Abstract
Researchers continue to question fathers’ willingness to report their biological children in surveys and the ability of surveys to adequately represent fathers. To address these concerns, this study evaluates the quality of men’s fertility data in the 1979 and 1997 cohorts of the National Longitudinal Survey of Youth (NLSY79 and NLSY97) and in the 2002 National Survey of Family Growth (NSFG). Comparing fertility rates in each survey to population rates based on data from the Vital Statistics and the U.S. Census Bureau, we document how the incomplete reporting of births in different surveys varies according to men’s characteristics, including their age, race, marital status, and birth cohort. In addition, we use Monte Carlo simulations based on the NSFG data to demonstrate how birth underreporting biases associations between early parenthood and its antecedents. We found that in the NSFG, roughly four out of five early births were reported, but in the NLSY79 and NLSY9, almost nine-tenths of early births were reported. In all three surveys, incomplete reporting was especially pronounced for nonmarital births. Our results suggest that the quality of male fertility data is strongly linked to survey design and that it has implications for models of early male fertility.
Concerns about the quality of male fertility data have a long history. For instance, Cherlin, Griffith, and McCarthy (1983) found that compared to women, men reported fewer children from prior marriages in both the 1970 and 1980 Current Population Surveys (CPS). This finding led the CPS to discontinue its collection of fertility histories from men (Byrne 1997). Researchers focusing on nonresident fathers in other surveys have similarly documented deficits in men’s reports of births from previous unions (Garfinkel, McLanahan, and Hanson 1998; Seltzer and Brandeth 1994; Sorensen 1997). These concerns raise serious questions about our ability to study the causes and consequences of male fertility.
Because of the importance of research on fathers in today’s policy debates, in the mid 1990s, the Federal Interagency Forum on Child and Family Statistics established working groups to develop recommendations for improving data and research on male fertility and fathering (Federal Interagency Forum on Child and Family Statistics 1998). This task force emphasized the need to include men in Cycle 6 of the National Survey of Family Growth (NSFG) and informed the collection of information on fathers in several recent surveys, including the 2002 NSFG and the 1997 cohort of the National Longitudinal Survey of Youth (NLSY97). These data sets are widely used today to study topics such as men’s transition to fatherhood (e.g., Hynes et al. 2008) and multi-partner fertility (e.g., Guzzo and Furstenberg 2007; Manlove et al. 2008).
This study evaluates the quality of men’s fertility data in the 1979 and 1997 cohorts of the NLSY and in the 2002 NSFG. As we discuss later, these three surveys differ in ways that allow us to identify aspects of surveys that improve data quality. We focus on early male fertility (fertility before the age of 25) because the majority of births in these early years are nonmarital (Morgan and Rindfuss 1999), and nonmarital births are less likely to be reported (Rendall et al. 1999). In addition, respondents in the NLSY97 are still young, making it impossible to study fertility rates for older men in this survey. We estimate fertility rates for men in the three surveys and compare them to population estimates of male fertility rates that we create by combining data from Vital Statistics and the U.S. Census Bureau. Calculating the ratio of survey rates to population rates, we document how the incomplete reporting of births to men in surveys differs according to men’s characteristics, including their age, race, marital status, and birth cohort. In addition, we use Monte Carlo simulations to demonstrate how incomplete reporting biases associations between early parenthood and other variables.
BACKGROUND
Previous studies have assessed male fertility data in surveys through either internal or external comparisons. For instance, studies have examined the consistency of men’s fertility reports across different waves of a given longitudinal survey based on within-person analyses (Boggess et al. 2007; Mott, Hurst, and Gryn 2007); they have compared men’s and women’s reports of births from a single survey (Sorensen 1997; Garfinkel, McLanahan, and Hanson 1998; Rendall et al. 1999); and they have obtained an external source of data to evaluate a particular survey (Lindberg et al. 1998; Martinez et al. 2006). We focus our review below on studies that have relied on women’s reports of births in some way (using both internal and external comparisons) to evaluate the quality of male fertility data, since our own study relies on women’s reports of fathers’ ages on birth certificates. We organize this review chronologically to highlight how the quality of male fertility data in surveys is linked to changes in survey design that have occurred over time.
Rendall and colleagues (1999) extended the within-survey methods used by Sorensen (1997) and Garfinkel, McLanahan, and Hanson (1998) in evaluating men’s birth reports in the British Household Panel Survey (BHPS) and the Panel Study of Income Dynamics (PSID). Specifically, they compared the number of births reported by men and women in the period from 1968 to 1991. They also compared men’s and women’s number of reported births during periods in which they were single versus married and further distinguished marital births by whether they were from dissolved versus current marriages at the time of survey. They found that one-third to one-half of men’s nonmarital births and births from previous marriages were missed. For the PSID, they were able to distinguish between men’s births reported retrospectively in 1985 and reports in each panel from 1986 to 1991 and found much lower underreporting for the panel-reporting years than for the retrospective-reporting years. These findings suggest that men’s willingness or ability to report births depends on their relationship with the mother at the time of birth, its subsequent stability, and the survey design (retrospective versus panel). No analysis of differences in this reporting by age of father was possible, however, as the women’s fertility reports in the PSID and BHPS did not include the father’s age.
A problem inherent to the within-survey comparison method explored in some detail by Rendall et al. (1999) is its vulnerability to survey underrepresentation of men or overrepresentation of women that is not adequately compensated for by the survey’s sample weights. The problem of differential representativeness, as well as the problem of survey sampling error, increases with the specificity of the group of men whose reports of fertility are being evaluated. This makes comparison of men’s survey reports to an external standard from population or large-scale survey data an attractive alternate method of evaluation.
The National Maternal and Infant Health Survey (NMIHS) asked a large representative sample of women with live births during the year of 1988 to report the age of the father of their child, allowing researchers to estimate the number of births to men of different ages during that calendar year. Lindberg and colleagues (1998) combined NMIHS information on fathers’ ages with data from the U.S. Census Bureau to estimate nonmarital fertility rates for teen men in the population. They used the NMIHS estimates to externally evaluate young men’s fertility reports from the 1988 and 1991 waves of the National Survey of Adolescent Males (NSAM). For the calendar year of 1988, they estimated a birth rate of 16.4 per 1000 unmarried teen men based on the NMIHS and 14.1 based on young men’s self-reports in the NSAM. The ratio of these rates indicates that as many as 87.8% of unmarried teen births were reported by men in the NSAM.
The more complete reports found in NSAM could reflect the fact that the interval between the birth and the interview in which fertility histories were first collected was considerably shorter than in other surveys. NSAM’s more complete reports could also reflect its innovative approach to collecting information about fertility. Respondents were first asked to recall various sexual partners and then asked whether they had children with each partner. Collecting fertility data within the context of relationship histories is intended to improve recall and appears to have been effective (Lindberg et al. 1998).
Cycle 6 (2002) of the NSFG implemented NSAM’s method of collecting information on male fertility within the context of relationship histories. But while both NSAM and the NSFG collected information about births within the contexts of relationships, NSAM obtained more complete reporting of births to teen men than did the NSFG. As part of a broader report on reproductive behavior based on the 2002 NSFG, Martinez et al. (2006:34) compared the weighted number of births reported by men in the NSFG to those estimated from Vital Statistics data from birth certificates. Their comparisons were limited to five calendar years of the birth (1997 to 2001) and to specific age groups of men (15 to 19; 20 to 24; 25 to 29; and 30 to 44). The only statistically significant difference found between the NSFG and Vital Statistics estimates was in the number of births to men at aged 15 to 19; the ratio of the NSFG estimate to the Vital Statistics birth estimate was 0.68, suggesting about a third of NSFG teen births were unreported. Their analysis, however, did not distinguish births according to the race or marital status of the parents. Especially important for the issue of examining retrospective versus panel collection, their comparisons extended only as far as five years before the interview.
While both NSAM and the NSFG collected information about births within the contexts of relationships, NSAM obtained more complete reporting of births to teen men than did the NSFG. However, the assessments of male fertility data in these two surveys differ in some important respects. For instance, Lindberg and colleagues (1998) relied on two waves of data to construct teen fertility rates.
By using parallel measures of male fertility across different surveys, a single method for estimating male fertility in the population, and analogous subgroups, we can make explicit comparisons of the quality of data across surveys. These comparisons allow us to identify aspects of surveys that improve the quality of male fertility data. Furthermore, these comparisons offer us indicators of data quality in the NLSY79 and NLSY97, data sets that are widely used but have not been evaluated against an external standard.
We expect the quality of male fertility data to be higher in the NLSY79 and NLSY97 than in the NSFG. Due to the cross-sectional design of the NSFG, the period of recall between the birth and survey administration for most men was considerably longer than in NLSY79 and NLSY97; respondents in these longitudinal surveys were interviewed yearly beginning in adolescence or young adulthood with fertility histories that were updated at each interview. The exclusion of certain groups of men from the household-based sampling frame of the NSFG may further compromise the quality of male fertility data. For instance, black men are disproportionately excluded from household-based surveys because of their higher rates of incarceration and military enlistment (Hernandez and Brandon 2002). In the NSFG, respondents were not interviewed if they were in jail or prison or if they were on a military base. In contrast, the NLSY79 and NLSY97 began with representative samples of noninstitutionalized civilian youth, and they followed respondents who subsequently became incarcerated or joined the military. The weights provided by all three surveys to adjust for nonresponse simply take into account sex, race, and age, but not the likelihood of being incarcerated or in the military.
Even a survey with adequate representation of men and closely-spaced interviews would have incomplete reporting of men’s births if some men do not know about the birth of their children. The Fragile Families and Child Wellbeing Study offers clues about men’s awareness of their paternity. For example, Fragile Families asked unmarried mothers about their relationship with the father at the time of the birth. Only 1% of women could not identify the father of the birth, and another 9% had little or no contact with the father; the remainder of women reported they were in a friendship, marriage, or cohabiting relationship at the time of the birth (Carlson, McLanahan, England, and Devaney 2005). If we take an extreme position and assume mothers with little or no contact with the biological father at the time of the birth did not ever tell the father about the pregnancy, these results suggest that, at most, 10% of biological fathers may not be aware that they have a child. It is likely that some of these mothers did tell the fathers about the pregnancy, bringing the true share below 10%.
DATA AND METHOD
In order to evaluate the quality of male fertility data, we compare age-specific fertility rates (ASFRs) in surveys to population rates based on data from the Vital Statistics and the U.S. Census Bureau. These rates are calculated similarly to those for women in the population (e.g., Hinde 1998; Morgan, Botev, Chen, and Huang 1999; Preston et al. 2001). We calculate the fertility rate at age x (Fx) for men as follows:
where Bx is the number of births to men age x, and Px is the number of men age x in the population. We calculate ASFRs that correspond to either periods or birth cohorts, depending on the focal survey, as we explain later. We estimate rates for all men and for different subgroups defined by race and marital status. We use birth certificate and population data that extend from 1972 to 2006 in order to cover the years that respondents in the surveys were at risk of having a birth.
While the calculation of ASFRs is relatively straightforward, generating reliable estimates of Bx and Px from the survey data and from the population data requires some assumptions. We first describe our methods for calculating the ASFRs from these data sources. We then discuss our assumptions and potential biases they may introduce.
Number of Births in Population or Bx
Birth certificate data include the race, age, and marital status of the mother, allowing us to estimate the number of births to women by these characteristics.1 Estimating the number of births to men of different ages in a given year is more complicated because in a substantial number of cases (e.g., one out of every six births in 1988), the age of the father is not reported on birth certificates (Landry and Forrest 1995). This is more likely to be true when the birth is to a young or unmarried mother; however, prior research indicates that it is possible to reliably impute this missing data. The NMIHS not only asked mothers about the ages of the fathers of their children, but also included information from their birth certificates. Using these data, Elo and colleagues (1999) found evidence that there was no systematic bias in the underreporting of fathers’ ages on birth certificates. Conditional on mothers’ age, the distribution of fathers’ ages based on mothers’ reports in the NMIHS interview did not differ by whether or not the mothers had reported the fathers’ age on the birth certificates. Therefore, imputation has been used routinely in population-level reports about male fertility, with researchers assuming that fathers’ ages are missing at random within five-year age groups among mothers of different racial groups (NCHS 2004).
We use multivariate imputation by chained equations (Allison 2001) to impute missing fathers’ ages for each calendar year of risk based on other information on the birth certificate: age of the mother, race of the mother, education of the mother, and race of the father. Typically, mothers who fail to report the age of fathers also fail to report their race. To impute the number of births to men of different racial groups, we assume that the race of the father, when missing, is the same as that of the mother.2
Number of Men in Population or Px
For our population estimates, we need counts of the total number of men of a given age in the population by race and by marital status for each year from 1972 to 2006. We use the U.S. Census intercensal population counts to calculate the denominator for overall and race-specific fertility rates (U.S. Census Bureau 2007, 2009). We use estimates for the resident population plus Armed Forces overseas to capture any men who may be at risk of fathering children born in the U.S. in any given year. Because the Census did not include a question on Hispanic origin prior to 1980, our measures of race simply distinguish the black and white populations. Hispanics may be included in either of these categories but are much more prevalent in the “white” group. This is likely to influence comparisons, especially between the NLSY79 and NLSY97, as Hispanics accounted for a larger share of “whites” in the period covered by the NLSY97. The Hispanic proportion of all births in the U.S. has risen rapidly over this period to 1 in 4 in 2006 (Hamilton, Martin, and Ventura 2007), and nonmarital fertility is higher among Hispanic than non-Hispanic whites. A relatively high proportion of nonmarital Hispanic births, however, are within cohabiting unions (Wildsmith and Raley 2006), and this can be expected to mitigate underreporting of nonmarital births.
Following the National Center for Health Statistics’ procedures used for women, we compute marital-specific estimates of the number of men in the population by applying marital rates from the Current Population Survey (CPS) to our Census estimates (NCHS 2004; King et al. 2009). For instance, to estimate the number of married black men aged 18 in 2002, we multiply the proportion that is married (using three-year moving averages from the CPS) by the number of men in this group (from the Census data).3
Age-Specific Fertility Rates in Surveys
Calculating fertility rates for the surveys similarly requires the number of births in the numerator and the number of men or women at risk in the denominator. For each survey, we create files of person-years lived by respondents to calculate fertility rates at each age from 15 to 24. The denominator includes the weighted number of respondents who contribute observations to a given age, and the numerator includes the weighted number of births to these respondents. In some analyses, we stratify age-specific fertility rates by race and marital status, using measures comparable to those used for the population. For instance, we estimate a nonmarital birth rate at the age of 18 by including the weighted number of single births to men at this age as the numerator and the weighted number of men who are single in the middle of this age interval in the denominator.
Surveys and Samples
We use data from the NLSY79, the NLSY97, and the 2002 NSFG. All three data sets include substantial minority over-samples that facilitate the estimation of race-specific fertility rates; sampling weights for all three surveys adjust statistics so that samples are representative of their sampling frames.
The NLSY79 is a longitudinal survey that began with a representative sample of 14 to 21-year-old men and women in 1979. Respondents were interviewed annually until 1996 and biennially since that time. Our analysis is based on data from the 1979–1995 interviews, allowing us to create person-years files for all respondents that extend through age 25. Although the initial sample represents the civilian, noninstitutionalized population, the NLSY79 follows respondents if they become incarcerated or join the military, whenever possible.
Both men and women in the NLSY79 were asked questions about their fertility behavior in each of the interviews. At the first interview, respondents were asked, “How many children, altogether, have (you ever given birth to/you ever had) at any time, not counting babies that were dead at birth?” Respondents who reported having any children were asked the dates of birth for each child. In subsequent rounds, the surveys alternated between updating from the previous survey (1980–1981; 1983–1994) and having respondents re-list any children they had ever had (1982; 1983 if missed the 1982 interview). The NLSY79 provides constructed fertility variables that incorporate several years of cleaning and editing (Center for Human Resource Research 2006).
Mott and colleagues (2007) checked the consistency in reports of fatherhood across waves of the 1979 NLSY cohort using the fertility reports and information from the household roster. They have created a set of public-use variables that indicate their level of confidence that the reported child is a biological child of the male respondent. In their analysis, men were more likely to over report fatherhood than to under report it, but this result is perhaps not surprising given that the fertility questions (‘how many children have you ever had’) are somewhat ambiguous about biological fatherhood. Based on Mott et al.’s variables, we have dropped births from men’s fertility records if the paternity confidence code indicates it is “reasonably” and “virtually” certain that the child is not a biological child of the respondent; however, we discuss results based on an alternative set of NLSY79 constructed variables that do not incorporate the changes Mott and colleagues made.
The NLSY79 also includes detailed information about each respondent’s marital history, including start and end dates of marriages and a measure of current marital status at each interview. The marital context of each birth is identified indirectly by examining respondents’ marital status in the month the child was born.
The NLSY97 began collecting data in 1997 from a household sample of respondents aged 12 to 16 and has interviewed them yearly since that time. At the first interview, NLSY97 respondents were asked if they had ever given birth to or fathered any children, and if applicable, the birth dates of each child. Subsequent interviews collected information on any children born since the previous interview. Note that the language about fathering children in the NLSY97 is less ambiguous than that for the 1979 cohort (‘ever fathered’ versus ‘ever had’ any children). Additionally, a detailed marital and cohabitation history is collected. Similar to the NLSY79, we determine the marital context of births indirectly by examining respondents’ marital status in the month of each birth. We use ten rounds of data to construct person-years’ files following each respondent from ages 15–24. Respondents who have not reached age 25 by their last interview contribute observations up to (but not including) their age at last interview.
Cycle 6 of the NSFG is, in contrast, a cross-sectional sample of women and men aged 15 to 45 in 2002 (Lepkowski, Mosher, and Davis 2006). This cycle of the NSFG was the first to include men, but collected information on fertility using different instruments for men and women. Cycle 6 collects information on male fertility with reference to specific sexual partners in hopes of eliciting higher reports of nonmarital fertility. Men who report births are asked a direct question about whether he and the partner were married, cohabiting, or single at the time of each birth. In contrast, for NSFG women, fertility, marriage, and cohabitation histories are collected separately, and the relationship context of the birth is determined by comparing dates, as we do in the NLSY data.
As noted above, we calculate age-specific fertility rates in the NLSY data sets that pertain to specific cohorts. Since the NSFG is cross-sectional and the composition of the population changes over time, we cannot be confident that the sample of older respondents in 2002 would actually be representative of their cohort in earlier years. For instance, many of the older Hispanic respondents may not have lived in the U.S. when they were 15–24. Therefore, we rely on the younger respondents to calculate fertility rates. Specifically, we create person-years files that represent respondents who were aged 15 to 24 in the 1991–2000 period. This minimizes not only the sample composition problem, but the recall problem. For these respondents, the minimum recall period is two years, and the maximum is 11 years. Respondents contribute observations for each completed age that falls in this period. Due to the smaller sample size in the NSFG, we use a broader period (ten calendar years versus five) than Martinez et al. (2006) in order to compute fertility rates for specific groups.
Assumptions and Potential Bias
In estimating both the birth and population counts, we make several assumptions. To the extent that these assumptions lead us to misestimate the number of births or number of individuals at risk of a birth, they create bias in the fertility rates. To the extent that we overestimate fertility rates in the population, surveys will appear worse than they actually are, whereas to the degree that we underestimate the population fertility rates, surveys will appear more favorable.
In estimating the number of births, we assume that father age is missing randomly on the birth certificates (controlling for demographic characteristics of the mother). Research by Elo and colleagues (1999) suggests this assumption is reasonable, but it needs to be verified with more recent data. We also assume that the father is the same race as mother when she fails to identify his race on the birth certificate. The bias posed by this assumption will depend on the degree to which births with missing father information are interracial and on the extent to which there are sex differences in interracial involvement within racial groups. Since studies documenting the extent of racial mixing in the population focus on cohabiting unions and marriage (Qian and Lichter 2007), it is difficult to predict the magnitude of this bias. To obtain an idea of the parameters of this bias, we conduct sensitivity tests that are discussed in the results section.
In estimating the number of men in the population, we assume that men who are in the armed forces overseas are at risk of having a birth in the United States. In results not presented here, we compared population ASFRs for black and white men estimated with and without overseas armed forces men in two different calendar years (1991 and 2000). At any given age, the largest difference between these two sets of estimates was 0.005. Presumably, the bias posed by including the overseas armed forces men is likely to be small because such a small percentage of young men fall into this category.
Our population counts by marital status assume that the proportion of men who are married in the civilian, noninstitutionalized population is equivalent to that of the overall population. Studies based on the NLSY79 suggest that the incarcerated population has lower rates of marriage than the civilian, noninstitutionalized population (Lopoo and Western 2005), while the military population has higher rates (Teachman 2007). Ultimately, the bias posed by our assumption depends on the percent of men who are outside of the civilian, noninstitutionalized population and their relative marriage rates. Some of our analyses based on surveys assess this bias by excluding specific groups of men (e.g., men who have ever been incarcerated).
RESULTS
Our analyses proceed as follows. First, we compare the fertility rates for men and women in the surveys to those estimated for the population. We include women in these analyses because previous studies evaluating female reports of fertility are based on older surveys (e.g., Bumpass 1983; Swicegood, Morgan, and Rindfuss 1984; Wu, Martin, and Long 2001), and we need a numerical benchmark for reasonable fertility counts (under circumstances in which reporting and representation are much less of an issue). Next, we examine how the underreporting of births in the different surveys varies according to race and marital status. Finally, we present results from our Monte Carlo simulations that address the implications of underreporting for conventional analyses of early fertility. The technical details of these analyses are discussed below.
Table 1 displays the ASFRs for three broad samples. Results for women appear on the left, and results for men are displayed on the right. For all six groups, we display at each age the survey fertility rate, the population rate (indicated by VS for Vital Statistics), the difference between the rates, and the ratio of the rates (i.e., the proportion of births reported). Below the ASFRs, we also present statistics that are averaged across five-year and ten-year age intervals. We weight each age equally in computing the averages for different age intervals, thus producing age-standardized ratios. For each ratio, we indicate whether the survey rate is significantly different from the population rate.
Table 1.
Ratio of Survey Fertility Rates to Population Fertility Rates (Using Multivariate Imputation by Chained Equations to Impute Missing Father Ages on the Birth Certificates)
| Women at Risk for Given Age | Men at Risk for Given Age | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| n | Survey Fertility Rate |
VS Fertility Rate |
Survey Minus VS |
Survey Vs Ratio |
n | Survey Fertility Rate |
VS Fertility Rate |
Survey Minus VS |
Survey VS Ratio |
|||
| NSFG Women (1991-2000 Period) | NSFG Men (1991-2000 Period) | |||||||||||
| 15 | 2601 | .013 | .015 | −.003 | 0.833 | 15 | 1885 | .003 | .003 | .001 | 1.216 | |
| 16 | 2607 | .028 | .032 | −.004 | 0.873 | 16 | 1814 | .007 | .008 | −.001 | 0.819 | |
| 17 | 2643 | .055 | .053 | .002 | 1.037 | 17 | 1708 | .015 | .019 | −.004 | 0.800 | |
| 18 | 2681 | .072 | .078 | −.006 | 0.918 | 18 | 1642 | .025 | .036 | −.011 | 0.699 | * |
| 19 | 2718 | .096 | .095 | .001 | 1.014 | 19 | 1596 | .032 | .051 | −.019 | 0.633 | * |
| 20 | 2720 | .112 | .104 | .008 | 1.079 | 20 | 1548 | .048 | .066 | −.018 | 0.733 | * |
| 21 | 2686 | .117 | .109 | .008 | 1.069 | 21 | 1505 | .054 | .079 | −.025 | 0.685 | * |
| 22 | 2680 | .104 | .115 | −.010 | 0.909 | 22 | 1459 | .069 | .091 | −.021 | 0.765 | * |
| 23 | 2657 | .111 | .115 | −.004 | 0.964 | 23 | 1436 | .089 | .099 | −.010 | 0.901 | |
| 24 | 2663 | .109 | .114 | −.005 | 0.958 | 24 | 1458 | .102 | .102 | .000 | 0.999 | |
|
| ||||||||||||
| 15-19 | 2601 | .053 | .055 | −.002 | 0.964 | 15-19 | 1596 | .016 | .023 | −.007 | 0.706 | * |
|
| ||||||||||||
| 20-24 | 2657 | .111 | .111 | −.001 | 0.994 | 20-24 | 1436 | .073 | .087 | −.015 | 0.831 | * |
|
| ||||||||||||
| 15-24 | 2601 | .082 | .083 | −.001 | 0.984 | 15-24 | 1436 | .044 | .055 | −.011 | 0.805 | * |
|
| ||||||||||||
| NLSY79 Women (1957−64 Birth Cohort) | NLSY79 Men (1957−64 Birth Cohort) | |||||||||||
| 15 | 4264 | .014 | .015 | −.001 | 0.927 | 15 | 4028 | .003 | .002 | .001 | 1.820 | |
| 16 | 4264 | .037 | .034 | .004 | 1.108 | 16 | 4028 | .007 | .006 | .002 | 1.340 | |
| 17 | 4264 | .056 | .054 | .002 | 1.039 | 17 | 4028 | .014 | .014 | −.001 | 0.942 | |
| 18 | 4264 | .070 | .073 | −.003 | 0.957 | 18 | 4028 | .025 | .030 | −.005 | 0.830 | * |
| 19 | 4264 | .090 | .088 | .002 | 1.022 | 19 | 4028 | .040 | .045 | −.005 | 0.892 | |
| 20 | 4264 | .100 | .098 | .002 | 1.018 | 20 | 4028 | .053 | .061 | −.008 | 0.874 | * |
| 21 | 4264 | .103 | .107 | −.004 | 0.960 | 21 | 4028 | .067 | .076 | −.009 | 0.876 | * |
| 22 | 4264 | .105 | .113 | −.008 | 0.929 | 22 | 4028 | .078 | .088 | −.010 | 0.887 | * |
| 23 | 4264 | .106 | .116 | −.010 | 0.917 | 23 | 4028 | .085 | .099 | −.014 | 0.855 | * |
| 24 | 4264 | .110 | .115 | −.005 | 0.956 | 24 | 4028 | .091 | .102 | −.011 | 0.888 | * |
|
| ||||||||||||
| 15-19 | 4264 | .053 | .053 | .001 | 1.013 | 15-19 | 4028 | .018 | .019 | −.001 | 0.923 | |
|
| ||||||||||||
| 20-24 | 4264 | .105 | .110 | −.005 | 0.954 | 20-24 | 4028 | .075 | .085 | −.011 | 0.876 | * |
|
| ||||||||||||
| 15-24 | 4264 | .079 | .081 | −.002 | 0.973 | 15-24 | 4028 | .046 | .052 | −.006 | 0.885 | |
|
| ||||||||||||
| NLSY97 Women (1980−84 Birth Cohort) | NLSY97 Men (1980−84 Birth Cohort) | |||||||||||
| 15 | 4336 | .013 | .014 | −.001 | 0.943 | 15 | 4545 | .003 | .002 | .001 | 1.271 | |
| 16 | 4300 | .028 | .029 | −.001 | 0.982 | 16 | 4518 | .008 | .007 | .001 | 1.164 | |
| 17 | 4266 | .044 | .046 | −.003 | 0.940 | 17 | 4472 | .016 | .016 | .000 | 1.025 | |
| 18 | 4221 | .063 | .067 | −.004 | 0.943 | 18 | 4418 | .029 | .030 | .000 | 0.989 | |
| 19 | 4164 | .087 | .087 | .001 | 1.009 | 19 | 4352 | .043 | .044 | −.001 | 0.969 | |
| 20 | 4087 | .088 | .096 | −.008 | 0.921 | 20 | 4266 | .052 | .056 | −.004 | 0.923 | |
| 21 | 3963 | .093 | .100 | −.007 | 0.929 | 21 | 4109 | .057 | .067 | −.009 | 0.862 | * |
| 22 | 3171 | .107 | .104 | .004 | 1.036 | 22 | 3240 | .064 | .077 | −.012 | 0.840 | * |
| 23 | 2329 | .104 | .104 | −.001 | 0.995 | 23 | 2359 | .074 | .085 | −.010 | 0.877 | |
| 24 | 1506 | .115 | .107 | .008 | 1.075 | 24 | 1476 | .074 | .091 | −.018 | 0.809 | * |
|
| ||||||||||||
| 15-19 | 4164 | .047 | .049 | −.001 | 0.970 | 15-19 | 4352 | .020 | .020 | .000 | 1.006 | |
|
| ||||||||||||
| 20-24 | 1506 | .101 | .102 | −.001 | 0.993 | 20-24 | 1476 | .064 | .075 | −.011 | 0.857 | |
|
| ||||||||||||
| 15-24 | 1506 | .074 | .075 | −.001 | 0.986 | 15-24 | 1476 | .042 | .048 | −.005 | 0.888 | |
Notes: Fertility rates for different age groups are computed by averaging the ASFRs comprising them (weighting each age equally).
p < .05 (two-tailed test of population / survey rate difference)
All three surveys obtain almost complete reporting of women’s fertility: the 10-year age ratios range from 0.973 to 0.986. The ratios for men are considerably lower and exhibit greater variability. The 10-year age ratio for NSFG is 0.805, while the ratios for NLSY79 and NLSY97 are 0.885 and 0.888 respectively. The ranking of these overall ratios for men is consistent with our hypothesis about the importance of longitudinal data, with the NLSYs capturing a greater share of male births than the NSFG. The NLSY97 does not appear to be substantially better than the NLSY79 at capturing early male fertility, but these first analyses do not distinguish marital and nonmarital births. For these broad age intervals, the only survey rate that differs significantly from the population rate is for the NSFG men.
Comparing the ratios averaged across ages 15–19 for the different surveys again suggests that the NLSY surveys do a better job of capturing births than the NSFG. The value for NSFG men indicates that this survey misses almost one-third of births to teen men. Differences for the group aged 20 to 24 are less pronounced, but reveal a similar pattern.
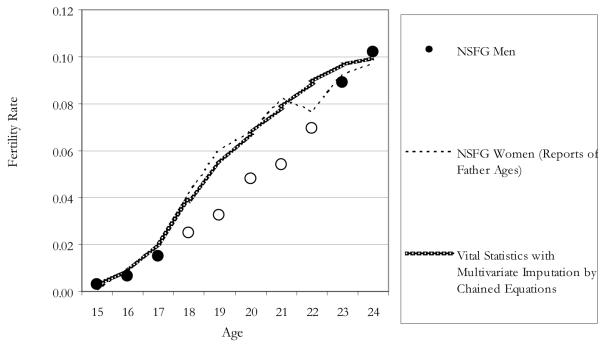
To facilitate interpretation, we present the remaining comparisons of survey and population ASFRs in graphs. In Figure 1, we compare Vital Statistics’ rates (using multiple imputation for missing father’s age) to estimates of male fertility based on the NSFG women’s reports of the fathers’ ages of their children born in this period (1991 to 2000). The NSFG rates include the weighted number of births to men of different ages (based on the NSFG female data) in the numerator and the weighted population of NSFG men at different ages (based on the NSFG male data) in the denominator. It is reassuring to note that the rates based on the NSFG women’s reports map closely to the population estimates. This suggests that our population-based rates are fairly accurate, and that any differences we see in the NSFG male ASFRs are due to differences in the reporting of births (the numerator) rather than the representation of men in the survey (the denominator).
Figure 1. Age-Specific Fertility Rates for Men by Data Source: 1991 to 2000 Period (Ratio of Rates r = .805*).
Note: Hollow circle means survey estimate different from population at p < .05 (two-tailed test).
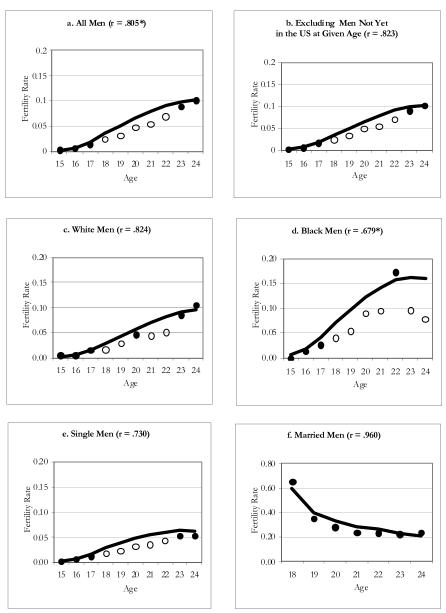
We now turn to analyses that pertain to specific subgroups of men, beginning with the NSFG. Figure 2 displays six separate graphs for NSFG men in the 1990s. The first graph (a) copies survey and population estimates from Table 1. The second graph (b) drops foreign-born men who were not yet in the U.S. at a given age of risk to better capture men who were actually at risk of having a birth in the period. In this graph, and other analyses based on the NSFG (not shown), we find that excluding this group does not make a substantive difference. In the remaining graphs, we include the foreign born because studies based on fatherhood in the NSFG typically include this group (e.g., Guzzo and Furstenberg 2007). The following graphs compare the ASFRs for four specific groups: white men (c), black men (d), single men (e), and married men (f). We do not take into account race and marital status simultaneously in our NSFG analyses because of small sample sizes for some subgroups, particularly black married men. Results indicate that for white men, there is some underreporting of births (r = .824). For black men, the gap between the population and survey estimates is much larger, and the gap increases with age. As expected, births to single men are underreported. In contrast, none of the survey estimates for married men differ significantly from the population estimates.
Figure 2. Age-Specific Fertility Rates for NSFG Men: 1991-2000 Period.
Notes: Line corresponds to rate estimated for the population using MICE; significant differences between the survey and population rate at any given age are indicated by the hollow circles.
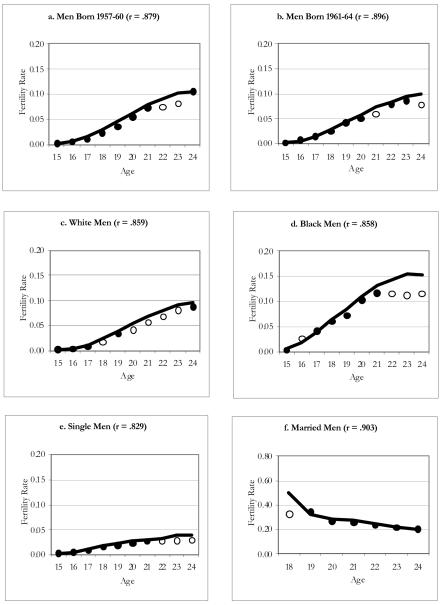
Figure 3 displays graphs for subgroups of men from the NLSY79; the top graphs (a and b) divide the sample into two smaller cohorts: men born 1957–60 versus 1961–64. We expected lower quality reporting for the earlier cohort for two reasons. First, recall was likely to be more of a problem (respondents were 18–21 at the start of the panel and thus had to recall births that could have occurred several years earlier). Second, they were less representative of their cohort than their counterparts born 1961–64 (the initial sampling frame of the NLSY79 excluded men not in households, and men 18 and older are more likely to be incarcerated or reside on a military base). Contrary to our expectations, the quality of male fertility data does not appear to be substantially better for men who were younger at the start of the panel than for men who were older when the survey began.
Figure 3. Age-Specific Fertility Rates for NLSY79 Men: 1957-64 Birth Cohort.
Notes: Line corresponds to rate estimated for the population using MICE; significant differences between the survey and population rate at any given age are indicated by the hollow circles.
The patterns for white men echo the patterns for men from the two cohorts combined (see Table 1): some differences are significant, but they are small in magnitude. As in the NSFG, births to black men are significantly underreported; however, in the NLSY79, the magnitude of underreporting is smaller, and it is statistically significant only in the early 20s. The results for single men resemble those for all men, with significant but small birth deficits among the three oldest ages. As expected, the ASFRs for married men from the NLSY79 do not significantly differ from those for the population, with the exception of the rate at age 18.
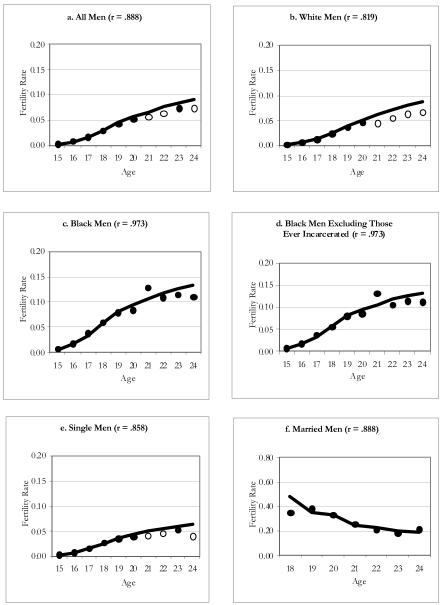
Figure 4 displays these graphs for men from the NLSY97. As in the case of the NSFG, the patterns for whites and single men from the NLSY97 closely resemble the overall patterns of men in this sample, with small deficits between the ages of 21 and 24. It is reassuring to observe once again that the ASFRs for men in the NLSY97 are close to those estimated for the population. In contrast to the other surveys, the NLSY97 does not show significant differences between the population estimates and the survey estimates for black men.
Figure 4. Age-Specific Fertility Rates for NLSY97 Men: 1980-84 Birth Cohort.
Notes: Line corresponds to rate estimated for the population using MICE; significant differences between the survey and population rate at any given age are indicated by the hollow circles.
To get an idea of the bias posed by excluding the institutionalized population in surveys such as the NSFG, we estimate ASFRs in the NLSY97, excluding those in institutions (e.g., Freedman et al. 2004). We were able to do this with the NLSY samples because they interviewed respondents, initially in households, as they moved to prisons or military barracks. In panel d, we show the ASFRs for black men, excluding men who were in jail or prison at any interview. We focus this graph on blacks from the NLSY97 because we observed the highest rates of incarceration for this group. The graphs for all black men and black men who have never been incarcerated are indistinguishable; dropping black men with any incarceration does not change the pattern of results; however, the prevalence of incarceration in our sample of black men from the NLSY97 appears low (less than 7%) in comparison to those estimated by Bonczar (2003) for a slightly younger age group in the U.S. as of 2001 (8.5% of black men aged 18–24).
In other analyses (results not shown) for blacks and whites from the NLSY79 and NLSY97, we excluded those who had ever been in military barracks. We alternatively ran analyses that excluded respondents who were in prison or on military barracks during a given age of year of risk. None of these exclusions changed the overall pattern of results for white and black men. This partly reflects the fact that respondents in these institutions comprised a small proportion of the entire sample.
Our imputation methods assume that fathers with missing information on race (who typically have missing information on age as well) are the same race as mothers. We conducted sensitivity tests for men in the population from the 1980–84 birth cohort (the group most likely to violate this assumption because of increases in interracial relationships) to obtain an idea of how much distortion our homogamy assumption may create in the race-specific population ASFRs (results not shown). First, we assumed that the racial mix of parents at each age was the same for births with and without information on birth certificates. Based on this assumption, we ended up reducing the number of births to white men at every age and increasing the number to black men, but the ASFRs for black and white men barely changed. Then, we repeated this exercise, assuming racial mixing was more common for births certificates with missing race. This alternative assumption did not dramatically change the patterns in these graphs.
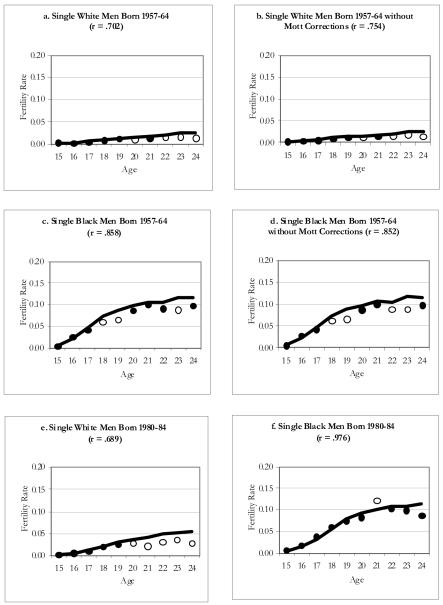
Because marital status differs considerably by race, the confounding of race and marital status in these graphs makes it difficult to identify whether black men or single men are responsible for the observed underreporting of births. Figure 5 presents nonmarital ASFRS for NLSY79 and NLSY97 by race. For men from the NLSY79, we present two sets of these graphs: one set is based on the Mott and colleagues’ data removing births with low confidence births paternity codes (a and c) and the other based on the set of constructed variables without the Mott and colleagues’ changes (b and d). As can be seen, the pattern of results does not differ according to which set we use; however, there is an increase in underreporting for white men with the Mott and colleague’s changes (i.e., r = .702 versus r = .754).
Figure 5. Age-Specific Fertility Rates for NLSY79 and NLSY97 Men.
Notes: Line corresponds to rate estimated for the population using MICE; significant differences between the survey and population rate at any given age are indicated by the hollow circles.
In all six graphs, reporting deficits are apparent in the early twenties, though to varying degrees. The overall deficit is greater for white men than for black men. These patterns are at odds with Rendall and colleagues (1999), who found blacks to have a greater deficit than whites, even after holding marital status constant. As we noted earlier, the white population includes a much larger Hispanic component at the time the NLSY97 was drawn than when either the NLSY79 or PSID samples were drawn. Further analysis of Hispanic/non-Hispanic differences are encouraged to understand better the sources of the relatively large levels of underreporting among single white men in their early 20s in the NLSY97. The NLSY97 nevertheless seems to be capturing the births of single black men unusually well, which is consistent with the expectation of improvement across the two NLSY cohorts. We are least confident about our population ASFRs estimated for these subgroups because the sample sizes are smaller, and because the bias is potentially compounded though the assumptions made.
Monte Carlo Simulations
Researchers have been concerned that incomplete reporting of men’s births in surveys will bias the coefficients in multivariate models of the antecedents and consequences of early fertility. Previous studies that have estimated parallel models of early parenthood find weaker effects of many family background variables for men compared to women (e.g., Hynes et al. 2008; Michael and Tuma 1985), but it is unclear whether these sex differences are due to differences in data quality or behavior.
To understand how underreporting biases results in conventional models of early parenthood, we conduct Monte Carlo experiments that allow us to compare the coefficients for models with full fertility data to models in which we simulate the underreporting of births (Mooney 1997). We use a sample of women for these simulations because we want to have initial models that are not biased by under-reporting or representation issues. We use the NSFG for these simulations. We limit the sample to women aged 20 or older at the time of the interview and estimate a logistic regression model predicting the likelihood of having a teen birth as a function of basic family background and demographic variables. We discard information on the exact timing of teen births in order to simplify our experiments. Odds ratios for this model appear in column one of Table 2.
Table 2.
Logistic Regression Models of Having a Teen Birth, Using Simulated Samples with Different Assumptions of Birth Underreporting: NSFG Women
| 100% of Teen Births Reported |
Models for 100 Simulated Samples with 30% of Teen Births Not Reported Random Underreporting |
Models for 100 Simulated Samples with 30% of Teen Births Not Reported: 43% of Births to Blacks Not Reported 23% of Births to Non-Blacks Not Reported |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| (1) | (2) | (3) % |
(4) %Models |
(5) | (6) % |
(7) %Models |
||||
| Difference | w/ Sig. | Difference | w/ Sig. | |||||||
| Average | between | Different | Average | between | Different | |||||
| Variable | β | β | (1) and (5) | β from (1) | β | (1) and (5) | β from (1) | |||
| Intercept | −2.563 | *** | −2.895 | *** | 13% | 71% | −2.812 | *** | 10% | 29% |
| Black | 1.072 | *** | 0.970 | *** | −10% | 9% | 0.573 | *** | −47% | 100% |
| Hispanic | 0.981 | *** | 0.893 | *** | −9% | 2% | 0.892 | *** | −9% | 1% |
| Mother high school dropout | 1.139 | *** | 1.030 | *** | −10% | 5% | 1.056 | *** | −7% | 1% |
| Mother has high school degree | 0.792 | *** | 0.731 | *** | −8% | 1% | 0.737 | *** | −7% | 0% |
| With single parent at 14 | 0.463 | *** | 0.403 | *** | −13% | 1% | 0.421 | *** | −9% | 0% |
| With stepparent at 14 | 0.749 | *** | 0.667 | *** | −11% | 4% | 0.701 | *** | −6% | 1% |
| No biological parent at 14 | 0.677 | *** | 0.588 | *** | −13% | 3% | 0.646 | *** | −5% | 2% |
| Foreign born | −0.610 | *** | −0.549 | *** | −10% | 1% | −0.511 | *** | −16% | 4% |
| Born 1960-64 | −0.042 | −0.048 | 13% | 0% | −0.045 | 8% | 0% | |||
| Born 1965-69 | −0.576 | *** | −0.537 | ** | −7% | 1% | −0.559 | ** | −3% | 0% |
| Born 1970-74 | 0.102 | 0.090 | −11% | 0% | 0.081 | −20% | 0% | |||
| Born 1975-79 | 0.321 | * | 0.278 | # | −13% | 0% | 0.279 | # | −13% | 0% |
| Born 1980-82 | −0.010 | −0.025 | 143% | 0% | −0.013 | 32% | 0% | |||
| N of cases | 6,449 | 6,449 | 6,449 | 6,449 | 6,449 | 6,449 | 6,449 | |||
Notes: We examine whether the 95% confidence interval corresponding to the estimate for each simulated includes the estimate from the model based on complete birth reports. Confidence intervals do not include the point estimate are classified signficantly different.
p < .10;
p < .05;
p < .01;
p < .001
In our first experiment, we randomly reclassify approximately 30% of women who reported a teen birth to the no birth category of the dependent variable, assuming that underreporting is random (roughly 30% of teen births to men in our NSFG sample and 32% in the Martinez et al. 2006 sample were underreported.) The values on the independent variables for these women remain the same. All other women in the sample retain their original values on both the dependent and independent variables. We then run models predicting the likelihood of having a teen birth. We record the coefficients and 95% confidence intervals based on this altered sample with 30% fewer earlier births (results not shown). Returning to the original data, we repeat this process 99 more times, so that we have 100 trials in this experiment, generating a sampling distribution of coefficients from teen birth models to take into account sampling variability.
In our second experiment, we once again change 30% of the teen births to the no birth category; however, we assume greater underreporting among blacks than among other respondents based on their respective proportions of teen births underreported for our NSFG sample (43% of teen births to black men and 23% for non-black men). All other steps remain the same. For each variable in the two experiments that we conduct, we present the average of the 100 coefficients in columns 2 and 5. In addition, we report the percent difference between the averaged coefficients and the original estimates (columns 3 and 6) and the percent of the 100 models with coefficients that are significantly different from the original estimates (columns 4 and 7).
The results for the first experiment reveal a decline in magnitude of coefficients for each of the family background variables, with an average decline of around 10%. Still, the vast majority of coefficients from the first experiment fail to significantly differ from the original estimates. In the second experiment, the coefficient for “black” declines by almost 50% and is significantly different from the original estimate in all of the simulated samples; this is consistent with the assumption of differential underreporting in this simulation. The attenuation of the other coefficients is generally weaker than in the first experiment. The results of these experiments suggest that underreporting attenuates coefficients in models of having a teen birth, especially the coefficients of variables associated with underreporting.
CONCLUSION
Researchers are increasingly emphasizing the importance of men in studies of family formation (Bachrach 2007; Forste 2002; Goldscheider and Kaufman 1996; Greene and Biddlecom 2000). At the same time, they continue to express concerns about the quality of male fertility data in recent surveys. In response to these concerns, we estimated age-specific fertility rates during the teens and early twenties for men in NLSY79, NLSY97, and NSFG 2002 and compared them to male fertility rates estimated for the U.S. population. We found that in the NSFG, roughly four out of five early births were reported, and in the NLSY79 and NLSY97, almost nine-tenths of early births were reported. In all three surveys, reporting deficits were especially pronounced for nonmarital births. Reporting of births to women was virtually complete in all three surveys.
There are two major reasons to suspect that the quality of early male fertility data would be lower in the NSFG than in the NLSY79 and NLSY97. Greater birth deficits in the NSFG could reflect its exclusion of men in prison/jail or on military bases, as well as its retrospective nature. While respondents in the longitudinal studies had the opportunity to report births shortly after they occurred, respondents in the NSFG had to report on births that happened up to a decade ago. For women, this is much less of a problem because they typically reside with their children; however, for men, particularly nonresident fathers who may not be actively involved with their children, fertility is clearly underreported.
In spite of this variation in overall quality, an important pattern can be seen for all three surveys and for black and white men alike. Reporting of nonmarital births is more complete in the teens than in the early twenties, a period of the life course found to be demographically dense. Fertility and geographic mobility rates are highest in the early twenties (Rindfuss 1991), and the risk of first time imprisonment is increasing in this period (Pettit and Western 2004). In both of the longitudinal surveys, nonresponse rates were higher in the early twenties than in the teens (results not shown). We suspect that these surveys may have greater difficulty including young fathers, particularly unwed fathers. This is consistent with findings in both the NLSY79 and NLSY97 suggesting youth from more disadvantaged backgrounds are more likely to leave the sample than their counterparts from more advantaged backgrounds (Aughinbaugh and Gardecki 2008; MaCurdy, Mroz, and Gritz 1998). Further undermining data quality, men who drop out of the surveys for one interview or more and subsequently return to the sample do not have an opportunity to report births soon after they occur, unlike their counterparts who remain continuously in the sample.
It could be the case that men who have nonmarital births in their twenties are less involved with their children than men in their teens, and consequently, less likely to subsequently report them. A study based on Fragile Families data found that fathers in their early twenties were less likely than fathers in other age groups (including the teens) to be in a relationship with the mother at the time of birth (Carlson and McLanahan 2004). Studies also find that among never married men, the percent not reporting on the number of children ever born increases with age (Bachu 1996; Lerman 1993); however, these age differences are not dramatic enough to explain the substantial underreporting of births to men in their early twenties. The primary drivers of incomplete reporting in this period of the life course appear to be selective nonresponse (for all three surveys) and sampling frame (in the case of the NSFG).
In spite of the fact that nonresponse rates were considerably higher in the NLSY97 than in the NLSY79 (Aughinbaugh and Gardecki 2008), we found a slightly greater proportion of nonmarital births reported for this more recent cohort (i.e., .858 versus .829). Several factors could have improved reporting of nonmarital births, including the development of the fatherhood movement, greater paternity establishment, and increases in the proportion of nonmarital births that were to cohabiting parents (Kennedy and Bumpass 2008; Marsiglio, Amato, Day, and Lamb 2000).
Results from our Monte Carlo experiments, which are based on levels of underreporting found for men in the NSFG, suggest that underreporting attenuates coefficients in models of early fatherhood. Furthermore, the magnitude of attenuation depends on how strongly a given variable is associated with underreporting; however, the attenuation of coefficients in models of teen births estimated for men from the NLSY79 and NLSY97 is likely to be smaller because a larger proportion of births in these surveys are reported.
While we were not able to conduct separate analyses of Hispanics due to limitations in the population data, we would be concerned about the reliability of these estimates nonetheless. The composition of the Hispanic population changes across cohorts and even within cohorts at different ages due to immigration (Portes and Rumbaut 1996). The further we follow NLSY Hispanics beyond the first interview, the less they will resemble Hispanics from the same birth cohorts in the general population.
This study makes an important contribution to our understanding of the quality of male fertility data in major U.S. surveys. In the period following the Nurturing Fatherhood Initiative, research on men’s fertility has proliferated. Yet, our understanding of the quality of male fertility data is based on studies that are now dated. Our results reveal that the quality of male fertility data in the NLSY97 and the NLSY79 is better than that in the NSFG, suggesting that survey design matters to the quality of these data. They suggest that surveys collecting information on fertility for men prospectively are likely to be more successful than studies that collect this information retrospectively.
Footnotes
Information on marital status in the Vital Statistics data has varied across states and over time. The majority of states ask directly whether the mother was married “at birth, conception, or any time in between,” or alternatively, whether she was married to the father of the child. A few states determine marital status using inferential procedures (See Hamilton, Martin and Ventura 2006 and Ventura and Bachrach 2000 for greater detail on how marital status is measured in the Vital Statistics.)
We also used the hotdeck method to impute missing fathers’ ages. First, we stratified the birth certificate data by calendar year, mother’s age, and father’s race. Then, for each stratum (e.g., births to black fathers and 18-year-old mothers in 1980), we randomly selected (with replacement) ages of fathers reported on birth certificates to fill in the father’s age when it is missing. This alternative method of imputation produced estimates of the number of births to men that are remarkably close to those based on multivariate imputation.
To develop comparable measures of race for data from years 2000 and beyond, we bridged the responses of those who reported more than one race to a single race using the largest group bridging method (NCHS 2004).
Contributor Information
Kara Joyner, Bowling Green State University.
H. Elizabeth Peters, Cornell University.
Kathryn Hynes, Pennsylvania State University.
Asia Sikora, Cornell University.
Jamie Rubenstein, Cornell University.
Michael S. Rendall, RAND Corporation
REFERENCES
- Allison P. Missing Data. Sage Publications; Thousand Oaks, CA: 2001. [Google Scholar]
- Aughinbaugh A, Gardecki RM. Attrition in the National Longitudinal Survey of Youth 1997. 2008. Working Paper.
- Bachrach C. Taking Stock: Do Surveys of Men’s Fertility Deliver? In: Hofferth S, Casper L, editors. Handbook of Measurement Issues in Family Research. Lawrence Erlbaum Associates; Mahwah, NJ: 2007. pp. 325–334. [Google Scholar]
- Bachrach C, Sonenstein F. In Federal Interagency Forum on Child and Family Statistics, Nurturing Fatherhood: Improving data and research on male fertility, family formation and fatherhood. U.S. Printing Office; Washington D.C.: 1998. Report of the Working Group on Male Fertility and Family Formation. [Google Scholar]
- Bachu A. Fertility of American Men. U.S. Census Bureau; Washington, DC: 1996. (Population Division Working Paper No. 14) [Google Scholar]
- Boggess S, Martinez G, Jasik CB, Lindberg LD. Counting Dads: Improving Estimates of Teen Fatherhood. In: Hofferth S, Casper L, editors. Handbook of Measurement Issues in Family Research. Lawrence Erlbaum Associates; Mahwah, NJ: 2007. pp. 285–302. [Google Scholar]
- Bonczar TP. Prevalence of Imprisonment in the U.S. Population, 1974–2001. U.S. Department of Justice Office of Justice Programs Bureau of Justice Statistics Special Report. 2003 [Google Scholar]
- Bumpass L. The Comparability of Marital and Fertility Histories across Fertility Surveys and June Current Population Surveys. 1983. CDE working paper 83-9.
- Byrne G. Father May Not Know Best, But What Does He Know? Population Today. 1997;25(10):1–3. [PubMed] [Google Scholar]
- Carlson M, McLanahan S, England P, Devaney B. What We Know about Unmarried Parents: Implications for Building Strong Families Programs. Mathematica Policy Research; Princeton, NJ: 2005. Building Strong Families Issue Brief, No. 3. [Google Scholar]
- Carlson M, McLanahan S. Fragile Families, Father Involvement and Public Policy. In: Tamis-LeMonda C, Cabrera N, editors. Handbook of Father Involvement: Multidisciplinary Perspectives. Lawrence Erlbaum Associates; Mahwah, NJ: 2004. pp. 461–488. [Google Scholar]
- Center for Human Resource Research 2006 Appendix 5 in NLSY79 Codebook Supplement. http://www.nlsinfo.org/nlsy79/docs/79html/codesup/appen5.htm.
- Center for Human Resource Research 2009 NLSY97 Codebook Supplement. http://www.nlsinfo.org/nlsy97/nlsdocs/nlsy97/codesup/maincstoc.html.
- Cherlin A, Griffith J, McCarthy J. A Note on Maritally-Disrupted Men’s Reports of Child Support in the June 1980 Current Population Survey. Demography. 1983;20:385–89. [PubMed] [Google Scholar]
- Elo IT, King RB, Furstenberg F. Adolescent Females: Their Sexual Partners and the Fathers of their Children. Journal of Marriage and the Family. 1999;61:74–84. [Google Scholar]
- Forste R. Where are All the Men? A Conceptual Analysis of the Role of Men in Family Formation. Journal of Family Issues. 2002;23(5):579–600. [Google Scholar]
- Federal Interagency Forum on Child and Family Statistics . Nurturing Fatherhood: Improving Data and Research on Male Fertility, Family formation and Fatherhood. U.S. Printing Office; Washington D.C.: 1998. [Google Scholar]
- Freedman VA, Crimmins E, Schoeni RF, Spillman B, Aykan H, Kramarow E, Land K, Lubitz J, Manton K, Martin L, Shinberg D, Waidmann T. Resolving Inconsistencies in Trends in Old-age Disability: Report from a Technical Working Group. Demography. 2004;41(3):417–441. doi: 10.1353/dem.2004.0022. [DOI] [PubMed] [Google Scholar]
- Garfinkel I, McLanahan S, Hanson T. A Patchwork Portrait of Nonresident Fathers. In: Garfinkel I, McLanahan S, Meyer D, Seltzer J, editors. Fathers Under Fire: The Revolution in Child Support Enforcement. Russell Sage Foundation; New York: 1998. pp. 31–59. [Google Scholar]
- Goldscheider FK, Kaufman G. Fertility and Commitment: Bringing Men Back In. Population and Development Review. 1996;22(Supplement):87–99. [Google Scholar]
- Greene ME, Biddlecom AE. Absent and Problematic Men: Demographic Accounts of Male Reproductive Roles. Population and Development Review. 2000;26(1):81–115. [Google Scholar]
- Guzzo KB, Furstenberg F. Multipartnered Fertility among American Men. Demography. 2007;44(3):583–601. doi: 10.1353/dem.2007.0027. [DOI] [PubMed] [Google Scholar]
- Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary Data for 2006. National Vital Statistics Reports. 2007;56(7) [PubMed] [Google Scholar]
- Hamilton BE, Martin JA, Ventura SJ. Birth: Preliminary Data for 2007. National Vital Statistics Reports. 2009;57(12) [PubMed] [Google Scholar]
- Hernandez DJ, Brandon PD. Who are the Fathers of the Today? In: Tamis-Lemonda CS, Cabrera N, editors. Handbook of Father Involvement: Multidisciplinary Perspectives. Lawrence Erlbaum Associates, Inc.; Mahwah, NJ: 2002. pp. 33–62. [Google Scholar]
- Hinde A. Demographic Methods. Arnold Publishers; New York: 1998. [Google Scholar]
- Hynes K, Joyner K, Peters HE, Yang F. The Transition to Early Fatherhood: National Estimates Based on Multiple Surveys. Demographic Research. 2008;18(12):337–376. [Google Scholar]
- Kennedy S, Bumpass L. Cohabitation and Children’s Living Arrangements: New Estimates from the United States. Demographic Research. 2008;19(47):1663–1692. doi: 10.4054/demres.2008.19.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MS, Ruggles T, Alexander D, Leicach, Sobek M. Integrated Public Use Microdata Series, Current Population Survey: Version 2.0. 2009 [Machine-readable database]. Minneapolis, MN: Minnesota Population Center [producer and distributor]. Available online at http://cps.ipums.org/cps/
- Landry DJ, Forrest JD. How Old are U.S. Fathers? Family Planning Perspective. 1995;27:159–165. [PubMed] [Google Scholar]
- Lepkowski JM, Mosher WD, Davis KE, et al. National Survey of Family Growth, Cycle 6: Sample Design, Weighting, Imputation, and Variance Estimation. National Center for Health Statistics. Vital Health Stat. 2006;2(142) [PubMed] [Google Scholar]
- Lerman RI. A National Profile of Young Unwed Fathers. In: Lerman RI, Ooms TJ, editors. Young Unwed Fathers: Changing Roles and Emerging Policies. Temple University Press; Philadelphia: 1993. pp. 27–51. [Google Scholar]
- Lindberg L, Sonenstein F, Martinez G, Marcotte J. Completeness of Young Fathers’ Reports of Fertility. Journal of Economic and Social Measurement. 1998;24(1):15–23. [Google Scholar]
- Lopoo LM, Western B. Incarceration and the Formation and Stability of Marital Unions. Journal of Marriage and Family. 2005;67(3):721–734. [Google Scholar]
- MaCurdy T, Mroz T, Gritz M. An Evaluation of the NLSY. Journal of Human Resources. 1998;33(2):345–436. [Google Scholar]
- Manlove J, Logan C, Ikramullah E, Holcombe E. Factors Associated With Multiple-Partner Fertility Among Fathers. Journal of Marriage and Family. 2008;70(2):536–548. doi: 10.1111/j.1741-3737.2008.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsiglio W, Amato PR, Day R, Lamb M. Fatherhood Scholarship in the 1990s: Past Impressions, Future Prospects. Journal of Marriage and te Family. 2000;62:1173–1191. [Google Scholar]
- Martinez GM, Chandra A, Abma J, Jones J, Mosher WD. “Fertility, Contraception, and Fatherhood: Data on Men and Women from Cycle 6 (2002) of the National Survey of Family Growth.” National Center for Health Statistics. Vital Health Statistics. 2006;23(26) [PubMed] [Google Scholar]
- Michael RT, Tuma NB. Entry into Marriage and Parenthood by Young Men and Women: The Influence of Family Background. Demography. 1985;22(4):515–544. [PubMed] [Google Scholar]
- Mooney CZ. Monte Carlo Simulation. Sage; Thousand Oaks, CA: 1997. [Google Scholar]
- Morgan SP, Botev N, Chen R, Huang J. White and Nonwhite Trends in First Birth Timing: Comparisons using Vital Registration and Current Population Surveys. Population Research and Policy Review. 1999;18:339–356. [Google Scholar]
- Morgan SP, Rindfuss RR. Re-examining the Link of Early Childbearing to Marriage and to Subsequent Fertility. Demography. 1999;36:59–75. [PubMed] [Google Scholar]
- Mott FC, Hurst DS, Gryn T. Male Relationship and Fertility Data in the NLSY. In: Hofferth S, Casper L, editors. Handbook of Measurement Issues in Family Research. Lawrence Erlbaum Associates; Mahwah, NJ: 2007. pp. 263–284. [Google Scholar]
- National Center for Health Statistics Vital Statistics of the United States, Volume I, Natality. 2004 http://www.cdc.gov/nchs/data/TechApp04.pdf.
- Pettit B, Western B. Mass Imprisonment and the Life Course: Race and Class Inequality in U.S. Incarceration. American Sociological Review. 2004;69:151–169. [Google Scholar]
- Portes A, Rumbaut R. Immigrant America: A Portrait. Second Edition University of California Press; Berkeley: 1996. [Google Scholar]
- Preston SH, Heuveline P, Guillot M. Demography: Measuring and Modeling Population Processes. Blackwell Publishers; Malden, Massachusetts: 2001. [Google Scholar]
- Qian Z, Lichter DT. Social Boundaries and Marital Assimilation: Interpreting Trends in Racial and Ethnic Intermarriage. American Sociological Review. 2007;72:68–94. [Google Scholar]
- Rendall MS, Clarke L, Peters HE, Ranjit N, Verropoulou G. Incomplete Reporting of Male Fertility in the United States and Britain: A Research Note. Demography. 1999;36(1):135–144. [PubMed] [Google Scholar]
- Rindfuss RR. The Young Adult Years: Diversity, Structural Change, and Fertility. Demography. 1991;28(4):493–512. [PubMed] [Google Scholar]
- Seltzer J, Brandeth Y. What Fathers Say About Involvement with Children After Separation. Journal of Family Issues. 1994;15(1):49–7. [Google Scholar]
- Sorensen E. A National Profile of Nonresident Fathers and Their Ability to Pay Child Support. Journal of Marriage and the Family. 1997;59(4):785–797. [Google Scholar]
- Swicegood G, Morgan SP, Rindfuss R. Measurement and Replication: Results from Eight U.S. Surveys Spanning a Quarter Century. Demography. 1984;21:19–34. [PubMed] [Google Scholar]
- Teachman J. Race, Military Service, and Marital Timing: Evidence from the NLSY-79. Demography. 2007;44:389–404. doi: 10.1353/dem.2007.0018. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau National Estimates by Age, Sex, Race. 1969–1999. 2007 Available online at http://www.census.gov/popest/archives.
- U.S. Census Bureau National Population Estimates for the 2000s. 2009 Available online at http://www.census.gov/popest/national/asrh/2006_nat_af.html.
- Ventura SJ, Bachrach CA. Nonmarital Childbearing in the United States, 1940-1999. National Vital Statistics Reports. 2000;48(16) [PubMed] [Google Scholar]
- Wildsmith E, Raley RK. Race-ethnic Differences in Nonmarital Fertility: A Focus on Mexican American Women. Journal of Marriage and Family. 2006;68:491–508. [Google Scholar]
- Wu L, Martin S, Long D. Comparing Data Quality of Fertility and First Sexual Intercourse Histories. The Journal of Human Resources. 2001;36(3):520–555. [Google Scholar]