Abstract
Background
Dyslipidemia and type 2 diabetes are two of the most significant risk factors for the development of cardiovascular disease. Measurement of lipoprotein subclasses provides important information about derangements in lipid metabolism and helps refine cardiovascular risk assessment. Exenatide, a glucagon-like peptide 1 receptor agonist, improved glycemic control, obesity, hypertension, and dyslipidemia in patients with type 2 diabetes in clinical trials.
Methods
In the DURATION-1 trial, patients with type 2 diabetes were treated with exenatide once weekly or twice daily for 30 weeks. This post hoc analysis evaluated the impact of exenatide on lipoprotein subclasses in 211 DURATION-1 patients using vertical auto profile methodology and the Statistical Package for the Social Sciences general linear model adjusted for glycosylated hemoglobin (HbA1c) and weight.
Results
Baseline lipids and high sensitivity C-reactive protein were normal overall based on the standard lipid panel. Once-weekly exenatide reduced apolipoprotein B and the apolipoprotein B to apolipoprotein A1 ratio (P < 0.05), independent of glycemic improvement and weight loss. A significant shift in lipoprotein pattern away from small, dense low-density lipoprotein-4 cholesterol was also observed (P < 0.05). Exenatide once weekly increased high-density lipoprotein-2 cholesterol, even after adjustment for changes in HbA1c and weight (P < 0.05). Triglycerides, very low-density lipoprotein cholesterol, and high sensitivity C-reactive protein were reduced with both the once-weekly and twice-daily exenatide regimens (P < 0.05).
Conclusion
In this post hoc analysis, exenatide significantly improved a number of cardiovascular risk markers. Continuous exenatide exposure with exenatide once weekly elicited a greater response than did immediate-release exenatide twice daily, generally independent of glycemic improvement and weight loss. Thus, in addition to improving glycemic control, exenatide induced favorable changes in lipid and lipoprotein metabolism and decreased systemic inflammation.
Keywords: glucagon-like protein-1 receptor agonist, incretin mimetic, dyslipidemia, type 2 diabetes mellitus
Introduction
Dyslipidemia in patients with type 2 diabetes increases the risk of cardiovascular disease.1–3 In insulin-resistant states, such as type 2 diabetes, there is greater constitutive mobilization of fatty acids from triglyceride stores in visceral adipose tissue, increased hepatic secretion of very low-density lipoprotein (VLDL) cholesterol, inhibition of lipoprotein lipase to some degree, and activation of cholesteryl ester transfer protein. Given these metabolic disturbances, the typical lipid profile in patients with type 2 diabetes includes elevated triglycerides and VLDL cholesterol, increased numbers of small, dense low-density lipoprotein (LDL) cholesterol and other atherogenic lipoproteins, and reduced high-density lipoprotein (HDL) cholesterol.2
Reducing LDL cholesterol is the primary target of dyslipidemia therapy in both primary and secondary prevention. However, LDL cholesterol incompletely assesses the contribution of all atherogenic lipoproteins, such as VLDL or intermediate-density lipoprotein (IDL), to cardiovascular disease risk. Direct measurement of circulating apolipoprotein B concentrations has been proposed as a better method for assessing this risk because it more accurately reflects the true burden of all atherogenic lipoproteins.4
Restoration of glycemic control and relieving insulin resistance promotes reductions in serum triglyceride levels, primarily by reducing free fatty acid and glucose levels, both of which fuel hepatic triglyceride production. However, lowering of glucose rarely results in pronounced improvements in LDL and HDL cholesterol or their subclasses. Given the increased burden of cardiovascular risk factors in patients with type 2 diabetes, it is critical to understand how current antihyperglycemic agents impact these risk factors and the progression of atherosclerosis, and to incorporate this knowledge into routine clinical practice. For example, the InterHeart study reported that smoking, dyslipidemia, hypertension, abdominal obesity, and diabetes account for 80% of the risk for an acute myocardial infarction,5 and that the strongest risk predictor is the ratio of apolipoprotein B to apolipoprotein A1. Also, the Quebec Cardiovascular Study found that the combination of diabetes, elevated small, dense LDL cholesterol, and elevated apolipoprotein B synergistically confer a 20-fold increased risk for cardiovascular events.6 Other studies suggested that high sensitivity C-reactive protein, a proinflammatory biomarker, helped to refine risk estimates beyond the measurement of established Framingham risk factors.7 Nissen et al8 demonstrated that progression of atherosclerosis is slower when atherogenic lipoprotein and high sensitivity C-reactive protein levels are both reduced.
Exenatide is a glucagon-like protein-1 receptor agonist that has been demonstrated to have multiple glucoregulatory effects in patients with type 2 diabetes, including glucose-dependent enhancement of insulin secretion, suppression of inappropriately high glucagon secretion, slowing of gastric emptying, and reduction of food intake, usually accompanied by weight loss.9 Additional effects of exenatide are under investigation, including improved endothelial function, reduced systolic blood pressure, decreased oxidative stress and inflammation, and improved myocardial bioenergetics.10–13 Preliminary investigation with nuclear magnetic resonance imaging demonstrated an exenatideinduced shift away from small LDL lipoproteins towards larger, more buoyant LDL lipoproteins (small LDL cholesterol change from baseline −146 ± 67 nmol/L, P = 0.037; large LDL cholesterol change from baseline 68 ± 27 nmol/L, P = 0.017) after 52 weeks of exenatide twice daily, compared with biphasic insulin aspart, on a background of metformin and sulfonylurea in patients with type 2 diabetes.14,15
In this post hoc analysis of data from the 30-week DURATION- 1 (Diabetes Therapy Utilization: Researching Changes in A1c, Weight and Other Factors Through Intervention with Exenatide Once Weekly) clinical trial,16 the effects of two exenatide formulations (immediate-release exenatide twice daily and extended-release exenatide once weekly) on lipoproteins and high sensitivity C-reactive protein were explored using a validated vertical auto profile ultracentrifuge methodology.17 This is the first comprehensive lipoprotein subclass analysis performed for a glucagon-like protein-1 receptor agonist in human patients with type 2 diabetes.
Materials and methods
Details on the DURATION-1 study (clinicaltrials.gov NCT00308139) have been reported previously.16 In brief, patients with type 2 diabetes were treated with exenatide 2 mg once weekly or exenatide 10 μg twice daily for 30 weeks. Of the 295 intent-to-treat patients in DURATION-1, 211 were assessed for lipid subclass concentrations at baseline and week 30 using vertical auto profile technology,17 and constitute the analysis cohort. The vertical auto profile method involves independent measurements of total cholesterol, HDL cholesterol, and triglycerides.17 The validated vertical auto profile technique is an inverted rate zonal, single vertical spin, density gradient ultracentrifugation technique that simultaneously and directly measures cholesterol concentrations of all five lipoprotein classes (HDL cholesterol, LDL cholesterol-real [LDL cholesterol without lipoprotein(a) and IDL cholesterol], VLDL cholesterol, IDL cholesterol, lipoprotein(a)) and their subclasses.17,18 The vertical auto profile separates all lipoproteins in three steps: a two-layer density gradient is prepared with the bottom layer containing a 1:40 serum dilution with KBr; the gradient is centrifuged at 65,000 rpm for approximately 45–60 minutes; and the layers are analyzed using a continuous flow cholesterol analyzer. Output has been validated against standardized reference tests from the Core Laboratory for Clinical Studies at Washington University School of Medicine in St Louis, MO, using beta quantification.19 High sensitivity C-reactive protein was measured using the Multigent CRP Vario™ assay (Ilex Medical Systems, Petach-Tikva, Israel) and the Architect c8000® system for quantitative immunoturbidometric determination (Abbott Diagnostics, Abbott Park, IL). Data were analyzed using the Statistical Package for Social Sciences version 17.0 (IBM, Armonk, NY) general linear model-univariate analysis. Outcome variables of interest at study end were treated as dependent variables, adjusted for baseline and week 30 change in glycosylated hemoglobin (HbA1c) and weight. The statistical significance level was set at P < 0.05.
Results
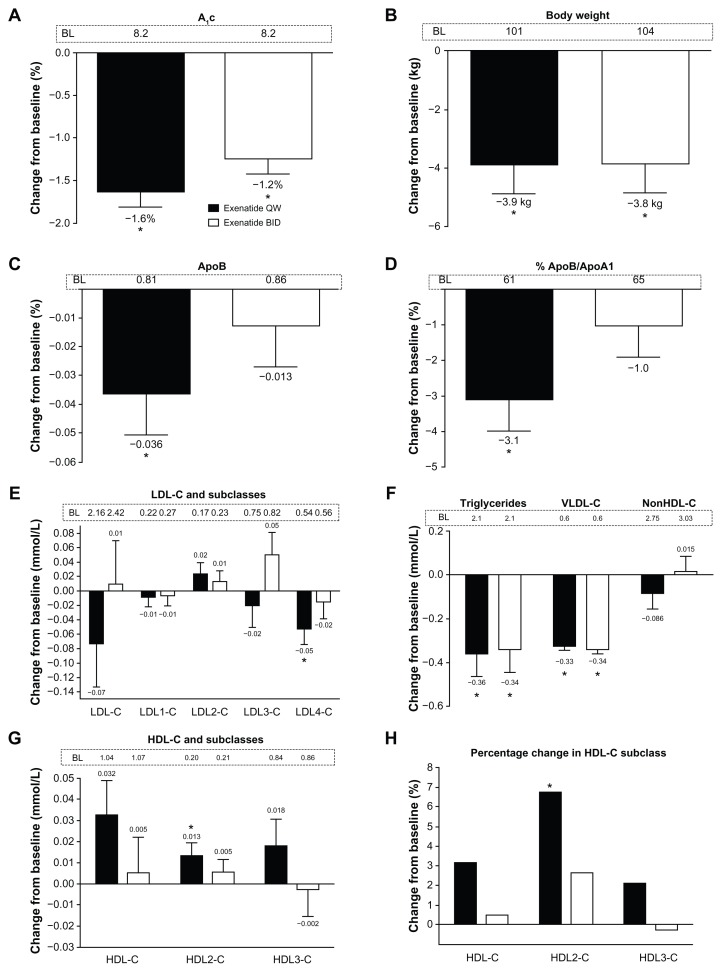
General demographics and baseline values for the total analysis cohort are shown in Table 1. The patients were generally middle-aged, obese Caucasians with a mean HbA1c of 8.2%. Approximately two thirds of the cohort were treated with metformin, and more than half with a statin. In the total analysis cohort, mean baseline lipid concentrations were generally within the normal range based on the standard lipid panel.1–3 However, as shown below, there was actually a preponderance of small, dense LDL cholesterol lipoproteins, and the LDL cholesterol distribution in both treatment groups (exenatide once weekly or twice daily) was skewed towards the smaller LDL3 cholesterol and LDL4 cholesterol. On average, the more buoyant HDL2 cholesterol was below the recommended target goal1–3 at baseline, with a resulting apolipoprotein B to apolipoprotein A1 ratio above 0.6 (60%). Exenatide treatment for 30 weeks significantly reduced mean HbA1c (exenatide once weekly −1.6%; exenatide twice daily −1.2%) and body weight from baseline (exenatide once weekly −3.9 kg; exenatide twice daily −3.8 kg, Figure 1A and B).
Table 1.
Demographics and baseline characteristics for the lipoprotein subclass analysis cohort
| Total cohort | ||
|---|---|---|
|
|
||
| ExQW n = 106 |
ExBID n = 105 |
|
| Age (years) | 56 ± 9 | 55 ± 10 |
| Female (%) | 36.8 | 47.6 |
| Caucasian (%) | 84.9 | 73.3 |
| Black/African American (%) | 4.7 | 11.4 |
| Hispanic (%) | 10.4 | 14.3 |
| Asian (%) | 0 | 1.0 |
| A1c (%) | 8.2 ± 0.9 | 8.2 ± 0.9 |
| Body weight (kg) | 101 ± 18 | 104 ± 22 |
| BMI (kg/m2) | 34.8 ± 5.0 | 34.9 ± 5.3 |
| Apolipoprotein A (g/L) | 1.3 ± 0.2 | 1.3 ± 0.2 |
| Apolipoprotein B (g/L) | 0.81 ± 0.21 | 0.86 ± 0.23 |
| Apolipoprotein B/A1 (%) | 61 ± 15 | 65 ± 17 |
| Triglycerides (mmol/L) | 2.14 ± 1.40 | 2.11 ± 1.44 |
| LDL-C (mmol/L) | 2.16 ± 0.80 | 2.42 ± 0.94 |
| LDL1-C (mmol/L) | 0.22 ± 0.17 | 0.27 ± 0.19 |
| LDL2-C (mmol/L) | 0.17 ± 0.19 | 0.23 ± 0.28 |
| LDL3-C (mmol/L) | 0.75 ± 0.36 | 0.82 ± 0.43 |
| LDL4-C (mmol/L) | 0.54 ± 0.29 | 0.56 ± 0.29 |
| VLDL-C (mmol/L) | 0.59 ± 0.20 | 0.60 ± 0.21 |
| HDL-C (mmol/L) | 1.04 ± 0.24 | 1.06 ± 0.27 |
| HDL-2 (mmol/L) | 0.20 ± 0.09 | 0.20 ± 0.09 |
| HDL-3 (mmol/L) | 0.84 ± 0.16 | 0.86 ± 0.20 |
| Diabetes medications (%) | ||
| None | 15.2 | 10.4 |
| MET alone | 34.9 | 33.3 |
| SFU alone | 2.8 | 6.7 |
| TZD alone | 1.9 | 14.3 |
| MET + TZD | 11.3 | 3.8 |
| MET + SFU | 29.2 | 26.7 |
| TZD + SFU | 3.8 | 2.9 |
| MET + TZD + SFU | 0.9 | 1.9 |
| Dyslipidemia medications (%) | ||
| Statin | 61.3 | 44.8 |
| Statin + CAI | 7.5 | 5.7 |
| Niacin | 2.8 | 4.8 |
| Fibrate | 7.5 | 9.5 |
| CAI | 8.5 | 4.8 |
| Fish oil | 7.5 | 5.7 |
Note: Values are mean ± SD.
Abbreviations: ApoB, apolipoprotein B; ApoA1, apolipoprotein A1; BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol; CAI, cholesterol absorption inhibitor; MET, metformin; SD, standard deviation; SFU, sulfonylurea; TZD, thiazolidinedione; A1c, glycosylated hemoglobin; ExQW, exenatide once weekly; ExBID, exenatide twice daily.
Figure 1.
Effects of exenatide on glycosylated hemoglobin, body weight, apolipoproteins, and lipoproteins in the total analysis cohort with an overall normal lipid profile at baseline. Week 30 change data are independent of glycemic improvement and weight loss. (A) Glycosylated hemoglobin. (B) Body weight. (C) Apolipoprotein B. (D) Percentage of apolipoprotein B/apolipoprotein A1. (E) Low-density lipoprotein cholesterol and its subclasses. (F) Triglycerides, very low-density lipoprotein cholesterol, and non-high-density lipoprotein cholesterol. (G) High-density lipoprotein cholesterol and its subclasses. (H) Percentage changes in high-density lipoprotein cholesterol and its subclasses. (Panels A and B) Least squares mean + 95% confidence intervals. *Change from baseline P < 0.0001. (Panels C–G) Adjusted mean + standard error of the mean.
Notes: *Week 30 change from baseline P < 0.05. Once weekly, n = 106, twice daily, n = 105.
Abbreviations: A1c, glycosylated hemoglobin; ApoB, apolipoprotein B; ApoA1, apolipoprotein A1; BID, twice daily; LDL-C, low-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; QW, once weekly.
Lipoprotein and apoprotein measurements for the total cohort are shown in Table 2, and week 30 changes after adjustment for HbA1c and weight changes are shown in Figure 1C–H. Exenatide once weekly significantly reduced triglycerides, apolipoprotein B, the apolipoprotein B to apolipoprotein A1 ratio, LDL4 cholesterol, and VLDL cholesterol (P < 0.05). Exenatide twice daily reduced triglycerides and VLDL cholesterol (P < 0.05). The changes in apolipoprotein A1, LDL cholesterol, LDL1 cholesterol, LDL2 cholesterol, LDL3 cholesterol, IDL cholesterol, VLDL3 cholesterol, VLDL1+2 cholesterol, non-HDL cholesterol, remnant lipoproteins, and lipoprotein(a) cholesterol were not significant with either treatment. Despite no statistically significant effect on total HDL cholesterol after adjustment for changes in HbA1c and weight, exenatide once weekly significantly increased HDL2 cholesterol (P < 0.05), but not HDL3 cholesterol. In contrast, twice-daily treatment with exenatide resulted in no change in HDL cholesterol subclasses.
Table 2.
Apolipoprotein and lipoprotein change from baseline in patients with type treated with exenatide for 30 weeks for the lipoprotein subclass analysis cohort
| ExQW (n = 106) |
ExBID (n = 105) |
|
|---|---|---|
| ApoA1 (g/L) | 0.016 (−0.011, 0.042) | −0.001 (−0.028, 0.025) |
| ApoB (g/L) | −0.036* (−0.065, −0.008) | −0.013 (−0.041, 0.015) |
| ApoB/ApoA1 (%) | −3.1 (−5.0, −1.3)* | −1.0 (−2.8, 0.9) |
| Triglycerides (mmol/L) | −0.361 (−0.566, −0.155)* | −0.339 (−0.546, −0.132)* |
| LDL-C (mmol/L) | −0.073 (−0.192, 0.028) | 0.009 (−0.111, 0.129) |
| LDL1-C (mmol/L) | −0.008 (−0.035, 0.019) | −0.006 (−0.034, 0.021) |
| LDL2-C (mmol/L) | 0.024 (−0.008, 0.055) | 0.012 (−0.019, 0.044) |
| LDL3-C (mmol/L) | −0.020 (−0.080, 0.040) | 0.050 (−0.011, 0.110) |
| LDL4-C (mmol/L) | −0.052 (−0.098, −0.006)* | −0.015 (−0.061, 0.031) |
| IDL-C (mmol/L) | −0.005 (−0.025, 0.015) | −0.005 (−0.025, 0.015) |
| VLDL-C (mmol/L) | −0.327 (−0.360, −0.295)* | −0.343 (−0.375, −0.310)* |
| VLDL3-C (mmol/L) | −0.007 (−0.022, 0.008) | 0.004 (−0.011, 0.019) |
| VLDL12-C (mmol/L) | −0.004 (−0.026, 0.018) | −0.007 (−0.029, 0.015) |
| nonHDL-C (mmol/L) | −0.086 (−0.220, 0.049) | 0.015 (−0.120, 0.151) |
| RLPs (mmol/L) | −0.012 (−0.044 0.020) | −0.001 (−0.034, 0.031) |
| Lp(a) (mmol/L) | −0.012 (−0.040, 0.016) | −0.023 (−0.051, 0.005) |
| HDL-C (mmol/L) | 0.032 (−0.000, 0.065) | 0.005 (−0.027, 0.038) |
| HDL2-C (mmol/L) | 0.013 (0.002, 0.025)* | 0.005 (−0.007, 0.017) |
| HDL3-C (mmol/L) | 0.018 (−0.007, 0.043) | −0.002 (−0.027, 0.023) |
Notes: Values are adjusted mean (95% CI).
P < 0.05 versus baseline. Adjusted model included changes from baseline in A1c and weight as covariates.
Abbreviations: ApoB, apolipoprotein B; ApoA1, apolipoprotein A1; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol; A1c, glycosylated hemoglobin; Lp(a), lipoprotein(a); RLPs, remnant lipoproteins; ExQW, exenatide once weekly; ExBID, exenatide twice daily.
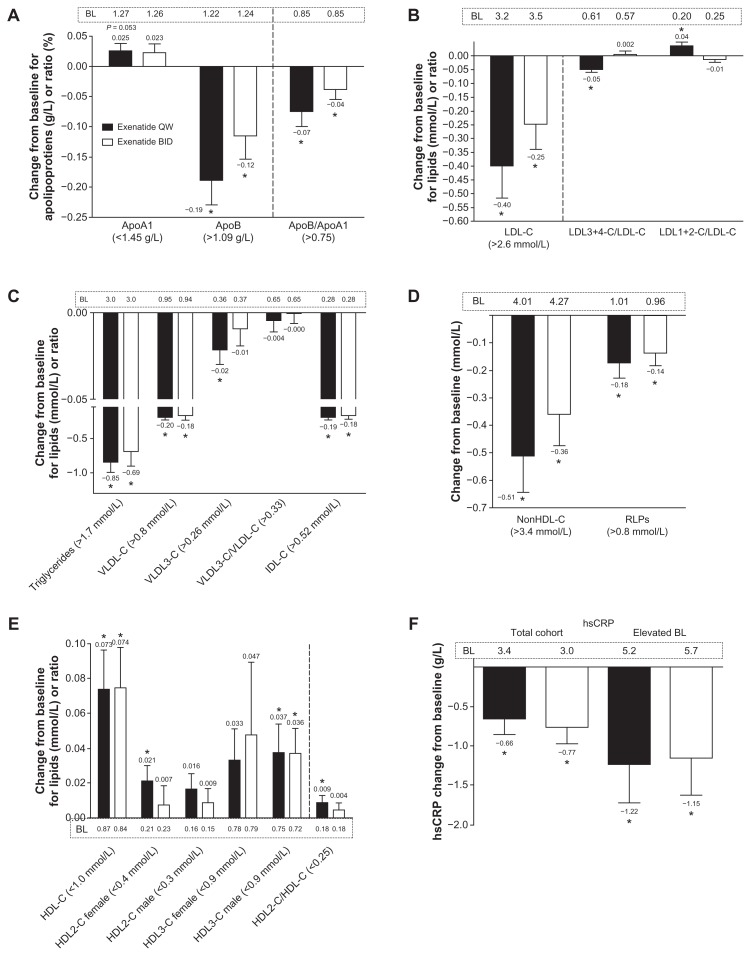
Among patients with abnormal baseline lipid values,1–3 exenatide once weekly significantly improved apolipoprotein B, the apolipoprotein B to apolipoprotein A1 ratio, LDL cholesterol, LDL3+4 cholesterol/LDL cholesterol, LDL1+2 cholesterol/ LDL cholesterol, triglycerides, VLDL cholesterol, VLDL3 cholesterol, IDL cholesterol, non-HDL cholesterol, and remnant lipoproteins (P < 0.05; Figure 2A–E). Exenatide twice daily improved apolipoprotein B, the apolipoprotein B to apolipoprotein A1 ratio, LDL cholesterol, triglycerides, VLDL cholesterol, IDL cholesterol, non-HDL cholesterol, and remnant lipoproteins (P < 0.05). Because recommended HDL cholesterol concentrations differ by gender (>1.0 mmol/L in men and >1.3 mmol/L in women),20 these variables were evaluated separately for men and women. Results of these analyses show that exenatide once weekly significantly improved HDL and HDL2 cholesterol in women, HDL and HDL3 cholesterol in men, and HDL2/HDL cholesterol in both men and women (P < 0.05); exenatide twice daily significantly improved HDL3 cholesterol in men and HDL cholesterol in both men and women (P < 0.05). In the exenatide once-weekly treatment group, the percentage of patients achieving lipoprotein normalization1–3 was 39% for LDL cholesterol, 36% for triglycerides, 50% for VLDL cholesterol, 42% for non-HDL cholesterol, and 20% for HDL cholesterol. In the exenatide twice-daily treatment group, the percentage of patients achieving lipoprotein normalization1–3 was 39% for triglycerides, 55% for VLDL cholesterol, 18% for non-HDL cholesterol, and 15% for HDL cholesterol. During the 30-week assessment period, doses of lipid-lowering agents remained generally stable in all patients in both treatment groups.
Figure 2.
Effects of exenatide on apolipoproteins, lipoproteins, and high sensitivity C-reactive protein in the subgroup of patients with abnormal lipid values1–3 at baseline. (A) Apolipoprotein A1, apolipoprotein B, and the ratio of these apolipoproteins; once weekly, n = 85, n = 9, and n = 22, respectively; twice daily, n = 78, 18, and 29, respectively. (B) Low-density lipoprotein cholesterol and subclass ratios; once weekly, n = 28, 28, and 28, respectively; twice daily, n = 36, 36, and 36, respectively. (C) Triglycerides, very low-density lipoprotein (VLDL) cholesterol, VLDL3 cholesterol, ratio of VLDL cholesterol/VLDL3 cholesterol, and intermediate-density lipoprotein cholesterol; once weekly, n = 55, 18, 77, 106, and 7, respectively; twice daily, n = 52, 20, 75, 104, and 6, respectively. (D) Non-high-density lipoprotein cholesterol and remnant lipoproteins; once weekly, n = 26 and n = 13, respectively; and twice daily, n = 34 and n = 21, respectively. (E) Total high-density lipoprotein cholesterol and its subclasses stratified by gender and overall subclass ratio; once weekly, n = 51, 39, 57, 22, 47, and 95, respectively; twice daily, n = 46, 39, 55, 17, 44, and 96, respectively. (F) High sensitivity C-reactive protein in the total cohort; once weekly, n = 104; twice daily, n = 103.
Notes: High sensitivity C-reactive protein in the subgroup with baseline > 3 mg/L and <10 mg/L; once weekly, n = 41; twice daily, n = 32. Adjusted mean + standard error of the mean. *Week 30 change from baseline P < 0.05.
Abbreviations: BL, baseline; ApoB, apolipoprotein B; ApoA1, apolipoprotein A1; LDL-C, low-density lipoprotein cholesterol; BID, twice daily; HDL-C, high-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol; IDL-C, intermediate-density lipoprotein cholesterol; RLPs, remnant lipoproteins; hsCRP, high sensitivity C-reactive protein; QW, once weekly.
In the total cohort, both exenatide formulations reduced high sensitivity C-reactive protein independent of glycemic control and weight loss (P < 0.05; Figure 2F). Among the patients with abnormal high sensitivity C-reactive protein at baseline, high sensitivity C-reactive protein was significantly reduced by 24% (P = 0.018). The percentages achieving high sensitivity C-reactive protein normalization (<3 mg/L) were 33% with exenatide once weekly and 38% with exenatide twice daily.
Discussion
Evidence garnered over the past two decades has revealed the failure of standard lipoprotein measurements (eg, triglycerides, total cholesterol, LDL cholesterol, and HDL cholesterol) to identify many of the lipoprotein abnormalities contributing to cardiovascular events.21 A recent meta-analysis of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B as markers of cardiovascular disease demonstrated that apolipoprotein B was the most reliable predictor of fatal or nonfatal ischemic events.22 Across all studies analyzed, the mean apolipoprotein B relative risk ratio was 12.0% greater than for LDL cholesterol (P < 0.0001) and 5.7% higher than for non-HDL cholesterol (P < 0.001). These head-to-head analyses also rank-ordered the three markers as apolipoprotein B > non-HDL cholesterol > LDL cholesterol. Over a 10-year period, an apolipoprotein B strategy would prevent 500,000 more cardiovascular events than a non-HDL cholesterol strategy, and a non-HDL cholesterol strategy would prevent 300,000 more cardiovascular events than an LDL cholesterol strategy.22
Comprehensive lipoprotein testing, such as the vertical auto profile, can better inform the clinician on the patient’s underlying lipid disorders, above and beyond the standard lipid panel, and can easily be incorporated into routine clinical management. This potential benefit was demonstrated in a cohort of patients with type 2 diabetes from the DURATION-1 exenatide clinical trial,16 in which there was a preponderance of small, dense LDL cholesterol lipoproteins (see Table 1) not apparent from the standard lipid panel.1–3 In fact, the LDL cholesterol distribution in both treatment groups (exenatide once weekly, exenatide twice daily) was skewed towards the smaller LDL3 cholesterol and LDL4 cholesterol. On average, the more buoyant HDL2 cholesterol was below the recommended target goal1–3 at baseline, with a resulting apolipoprotein B to apolipoprotein A1 ratio above 0.6 (60%). Clearly, the most widely used clinical test failed to capture the level of cardiovascular risk accurately in these patients.
Despite the appearance of a benign lipid profile at baseline based on the standard lipid panel,1–3 a clinically important shift in lipoprotein pattern away from small, dense LDL4 cholesterol particles was observed with once-weekly exenatide treatment. This is consistent with the reduction in serum triglycerides that was also observed in patients treated with exenatide once weekly. As triglyceride availability in serum decreases, there is less cholesteryl ester transfer protein-dependent loading of LDL cholesterol particles with triglyceride. This, is turn, results in less hepatic lipase-dependent lipolysis of LDL cholesterol particles, allowing for them to remain larger, more buoyant, and possibly less atherogenic. Exenatide once weekly also significantly increased the levels of more buoyant HDL2 cholesterol particles, even after adjustment for treatment effects on HbA1c and body weight. This shift suggests that HDL cholesterol particles in exenatide-treated patients are maturing, and may not be as vulnerable to lipolysis and catabolism by hepatic lipase. Although the subject of debate, investigations have suggested that the HDL2 cholesterol subtype may have a greater cardioprotective effect in type 2 diabetes.23 Given that the pathogenic potential of small dense LDL cholesterol and apolipoprotein B may be further amplified by insulin resistance and the hyperglycemia of type 2 diabetes, the additional 4% reduction in apolipoprotein B, 1.6% reduction in HbA1c, and 4 kg weight loss observed with exenatide therapy in this study would be expected to contribute to overall risk reduction. Considering the changes in serum levels of apolipoproteins, lipids, and lipoproteins observed in this cohort, at a minimum, exenatide therapy was associated with improvements in lipoprotein metabolism.
Hypertriglyceridemia is a marker of metabolic disease, and is assuming an increasingly important role in the assessment and management of cardiovascular disease risk.24 Prospective studies support a strong link between triglyceride concentrations and cardiovascular risk in patients with type 2 diabetes, and in individuals with lower levels of HDL and LDL cholesterol. The incomplete hydrolysis of triglyceride-rich chylomicrons and VLDL particles results in atherogenic cholesterol-enriched remnant lipoproteins and elevated nonfasting triglycerides that have been strongly correlated with high levels of remnant lipoproteins. In our post hoc analysis, both exenatide formulations significantly reduced circulating concentrations of triglycerides and VLDL cholesterol. The beneficial effects of exenatide on the entire spectrum of apolipoproteins, lipoproteins, and lipoprotein subclasses were especially apparent in patients with abnormal baseline values. Exenatide treatment improved apolipoprotein B, LDL cholesterol, and several subclass risk indicators, including a number of other atherogenic cholesterol-rich lipoproteins, triglycerides, and antiatherogenic HDL cholesterol, HDL2 cholesterol, and HDL3 cholesterol.
Several previous studies offer additional insights into the effects of exenatide on circulating lipids and lipoproteins.10–12,25–29 In an open-label extension of the first three Phase III exenatide twice daily clinical trials, 314 patients with type 2 diabetes treated with metformin and/or sulfonylurea plus exenatide twice daily for 82 weeks had significantly reduced triglycerides (−0.43 mmol/L) and elevated HDL cholesterol (+0.12 mmol/L).25 There were trends for reductions in total cholesterol, apolipoprotein B, and LDL cholesterol. By 3.5 years of twice-daily exenatide treatment, the 151 evaluable patients had significant reductions from baseline in triglycerides (−12%), total cholesterol (−5%), and LDL cholesterol (−6%).26 In addition, HDL cholesterol was significantly increased by 24%. Although the 25% of patients who lost the most weight had the greatest improvements in triglycerides and HDL cholesterol, there was minimal correlation between weight change and lipid improvements for the total cohort. Correlations between HbA1c or fasting plasma glucose and serum lipid concentrations were similarly low. In a clinical practice setting, Bhushan et al10 reported a retrospective analysis of the laboratory and medical records of 176 adults with both type 2 diabetes and metabolic syndrome treated with exenatide twice daily for 16 weeks. Exenatide significantly reduced total cholesterol and LDL cholesterol, but not triglycerides. Of interest, these results were not attributable to changes in concomitant dyslipidemia medications. In a later clinical trial, Bunck et al11 evaluated 69 patients with type 2 diabetes treated chronically with metformin and exenatide twice daily or insulin glargine for a year. Compared with insulin, exenatide twice daily significantly reduced post-meal excursions in triglycerides, apolipoprotein B48, VLDL cholesterol, and free fatty acids, and increased HDL cholesterol. Finally, acute exenatide administration suppressed postprandial excursions of proatherogenic lipoproteins in overweight/ obese adults with impaired glucose tolerance or recent onset type 2 diabetes (57% treated with statins).12,27 One injection of exenatide markedly reduced postprandial elevation of triglycerides, apolipoprotein B-48, apolipoprotein CIII, remnant lipoprotein cholesterol, and remnant lipoprotein triglyceride (each P < 0.05 versus placebo). A subgroup analysis found that postprandial endothelial function was higher after exenatide than after placebo (P = 0.0002) and that exenatide-induced changes in postprandial triglyceride concentrations explained 64% of this effect. Further, the effects of exenatide on postprandial lipoproteins were not affected by the degree of loss of glucose control nor by dyslipidemia treatment with statins. In the DURATION-2 study, patients with type 2 diabetes suboptimally controlled with metformin and treated with exenatide once weekly for 26 weeks had significantly increased HDL cholesterol compared with baseline (P < 0.05),28 and this improvement was maintained out to 52 weeks.29 Taken together, these data lend further support to a role for exenatide in improving the typical diabetic proatherogenic profile that is at least partially independent of the effects of exenatide on glycemic control and weight loss. Although little is known concerning potential mechanisms to explain the effects of exenatide on lipoproteins, in a recent study, exenatide acutely suppressed intestinal lipoprotein production, possibly through a direct effect on intestinal lipoprotein production, but independent of changes in body weight, satiety, gastric emptying, glucagon, and circulating free fatty acid concentrations.30
The interplay between proinflammatory cytokines and lipid homeostasis has been well described.31 Type 2 diabetes is associated with a chronically heightened level of systemic inflammation characterized by increased plasma levels of numerous inflammatory biomarkers, including high sensitivity C-reactive protein. In a large, representative study, the relationship between circulating levels of high sensitivity C-reactive protein and cardiovascular disease mortality was tracked over 7 years.32 In these patients, individuals with a baseline high sensitivity C-reactive protein > 3 mg/L were significantly more likely to die from cardiovascular disease (about 1.5- fold) than were individuals with high sensitivity C-reactive protein ≤ 3 mg/L (P < 0.004). Furthermore, this association remained even after adjustment for age, gender, total cholesterol, HDL cholesterol, triglycerides, diabetes duration, HbA1c, hypertension, smoking, residence area, and body mass index, and was independent of pre-existing myocardial infarction events.
Exenatide treatment was observed to reduce high sensitivity C-reactive protein significantly, independent of glycemic control and weight loss in the total lipid-analysis cohort. When patients with type 2 diabetes suboptimally controlled with metformin and/or sulfonylurea were treated with exenatide twice daily for 16 weeks, high sensitivity C-reactive protein was significantly reduced (from 0.4 ± 0.5 to 0.2 ± 0.3 mg/L) compared with placebo (increased from 0.6 ± 0.4 to 1.4 ± 1.6 mg/L; P < 0.05).33 In the DURATION- 2 study, patients whose type 2 diabetes was suboptimally controlled with metformin and who were treated with exenatide once weekly for 26 weeks had significantly reduced high sensitivity C-reactive protein (P < 0.05),28 and this improvement reached −25% by week 52.29
The limitations of this study include the post hoc nature of the analysis, the small number of patients, especially those with abnormal baseline values, and the open-label treatment design. Furthermore, these analyses were exploratory in nature, and the results should be considered primarily as hypothesis generating.
Conclusion
This post hoc analysis demonstrated that exenatide therapy may improve a wide spectrum of cardiovascular disease risk markers. Continuous exenatide exposure with once-weekly administration elicited a greater response than exenatide twice daily. Importantly, these improvements were at least partially mediated through mechanisms distinct from the effects of exenatide on hyperglycemia and obesity. Thus, administration of once-weekly exenatide has the potential to modify cardiovascular disease risk factors beneficially in patients with type 2 diabetes and, thus, warrants further clinical investigation in prospective studies. An ongoing, prospective morbidity and mortality outcomes study (EXSCEL, clinicaltrials.gov NCT01144338) is expected to elucidate further the potential cardiovascular benefits stemming from the effect of exenatide once weekly on dyslipidemia.
Acknowledgments
The authors thank and acknowledge medical writing and data analyses by Loretta L Nielsen, LLNielsen Medical Writing, and data compilation by Carl LaCerte, Amylin Pharmaceuticals Inc.
Footnotes
Disclosure
EC is an employee and stockholder of Amylin Pharmaceuticals Inc. PT is a consultant for AstraZeneca, Abbott Laboratories, Genzyme, Genentech, Kowa, and Merck, and a member of the speakers’ bureau for AstraZeneca, Abbott Laboratories, Boehringer-Ingelheim, GSK, Kowa, Merck, and Takeda. GR is an employee of Florida International University and has no other financial conflicts of interest to disclose. MC is a member of the speakers’ bureau or advisory boards for Abbott Laboratories, AstraZeneca, Boehringer-Ingelheim, Bristol- Meyers-Squib, Eli Lilly and Company, Forest Pharmaceuticals, Genetech/Roche, and Kowa. He has served as a continuing medical education speaker for PriMed, and is a board member for the not-for-profit Utah Healthy Living Foundation. MC is also the chief medical officer for Atherotech Cardiometabolic Diagnostic Laboratory and has received research funding from the Johnson and Johnson Research Institute. RC is associated with the following companies as a consultant: Amylin Pharmaceuticals Inc, Eli Lilly and Company, Hoffman- La-Roche, Pfizer, AstraZeneca, Boehringer- Ingelheim, Boston Scientific, Endosonics, Medicines Company, Forest Pharmaceuticals, Medtronics, and Novo Nordisk. Parts of this study were presented in abstract form at the 47th Annual Meeting of the European Association for the Study of Diabetes, Lisbon, Portugal, September 12–16, 2011.
References
- 1.NCEP Expert Panel. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 2.Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31(4):811–822. doi: 10.2337/dc08-9018. [DOI] [PubMed] [Google Scholar]
- 3.Catapano AL, Reiner Z, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias. The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Atherosclerosis. 2011;217S:S1–S44. doi: 10.1016/j.atherosclerosis.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Contois JH, McConnell JP, Sethi AA, et al. Apolipoprotein B and cardiovascular disease risk: position statement from the AACC Lipoproteins and Vascular Diseases Division Working Group on Best Practices. Clin Chem. 2009;55(3):407–419. doi: 10.1373/clinchem.2008.118356. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 6.St-Pierre AC, Cantin B, Dagenais GR, et al. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 2005;25(3):553–559. doi: 10.1161/01.ATV.0000154144.73236.f4. [DOI] [PubMed] [Google Scholar]
- 7.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Inflammatory markers, lipoprotein components and risk of major cardiovascular events in 65,005 men and women in the Apolipoprotein MOrtality RISk study (AMORIS) Atherosclerosis. 2010;213(1):299–305. doi: 10.1016/j.atherosclerosis.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 8.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352(1):29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 9.Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, Buse JB. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care. 2010;33(2):428–433. doi: 10.2337/dc09-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhushan R, Elkind-Hirsch KE, Bhushan M, Butler WJ, Duncan K, Marrioneaux O. Improved glycemic control and reduction of cardiometabolic risk factors in subjects with type 2 diabetes and metabolic syndrome treated with exenatide in a clinical practice setting. Diabetes Technol Ther. 2009;11(6):353–359. doi: 10.1089/dia.2008.0090. [DOI] [PubMed] [Google Scholar]
- 11.Bunck MC, Cornér A, Eliasson B, et al. One-year treatment with exenatide vs insulin glargine: effects on postprandial glycemia, lipid profiles, and oxidative stress. Atherosclerosis. 2010;212(1):223–229. doi: 10.1016/j.atherosclerosis.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Koska J, Schwartz EA, Mullin MP, Schwenke DC, Reaven PD. Improvement of postprandial endothelial function after a single dose of exenatide in individuals with impaired glucose tolerance and recent-onset type 2 diabetes. Diabetes Care. 2010;33(5):1028–1030. doi: 10.2337/dc09-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okerson T, Yan P, Stonehouse A, Brodows R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am J Hypertens. 2010;23(3):334–339. doi: 10.1038/ajh.2009.245. [DOI] [PubMed] [Google Scholar]
- 14.Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50(2):259–267. doi: 10.1007/s00125-006-0510-2. [DOI] [PubMed] [Google Scholar]
- 15.Kapitza C, Nauck M, Trautmann M, et al. Long-term treatment with exenatide was associated with improved post-prandial glycaemic control and a shift from small to large HDL and LDL particles. Diabet Med. 2006;23(Suppl 4):S4. [Google Scholar]
- 16.Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni KR. Cholesterol profile measurement by vertical auto profile method. Clin Lab Med. 2006;2694:787–802. doi: 10.1016/j.cll.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni KR, Garber DW, Marcovina SM, Segrest JP. Quantification of cholesterol in all lipoprotein classes by the VAP-II method. J Lipid Res. 1994;35(1):159–168. [PubMed] [Google Scholar]
- 19.Atherotech Diagnostics Lab. The VAP cholesterol test. 2012. [Accessed October 14, 2011]. Available from: http://www.atherotech.com.
- 20.Standards of medical care in diabetes – 2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [No authors listed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Superko HR. Advanced lipoprotein testing and subfractionation are clinically useful. Circulation. 2009;119(17):2383–2395. doi: 10.1161/CIRCULATIONAHA.108.809582. [DOI] [PubMed] [Google Scholar]
- 22.Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4(3):337–345. doi: 10.1161/CIRCOUTCOMES.110.959247. [DOI] [PubMed] [Google Scholar]
- 23.Franceschini G, Bondioli A, Granata D, et al. Reduced HDL2 levels in myocardial infarction patients without risk factors for atherosclerosis. Atherosclerosis. 1987;68(3):213–219. doi: 10.1016/0021-9150(87)90200-0. [DOI] [PubMed] [Google Scholar]
- 24.Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 25.Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1c, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8(4):436–447. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 26.Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24(1):275–286. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz EA, Koska J, Mullin MP, Syoufi I, Schwenke DC, Reaven PD. Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis. 2010;212(1):217–222. doi: 10.1016/j.atherosclerosis.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 28.Bergenstal RM, Wysham C, Macconell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376(9739):431–439. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 29.Wysham C, Bergenstal R, Malloy J, et al. DURATION-2: efficacy and safety of switching from maximum daily sitagliptin or pioglitazone to once-weekly exenatide. Diabet Med. 2011;28(6):705–714. doi: 10.1111/j.1464-5491.2011.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao C, Bandsma RH, Dash S, Szeto L, Lewis GF. Exenatide, a glucagon- like peptide receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol. 2012;32(6):1513–1519. doi: 10.1161/ATVBAHA.112.246207. [DOI] [PubMed] [Google Scholar]
- 31.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89(2):463–478. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 32.Soinio M, Marniemi J, Laakso M, Lehto S, Ronnemaa T. High-sensitivity C-reactive protein and coronary heart disease mortality in patients with type 2 diabetes: a 7-year follow-up study. Diabetes Care. 2006;29(2):329–333. doi: 10.2337/diacare.29.02.06.dc05-1700. [DOI] [PubMed] [Google Scholar]
- 33.Wu JD, Xu XH, Zhu J, et al. Effect of exenatide on inflammatory and oxidative stress markers in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2011;13(2):143–148. doi: 10.1089/dia.2010.0048. [DOI] [PubMed] [Google Scholar]