Abstract
Tetrabromobisphenol-A (TBBP-A) and hexabromocyclododecanes (HBCDs) are brominated flame retardants that have been found in human milk and serum throughout the world, but have received comparatively little attention in the United States. The objective of this study is to determine concentrations of these analytes in samples of breast milk collected from first-time mothers in the Greater Boston, Massachusetts area and to explore predictors of exposure. Human milk samples were analyzed by LC-ESI-MS/MS for TBBP-A, HBCDs (the α, β and γ diastereomers), and HBCD degradation products: pentabromocyclododecanes (PBCDs) and tetrabromocyclododecadienes (TBCDs). HBCD diastereomers were detected in all samples with α-HBCD present in the highest proportion. TBBP-A, PBCDs and TBCDs were detected in 35%, 42% and 56% of the analyzed samples, respectively. Self-reported demographic, dietary and behavioral data were examined as predictors of HBCD levels. Levels of HBCD were significantly, positively associated with the number of stereo and video electronics in the home (17% increase/item; 95% Confidence Interval (CI)=4%–31%) and reduced in participants who regularly chose organic foods compared to those who did not (0.51, 95% CI=0.32 to 0.82). These results suggest that lifestyle factors are related to body burdens of HBCD and that domestic electronics may be an important source of HBCD exposure in the indoor environment.
INTRODUCTION
Tetrabromobisphenol-A (TBBP-A) and hexabromocyclododecanes (HBCDs) are widely used brominated flame retardants (BFRs) [1]. Over the past decade global production and environmental concentrations of these BFRs has increased [2, 3]. Both are stable, lipophilic/hydrophobic compounds that persist in the environment [4, 5]. HBCD is on the U.S. Environmental Protection Agency’s list of Chemicals of Concern and a global phase-out will be reviewed by the Stockholm Convention in 2013 [6, 7].
The main use of TBBP-A is as a reactive flame retardant in epoxy resins for printed circuit boards in computers, telecommunications equipment, industrial controls and automotive electronics [8]. In reactive applications, flame retardants are not expected to migrate from the product. However, TBBP-A is also used as an additive to circuit boards for low energy applications such as remote controls and video recorders as well as the plastic housing for electrical and electronic equipment, mainly computer monitors and printers [2]. In additive applications, there is potential for the flame retardant to escape from the product and enter the air and dust of the indoor environment. The major use of HBCDs is as an additive to expanded and extruded polystyrene foam used to thermally insulate buildings. It is also added to the back-coating of textiles of upholstered furniture as well as the high impact polystyrene housing of electrical and electronic equipment and appliances [9].
TBBP-A has an estimated biological half-life of 2–6 days based on measurements in human serum [10]. It structurally resembles the thyroid hormone thyroxine, and is characterized as a suspected endocrine disruptor due to its activity in multiple in vitro assays [11, 12]. These include competitive binding of transthyretin, activation of peroxisome proliferator-activated receptors α, β and γ, and activation of estrogen receptors α and β [13–15]. In vitro studies suggest potential immuno- and neurotoxic effects [16, 17]. Rodent studies have failed to identify neurodevelopmental effects with perinatal doses as low as 10 mg/kg-day [18]. However, given the suspected endocrine disrupting effects of TBBP-A and the finding of a dose-unrelated decrease in T3 [18] additional studies using lower doses are needed.
HBCD has been shown to bioconcentrate and biomagnify in fish and wildlife [4, 5, 19]. The HBCD technical mixture consists of mainly the α- β- and γ-diastereomers and is dominated by γ-HBCD, whereas biological samples are dominated by α-HBCD [20]. This may be due to more rapid stereoisomerization to α- and β-HBCD or preferential metabolism and excretion of β- and γ-HBCD in vivo [21]. The biological half-life of HBCD is unknown but has been estimated to be around 64 days in human adipose tissue [22]. Pre-natal and neonatal exposure to HBCDs in rodents has been shown to decrease TSH levels and alter spontaneous behavior in the lowest dose groups [18, 23, 24]; demonstrating the need for lower dose studies. Biotransformation to pentabromocyclododecenes (PBCDs) and potentially tetrabromocyclododecadienes (TBCDs) occurs through dehydrobromination of HBCD, with possible hydroxylation to mono- and dihydroxy metabolites [25]. PBCDs and TBCDs can also be impurities in the HBCD technical product [25].
Both TBBP-A and HBCD accumulate in lipid-rich tissue [25, 26] and have been measured in human milk and serum [27–30]. Estimates of infant exposure to TBBP-A and HBCD are driven by consumption of human milk and exceed estimates of both adult and toddler exposure [28]. A recent study found HBCDs in dust to be a significant predictor of levels in human serum but failed to find similar results for diet [31]. No epidemiologic studies have investigated human exposure to TBBP-A.
To date, pentaBDE has been a focus of environmental health efforts in the USA due to widespread, high exposures compared to other countries [32]. TBBP-A and HBCDs were both in use prior to the manufacturing phase-out of pentaBDE and octaBDE in the USA in 2005 [20, 33]. Like octaBDE and decaBDE they can be used as additives to the plastic housing of electronics.
Very little biomonitoring data exist for TBBP-A or HBCDs in human populations from the USA [19, 27]. Here we report concentrations of TBBP-A and HBCDs in human milk collected in 2004–2005 from a population of first time mothers in Boston, Massachusetts, USA and assess predictors of exposure. PentaBDE concentrations for this cohort were reported previously [34].
METHODS
Study Participants and Sampling
Study participants included 43 first-time mothers, 18 years or older, who had lived in the Greater Boston area for at least 3 years at the time of delivery. Participants spoke English or Spanish and had pregnancies that were healthy and singlet. Eligibility for participation was based on a World Health Organization protocol for human milk monitoring [35]. We recruited participants from three sites in or near Boston, Massachusetts, USA. The study protocol was approved by the Institutional Review Boards at Boston University Medical Center and University of Massachusetts Lowell. All participants gave informed consent prior to enrollment. See the Supplemental Information (SI) and Wu et al. [34] for additional details.
Questionnaires were administered to each participant in person. A single human milk sample was collected from each participant 2 to 8 weeks post-partum between April 2004 and January 2005. Most women used an electric or manual milk pump to collect the sample, pumping directly into glass storage jars that had been rinsed with analytical grade solvents and fitted with a Teflon cap liner [34]. Samples were stored at −20 °C and shipped to the University of Birmingham in 2010 for analysis of TBBP-A, α-, β-, and γ-HBCD, PBCDs and TBCDs using a previously published method [28].
Sample extraction
Accurately-weighed aliquots of the freeze-dried samples (~ 2 g) were loaded into pre-cleaned 66 mL Accelerated Solvent Extraction (ASE 300, Dionex Inc., UK) cells containing 1.5 g florisil, 3 g alumina, 5 g anhydrous Na2SO4 and hydromatrix (Varian Inc., UK) to fill the void volume of the cells, and spiked with 25 ng of each of 13C-labelled TBBP-A, a-, b- and g-HBCD as internal standards. The ASE cells were extracted with hexane:dichloromethane (1:9, v/v) at 90 °C and 1500 psi. The heating time was 5 min, static time 4 min, purge time 90 s, flush volume 50%, with three static cycles [28]. The lipid weight of the studied samples was determined gravimetrically on separate aliquots using a standard procedure (the European Standard EN 1528-2, 1996; See SI for more details).
Sample Clean-up
The crude extracts were concentrated to 0.5 mL using a Zymark Turbovap® II (Hopkinton, MA, USA) then washed with 3 mL of 98% sulfuric acid. After phase separation, the hexane layer was transferred onto a florisil column topped with sodium sulfate and eluted with 25 mL of hexane:dichloromethane (1:1, v/v). The eluate was evaporated to dryness under a gentle stream of N2 and the dried extract was reconstituted in 200 μL of d18-γ-HBCD (25 pg μL−1 in methanol) used as recovery determination (or syringe) standard to determine the recoveries of internal standards for QA/QC purposes [28].
LC-ESI-MS/MS analysis
Separation of TBCDs, PBCDs, HBCDs and TBBP-A was achieved using a dual pump Shimadzu LC-20AB Prominence liquid chromatograph (Kyoto, Japan) equipped with SIL-20A autosampler, a DGU-20A3 vacuum degasser and a Varian Pursuit XRS3 C18 analytical column (150 mm × 2 mm I.D., 3 μm particle size). A mobile phase of (a) 1:1 methanol/water with 2 mM ammonium acetate and (b) methanol at a flow rate of 120 μL min−1 was applied for elution of the target compounds: starting at 50 % (b) then increased linearly to 100 % (b) over 3 min, held for 5 min, followed by a linear decrease to 65 % (b) over 2.5 min and held for 5.5 min. TBBP-A was eluted as a single peak at 9 min. The three main HBCD diastereomers were baseline separated with retention times of 12.3, 12.9 and 13.3 min for a-, b- and g- HBCD, respectively. Three well-resolved peaks were obtained for PBCD isomers at retention times 11.0, 11.6 and 11.9 min (SI Figure S1a) while 2 TBCD isomers eluted at retention times 9.2 and 9.7 min (SI Figure S1b).
Separation of HBCD enantiomers was performed on a chiral permethylated β-cyclodextrin LC column (200 mm × 4 mm I.D., 5 μm particle size) (NUCLEODEX beta-PM, Macherey-Nagel; GmbH & Co, Düren, Germany). A mobile phase of (a) 1:1 methanol/water with 2 mM ammonium acetate and (b) 3:7 methanol/acetonitrile at a flow rate of 500 μL min−1 was applied for elution of the target compounds. Full details of the chiral separation method can be found elsewhere [28].
Mass spectrometric analysis was performed using a Sciex API 2000 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA, USA) operated in electrospray negative ionization (ESI) mode. MS/MS detection operated in the multiple reaction monitoring (MRM) mode was used for quantitative determination of HBCD isomers based on m/z 640.6→79, m/z 652.4→79 and m/z 657.7→ 79 for the native, 13C-labelled and d18-labelled diastereomers, respectively. TBCDs and PBCDs were monitored via the transitions m/z 480.4→79 and m/z 560.8→79 respectively, while m/z 540.8→79, m/z 552.8→79 were used to monitor native and 13C-labelled TBBP-A. Further details of the multi-residue analytical methodology used for separation and quantification of the studied BFRs can be found elsewhere [28].
Quality assurance/Quality control
Good recoveries (72–106%) of the 13C-labelled internal standards were obtained for all the studied compounds (SI Table S1). Further assessment of the method extraction/clean up performance was achieved by spiking milk samples (n=5) with d18-α-HBCD prior to freeze-drying and excellent recoveries (>85%) were obtained (SI Table S2).
Neither TBBP-A or HBCDs were detected in method blanks (n=5; consisting of 2 g pre-extracted anhydrous sodium sulfate treated exactly as a sample) or field blanks (n=5; consisting of ~2 g of broken pieces of the milk containers treated exactly as a sample). Therefore, there was no need for blank correction of the results and method limits of quantification (LOQ) were estimated based on 10:1 S:N ratios and were 30, 36, 26 and 31 pg g−1 lipid weight (lw) for TBBP-A, HBCDs, TBCDs and PBCDs respectively.
In the absence of an appropriate standard reference material for TBBP-A and HBCDs, the accuracy and precision of the analytical method for HBCDs was assessed via replicate analysis (n=7) of NIST SRM 2585 (organics in indoor dust). The results obtained compared favorably with the indicative values reported elsewhere [36] (SI Table S3a). For TBBP-A, a standard addition or “matrix spike” method at 3 concentration levels (n=5 at each level) was used to assess the accuracy and precision of the method and good results were obtained (SI Table S3b).
Data Analysis
Concentrations of the target compounds detected in milk were lipid-adjusted. Concentrations below the LOQ were substituted with a value of ½ the limit of detection. ΣHBCD was calculated as the sum of α-, β- and γ–HBCD. Average concentrations were not calculated for compounds with <50% detection frequency. Log-normality of the target compounds was assessed and confirmed using quantile-quantile plots and Shapiro-Wilks tests. Accordingly, concentrations of HBCDs were natural log-transformed and analyzed as continuous variables in the statistical models. Results of regression models using this outcome were exponentiated, yielding the percent change in ΣHBCD per unit of predictor variable.
Due to lower detection frequencies, concentrations of TBBP-A, PBCDs, and TBCDs were categorized as dichotomous variables (detect/non-detect) for data analysis. For comparison to these compounds, HBCDs were divided on the median and categorized as high and low. The resulting binary variables were analyzed using logistic regression. Results are provided as odds ratios (ORs). For example, the odds of detecting PBCDs in milk samples in which TBBP-A was detected compared to the odds of detecting PBCDs in samples in which TBBP-A was not detected.
Potential associations of the target compounds and the predictors were explored using scatter plots, box plots, Spearman’s correlation, and regression analyses using Microsoft Excel (Version 12.3.2) and SAS (Version 9.1.3). Food frequency data were converted to a linear scale. Statistical analyses used the criteria of α=0.05 for statistical significance. Potential confounding was evaluated as a greater than 10% change in effect estimate.
Daily intake for infants to BFRs in our human milk samples was estimated using the following equation:
where Di is the estimated daily breast milk intake (ng kg-day−1); CBFR is the concentration of BFR in milk lipid (ng g-lw−1); is the intake rate of breast milk per kilogram body weight per day (100 ml kg-day−1); and flipid is the lipid fraction in breast milk (g-lw 100 ml−1). Estimates of intake were based on both geometric mean (GM) and maximum concentrations of BFRs (CBFR). We assumed an average breast milk intake rate of 140 ml kg-day−1 and an upper bound of 190 ml kg-day−1 for an exclusively breastfeeding infant, ages 1 to <3 months [37]. This age range was selected because 90% of our samples were collected from mothers with 4 to 8 week old infants. The lipid fraction of consumed milk was assumed to be 4.0 g-lw 100 ml−1 [37]. Absorption was assumed to be 100%.
RESULTS
Fifty women enrolled in the study and completed the questionnaire. Forty-six women provided a human milk sample [34] and 43 samples had sufficient volume for the current chemical analysis. Despite efforts to recruit a diverse population, the majority of participants were from Brookline and Cambridge. All participants from the Brookline and Cambridge locations were college educated whereas those from Lowell were not (SI Table S4).
Analyte Concentrations in Human Milk Samples
We detected TBBP-A in 35% of the 43 analyzed samples with concentrations ranging from the LOQ of 30 to 550 pg g-lw−1 (Table 1) (SI table S5). We detected α- β- and γ-HBCDs in all samples. α-HBCD was the dominant diastereomer comprising an average of 74% (range: 40–84%) of ΣHBCDs, while β- and γ-HBCD comprised 9% (range: 3–20%) and 20% (range: 9–44%) of ΣHBCDs, respectively. Levels of ΣHBCDs ranged from 360 to 8100 pg g-lw−1 with a GM of 1020 pg g-lw−1. We observed a slight enrichment of the (−)α-HBCD enantiomer in our samples, whereas the enantiomer fractions of β-HBCD and γ-HBCD showed little deviation from a racemic composition. We detected PBCDs and TBCDs in 42% and 56% of analyzed samples; the GM of TBCD was 50 pg g-lw−1. Lipid content of the milk samples ranged from 0.57 to 4.69 g-lw 100 ml−1 with a GM of 1.58 g-lw 100 ml−1.
Table 1.
Concentrations of target compounds, lipids and enantiomer fractions of HBCD in the 43 analyzed human milk samples.
| Analyte (pg g-lw−1) | % Detect | Geometric Mean (Geometric SD) | Range |
|---|---|---|---|
| TBBP-A | 35% | NR | <30 – 550 |
| ΣHBCDs | 100% | 1020 (2.2) | 360 – 8100 |
| α-HBCD | 100% | 710 (2.2) | 250 – 4430 |
| β-HBCD | 100% | 80 (2.3) | 30 – 1640 |
| γ-HBCD | 100% | 200 (2.4) | 70 – 3200 |
| PBCDs | 42% | NR | <30 – 320 |
| TBCDs | 56% | 50 (3.7) | <30 – 530 |
|
| |||
| Lipid content (g 100 ml−1) | 1.58 (1.65) | 0.57 – 4.69 | |
|
| |||
| Enantiomer Fraction | Mean (SD) | Range | |
|
| |||
| α-HBCD | 0.44 (0.05) | 0.36 – 0.53 | |
| β-HBCD1 | 0.51 (0.02) | 0.47 – 0.54 | |
| γ-HBCD | 0.52 (0.03) | 0.48 – 0.57 | |
Enantiomer fraction determined for 49% of samples.
NR: not reported due to low detection frequency (<50%)
Relationships between Analytes
The HBCD diastereomers in the human milk samples were significantly correlated with one another (α-HBCD to β- and γ-HBCD rs=0.84; β- and γ-HBCD rs=0.75; p<0.001). Milk samples were previously analyzed for major congeners in the penta- and decaBDE mixtures. PentaBDE was not correlated with HBCDs (rs=−0.15, p=0.33). Correlations were not performed for TBBP-A or decaBDE due to low detection frequencies (<50%).
Samples with high concentrations of ΣHBCDs (e.g., greater than median concentrations) were more likely to have detectable levels of the putative metabolites PBCDs or TBCDs compared to those with low levels of ΣHBCDs (OR=12.9; 95% CI=2.82 to 58.6 and OR=20.3; 95% CI=4.18 to 98.2, respectively) (SI Table S6). Similarly, samples with detectable levels of PBCDs were more likely to have detectable levels of TBCDs (OR=43.7; 95% CI=4.86–394). Samples with high levels of ΣHBCDs were more likely to have detectable concentrations of TBBP-A than samples with low levels of ΣHBCDs (OR=4.25; 95% CI=1.08–16.8).
Predictors of Analytes in Human Milk
We used regression analyses to evaluate the relationships between concentrations of the target analytes in human milk and possible predictors of exposure from the questionnaire, including self-reported pre-pregnancy consumption of animal products (meat, fish, eggs, dairy, dairy fat), regularly choosing organic foods, use and possession of home electronics, home carpeting, typical methods of transportation, maternal age, pre-pregnancy body mass index, clinic location and highest completed education (SI Tables S7 and S8).
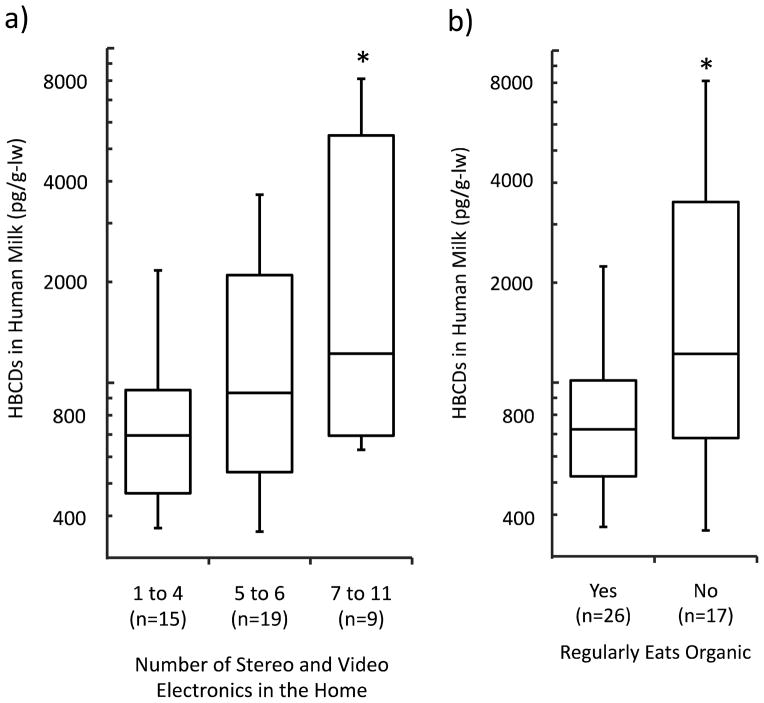
The number of stereo and video electronics (e.g., TVs, CD player, DVD player, stereos, etc.) in the home was positively associated with body burdens of ΣHBCDs (Figure 1a). Linear regression showed that the average concentration of ΣHBCDs in human milk increased 17% (95% CI=4–31%) for every additional stereo or video item in the home (Table 2). This effect was stronger when restricted to stereo and video items other than televisions (22%/item; 95% CI=5–43%). We did not find associations with the number of computers or other small appliances, or reported hours of use.
Figure 1.
a) Mothers who had 7 or more stereo and video electronics in their home (e.g., TVs, CD players, DVD players, stereos, etc.) had higher average levels of ΣHBCDs in their milk than those who had 4 or fewer (153%, 95% CI=34%–375%). b) Mothers who reported regularly choosing organic foods had lower average levels of ΣHBCDs in their milk than those who did not (51%, 95% CI=32%–82%).
Table 2.
Univariate linear regression of ΣHBCDs human milk samples potential predictors from the questionnaire.
| Predictor | Full Dataset
|
College Graduates Only
|
||
|---|---|---|---|---|
| Multiplicative Increase (95% CI) | n | Multiplicative Increase (95% CI) | n | |
| Number of Electronics in the Home | ||||
| Stereo & Video Items | 1.17 (1.04 – 1.31) | 43 | 1.17 (1.03 – 1.34) | 39 |
| Televisions | 1.18 (0.93 – 1.49) | 43 | 1.21 (0.92 – 1.56) | 39 |
| Stereo & Video Items other than Televisions | 1.22 (1.05 – 1.43)* | 43 | 1.19 (1.01 – 1.41)* | 39 |
| Carpeted home | ||||
| No | Reference | 17 | Reference | 15 |
| Yes | 1.39 (0.84 – 2.29) | 26 | 1.35 (0.80 – 2.27) | 24 |
| Regularly uses public transportation | ||||
| No | Reference | 28 | Reference | 26 |
| Yes | 0.71 (0.43 – 1.18) | 15 | 0.67 (0.40 – 1.14) | 13 |
| Regularly eats organic | ||||
| No | Reference | 17 | Reference | 13 |
| Yes | 0.51 (0.32 – 0.82)* | 26 | 0.55 (0.33 – 0.91) | 26 |
| Race | ||||
| Not White | Reference | 5 | Reference | 3 |
| White | 0.51 (0.24 – 1.07) | 38 | 0.62 (0.24 – 1.59) | 36 |
| Highest completed education | ||||
| High school | Reference | 4 | NA | 0 |
| College | 0.50 (0.22 – 1.44) | 39 | NA | 39 |
Statistically significant at the α = 0.05 level.
Coefficients represent the multiplicative increase in breast milk concentrations per item (for continuous variables) or relative to the reference group (for categorical variables).
Mothers who reported they regularly chose organic foods had ΣHBCDs levels in their milk that were 51% (95% CI=32–82%) of those who did not (Figure 1b). We found no association with consumption frequencies of animal products in univariate analyses or while controlling for regularly choosing organic foods (SI Table S8). All mothers who indicated they regularly ate organic foods chose organic vegetables at least some of the time (n=29). Most of these mothers also ate organic meat or dairy at least some of the time (n=25).
Mothers who reported regularly choosing organic foods had, on average, one fewer stereo or video electronic item in the home (p<0.0001). When included together in a multivariate model, effect estimates for these predictors decreased slightly suggesting minimal confounding (Tables 2, 3). Together they explain 22% of the variability in ΣHBCD body burden of Boston mothers (R2=0.22; p=0.006).
Table 3.
Multivariate linear regression for average levels of ΣHBCDs in the analyzed human milk samples.
| Predictor | Full Dataset
|
College Graduates Only
|
|
|---|---|---|---|
| Multiplicative Increase (95% CI) | Multiplicative Increase (95% CI) | ||
| Number of Electronics in the Home | |||
| Stereo & Video Electronics other than Televisions | 1.14/item (0.97 – 1.35) | 1.13/item (0.95 – 1.35) | |
| Regularly eats organic | |||
| No | Reference | Reference | |
| Yes | 0.61 (0.37 – 1.01) | 0.62 (0.37 – 1.06) | |
|
| |||
| R2 | 0.22 (p=0.006)* | 0.18 (p=0.03)* | |
Statistically significant at the α = 0.05 level.
Coefficients represent the multiplicative increase in breast milk concentrations per item (for continuous variables) or relative to the reference group (for categorical variables).
Choice of organic food and numbers of stereo and video electronics in the home may be related to socioeconomic status (SES). As only four mothers had less than a college education, we repeated the regression analyses restricting our sample to mothers with a college education. This did not substantially change effect estimates for the univariate or multivariate models (Tables 2,3).
Although not statistically significant, we found that mothers who reported regularly using public transportation had lower average levels of ΣHBCDs in their milk (0.71; 95% CI=0.43–1.18) and mothers whose homes were carpeted had higher average levels of ΣHBCDs in their milk (1.39; 95% CI=0.84–2.29). There were no significant associations between ΣHBCDs in breast milk and any of the other potential predictors of exposure.
Using logistic regression, we found that the odds of detecting TBBP-A in milk was lower for mothers who reported regularly using public transportation compared to those who did not (OR=0.18, 95% CI=0.03–0.94) (SI Table S7). There were no significant associations between TBBP-A detection in breast milk and any of the other potential predictors of exposure.
DISCUSSION
Analyte Concentrations in Human Milk Samples
We detected TBBP-A in about a third of our human milk samples, whereas HBCDs were detected in all samples and at higher concentrations. The low detection frequency of TBBP-A may be due to preferential partitioning to serum and its relatively short half-life, which reflects recent rather than cumulative exposure. Lower concentrations and detection frequencies of TBBP-A compared to HBCDs were also found in samples of human adipose tissue collected from New York City liposuction patients [19]. This finding is also consistent with human milk from the UK (analyzed by the same laboratory) and France (SI Table S9) [28, 38].
Consistent with observations from around the world, the predominant HBCD diastereomer in our samples was α-HBCD [28, 39–43]. Our median concentration of α-HBCD (620 pg g-lw−1) was similar to that reported previously for Texas, USA (500 pg g-lw−1) and lower than reported for Ontario, Canada (1600 pg g-lw−1) (SI Table S10) [27]. We know of no measurements of other HBCDs in human milk or serum from the USA, and the Canadian study did not report concentrations of β- or γ-HBCD. Our median concentration of ΣHBCD (790 pg g-lw−1) is higher than reported from Sweden (250 pg g-lw−1), similar to reported from Russia, the Philippines and Norway (450 to 860 pg g-lw−1), lower than reported from China, Ghana, Belgium, Canada, Vietnam, Mexico, and the UK (1000 to 3830 pg g-lw−1), and much lower than reported from Spain (27000 pg g-lw−1) [27, 28, 41–50]. Caution should be used when interpreting these types of comparisons, as the populations were not sampled in a manner designed to be representative and analytical methods can differ between studies. However, our results appear generally consistent with lower production of HBCD in the USA compared to Europe and Asia [1].
Enrichment of our milk samples with the (−)-α-HBCD enantiomer (EF average = 0.44) is less than that observed in samples from the UK (EF average = 0.29) [28]. Nevertheless, such enrichment with (−)-α-HBCD may indicate the presence of potential enantioselective processes involved with the absorption, metabolism and/or excretion of HBCDs. This hypothesis is supported by the previous reports of racemic HBCD chiral signatures in indoor dust and diet [31, 51]. Enrichment of both (−)α-HBCD and (+)α-HBCD have been found in various species of fish and predatory birds, suggesting the influence of some enantioselective process [52, 53].
The UK study is the only other known study to report levels of the HBCD degradation products in human milk [28]. The two studies had similar concentrations of TBCDs and both studies detected PBCDs less frequently than TBCDs (SI Tables S11 and S12). Possible explanations for the latter include in vivo biotransformation, intake of dust contaminated with PBCDs and TBCDs, and/or reflect the ratio of impurities in the technical mixture [25, 28].
Relationships Between Analytes
The correlation between HBCD diastereomers in our human milk samples was expected and is consistent with a common source of exposure. The lack of correlation between pentaBDE and ΣHBCDs is consistent with differences in exposures sources, as the major use of pentaBDE was as an additive to polyurethane foam (e.g., couches, chairs).
Our findings support the hypothesis that PBCDs and TBCDs are degradation products of HBCDs and/or trace compounds in the technical mixture. The weak, positive relationship between TBBP-A and HBCDs suggests related sources or routes of exposure, however more research is needed to follow-up on this finding.
Predictors of Analytes in Human Milk
The number of domestic stereo and video electronics was positively associated with HBCD body burdens in our population. A Norwegian study also found a positive, although statistically insignificant, trend between levels of ΣHBCDs in mother’s milk and the number of televisions in the home [46]. In our population, the association was stronger for stereo and video electronics other than televisions (e.g., CD players, DVD players, stereos, etc.). These findings are consistent with reported use of HBCDs in the plastic housing of consumer electronics, its identification in indoor dust, and the positive correlation between levels in dust and serum [31, 51, 54]. To our knowledge it is not known if HBCD is used to a greater extent in other stereo and video electronics compared to televisions. Previous work in the USA identified decaBDE in televisions [55], suggesting this may be true. However, a study from the UK found increasing concentrations of HBCD in dust with decreasing distance from a television set [51].
Mothers who reported regularly choosing organic foods had lower levels of ΣHBCDs in their milk than those who did not. This association is likely not due to differences in consumption of animal products as, similar to a Belgian duplicate diet study and a Norwegian dietary questionnaire study, we failed to find associations between consumption of meat, fish or dairy and ΣHBCDs in human milk [31, 46]. It is possible that organic foods consumed by mothers in our study contained lower levels of HBCD than non-organic foods. No known data exist for levels of HBCD in organic vs. non-organic foods and we are aware of no studies reporting levels of HBCD in US foods during our study period (2004–2005). A 2009 market basket survey conducted in Dallas, Texas identified HBCD in fish, red meat, poultry and peanut butter but not dairy, eggs, potatoes or apples [56]. The study did not report whether any of the food items were certified USDA Organic.
Alternatively, the associations we found with organic food choice and numbers of electronics may be confounded by unmeasured lifestyle choices and behaviors. We attempted to control for SES by restricting our analysis to college-educated mothers. While this reduced the observed associations only slightly (Tables 2, 3), there remains the potential for residual uncontrolled confounding by SES within this group.
Although not statistically significant, mothers who reported regularly using public transportation had lower average levels of ΣHBCDs in their milk. While these mothers also spent less time in a personal vehicle per week, we did not find an association between this predictor and HBCD body burden. Possible explanations include exposure misclassification, which tends to bias results towards the null, or confounding by an unmeasured lifestyle factor. HBCDs have been detected in car dust at levels in the UK that exceed significantly those in home and office dust [57]. We are not aware of reported levels for other modes of transportation (e.g., trains, buses).
As study participants were all first-time mothers, our findings may not be generalizable to the overall population. For example, women at this stage of their lives may make different food selections. However, these women represent a population of particular importance, as these measurements are reflective of their children’s exposures during a developmentally sensitive period. Daily intake estimates for infants (1 to <3 months) are presented in SI Table S13 for ΣHBCDs (GM=5.7 ng kg-day−1) and TBBP-A (range: <0.17 to 4.2 ng kg-day−1). As human milk is an ideal food for infants our findings do not negate the healthfulness and importance of breastfeeding, rather highlight the importance of considering infant exposure in the management of organic pollutants such as TBBP-A and HBCDs.
Strengths of our study include the measurement of less studied xenobiotics in a potentially vulnerable population and the use of a detailed exposure questionnaire. Limitations include a relatively small sample size as well as a lack of measurements of the target compounds in dust, electronics and various types of food. Self-reporting of ‘regularly eating organic foods’ is imprecise and may introduce some bias. Absence of a more sensitive measure of SES may mean there is residual confounding. As we examined many potential associations, it is possible that some findings are due to chance. Future research on human exposure to TBBP-A and HBCD should measure these analytes in dust (and possibly air) of participant microenvironments (home, workplace, vehicle) at the same time as collecting detailed measures of socioeconomic status and related lifestyle factors. Additional research is needed on HBCDs in organic vs. non-organic food.
In summary, we found TBBP-A and HBCDs in the milk of first-time mothers from the Boston, MA area during 2004–2005 at concentrations within the range of those measured in several other countries. Our results suggest that body burdens of these BFRs are related to lifestyle factors, potentially including diet and domestic electronics. Further research is needed to examine these findings and monitor future trends.
Supplementary Material
Acknowledgments
We thank the study participants. This research was supported in part by grants R01ES015829 and T32ES014562 from the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences.
Footnotes
SUPPORTING INFORMATION AVAILABLE. Tables S1–S13 and Figure S1 are available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Courtney C. Carignan, Email: cwalker@bu.edu.
Mohamed Abou-Elwafa Abdallah, Email: mae_abdallah@yahoo.co.uk.
Nerissa Wu, Email: nerissa.wu@cdph.ca.gov.
Wendy Heiger-Bernays, Email: whb@bu.edu.
Michael D. McClean, Email: mmcclean@bu.edu.
Stuart Harrad, Email: s.j.harrad@bham.ac.uk.
Thomas F. Webster, Email: twebster@bu.edu.
References
- 1.Law RJ, Allchin CR, de Boer J, Covaci A, Herzke D, Lepom P, Morris S, Tronczynski J, de Wit CA. Levels and trends of brominated flame retardants in the European environment. Chemosphere. 2006;64(2):187–208. doi: 10.1016/j.chemosphere.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 2.European Risk Assessment Report: 2,2′,6,6′-tetrabromo-4,4′-isopropylidenediphenol (tetrabromobisphenol-A or TBBPA-A), Part II, Human health. European Chemicals Bureau, Institute for Health and Consumer Protection; 2006. www.bsef.com/uploads/library/final_tbbpa_human_health_report.pdf. [Google Scholar]
- 3.Law RJ, Herzke D, Harrad S, Morris S, Bersuder P, Allchin CR. Levels and trends of HBCD and BDEs in the European and Asian environments, with some information for other BFRs. Chemosphere. 2008;73(2):223–241. doi: 10.1016/j.chemosphere.2008.02.066. [DOI] [PubMed] [Google Scholar]
- 4.Covaci A, Voorspoels S, Abdallah MA, Geens T, Harrad S, Law RJ. Analytical and environmental aspects of the flame retardant tetrabromobisphenol-A and its derivatives. J Chromatogr A. 2009;1216(3):346–63. doi: 10.1016/j.chroma.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 5.Harrad S, Abdallah MA-E, Rose NL, Turner SD, Davidson TA. Current-use brominated flame retardants in water, sediment, and fish from English lakes. Environ Sci Technol. 2009;43(24):9077–9083. doi: 10.1021/es902185u. [DOI] [PubMed] [Google Scholar]
- 6.Exisiting Chemicals Action Plan. U.S. Environmental Protection Agency; 2012. http://www.epa.gov/oppt/existingchemicals/pubs/ecactionpln.html. [Google Scholar]
- 7.Stockholm Convension on Persistant Organic Pollutants (POPs): Newly proposed chemicals. 2012 http://chm.pops.int.
- 8.Leisewitz A, Kruse H, Schramm E. Results and Summary Overview. Research Report 297 44 542. Vol. 1. German Federal Environmental Agency (Umweltbundesamt); 2000. Substituting environmentally relevant flame retardants: assessment fundamentals. [Google Scholar]
- 9.Bromine Science and Environmental Forum. 2012 http://www.bsef.com/our-substances/hbcd/applications-3.
- 10.Hagmar L, Sjodin A, Haglund P, Thuresson K, Rylander L, Bergman A. Biological half-lives of polybrominated diphenyl ethers and tetrabromobisphenol A in exposed workers. Organohalogen Compd. 2000;47:198–201. [Google Scholar]
- 11.Meerts IATM, van Zanden JJ, Luijks EAC, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56(1):95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- 12.Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A, Perdu E, Zalko D, Bourguet W, Balaguer P. Peroxisome proliferator-activated receptor γ Is a target for halogenated analogs of bisphenol A. Environ Health Perspect. 2011;119(9):1227–1232. doi: 10.1289/ehp.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MHA, Andersson PL, Legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92(1):157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura S, Jinno N, Ohta S, Kuroki H, Fujimoto N. Thyroid hormonal activity of the flame retardants tetrabromobisphenol A and tetrachlorobisphenol A. Biochem Biophys Res Commun. 2002;293(1):554–559. doi: 10.1016/S0006-291X(02)00262-0. [DOI] [PubMed] [Google Scholar]
- 15.Ghisari M, Bonefeld-Jorgensen EC. Impact of environmental chemicals on the thyroid hormone function in pituitary rat GH3 cells. Mol Cell Endocrinol. 2005;244(1–2):31–41. doi: 10.1016/j.mce.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Pullen S, Boecker R, Tiegs G. The flame retardants tetrabromobisphenol A and tetrabromobisphenol A-bisallylether suppress the induction of interleukin-2 receptor alpha chain (CD25) in murine splenocytes. Toxicology. 2003;184(1):11–22. doi: 10.1016/s0300-483x(02)00442-0. [DOI] [PubMed] [Google Scholar]
- 17.Mariussen E, Fonnum F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochem Int. 2003;43(4–5):533–542. doi: 10.1016/s0197-0186(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 18.Saegusa Y, Fujimoto H, Woo GH, Inoue K, Takahashi M, Mitsumori K, Hirose M, Nishikawa A, Shibutani M. Developmental toxicity of brominated flame retardants, tetrabromobisphenol A and 1,2,5,6,9,10-hexabromocyclododecane, in rat offspring after maternal exposure from mid-gestation through lactation. Reprod Toxicol. 2009;28(4):456–467. doi: 10.1016/j.reprotox.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Johnson-Restrepo B, Adams DH, Kannan K. Tetrabromobisphenol A (TBBPA) and hexabromocyclododecanes (HBCDs) in tissues of humans, dolphins, and sharks from the United States. Chemosphere. 2008;70(11):1935–1944. doi: 10.1016/j.chemosphere.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Covaci A, Gerecke AC, Law RJ, Voorspoels S, Kohler M, Heeb NV, Leslie H, Allchin CR, de Boer J. Hexabromocyclododecanes (HBCDs) in the environment and humans: a review. Environ Sci Technol. 2006;40(12):3679–3688. doi: 10.1021/es0602492. [DOI] [PubMed] [Google Scholar]
- 21.Szabo DT, Diliberto JJ, Hakk H, Huwe JK, Birnbaum LS. Toxicokinetics of the flame retardant hexabromocyclododecane gamma: Effect of dose, timing, route, repeated exposure, and metabolism. Toxicol Sci. 2011;117(2):282–293. doi: 10.1093/toxsci/kfq183. [DOI] [PubMed] [Google Scholar]
- 22.Geyer HJ, Schramm K-W, Darnerud PO, Aune M, Feicht E, Fried KW, Henkelmann B, Lenoir D, Schmid P, McDonald TA. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compd. 2004;66:3820–3825. [Google Scholar]
- 23.Ema M, Fujii S, Hirata-Koizumi M, Matsumoto M. Two-generation reproductive toxicity study of the flame retardant hexabromocyclododecane in rats. Reprod Toxicol. 2008;25(3):335–351. doi: 10.1016/j.reprotox.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson P, Fischer C, Wallin M, Jakobsson E, Fredriksson A. Impaired behaviour, learning and memory, in adult mice neonatally exposed to hexabromocyclododecane (HBCDD) Environ Toxicol Pharmacol. 2006;21(3):317–322. doi: 10.1016/j.etap.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Brandsma SH, van der Ven LTM, de Boer J, Leonards PEG. Identification of hydroxylated metabolites of hexabromocyclododecane in wildlife and 28-days exposed Wistar rats. Environ Sci Technol. 2009;43(15):6058–6063. doi: 10.1021/es900879k. [DOI] [PubMed] [Google Scholar]
- 26.Szymanska J, Spta A, Frydrych B. The deposition and metabolism of tetrabromobisphenol-A after a single i.p dose in the rat. Chemosphere. 2001;45:693–700. doi: 10.1016/s0045-6535(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 27.Ryan JJ, Wainman BC, Schecter A, Moisey J, Kosarac I, Sun WF. Trends of the brominated flame retardants, PBDEs and HBCD, in human milks from North America. Organohalogen Compd. 2006;68:778–781. [Google Scholar]
- 28.Abdallah MA, Harrad S. Tetrabromobisphenol-A, hexabromocyclododecane and its degradation products in UK human milk: Relationship to external exposure. Environ Int. 2011;37(2):443–448. doi: 10.1016/j.envint.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Weiss J, Meijer L, Sauer P, Linderholm L, Athanasiadis I, Bergman A. PBDE and HBCDD levels in blood from Dutch mothers and infants - analysis of a Dutch Groningen infant cohort. Organohalogen Compd. 2004;66:2677. [Google Scholar]
- 30.Hagmar L, Jakobsson K, Thuresson K, Rylander L, Sjodin A, Bergman A. Computer technicians are occupationally exposed to polybrominated diphenyl ethers and tetrabromobisphenol A. Organohalogen Compd. 2000;47:202–205. [Google Scholar]
- 31.Roosens L, Abdallah MAE, Harrad S, Neels H, Covaci A. Exposure to hexabromocyclododecanes (HBCDs) via dust ingestion, but not diet, correlates with concentrations in human serum: preliminary results. Environ Health Perspect. 2009;117(11):1707–1712. doi: 10.1289/ehp.0900869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38(4):945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 33.Law RJ. Tetrabromobisphenol A: Investigating the worst-case scenario. Mar Pollut Bull. 2009;58(4):459–460. doi: 10.1016/j.marpolbul.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 34.Wu N, Herrmann T, Paepke O, Tickner J, Hale R, Harvey E, La Guardia M, McClean MD, Webster TF. Human exposure to PBDEs: associations of PBDE body burdens with food consumption and house dust concentrations. Environ Sci Technol. 2007;41(5):1584–1589. doi: 10.1021/es0620282. [DOI] [PubMed] [Google Scholar]
- 35.Environmental Health Series Report #34. World Health Organization: Regional Office for Europe; 1989. Levels of PCBs, PCDDs, and PCDFs in breast milk: Second round of quality control studies. [Google Scholar]
- 36.Keller JM, Stapleton HM, Heltsley R, Peck A, Kucklick JR, Schantz M, Wise SA. SRMs available from NIST for the analysis of brominated flame retardants. Poster presented at BFR2007; Amsterdam, The Netherlands. 2007. [Google Scholar]
- 37.Exposure Factors Handbook: 2011 Edition. U.S. Environmental Protection Agency, National Center for Environmental Assessment, Office of Research and Development; Washington, D.C: 2011. http://www.epa.gov/ncea/efh/report.html. [Google Scholar]
- 38.Cariou R, Antignac JP, Zalko D, Berrebi A, Cravedi JP, Maume D, Marchand P, Monteau F, Riu A, Andre F, Le bizec B. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: Occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere. 2008;73(7):1036–1041. doi: 10.1016/j.chemosphere.2008.07.084. [DOI] [PubMed] [Google Scholar]
- 39.Kakimoto K, Akutsu K, Konishi Y, Tanaka Y. Time trend of hexabromocyclododecane in the breast milk of Japanese women. Chemosphere. 2008;71(6):1110–1114. doi: 10.1016/j.chemosphere.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 40.Antignac J-P, Cariou R, Daniel M, Philippe M, Fabrice M, Daniel Z, Alain B, Jean-Pierre C, Francois A, Bizec BL. Exposure assessment of fetus and newborn to brominated flame retardants in France: preliminary data. Mol Nutr Food Res. 2008;52(2):258–265. doi: 10.1002/mnfr.200700077. [DOI] [PubMed] [Google Scholar]
- 41.Polder A, Gabrielsen GW, Odland JO, Savinova TN, Tkachev A, Loken KB, Skaare JU. Spatial and temporal changes of chlorinated pesticides, PCBs, dioxins (PCDDs/PCDFs) and brominated flame retardants in human breast milk from Northern Russia. Sci Total Environ. 2008;391(1):41–54. doi: 10.1016/j.scitotenv.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 42.Shi Z-X, Wu Y-N, Li J-G, Zhao Y-F, Feng J-F. Dietary exposure assessment of Chinese adults and nursing infants to tetrabromobisphenol-A and hexabromocyclododecanes: Occurrence measurements in foods and human milk. Environ Sci Technol. 2009;43(12):4314–4319. doi: 10.1021/es8035626. [DOI] [PubMed] [Google Scholar]
- 43.Eljarrat E, Guerra P, Martinez E, Farre M, Alvarez JG, Lopez-Teijon M, Barcelo D. Hexabromocyclododecane in human breast milk: Levels and enantiomeric patterns. Environ Sci Technol. 2009;43(6):1940–1946. doi: 10.1021/es802919e. [DOI] [PubMed] [Google Scholar]
- 44.Lignell S, Aune M, Darnerud PO, Glynn A. Report to the Swedish Environmental Protection Agency. 2008. Brominated flame retardants in mother’s milk from primiparae women in Uppsala County, Sweden - updated temportal trends 1996–2006. [Google Scholar]
- 45.Malarvannan G, Kunisue T, Isobe T, Sudaryanto A, Takahashi S, Prudente M, Subramanian A, Tanabe S. Organohalogen compounds in human breast milk from mothers living in Payatas and Malate, the Philippines: Levels, accumulation kinetics and infant health risk. Environ Pollut. 2009;157(6):1924–1932. doi: 10.1016/j.envpol.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 46.Thomsen C, Stigum H, Froshaug M, Broadwell SL, Becher G, Eggesbo M. Determinants of brominated flame retardants in breast milk from a large scale Norwegian study. Environ Int. 2010;36(1):68–74. doi: 10.1016/j.envint.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Asante KA, Adu-Kumi S, Nakahiro K, Takahashi S, Isobe T, Sudaryanto A, Devanathan G, Clarke E, Ansa-Asare OD, Dapaah-Siakwan S, Tanabe S. Human exposure to PCBs, PBDEs and HBCDs in Ghana: Temporal variation, sources of exposure and estimation of daily intakes by infants. Environ Int. 2011;37(5):921–928. doi: 10.1016/j.envint.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Colles A, Koppen G, Hanot V, Nelen V, Dewolf MC, Noel E, Malisch R, Kotz A, Kypke K, Biot P, Vinkx C, Schoeters G. Fourth WHO-coordinated survey of human milk for persistent organic pollutants (POPs): Belgian results. Chemosphere. 2008;73(6):907–914. doi: 10.1016/j.chemosphere.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Tue NM, Sudaryanto A, Tu BM, Isobe T, Takahashi S, Pham HV, Tanabe S. Accumulation of polychlorinated biphenyls and brominated flame retardants in breast milk from women living in Vietnamese e-waste recycling sites. Sci Total Environ. 2010;408(9):2155–2162. doi: 10.1016/j.scitotenv.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Lopez D, Athanasiadou M, Athanasiadis I, Yanezestrada L, Diaz-Barriga F, Bergman A. Third annual workshop on brominated flame retardants; Toronto, Canada. 2004. pp. 482–487. [Google Scholar]
- 51.Harrad S, Abdallah MAE, Covaci A. Causes of variability in concentrations and diastereomer patterns of hexabromocyclododecanes in indoor dust. Environ Int. 2009;35(3):573–579. doi: 10.1016/j.envint.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Janak K, Covaci A, Voorspoels S, Becher G. Hexabromocyclododecane in marine species from the Western Scheldt Estuary: Diastereoisomer- and enantiomer-specific accumulation. Environ Sci Technol. 2005;39(7):1987–1994. doi: 10.1021/es0484909. [DOI] [PubMed] [Google Scholar]
- 53.Janak K, Sellstrom U, Johansson AK, Becher G, de Wit CA, Lindberg P, Helander B. Enantiomer-specific accumulation of hexabromocyclododecanes in eggs of predatory birds. Chemosphere. 2008;73(1):S193–S200. doi: 10.1016/j.chemosphere.2007.03.077. [DOI] [PubMed] [Google Scholar]
- 54.Abdallah MAE, Harrad S, Ibarra C, Diamond M, Melymuk L, Robson M, Covaci A. Hexabromocyclododecanes in indoor dust from Canada, the United Kingdom, and-the United States. Environ Sci Technol. 2008;42(2):459–464. doi: 10.1021/es702378t. [DOI] [PubMed] [Google Scholar]
- 55.Allen JG, McClean MD, Stapleton HM, Webster TF. Linking PBDEs in house dust to consumer products using x-ray fluorescence. Environ Sci Technol. 2008;42(11):4222–4228. doi: 10.1021/es702964a. [DOI] [PubMed] [Google Scholar]
- 56.Schecter A, Haffner D, Colacino J, Patel K, Papke O, Opel M, Birnbaum L. Polybrominated diphenyl ethers (PBDEs) and hexabromocyclodecane (HBCD) in composite U.S. food samples. Environ Health Perspect. 2010;118(3):357–362. doi: 10.1289/ehp.0901345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abdallah MAE, Harrad S, Covaci A. Hexabromocyclododecanes and tetrabromobisphenol-A in indoor air and dust in Birmingham, UK: Implications for human exposure. Environ Sci Technol. 2008;42(18):6855–6861. doi: 10.1021/es801110a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.