google Scholar]
- (14).Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain. 2001;124:457–467. doi: 10.1093/brain/124.3.457. [DOI] [PubMed] [Google Scholar]
- (15).Ogasawara K, Ogawa A, Terasaki K, Shimizu H, Tominaga T, Yoshimoto T. Use of cerebrovascular reactivity in patients with symptomatic major cerebral artery occlusion to predict 5-year outcome: comparison of xenon-133 and iodine-123-IMP single-photon emission computed tomography. J Cereb Blood Flow Metab. 2002;22:1142–1148. doi: 10.1097/00004647-200209000-00012. [DOI] [PubMed] [Google Scholar]
- (16).Reinhard M, Gerds TA, Grabiak D, Zimmermann PR, Roth M, Guschlbauer B, et al. Cerebral dysautoregulation and the risk of ischemic events in occlusive carotid artery disease. J Neurol. 2008;255:1182–1189. doi: 10.1007/s00415-008-0865-z. [DOI] [PubMed] [Google Scholar]
- (17).Silvestrini M, Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA. 2000;283:2122–2127. doi: 10.1001/jama.283.16.2122. [DOI] [PubMed] [Google Scholar]
- (18).Vernieri F, Pasqualetti P, Passarelli F, Rossini PM, Silvestrini M. Outcome of carotid artery occlusion is predicted by cerebrovascular reactivity. Stroke. 1999;30:593–598. doi: 10.1161/01.str.30.3.593. [DOI] [PubMed] [Google Scholar]
- (19).Webster MW, Makaroun MS, Steed DL, Smith HA, Johnson DW, Yonas H. Compromised cerebral blood flow reactivity is a predictor of stroke in patients with symptomatic carotid artery occlusive disease. J Vasc Surg. 1995;21:338–44. doi: 10.1016/s0741-5214(95)70274-1. discussion 344–5. [DOI] [PubMed] [Google Scholar]
- (20).Yamamoto KK, Miyata T, Momose T, Nagayoshi M, Akagi D, Hosaka A, et al. Reduced vascular reserve measured by stressed single photon emission computed tomography carries a high risk for stroke in patients with carotid stenosis. Int Angiol. 2006;25:385–388. [PubMed] [Google Scholar]
- (21).Flaherty ML, Flemming KD, McClelland R, Jorgensen NW, Brown RD., Jr Population-based study of symptomatic internal carotid artery occlusion: incidence and long-term follow-up. Stroke. 2004;35:e349–52. doi: 10.1161/01.STR.0000135024.54608.3f. [DOI] [PubMed] [Google Scholar]
- (22).Adams RJ, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, et al. Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2008;39:1647–1652. doi: 10.1161/STROKEAHA.107.189063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


