Abstract
This review discusses the occurrence and distribution (within a plant) of methyl eugenol in different plant species (> 450) from 80 families spanning many plant orders, as well as various roles this chemical plays in nature, especially in the interactions between tephritid fruit flies and plants.
Keywords : allomone, attractant, Bactrocera, chemical ecology, floral fragrance, insect pollinators, plant-insect interactions, plant semiochemicals, sex pheromone, synomone, tephritid fruit flies
1. Introduction
Plants produce a huge array of chemicals, numbering tens of thousands, primarily for defense against herbivores and pathogens as well as for production of floral fragrance to attract pollinators. Among them is a class of phenolics that consists of a group of compounds known as phenylpropanoids. The phenylpropanoids have numerous functions in plants, ranging from structural constituent, growth, and reproductive biochemistry and physiology to chemoecological interactions with microbes, animals (particularly insects), and neighboring plants.
Methyl eugenol (ME) CAS No. 93-15-12 (Figure 1) is a phenylpropanoid chemical with many synonyms: 4-allylveratrole; 4-allyl-l,2-dimethoxybenzene; eugenyl methyl ether; 1,2-dimethoxy-4-(2-propenyl)benzene; 3,4-dimethoxy-allylbenzene; 3-(3,4-dimethoxyphenyl)prop-l-ene; O-methyleugenol; and methyl eugenol ether. It is directly derived from eugenol, a product from phenylalanine (an essential amino acid) through caffeic acid and ferulic acid via ‘the shikimate pathway’ (Herrmann and Weaver 1999). It is a common phenylpropanoid found in many plant species, particularly in spices and medicinal plants. Furthermore, this chemical can be converted to other useful phenylpropanoids either to elemicin or myristicin, and then, in the latter compound, to dillapiole, via the regulation of two genes in Perilla frutescens (Lamiaceae) (Koezuka et al. 1986).
Figure 1.
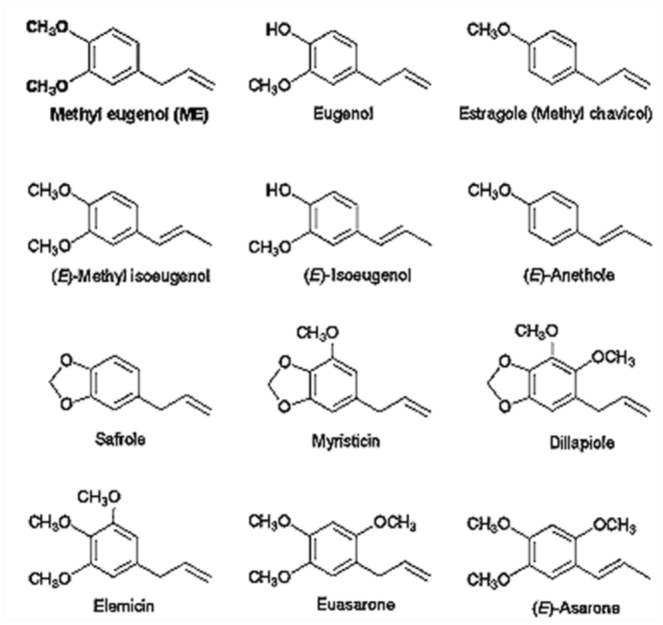
Chemical structures of methyl eugenol (ME) and its analogs. High quality figures are available online.
Synthetic ME has been used extensively: a) as a flavoring agent in many types of processed food, soft drinks, and sauces; b) in perfumery; and c) as an essential oil in aromatherapy. From an entomological perspective, synthetic ME has been successfully used in: a) fruit fly surveys (Tan and Lee 1982) and quarantine detection (see reviews by Metcalf and Metcalf 1992; Vargas et al. 2010); b) estimation of native fruit fly populations (Steiner 1969; Newell and Haramoto 1968) and survival rates in natural ecosystems (Tan 1985; Tan and Jaal 1986); c) determining the relationship between fruit phenology and native fruit fly population dynamics (Tan and Serit 1994); d) monitoring movement of native fruit flies between different ecosystems (Tan and Serit 1988); and e) control of tephritid fruit flies (Diptera: Tephritidae) via male annihilation technique through mass trapping (see review by Vargas et al. 2010).
2. Methyl eugenol in nature
The role of ME in citronella grass, Cymbopogon nardus (Poaceae), in the strong attraction of Dacus (currently Bactrocera) fruit flies which also visited other plant species including flowers of papaya and Colocasia antiquorum, was first discovered almost a century ago (Howlett 1915). Sixty years later, ME was found to be the most active attractant for the oriental fruit fly, Bactrocera dor salis, when compared with 34 chemical analogs (Metcalf et al. 1975). Since then, about 20 plant species from 16 families were reported to contain ME, and the role of chemicals as plant kairomone in dacine fruit fly ecology has been discussed (Metcalf 1990; Metcalf and Metcalf 1992). Additionally, eight plant species containing 0.1–17.9% ME as a natural constituent, and another seven plant species with ME but without quantitative data, were reported by De Vincenzi et al. (2000). Prior to this review, it was reported that a) ME was present in 20 angiosperm and 3 gymnosperm families (Schiestl 2010); and b) ∼350 plant species belonging to 61 families possessed ME as a constituent component and/or as a component of floral fragrance (Tan et al. 2011).
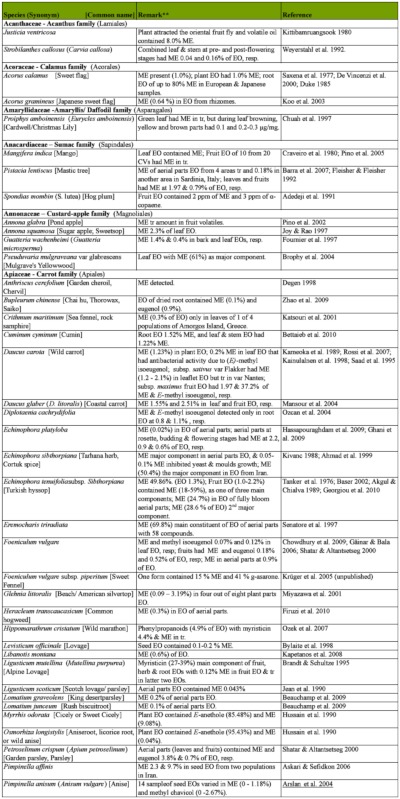
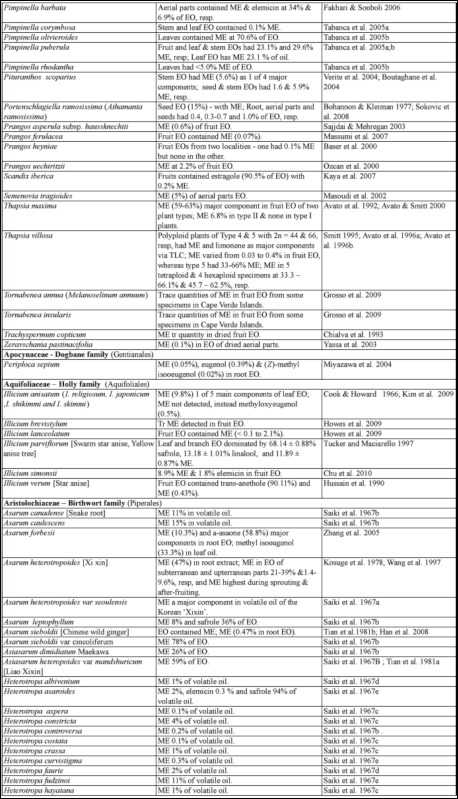
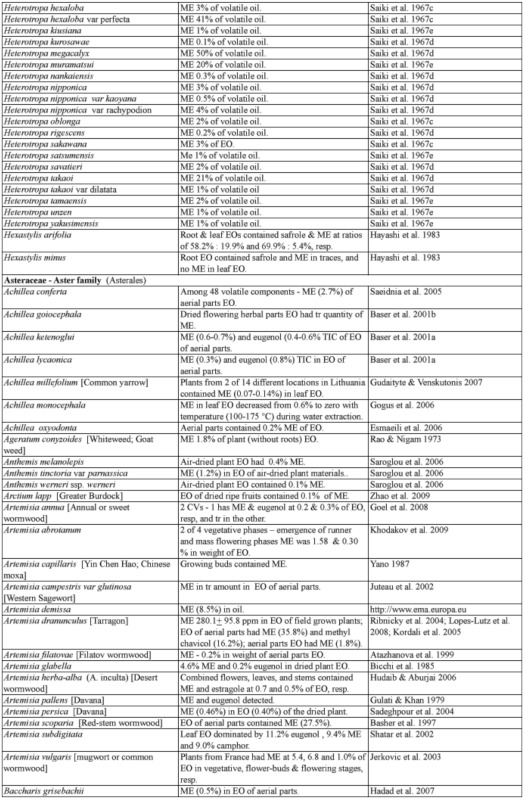
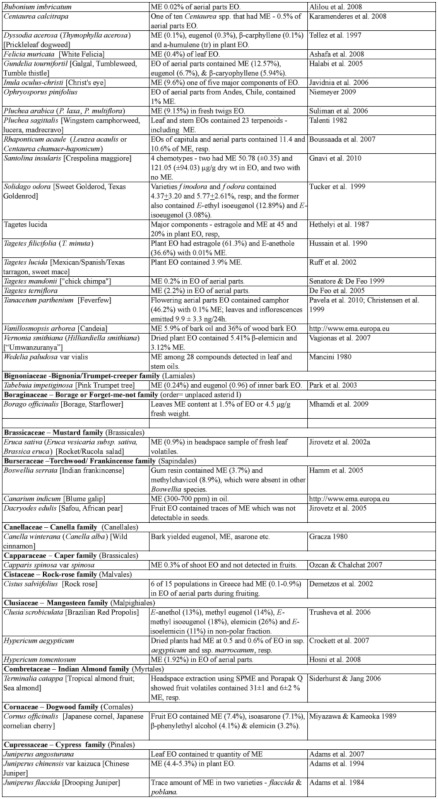
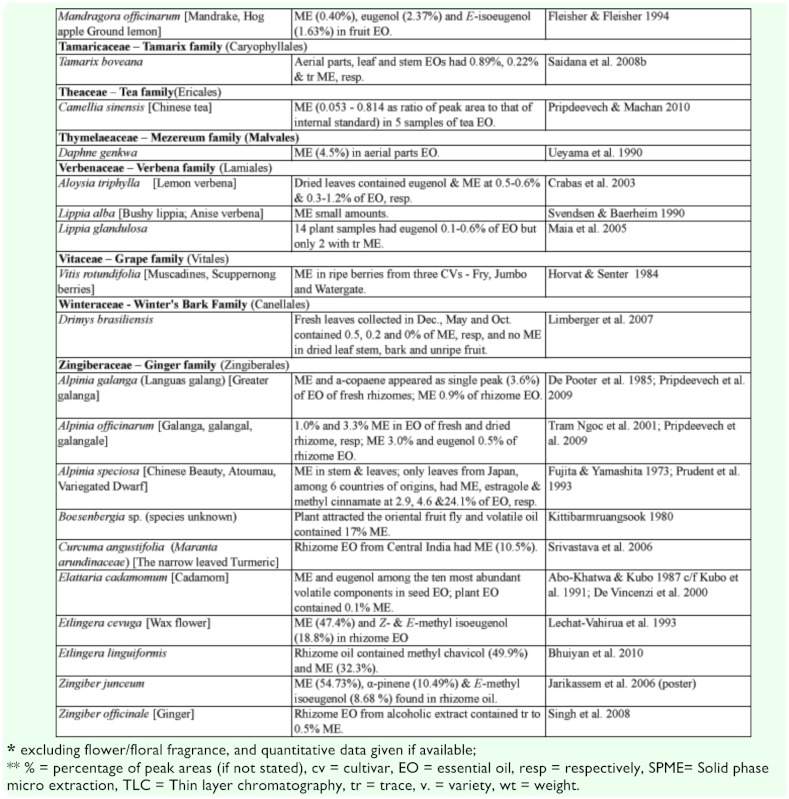
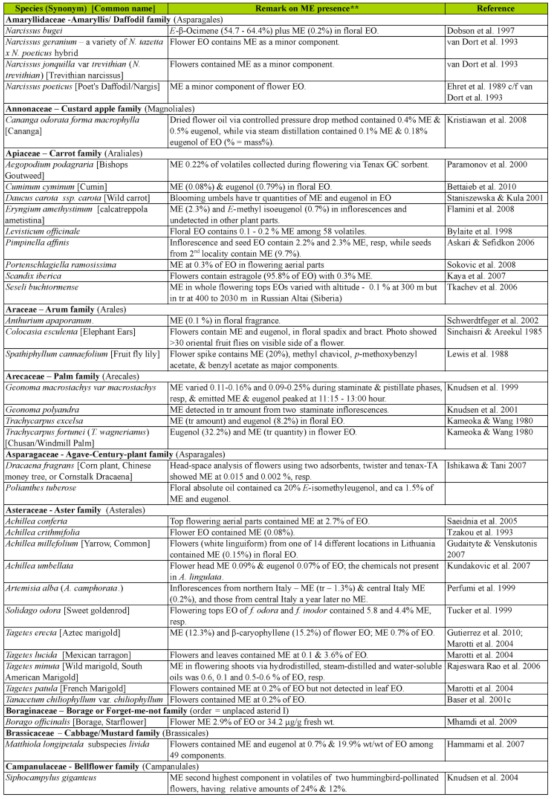
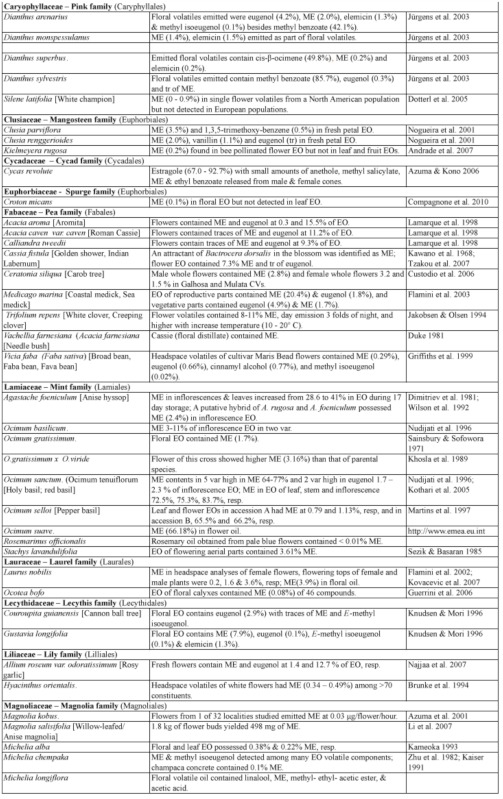
2.1. Occurrence of methyl eugenol
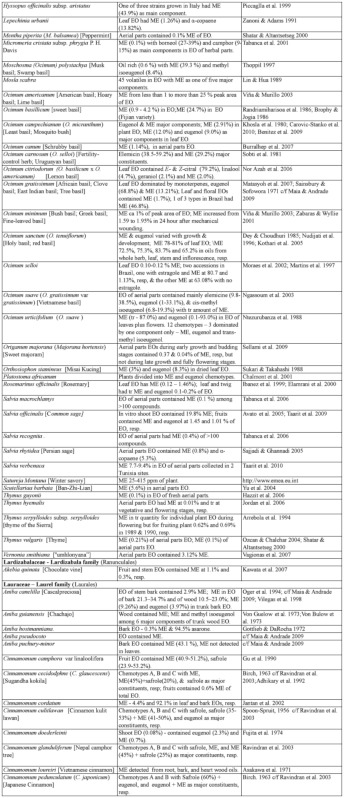
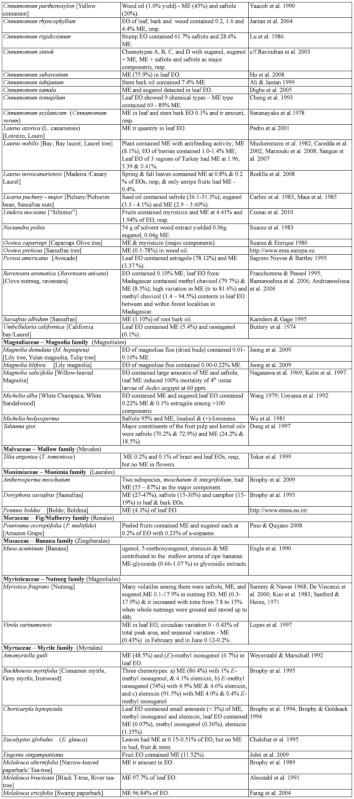
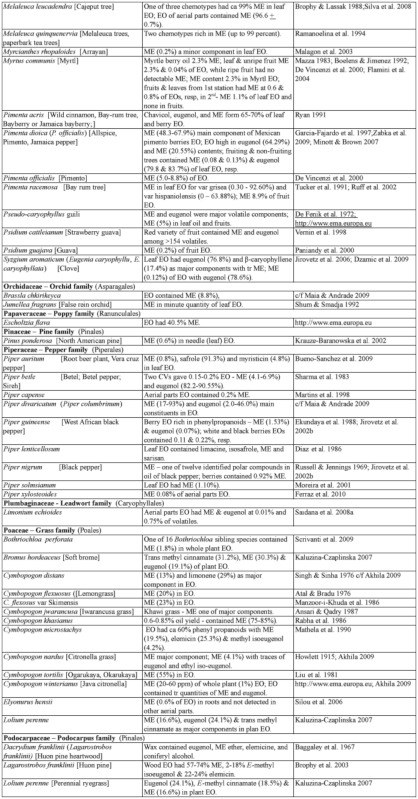
From an intensive literature search conducted over the first half of 2011, an additional ∼100 were added to the 350 plant species to yield a total of over 450 species from 80 families spanning 38 plant orders that contain varying amounts of ME in essential oils from leaves, roots, stems, flowers, or whole plant extracts. The compiled species are presented here in two separate tables. Table 1 shows over 370 species of plants listed alphabetically from 62 families (one fern, two gymnosperms, four monocots, and 55 dicots) having ME content varying from a trace quantity to 99% of essential oils detected in various plant organs, except flowers (which will be presented in Table 2 in section 3.4). The large number of families involved indicates that biosynthesis of ME evolved independently in many of the Plantae orders and families. Families that are represented by 10 or more species in Table 1, in decreasing order, are Asteraceae (47), Apiaceae (44), Lamiaceae (38), Lauraceae (34), Aristolochiaceae (32), Rutaceae (23), Myrtaceae (20), Poaceae (12), Cupressaceae (10), Euphorbiaceae (10), and Zingiberaceae (10). The ME content varies greatly within and between species as well as within and between the plant families. Several species have ME content over 90% in essential oils, namely Croton malambo (Euphorbiaceae), Cinnamomum cordatum (Lauraceae), Melaleuca bracteata, M. ericifolia, M. leucadendra, M. quinquenervia, Pimenta racemosa (all Myrtaceae), Piper divaricatum (Piperaceae), and Clusena anisata (Rutaceae). Furthermore, 68 species possess ME content between 20 and 90% in essential oils of either a whole plant or a part thereof (Table 1). These plant species are likely to involve ME in their chemical defense against pathogens and/or insect herbivores. Most of the plant species listed in the table are either spices, medicinal plants (many with ethnopharmacological properties), or plants of economic importance, especially in the production of essential oils for aromatherapy and perfumery. As such, many more plant species, currently with little or no anthropocentric importance, may contain ME and await discovery and/or chemical analysis.
Table 1.
Plant family (order) and species containing methyl eugenol (ME)*.
Continued.
Continued.
Continued.
Continued.
Continued.
Continued.
Continued.
Continued.
Continued.
Table 2.
Plant family (order) and species containing methyl eugenol [ME] in flowers*.
Continued.
Continued.
Continued.
Methyl eugenol, as a constituent in leaves, fruits, stems, and/or roots, may be released when that corresponding part of a plant is damaged as a result of feeding by an herbivore. If present in sufficiently high concentration, it will immediately deter the herbivore from further feeding on the affected part (see section 3.2.3). In this case, ME acts as a deterrant or repellant. In many plant species, ME is present along with varying amounts of eugenol—ME's immediate precursor (see section 3.4.2.2 B). Both the compounds are found in most spices.
For plant species with low ME content, this component may be detected only in certain developmental stages. This is demonstrated by the sweet marjoram, Origanum majorana (Lamiaceae), in which ME was detected during the early vegetative and budding stages of four growth stages investigated (Seilami et al. 2009). Similarly, ME was detected in Artemisia abrotanum (Asteraceae) only during the emergence of runners and mass flowering phases among four studied (Table 1). Nevertheless, in Artemisia dracunculus ME was detected at 6.06, 6.40, 38.16, and 7.82 % of essential oil weight during emergence of runners, budding, mass flowering, and seed ripening phases, respectively (Khodakov et al. 2009).
A native Mediterranean plant species with ethnopharmacological properties, Erodium cicutarium (Geraniaceae), was shown to contain a relatively high content of ME (10.6%) in leaf hexane extract (Lis-Balchin 1993). Nevertheless, out of approximately 170 chemical components, many of which existed in trace quantities, ME was not detected in some specimens of the same species (Radulovic et al. 2009). This finding probably reflects geographical variation among varieties or populations and not different extraction methods or chemical analyses.
High variation within a plant species in terms of ME content may lead to the identification of distinct chemotypes. To further illustrate varietal differences in plant species, two common Ocimum species (Lamiaceae), O. basilicum and O. sanctum, which are frequently used for culinary and medicinal purposes in Southeast Asian countries in particular, show distinct variations in terms of ME content. 19 accessions/varieties of O. basilicum (sweet basil), two wild and 14 cultivated as ornamentals in Sudan, two from Germany, and one from United Arab Emirates, had varying contents of phenylpropanoids—eugenol, ME, and methyl cinnamate—in combined leaf and flower essential oils. As indicated by peak area in essential oils, 12 varieties had highly variable content of eugenol from 0.05 to 43.3%, and for methyl cinnamate, 11 varieties had content from 1.9 to 42.4%, of which seven had over 15%. However, only one variety had 8.7% ME without the other two phenylpropanoids, and three had ME in trace amounts (Abduelrahman et al. 2009). Nevertheless, two varieties of the sweet basil found in Malaysia had no eugenol, but ME content was at 5.6-12.3% in leaf and 3.2-11.1% in inflorescence essential oils (Nurdijati et al. 1996).
Ocimum sanctum (holy basil) also varies considerably in terms of ME and eugenol contents in leaf and inflorescence essential oils. Seven varieties of holy basil in Malaysia and Indonesia can be grouped into three chemotypes based on the phenylpropanoid content in leaf essential oils: two as eugenol chemotypes with 66–73% eugenol and 0.5–3.1 % ME, four ME chemotypes with 78–81% ME and 2.7–5.8 % eugenol, and one ME—eugenol chemotype with 52% ME and 27% eugenol (Nurdijati et al. 1996). The phenylpropanoids in the leaves of both sweet and holy basils are not released naturally. They are stored in the numerous oily glands (characteristic of Lamiaceae (formerly Labiatae)). More glands per unit surface area are found on the lower surfaces of leaves in the basils. Healthy leaves on a plant do not attract male Bactrocera fruit flies (see 3.3.1. Insect attractant). However, when any part of the plant (especially the leaves) is damaged or squashed, many male fruit flies are attracted to the damaged part, indicating the release of ME and eugenol. Further, it is very interesting to note that the O. sanctum leaf (chemotype unspecified) essential oil has lipid–lowering and anti—oxidative effects that protect the heart against hypercholesterolemia in rats fed with a high cholesterol diet (Suanarunsawat et al. 2010).
Additionally, another species of Ocimum in Brazil, O. selloi, has two chemotypes. Leaf and flower essential oils of chemotype A contained estragole (methyl chavicol) at 80.7 and 81.8% with ME at 0.79 and 1.13% of peak area, respectively, while chemotype B had ME as the major component at 65.5 and 66.2% of peak area in leaf and flower essential oils, respectively, and with no trace of estragole (Martins et al. 1997).
The same species of plant grown in different countries may show high variation in chemical constituents. This was well illustrated by Alpinia speciosa (Zingiberaceae) in which leaves collected from Japan contained ME, estragole, and (E)methyl cinnamate at 2.9, 4.6, and 24.1% of essential oil. The phenylpropanoids were not detected in leaves that originated from Amazonia (Brazil), Martinique (French West Indies), Rio Grande (USA), and China and Egypt (Prudent et al. 1993).
Furthermore, within a variety of a plant species, the quantity of ME may also vary depending on the plant tissue and on the time of harvest. This is elucidated by Myrtus communis var. italica (Myrtaceae) grown in Tunisia. The quantity of ME varied from 0.4 to 1.9% of leaf essential oil, with > 1% for October, November, and March over a period of 12 months. The monthly ME content of stem oil varied between 0.8 and 3.6%, with January and April > 3%. However, fruits had monthly ME content of 1.1–1.3% for August and September, which then rose to 3% in subsequent months and remained between 3.1–3.6% from October to January (Wannes et al. 2010).
Even during storage, the major components of essential oils may change considerably. This is shown by Agastache foeniculum (Lamiaceae), which contained five major components. During storage of the plants for 17 days, estragole decreased from 63.2 to 50%, with a corresponding increase of ME from 28.6 to 41% in plant essential oil (Dimitriev et al. 1981).
It was shown that green parts of Proiphys amboinensis (Amaryllidaceae) leaves contained a trace quantity of ME, and during browning of a leaf, the yellow and brown parts contained 0.1 and 0.2–0.3 µg/mg of leaf, respectively, that attracted many male fruit flies (Chuah et al. 1997). The attraction phenomenon has never been observed in the normal browning of the leaves, except on one occasion after a raining shower when an infected leaf attracted many male fruit flies(ME—sensitive Bactrocera species) that fed along a yellow—brown border between the green and yellow to brown parts (Figure 2, unpublished observation). The attractant in the browning phenomenon may be induced or produced by microbes as a result of an infection, and this certainly warrants further investigation.
Figure 2.

Male fruit flies (Bactrocera dorsalis and Bactrocera umbrosa) feeding along yellow—brown border of an infected leaf of Proiphys amboinensis. High quality figures are available online.
Besides large variation within species, differences between species within a genus frequently occur. For example, the genus Heterotropa (Aristolochiaceae) possesses species with ME content ranging from 0.1 to 50% of volatile oil. Many of the 27 species have ME content below 5% of volatile oil, except for H. fudzinoi (11%), H. muramatsui (20%), and H. megacalyx (50%). Eleven species of Artemisia (Asteraceae) have ME in trace quantities (e.g., A. campestris), whereas A. dranunculus has an ME content of 35.8%. Similarly, high variation in ME content exists for genera Ocimum (Lamiaceae), Cinnamomum (Lauraceae), and Melaleuca (Myrtaceae), in which most species are known to have relatively high ME content (Table 1). Strangely, many species in the genus Croton (Euphorbiaceae) contain ME in aerial parts (stems and leaves) except Croton microns, which has ME in flowers but not in leaves (Compagnone et al. 2010). It was found that shading from the direct sunlight also affected the content of phenylpropanoids in leaves. Ocimum selloi seedlings from the same population grown under normal sunlight and two different shadings, blue and red, showed a change in two phenylpropanoids, estragole and ME. The leaf estragole content under full sunlight, blue shading (with transmittance of 400–540 nm), and red shading (with transmittance of > 590 nm), was 93.2, 87.6, and 86.1% (relative percentage of peak area), respectively. While for leaves, the ME content was 0.6% under full sunlight and 1.1% under both types of shading (Costa et al. 2010).
2.2 Distribution of ME in various plant organs
The distribution of ME among plant organs is never even as illustrated by many of the species listed in Table 1. A Brazilian folk medicine plant, Kielmeyera rugosa (Caryophyllaceae), possesses ME only in flowers and not in leaves and fruits; the showy flowers are pollinated by large bees (Andrade et al. 2007). Valeriana tuberosa (Valerianaceae), a medicinal plant used as a mild sedative, commonly found in Greece, has eugenol and ME in similar quantities (∼0.45% of oil) in inflorescences but none in roots, stems, or leaves (Fokialakis et al. 2002).
Another medicinal plant, bay laurel Laurus nobilis (Lauraceae), is known to have antibacterial, antifungal, anti-inflammatory, and anti—oxidative properties. It was reported to contain ME in all its aerial parts but in different quantities, such as 3.1, 11.8, 4.7, and 16 % of flower, leaf, bark, and wood essential oils, respectively (Fiorini et al. 1997). Recently, 10 populations of wild bay laurel found in Tunisia had ME at 13.1–33.6, 6.6– 17.8, 1.0–16.8, and 3.9–14.3 percentage composition of essential oil in stems, leaves, buds, and flowers, respectively (Marzouki et al. 2009). In another study on the same species, plants from Turkey had ME content that varied considerably between old and young leaves at 1.2 and 0.2% of volatile composition, respectively, while buds had 0.3% and fruits had 0.1% ME, with no ME detected in flowers (Kilic et al. 2004). Additionally, flowers of Myrtus communis var. italica (Myrtaceae) contained ME at 4.02% of the essential oil as one of seven major components, but as a minor component in leaves and stems at 0.38 and 0.22% of the essential oil, respectively (Wannes et al. 2010)
The amount of ME emitted from flowers of carob tree, Ceratonia siliqua (Fabaceae), varies considerably. Whole hermaphrodite flowers did not emit ME, male flowers emitted 2.8% ME of total volatiles, and female flowers of cultivars Galhosa and Mulata emitted 32 and 1.5% of total volatiles, respectively. In this species, the stamens and stigmas did not emit ME, but the nectar disk (source of most volatiles) of hermaphrodite, male, and female flowers emitted 0.8, 1.7, and 4.7–5.7% ME of total volatiles, respectively (Custodio et al. 2006). Whole flowers of Clarkia breweri from some plants emit eugenol, isoeugenol, ME, and methyl isoeugenol, while those for other plants do not emit ME and methyl isoeugenol. For flowers that emit all the four phenylpropanoids, the petals emit on average ME, methyl isoeugenol, and eugenol approximately 2.5, 1.8, and 0.5 µg/flower/24 hours, respectively, without any isoeugenol. In contrast, pistils and stamens emit only a single component of methyl isoeugenol and ME in very low quantities (Wang et al. 1997). This and the preceeding examples clearly show that the phenylpropanoids are distributed or released unevenly among different parts of individual flowers. All these species show that distribution or release of ME varies even in different parts of individual flowers.
Fruit of Myrtus communis var. italica showed variation in many of its 48 volatile components during development and ripening. As to its ME content, it increased slightly during the initial stage of development when the fruit was green in color from 1.14 to 1.26 % (wt/wt) during 30 to 60 days after flowering. Then, ME concentration increased two-fold when the fruits were pale yellow from 3.05–3.30% during 90–120 days after flowering. A slight increase was noted when the fruits ripened and turned dark blue (Wannes et al. 2009).
Calamus or sweet flag, Acorus calamus (Acoraceae), is a unique medicinal plant in that, unlike many other species in which ME is mainly found in aerial parts, it has ME in the roots. In this species, aerial parts contained only about 1% ME but root essential oil contained up to 80% ME, particularly in the European and Japanese samples (Duke 1985). In this species, the high ME content may be used as chemical defense against root-feeding insects or nematodes.
The distribution of ME within a plant is clearly uneven. In many species, ME may be detected in a specific plant part but not in other parts. Intraspecific chemical variation may be the result of several phenomena, namely: a) adaptation to different pollinator species, b) random genetic drift, c) adaptation to disruptive learning processes in pollinators among non—rewarding flowers, and d) introgression effects involved in hybridization (Barkman et al. 1997). Another possible phenomenon is the selection pressure exerted by herbivores, microbes, and nematodes in their interactions with plants (see section 3.2).
3. Role of methyl eugenol in plants
There are two main theories on the evolution of secondary plant metabolites. First, due to oxidative pressure and the possibility of photo—damage, plants might have developed secondary plant metabolites with antioxidant properties, namely flavonoids, to prevent cellular damage by highly reactive chemicals (Close and McArthur 2002; Treutter 2005). The second theory states that it arose from the relationship between plants and various groups of herbivores or pathogens (Dicke and Hilker 2003; Franceschi et al. 2005), and this latter view is further substantiated in this review.
3.1. Induction of phenylpropanoid biosynthesis due to stress
Phenylpropanoids form a large subclass of chemical compounds within the class of phenolics. All of them are derived from cinnamic acid/p—coumaric acid, which in turn is derived from phenylalanine, an essential amino acid, catalyzed by an enzyme, phenylalanine ammonia lyase (see 3.4.2.B below). This enzyme is the branch—point enzyme between primary (shikimate pathway) and secondary (phenylpropanoid) metabolisms. Many simple and complex phenylpropanoids may be induced in plants by external stresses, such as high ultra—violet light, pathogen attack, and physical —wounding, such as that caused by herbivory (see review by Dixon and Palva 1995). The cytochromecytochrome-p450s-dependentp450s-dependent oxygenases, belonging to a large plant gene family, are involved in primary metabolism, such as in steroid and phenylpropanoid biosynthesis, and secondary metabolism. A similar phenomenon also exists for O-methyltransferase enzymes that are involved in primary metabolism, namely lignin synthesis and secondary metabolism, such as phenylpropanoid biosynthesis (Pichersky and Gang 2000).
Essential oils of three untreated orange varieties of Citrus sinensis (Rutaceae)— Hamlin, Pineapple and Valencia—did not contain any ME. But, when treated with abscission agents to loosen fruits for mechanical harvesting, six phenylpropanoids, namely eugenol, ME, (E)- and (Z)-methyl isoeugenol, elemicin, and isoelemicin, were detected for the first time. Among these compounds, ME was the most abundant component present at 42 ppb in orange juice from the treated fruits (Moshonas and Shaw 1978). This study clearly shows induction of phenylpropanoid biosynthesis in fruit under stress. The role of ME in the treated orange is unclear, however.
3.2. Defense
Plants produce a large diversity of chemical compounds to deter phytophagous organisms, especially against insect herbivores and/or pathogens. These chemicals may exist as plant primary constituents or as secondary byproducts/metabolites. They have diverse biochemical and physiological activities against a) pathogenic microbes, b) competitive/neighboring plant species, and c) herbivores. Plant chemical constituents that are not secreted naturally, and affect animal behavior in self—defense by acting as a toxicant, antifeedant, deterrant, irritant, repellant, and/or growth regulator, act as para—allomones (an allomone is a naturally secreted chemical that benefits only the releaser in an interaction between two species of organisms).
3.2.1. Microbes.
Essential oils and ME have been known for a long time to possess antifungal activity. ME and eugenol have similar antifungal activity against seven species of fungus at 2.0 mM concentration (Kurita et al. 1981). The essential oil of Echinophora sibthorpiana (Apiaceae) contains ME, and the oil (∼0.1%) or ME alone (at 0.05–0.1%) showed some inhibitory activity against fungi and bacteria (Kivanc 1988). At temperatures 5–15 °C, 1000 ppm ME delayed mold's initiation of mycelium and spore development in 32 strains: four of Aspergillus ochraceus, two A. niger, 16 Penicillium clavigerum, and 10 P. expansum (Kivanc and Akgul 1990). Furthermore, sprays of 0.5% ME on peanut pods and kernels prevented colonization of Aspergillus flavus, common mold, and inhibited aflatoxin synthesis in the fungus. Consequently, it was suggested that ME be used to prevent infestation of the fungus in peanuts (Sudhakar et al. 2009).
Fruit essential oil of emblica, Phyllanthus emblica (Euphorbiaceae), that contained 1.25% ME among eight major components had high antimicrobial activity against contaminating microbes, such as: a) Gram— positive bacteria, e.g., Bacillus subtilis and Staphylococcus aureus; b) Gram—negative bacteria, e.g., Escherichia coli, and Salmonella; c) molds, e.g., Aspergillus niger and A. oryzae; and d) the budding yeast, Saccharomyces cerevisiae. The antimicrobial activity of the oil was mainly due to the presence of ME, β-caryophyllene, βbourbonene, and thymol (Zhao et al. 2007). Recently, another fruit essential oil of Eugenia singampattiana (Myrtaceae) had major constituents, namely, a-terpineol (59.6%), camphene (12.1%), ME (11.5%), and αpinene (4.7%). A minimum inhibitory concentration (MIC) at 0.2 µL/mL of the essential oil yielded complete inhibition against Candida albicans (a form of yeast that causes infections such as “thrush”) (Jeya Johti et al. 2009).
The growth of a strain of Campylobacter jejuni, a major bacteria species causing gastroenteritis in humans worldwide, was inhibited by essential oil of carrot, Daucus carota (Apiaceae), as well as individual component of ME and elemicin at a MIC of 250 µg/mL, which was slightly less effective than methyl isoeugenol at MIC of 125 µg/mL (Rossi et al. 2007).
3.2.2. Nematodes.
The pinewood or pine wilt nematode, Bursaphelenchus xylophilus, is very damaging to matsutake mushroom cultivation in addition to causing pine wilt. Nematicidal activities against the nematode were demonstrated with LC50 (lethal concentration that induces mortality in 50% of test organisms) values for geranial, isoeugenol, methyl isoeugenol, eugenol, and ME at concentration of 0.120, 0.200, 0.210, 0.480, and 0.517 mg/mL, respectively (Park et al. 2007).
3.2.3. Antifeedant.
Plant ME in the growing bud of Artemisia capillaries was found to inhibit feeding (100% antifeeding activity on 2 cm diameter leaf disc) by larvae of the cabbage butterfly, Pieris rapae subspecies crucuvera (Katsumi 1987). In addition, ME was the most potent of seven eugenol analogs in essential oil of Laurus nobilis against a noctuid moth white—speck, Mythimna unipuncta (Muckensturm et al. 1982).
A fresh water aquatic plant Micranthemum umbrosum (Scrophulariaceae) possesses elemicin, a phenylpropanoid as one of two chemicals used in chemical defenses against herbivores, which acts as an antifeedant against generalist consumers such as crayfish (Procambarus acutus). To determine the structure—activity relationship among eight naturally occurring phenylpropanoids, bioassays were conducted and showed that ME was most active and much more effective than either eugenol or elemicin in deterring feeding by crayfish (Lane and Kubanek 2006).
3.2.4. Insects.
Of the nine major constituents of essential oils, benzene derivatives (eugenol, isoeugenol, ME, safrole, and isosafrole) are generally more toxic and repellent to the American cockroach, Periplaneta americana, than the terpenes (cineole, limonene, pcymene, and a-pinene). Furthermore, ME was most effective in terms of knockdown activity, as well as repelling and killing effects (Ngoh et al. 1998).
Toxicity of ME against larvae of the tobacco armyworm, Spodoptera litura, was found to be significant. Larvicidal activity of a residual ME (15 µg/leaf cm2) was 36.0 ±15.3% and 76.6 ±11.5% for 24 and 48 hours of exposure, respectively (Bhardwaj et al. 2010). However, as to mosquitocidal impact, ME, found only in leaves of Magnolia salicifolia (Magnoliaceae), induced 100% mortality at 60 ppm against 4th instar larvae of the yellow fever mosquito, Aedes aegypti, which is responsible for the spread of dengue fever and Chikungnya viruses (Kelm et al. 1997).
In a fumigation study comparing the toxicity of more than a dozen monoterpenes against the rice weevil, Si tophi lus oryzae (Coleoptera: Curculionidae), ME and eugenol were moderately toxic compared to the most toxic compound tested, menthone (Lee et al. 2001). The latter was the main chemical component in Mentha arvensis (Lamiaceae) var. piperascens essential oil, which in turn was the most toxic among 16 medicinal and spice plants tested. Nonetheless, ME was the most potent inhibitor against the acetylcholine esterase (Lee et al. 2001), an enzyme responsible for the hydrolysis of the neurotransmitter acetylcholine, which can eventually lead to paralysis. Similarly, fruit essential oil of Illicium simonsii (Aquifoliaceae) that contained βcaryophyllene (10.30%), δ-cadinene (9.52%), and ME (8.94%) as major components had strong fumigant and contact toxicities against adults of the maize weevil, Sitophilus zeamais, with LC50 values of 14.95 mg/L air and 112.74 µg/adult, respectively (Chu et al. 2010). Fumigant and repellant effects, leading to almost 100% mortality within 24 hours, were observed on adult brown plant hoppers, Nilaparvata lugens, feeding on rice seedlings placed over a filter paper containing ME residue at ∼0.15mg/cm2 (Tan, unpublished data).
It is interesting to note that ME as a fumigant was also very toxic to two global pest fruit fly species—the Mediterranean fruit fly, Ceratitis capitata, and the melon fly, Bactrocera Cucurbitae (a cue—lure/ raspberry ketone [RK] responsive species)—compared with basil oil, linalool, estragole, and (E)-anethole, all of which showed no knockdown effect at 0.75% concentration (Chang et al. 2009). After two hours of exposure to ME at concentrations of 0.5 and 0.75%, mortality/ knockdown was 96 and 100% against C. capitata and 98 and 97% against Ba. Cucurbitae. However, ME was less toxic as a fumigant, even though it was a strong attractant, to the oriental fruit fly, Ba. dorsalis. Concentrations of 10–100 % induced 35–53% mortality/knockdown against this species (Chang et al. 2009).
3.3. Chemical cue
Certain insect species have adapted to using ME as a stimulant or attractant to locate plant host or source for pharmacophagy (consumption of non—nutritive and nonessential chemicals).
3.3.1. Insect attractant.
Some insect species are known to be attracted to ME for unknown reasons, while others may be attracted and stimulated to undergo pharmacophagous feeding.
3.3.1.1. Pest insect species.
Two scarabid pest species, Cetonia aurata aurata and Potosia cuprea, were captured in traps baited with a known attractant consisting of ME, 1-phenylethanol, and (E)-anethole (1:1:1). However, the numbers trapped were significantly increased for both the species with the addition of a synergist, either geraniol or (+)-lavandulol (Vuts et al. 2010). Larvae of the rice stem borer, Chilo suppressalis, are attracted to “oryzanone” (pmethylacetophenone), and ME among 30 compounds related to the “oryzanone” also attracted the larvae (Kawano and Saito 1968). Although ME is not present in rice plants, it may be interesting to evaluate the impact of ME on stem borer physiology and behavior.
Two Dacus (currently Bactrocera) (Diptera: Tephritidae) species of fruit flies were first discovered to be attracted to citronella grass Cymbopogon nardus used as a mosquito repellant (Howlett 1912). Subsequently, ME was positively demonstrated to be solely responsible for the attraction (Howlett 1915). Since then, voluminous publications related to fruit fly attraction to ME have appeared. It should be pointed out at this juncture that all Bactrocera species may be categorized into three groups based on their response to two potent attractants: cue—lure, a synthetic analog of RK (195 species cue—lure responders, this chemical being a synthetic of RK) and ME (∼84 ME responders), and non—responders to the attractants (28 species confirmed and 258 species listed under “lures unknown”) (IAEA 2003). The effects of the attractants on sexual behavior of Bactrocera fruit flies have recently been reviewed (Shelly 2010).
ME acts as a precursor or booster to male fruit fly sex pheromonal component(s) in the rectal gland of certain Bactrocera species (Nishida et al. 1988, 1990, 1993; Tan and Nishida 1995, 1996, 1998). Plant ME, when released, attracts only male fruit flies, although there are two reports of wild females being attracted into traps baited with poisoned synthetic ME (Steiner et al. 1965; Verghese 1998). The attraction of females was probably due to a chemical contamination—perhaps male sex pheromonal components from spontaneous ejaculation induced by the poisoned bait prior to death of captured males. In contrast, no female Bactrocera dorsalis or Ba. umbrosa was ever attracted to or captured in ME— baited clear—traps, without an insecticide, used in the ‘capture—mark—release—recapture’ technique to capture thousands of live wild males for ecological and population studies in areas with high fruit fly infestation (Tan 1985; Tan and Jaal 1986; Tan and Serit 1988, 1994). These field studies further confirm that pure ME is a male attractant, although ME did induce an electrophysiological response in the antennae of Ba. dorsalis females (Siderhurst and Jang 2006) that may be translated into a negative rather than positive attraction response under natural conditions. Male fruit flies do not directly cause harm or damage to plants by just feeding on ME.
Several putative and ME—sensitive sibling species of the Bactrocera dorsalis complex, such as Ba. carambolae, Ba. caryeae, Ba. dorsalis, Ba. invadens, Ba. kandiensis, Ba. occipitalis, Ba. papayae, and Ba. philippinensis form the most serious group of pests of fruits and vegetables. Males are strongly attracted to and compulsively feed on ME, which acts as a) a sex pheromone precursor in Ba. dorsalis and Ba. papayae— the latter shown to be neither distinct biological nor genetic species from the former (Naeole and Haymer 2003; Tan 2003; Zimowska and Handler 2005), in which ME is converted mainly to (E)-coniferyl alcohol and 2-allyl-4,5-dimethoxyphenol (Nishida et al. 1988; Tan and Nishida 1996, 1998; Hee and Tan 2004); and b) a booster component to endogenously produced sex pheromone in Ba. carambolae, where it is biotransformed to only (E)-coniferyl alcohol (Tan and Nishida 1998; Wee et al. 2007). Recently, it was reported that the extremely invasive species in Africa, Ba. invadens, and in the Philippines, Ba. philippinensis, convert consumed ME to the same ME metabolites in similar ratio as Ba. dorsalis, and they belong to the same species clade, while Ba. zonata biotransformed ME to 2-allyl-4,5-dimethoxyphenol and (Z)-coniferyl alcohol, and Ba. correcta to (Z)-3,4-dimethoxycinnamyl alcohol and (Z)-coniferyl alcohol (Tan et al. 2011 a,b).
Consumption of ME has been shown to significantly improve male mating competitiveness in Ba. dorsalis (Shelly and Dewire 1994, 2000; Tan and Nishida 1996, 1998), Ba. carambolae (Wee et al. 2007), Ba. correcta (Orankanok et al. 2009), and Ba. zonata (Quilici et al. 2004; Sookar et al. 2009). Wild fruit fly males have easy access to natural sources of ME (Tan 2009). Therefore, it would be desirable to feed sterile males with ME in order to compete with wild males “on a level playing field”, before mass release so as to enhance mating success in a sterile insect technique (SIT) program (Shelly et al. 2010).
3.3.1.2. Beneficial insect species.
The green lacewing, Ankylopteryx exquisite, was attracted to ME—baited traps set up in two locations in central Taiwan in large numbers (350–800 adults/trap/two weeks during July) (Pai et al. 2004). Additionally, adults of another lacewing, Chrysopa basalis, were captured in plastic traps containing ME (Suda and Cunningham 1970). The reason for their attraction to ME for these predatory insects is still unclear. This is also the case for the weak attraction of honeybees, Apis mellifera, to traps baited with ME in high elevation native forest in Hawaii. The number captured varied with seasons, and it was found that more honeybees were captured in March and between June and August (Asquith and Burny 1998). The numbers trapped certainly did not reflect capture due to chance. Therefore, could the worker honeybees be mistakenly guided into ME traps through previously learned odor of ME resembling floral fragrance of golden shower or other flowers (see below)? Perhaps this question may be satisfactorily answered through proper electrophysiological and chemoecological investigations.
3.4 Methyl eugenol in flowers—ME as attractant and floral reward
Many plants, besides fending off insect herbivores, may require insects to assist in pollination. Recently, Knudsen et al. (2006) reviewed many aspects of floral scent with respect to variation within and between congeneric species belonging to a genus. They listed 12 common compounds, namely limonene, (E)-ocimene, myrcene, linalool. aand b-pinene, benzaldehyde, methyl 2-hydroxybenzoate, benzyl alcohol, 2-phenylethanol, caryophyllene, and 6-methyl-5-hepten-2-one that are detected in floral scent from over 50% of seed plant families, and also provided a list of 1719 compounds identified from floral fragrances. ME was among the compounds listed and was detected in 21 plant families. Nonetheless, many more plant species produce flowers that possess ME that may be released as a component in floral fragrance. Table 2 shows ∼122 species from 42 plant families, many of which (∼85 species from 22 families) have ME detected exclusively in flowers or floral fragrances. This further substantiates the notion that synthesis of floral ME evolved independently in different plant families and orders. However, 27 species, namely Cuminum cyminum, Daucus carota, Pimpinella affinis, and Scandix iberica (Apiaceae), Achillea conferta, Solidago odora, and Tagetes lucida, (Asteraceae), Borago officialis (Boraginaceae), Medicago marina (Fabaceae), Agastache foeniculum, Ocimum basilicum, O. gratissimum, O. sanctum, O. selloi, O. suave, and Rosemarinus officionalis (Lamiaceae), Laurus nobilis (Lauraceae), Michelia alba (Magnoliaceae), Myrtus communisand and Syzygium aromaticum (Myrtaceae), Piper betel (Piperaceae), Cymbopogon flesuosus (Poaceae), Rosa damascena and R. hybrida (Rosaceae), Tamarix boveana (Tamaricaceae), Daphne genkwa (Thymelaceae), and Lippia alba and Lippia schomburgkiiana (Verbenaceae) also have ME detected in other plant parts (Tables 1 and 2).
Except for several species, neither the role of ME in flowers nor the attraction of fruit flies was mentioned in the published articles. However, if ME is released naturally in an area where Bactrocera fruit flies are present, the flowers would have attracted the ME— responsive Bactrocera species.
Much of the published work on floral chemical composition with detected ME did not indicate the type of floral visitors or pollinators. While some species of Dianthus (Caryophyllaceae) had flowers that bloom at night, these flowers attracted nocturnal insects, such as moths, and bats as visitors/pollinators (Jurgens et al. 2003). Mediterranean flowers of Dianthus arenarius, D. monspessulanus, D. superbus, and Silene officinalis are whitish in color and strongly scented (especially during the night), indicating pollination by night—active flower visitors. Another species, Silene latifora, in the same family bears night flowers. The flowers from a European population had no detectable ME, whereas those collected from some plants in a North American population had detectable ME. However, the flowers did not exclude diurnal flower visitors, because unlike some nocturnal Silene species, they did not close or wilt during the day following anthesis. Nevertheless, there were clear differences in the floral scent of diurnal butterfly—flowers and moth— or hawkmoth— pollinated nocturnal species. According to Jurgens et al. (2003), the phenylpropanoids such as ME, methyl isoeugenol, elemicin, (Z)-asarone, and (E)-asarone were only found in the nocturnal Dianthus species.
Flowers from other families, similar to those of the family Caryophyllaceae, may attract other insects in regions/countries without ME—responsive Bactrocera species. Therefore, these flowers are not specifically adapted to fruit fly pollinators even though they possess ME.
3.4.1. ME in flowers with unknown purpose.
From 16 Clusia species (Clusiaceae) under four different taxonomic sections, only two species, C. parviflora (section Criuva) and C. renggerrioides (section Corylandra) possessed floral ME (Nogueira et al. 2001). The role of ME in the two species is still unknown. This is similar to the often—cited flowers of golden shower or Indian labernum, Cassia fistula, that contained ME and attracted the oriental fruit fly, Ba. dorsalis (Kawano et al. 1968). Recently, the flower essential oil was reported to contain ME at 7.3% of peak areas and trace amount of eugenol; these compounds were not detected in leaf oil (Tzakou et al. 2007). Unfortunately, there is still no report that the attracted fruit flies are either potential pollinators or just visitors.
Cymbopogon flexuosus (Poaceae) exists as four varieties based on the major component among approximately 75 constituents in inflorescence essential oils. The varieties of C. flesuosus (var. arunachalis, var. assamensis, and var. sikkimensis) had citral, citronellol, elemicin, and ME as the major component, respectively. The first two varieties did not possess floral ME. The var. sikkimensis had 32–34% floral ME, while var. assamensis had 0.2–0.4% of essential oils (Nath et al. 2002). As such, the former variety would be more attractive to ME—responsive Bactrocera species than the latter. Nevertheless, this attraction of fruit flies as either pollinators or visitors remains to be determined for the two varieties. This is expected as most floral fragrances contain many chemical components (sometimes well over a hundred), and to ascribe the actual role for each of the ingredients, especially those in trace quantities, is extremely difficult, time consuming, and often unrewarding.
In the family Orchidaceae, many species are known to have trace quantities of ME. Since some of them are known to exist in regions with no insect species that are specifically attracted to ME or flowers in the night (Table 2), it is obvious that the ME—sensitive Bactrocera species play no role in pollination. However, flowers of the Malayan type of Phalaenopsis violacea possess trace quantities of ME and eugenol (Kaiser 1993), and usually attract one to several fruit flies per flower. The trace amount of floral ME is sufficient to attract fruit flies, since ∼ 1 nanogram (10-9g) of ME spotted on a silica gel TLC plate placed in the field can attract native male flies of the ME—sensitive species, such as Ba. dorsalis (Tan and Nishida 2000). The Bornean type of this orchid species, which is currently placed as a different species, P. bellina, has none of the phenylpropanoids (Kaiser 1993), although their flowers appear very similar in terms of color pattern and morphology to the untrained eye. As such, the observed attraction of fruit flies to P. bellina was probably due to the presence of 2,6-dimethoxy-4-(2-propenyl)-phenol. This compound was emitted as a component of floral fragrance at a rate of 12.0 ± 8.5 ng/flower/hour (Hsiao et al. 2006). It is an isomer of 2-allyl-4,5-dimethoxyphenol, which is a relatively strong fruit fly attractant and a component of the oriental fruit fly sex pheromone after ME consumption. Interestingly, P. violacea has no special adaptation, such as a movable lip as in Bulbophyllum orchids (see section 3.4.2.2 B), to aid in the removal of pollinarium (a composite structure of pollinia containing numerous pollens, a tegula/hamulus stipe, and visidium). This is further substantiated by our observations that the ME—sensitive fruit fly males never removed pollinarium from flowers of P. violaceae, are mere visitors, and thus do not assist in pollination for this orchid species.
It has been proposed that an additional role of floral fragrance may be in defense to deter or repel insect herbivores/florivores, as many of the floral volatile compounds are also released from leaves in response to herbivore damage (Kessler and Baldwin 2001). This is further substantiated by ME, which is used by plants as a chemical defense as previously discussed in section 3.2. Therefore, floral ME, which appears not to have any specific function in pollination, may be playing a ‘silent’ role in deterring and/or repelling possible insect florivores.
3.4.2. In pollination.
Floral fragrance is presumably for the sole purpose of guiding potential pollinators to perform pollination that results in fertilization of flowers. The presence of ME in floral fragrances, even in trace quantities, may be responsible for attracting potential Bactrocera pollinators in the tropical/subtropical regions where the ME—responsive species of fruit flies are endemic.
3.4.2.1. For non—orchid flowers
The fruit fly lily Spathiphyllum cannaefolium (Araceae) floral spadix has a high content of ME (Lewis et al. 1988), which attracts many ME—sensitive Bactrocera male flies to visit and pollinate the flower by transferring white powdery pollens as the flies feed on the spadix. Plants grown in Penang (Malaysia) often attract one or two fruit fly males (Figure 3) as well as stingless bees (Trigona species) for pollination (unpublished observation).
Figure 3.

A male Bactrocera umbrosa feeding on Spathiphyllum cannaefolium spadix. High quality figures are available online.
Another Araceae species, Colocasia esculenta, which contained ME and eugenol (relative quantities not provided), attracted many male Ba. dorsalis fruit flies (> 40) to the spadix and bract (Sinchaisri and Areekul 1985). In this species, only the fruit flies feeding on the spadix will pick up powdery pollens and transfer them to the stigmas on the radix.
Flowers of the cannon ball tree Couroupita guinanensis (Lecythidaceae) contained 3% eugenol with a trace quantity of ME in floral essential oil (Knudsen and Mori 1996). Flowers in tropical South America have been observed to attract many male Ba. carambolae fruit flies in Suriname (photograph shown by van Sauers-Muller, personal communication, 2010). However, the flowers obtained from trees grown in the Botanical Garden in Penang have eugenol and no detectable ME, and they attract many stingless bees (Trigona species) with an occasional Ba. dorsalis as a visitor (unpublished observation).
Paraguay jasmine, Brunfelsia australis (Solanaceae), commonly known as “Yesterday—Today—and—Tomorrow”, has floral fragrances comprised of monoterpenoids (81% of the identified volatile compounds), with ME in trace quantity in young flowers and 0.1% content of mature flowers. But in the scentless mature flowers of a closely related species, Brunfelsia pauciflora (Fabaceae), two sesquiterpenes (γmuurolene and α-copaene) were present with no detectable ME (Bertrand et al. 2006). Similarly, the only species in the Onagraceae family that emits a floral scent containing substantial ME is Clarkia breweri (Table 2); its closely related Clarkia concinna is virtually scentless with no detectable ME (Raguso and Pichersky 1995).
3.4.2.2. For orchid flowers
Orchids have evolved highly diverse and fascinating mechanisms to attract and entice animals, especially insects, to assist in cross— pollination. In this section, discussion will be confined to orchid flowers that possess or secrete ME that attracts insects to be pollen vectors.
3.4.2.2a. Orchids excluding
Bulbophyttum. Orchid flowers of Satyrium microrrhynchum produce nectar and are visited by several species of flower—visiting insects such as beetles, wasps, and flies, but not various honeybees and solitary bees that are commonly present at the study sites. Two insect species, cetoniid beetles, Atrichelaphinus tigrina (both sexes) and a pompilid wasp, Hemipepsis hilaris (males), have been shown to be pollinators while the other insect visitors do not carry any pollinarium (Johnson et al. 2007). Linalool is the major chemical component in the orchid fragrance and has been shown to attract the pollinators. Although seven phenylpropanoids with ME (at 1.83–4.51%) as the highest component were detected in the flowers from one of three populations studied in South Africa, there was no difference in the type of insect visitors/pollinators observed, as ME also stimulated an electrophysiological response in antennae of the cetoniid beetle (Johnson et al. 2007).
The inflorescence of an orchid species, Gymnadenia conopea, emits both eugenol and ME at different relative quantities during the day and night (Table 2). It attracts six lepidopteran taxa: three species each of butterflies and moths. Among the lepidopteran visitors caught, two species each of butterflies and moths bore pollinia. This indicates that pollination occurs during the day as well as at night (Huber et al. 2005). Similarly, a closely related species, Gymnadenia odoratissima, has 10 lepidopteran taxa, six moth, and four butterfly species as floral visitors, and all the species have been observed to be pollinators confirmed via their bearing of pollinia. There is no overlap of pollinator species between the two orchid species, and eugenol and benzyl acetate, which are among several of the 44–45 volatiles, are physiologically active components in the floral scent of the two species (Huber et al. 2005). In these orchid species, ME is not physiologically active against the lepidopteran species attracted to the orchid flowers and may instead be playing a role in deterring florivores. This certainly warrants further investigation.
3.4.2.2b. Bactrocerophilous Bulbophyttum orchids.
There are nearly 2000 recognized species of Bulbophyllum (Orchidaceae) worldwide. Some species (∼30) are known to have adapted to, and are entirely dependent on, Bactrocera (Tephritidae: Diptera) fruit flies for pollination without offering the usual nectar as floral reward. These bactrocerophilous Bulbophyllum species might have coevolved with the tephritid fruit flies. They basically make use of either RK, detected in Bu. apertum (syn. Bu. ecornutum) (Tan and Nishida 2005), zingerone in Bu. patens and Bu. baileyi (Tan and Nishida 2000, 2007), or ME (examples given below) as a floral attractant and reward for male Bactrocera fruit flies (Tan 2009). It is interesting to note that zingerone is the only known compound to attract both RK— and ME—responsive Bactrocera species, although it is a relatively weak attractant due to its resemblance to both RK and ME chemical structures (Tan and Nishida 2000).
The possible pathway for the biosynthesis of ME found in Bulbophyllum is shown in Figure 4. Starting from phenylalanine, it undergoes a series of intermediary steps involving cinnamic acid, ferulic acid, coniferyl alcohol, coniferyl acetate, and eugenol (Figure 4) (Kapteyn et al. 2007; Ferrer et al. 2008). The eugenol is ultimately biotransformed to ME by the addition of a methyl group to the ‘para— hydroxy’ group of eugenol catalyzed by an O-methyltransferase (Lewinsohn et al. 2000; Pichersky and Gang 2000).
Figure 4.
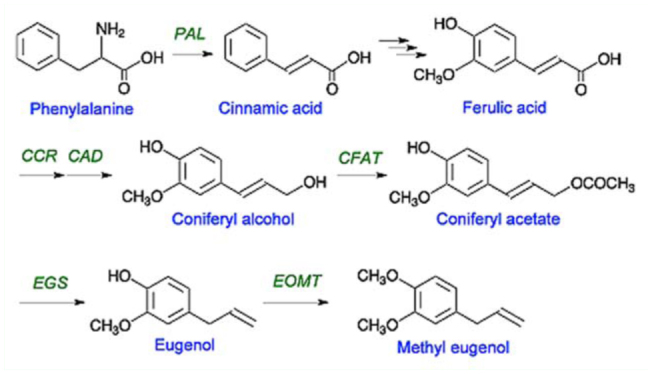
A possible biosynthetic pathway of methyl eugenol in an orchid flower of a bactrocerophilous Bulbophyllum species. PAL, phenylalanine ammonia lyase; CCR, cinnamoyl-CoA reductase; CAD, cinnamyl alcohol dehydrogenase; CFAT, coniferyl alcohol acyltransferase; EGS, eugenol synthase; EOMT, eugenol O-methyltransferase. High quality figures are available online.
Here only Bulbophyllum flowers that possess and release ME as a component of floral fragrance will be discussed to show that the flowers of some species have coevolved, via special floral architectural modifications to enhance fly pollination, with Bactrocera male flies. A nonresupinate flower (with lip/labellum above the floral column) of the ginger orchid, Bu. patens, possesses a major component of a fruit fly attractant, zingerone, which is weakly attractive to Bactrocera males from both ME—responsive species, such as Ba, carambolae, Ba. dorsalis and Ba. umbrosa, as well as RK—responsive species, namely Ba. caudata, Ba. Cucurbitae, and Ba. tau, with trace amounts of ME (Tan and Nishida 2000). It has a see—saw lip that is positioned in a plane above the floral column. When an attracted male Ba. dorsalis alights on and continues feeding along the lip, an imbalance will occur, and the fly will suddenly be tipped into the column cavity head first. The fly immediately retreats by moving backwards along the lip still in a closed position, and during this movement it removes the pollinia to initiate pollination. This process is repeated when a fly bearing pollinia lands on another flower (Figure 5) to initiate fertilization by depositing the pollinia onto the stigma.
Figure 5.

A male Bactrocera dorsalis bearing pollinia on see—saw lip of Bulbophyllum patens. High quality figures are available online.
The fruit fly orchid, Bulbophyllum cheiri, with non—resupinate and a solitary flower, does not have its sepals and petals fully spread out but just slightly parted when fully in bloom (Figure 6). It releases ME as its sole major volatile component in its floral fragrance, which attracts only male fruit flies (Tan et al. 2002). The concentration of ME in the various floral parts varies from 107, 95, 91, 44, and 41 ppm for lateral sepals, lip, petals, median sepal, and column, respectively (Tan et al. 2002). Further surveys identified seven more related analogs, including eugenol, (Z)-methyl isoeugenol, (E)-methyl isoeugenol, (E)-coniferyl alcohol (CF), 2-allyl-4,5-dimethoxyphenol (DMP), 5-allyl-1,2,4-trimethoxybenzene (euasarone), and (E)-3,4-dimethoxycinnamyl acetate (Nishida et al. 2004). It is interesting that the two major sex pheromonal components of Ba. dorsalis, CF and DMP, are also found in the orchid flowers. Many male flies of Ba. dorsalis with one or two Ba. umbrosa visit a newly bloomed flower in the morning. Usually, the first fly visitor removes the pollinia from the flower (Figures 6 and 7). Here the movable floral see—saw lip plays an important role in suddenly tipping a probing fly into the floral column cavity when an imbalance occurs due to the shifting of the fly's weight. This way the fly, during its retreat, either removes or deposits pollinia on the floral stigma. Headspace analysis of the flower indicates a high ME peak in the morning, a much smaller one between 12:00 and 14:00, and no ME detected after 14:00 (Tan et al. 2002). In spite of this, one or two male Ba. dorsalis flies can still be seen on a Bu. cheiri flower up until approximately 18:30 (personal observations).
Figure 6.

Male fruit flies, Bactrocera dorsalis, congregating and licking on a fully bloomed Bulbophyllum cheiri flower. High quality figures are available online.
Figure 7.

Male Bactrocera dorsalis bearing pollinia of Bulbophyllum cheiri. High quality figures are available online.
The wine red orchid, Bu. vinaceum, bears resupinate (lip/labellum below the floral column) and a solitary flower, which has a spring—loaded lip kept in a closed position to protect its sexual organs, especially the pollinarium with a stiff hamulus (derived from the entire distal portion of the rostellum that is prolonged into a stalk). The major floral volatile components identified are ME, CF, DMP, and (E)-3,4-dimethoxycinnamyl acetate, whereas the minor components are eugenol, euasarone, (E)-3,4-dimethoxy cinnamyl alcohol, and (Z)-coniferyl alcohol. The bouquet of floral phenylpropanoids attracts ME—sensitive species, particularly Ba. dorsalis with one or two Ba. unimacula in the highlands of Sabah (Tan et al. 2006). An attracted male fly normally lands on one of the petals before climbing onto and forcing the “spring loaded” floral lip that has the highest concentration of the phenylpropanoids, into the open position. This action reveals the floral sexual organs. The architecture of the lip and location of attractants compel the fly to align itself precisely along the lip's longitudinal axis. As the fly probes and feeds, it passes the point of imbalance, causing the lip to spring back to its normal closed position. This catapults the fly head first into the column cavity, and its dorsum strikes the protruding sticky base of the hamulus and adheres to it. The momentum of the fly and the structural morphology of the long stiff hamulus act in tandem to pry out the pollinia from its anther cover. Pollinarium removal (Figure 8) is a precise and very quick process assisted by the specially modified spring lip, which plays an essential and important role in pollination. In this orchid species, ME is the main component in the floral fragrance and plays a pivotal role in the true mutualism between the flower and fruit fly pollinator, in which both receive reproductive benefits. Interestingly, both CF and DMP detected in the flowers are also sex pheromonal components of male Ba. dorsalis after consuming ME. Although CF and DMP attract and arrest females during courtship at dusk, and thus would serve as specific female attractants, the flower has never been observed to attract female fruit flies, not even during dusk when they are most sensitive to these chemicals (Tan et al. 2006). This evidence, and that of Bu. cheiri, may substantiate and indicate the outcome or culmination of a co—evolutionary process between the orchid species and Bactrocera pollinators.
Figure 8.

Flower of Bulbophyllum vinaceum with its spring— loaded lip in a closed position and a pollinarium—bearing fruit fly, Bactrocera dorsalis. High quality figures are available online.
The ‘raised dot Bulbophyllum’, Bu. elevatopunctatum, has relatively high content of ME 78.5 ±+ 21.6 mg (mean + standard deviation; n= 10) per flower as a major floral volatile (unpublished data). The solitary and resupinate flower does not have a spring— loaded lip like that present in Bu. vinaceum, but a simple hinged one kept at an acute angle with respect to the floral column by the fused lateral sepals. When an attracted male fruit fly moves on to the lip that is prevented from moving away from the column to a fully opened position, it will very quickly be jerked into the floral column cavity, thereby hitting the hamulus and dislodging the pollinia from the anther and its cover. Upon its retreat, the fly removes the pollinarium to initiate pollination (Figure 9).
Figure 9.

A male Bactrocera dorsalis bearing a pollinarium just removed from the Bulbophyllum elevatopuntatum flower (P.T. Ong). High quality figures are available online.
In the aforementioned Bulbophyllum— Bactrocera association, each Bulbophyllum species has specifically adapted and evolved precise lip mechanism to entice fruit flies and enhance pollination through the offer of ME as an attractant as well as a floral reward. Furthermore, both organisms gain direct reproductive benefits, exhibiting a true mutualism; the orchid flower gets pollinated without having to offer nectar as reward, and the fruit fly boosts its pheromone and defense system as well as its sexual competitiveness by feeding on the ME produced by the flower as floral reward to its potential pollinator.
4. Methyl eugenol and human health
When present in human blood serum after a meal, ME is rapidly eliminated and excreted (Schecter et al. 2004). ME has ill effects on human health as a known carcinogen and mutagen, probably because of its conversion to a hydroxy analog at the allylic position (De Vincenzi et al. 2000). Further, safrole, estragole, and ME found in herbs and spices are weak animal carcinogens as demonstrated by the formation of DNA adducts in cultured human cells (Zhou et al. 2007).
Recent research by Choi et al. (2010) indicated that ME may have positive effects on human health as well. Based on their studies, ME may reduce cerebral ischemic injury through suppression of oxidative injury and inflammation (Choi et al. 2010). The chemical also decreased activation of an enzyme, caspase-3, and the death of cultured cerebral cortical neurons through oxygen— glucose deprivation for one hour. Additionally, it was shown that ME elevated the activities of superoxide dismutase and catalase, thereby markedly reducing superoxide generation in the ischemic brain and decreasing intracellular oxidative stress. Furthermore, ME also reduced the production of pro—inflammatory cytokines in the ischemic brain (Choi et al. 2010).
Studies on rodents showed that minimal ME within a dose range of 1–10 mg/kg body weight, which is about 100–1000 times the anticipated human exposure to ME as a result of spiced and/or flavored food consumption, did not pose a significant cancer risk (Smith et al. 2002). Further, toxicological studies in animals demonstrated that orally administered relatively high—bolus doses of ME resulted in hepatic neoplasms. Nevertheless, the detected level of ME in biomonitoring studies indicated that human exposure was several orders of magnitude lower than the lowest dose utilized in the bioassay (Robison and Barr 2006). Arguably, a single high dose may cause any number of ill or side effects in animals.
Conclusions
In this review, the occurrence of ME in over 450 species of plants belonging to 80 families under 48 orders compiled from numerous published papers is listed. The distribution of ME in various plant organs within a species is definitely uneven and varies greatly according to growth stage as well as plant variety/chemotype. Similarly, even in flowers, the distribution and release of ME by various floral parts can vary considerably depending on the physiological stage and time of day.
The various roles of ME in nature especially related to the chemical defense of plants, such as antifungal, antibacterial, antinematodal, or toxicant roles against pathogens and insect herbivores, as well as its functions as an insect antifeedant/repellant and in pollination are reviewed. In particular, ME has been shown to act as floral synomone in the coevolution of orchid species in the genus Bulbophyllum with fruit flies. More research should be conducted to fully understand the biochemical, physiological, and/or chemoecological basis for these bitrophic interactions between plants and insects mediated by ME.
Acknowledgements
We wish to gratefully thank TE. Shelly (APHIS, USDA, Waimanalo) for encouragement and assistance in reviewing this manuscript, and L.T. Tan of University College London for reading through the initial draft, and A.K.W. Hee for assistance in providing some reference materials.
Glossary
Abbreviations
- ME,
methyl eugenol;
- RK
raspberry ketone
References
- Abdon APV, Leal-Cardoso JH, Coelho-de-Souza AN, Morais SM, Santos CF. Antinociceptive effects of the essential oil of Croton nepetaefolius on mice. Brazilian Journal Medical and Biological Research. 2002;35:1215–1219. doi: 10.1590/s0100-879x2002001000015. [DOI] [PubMed] [Google Scholar]
- Abduelrahman AHN, Elhussein SA, Osman NA, Nour AH. Morphological variability and chemical composition of essential oils from nineteen varieties of Basil (Ocimum basilicum L.) growing in Sudan. International journal of Chemical Technology. 2009;1:1–10. [Google Scholar]
- Aboutabl EA, Tohamy SFE, De Pooter HL, De Buyck LF. A comparative study of the essential oils from three Melaleuca species growing in Egypt. Flavour and Fragrance Journal . 1991;6:139–141. [Google Scholar]
- Acharya RN, Chaubal MG. Essential oil of Anemopsis californica. Journal of Pharmaceutical Sciences. 1968;57:1020–1022. doi: 10.1002/jps.2600570622. [DOI] [PubMed] [Google Scholar]
- Adams R.P. The leaf oil of Juniperus gracilior Pilger var. urbaniana (Pilger & Ekman) R.P. Adams: Comparison with other Caribbean Juniperus species. Journal of Essential Oil Research . 1997;9:641–647. [Google Scholar]
- Adams RP, Almirall AL, Hogge L. The volatile leaf oils of the Junipers of Cuba: Juniperus lucayana Britton and Juniperus saxicola Britton and Wilson. Flavour and Fragrance Journal. 1987;2:33–36. [Google Scholar]
- Adams RP, Chu GL, Zhang SZ. Comparison of the volatile leaf oils of Juniperus chinensis L., J. chinensis var. kaizuca Hort, and cv. pyramidalis from China. Journal of Essential Oil Research. 1994;6:149–154. [Google Scholar]
- Adams RP, Von Rudioff E, Hogue L, Zanoni TA. The volatile terpenoids of Juniperus blancoi and its affinities with other entire leaf margin of Junipers of North America. Journal of Natural Products. 1981;44:21–26. [Google Scholar]
- Adams RP, Zanoni TH, Hogge L. The volatile leaf oils of Juniperus flaccida var. flaccida and var. poblana. . Journal of Natural Products. 1984;47:1064–1065. [Google Scholar]
- Adedeji J, Hartman TG, Rosen RT, Ho C-T. Free and glycosidically bound aroma compounds in hog plum (Spondias mombins L.). Journal of Agricultural Chemistry. 1991;39:1494–1497. [Google Scholar]
- Adhikary SR, Tuladhar BS, Sheak A, van Beek TA, Posthumus MA, Lelyveld GP. Investigation of Nepalese essential oils. I. The oil of Cinnamomum glaucescens (Sugandha Kokila). Journal of Essential Oil Research. 1992;4:151–159. [Google Scholar]
- Aggarwal VK, Aggarwal SG, Thappa RK, Mehra MS, Dhar KL. Chemical constituents of Lovunga scandens Buch & Ham. Indian Perfumer. 1983;27:163–165. [Google Scholar]
- Ahmad VU, Jassbi AR, Pannahi MCS. Analysis of the essential oil of Echinophora sibthorpiana Guss. by means of GC, GC/MS and 13C-NMR techniques. Journal of Essential Oil Research. 1999;11:107–108. [Google Scholar]
- Akgul A, Chialva F. Constituents of the essential oil of Echinophora tenuifolia L. subsp. sibthorpiana (Guss.) Tutin from Turkey. Flavour and Fragrance Journal. 1989;4:67–68. [Google Scholar]
- Akhila A. Chapter 2. Chemistry and biogenesis of essential oil from the genus Cymbopogon. In: Akhila A, editor. Essential oil bearing grasses: The genus Cymbopogon. CRC Press; 2009. pp. 20–106. [Google Scholar]
- Ali NA, Jantan I. Essential oil of Cinnamomum tahijanum Kost. from Sarawak. ASEAN Review of Biodiversity and Environment Conservation (ARBEC) 1999. Nov.Dec. 1999. Available online, http://www.arbec.com.my/pdf/art5novdec99.pdf.
- Alilou H, Akssirai M, Hassani LMI, Chebli B, El Hak-Moui A, Mellouki F, Rouhi R, Boira H, Blazquez MAB. Chemical composition and antifungal activity of Bubonium imbricatum volatile oil. Phytopathologia Mediterranea. 2008;47:3–10. [Google Scholar]
- Alves RJV, Pinto AC, da Costa AVM, Rezende CM. Zizyphus mauritiana Lam. (Rhamnaceae) and the chemical composition of its floral fecal odor. Journal of Brazilian Chemical Society. 2005;16:654–656. [Google Scholar]
- Anasari SH, Qadry JS. TLC and GLC studies on khawi grass oil. Indian Journal of Natural Products. 1987;3:10–12. [Google Scholar]
- Andrade EH, Zoghbi MDG, Maia JG. Volatiles from the leaves and flowers of Carapa guinensis Aubl. Journal of Essential Oil Research. 2001;13:436–438. [Google Scholar]
- Andrade MS, Sampaio TS, Nogueira PCL, Ribeiro AS, Bittrich V, Amaral MCE. Volatile compounds of the leaves, flowers and fruits of Kielmeyera rugosa Choisy (Clusiaceae). Flavour and Fragrance Journal. 2007;22:49–52. [Google Scholar]
- Andrianoelisoa HS, Menut C, de Chatelperron PC, Saracco J, Ramanoelina P, Danthu P. Intraspecific chemical variability and highlighting of chemotypes of leaf essential oils from Ravensara aromatica Sonnerat, a tree endemic to Madagascar. Flavour and Fragrance Journal. 2006;21:833–838. [Google Scholar]
- Arrebola ML, Navarro M, Jimenez J, Ocana FA. Yield and composition of the essential oil of Thymus serpylloides subsp. serpylloides. Phytochemistry. 1994;36:67–72. [Google Scholar]
- Arslan N, Gurbuz B, Sarihan EO. Variation in essential oil content and composition in Turkish anise (Pimpinella anisum L.) populations. Turkish Journal of Agriculture and Forestry. 2004;28:173–177. [Google Scholar]
- Asakawa Y, Komatsu T, Hayashi S, Matsura T. Chemical components of the benzene extract of Cinnamomum loureiri. Flavours of India. 1971;2:114–119. [Google Scholar]
- Ashafa AOT, Grierson DS, Afolayan AJ. Effects of drying methods on the chemical composition of essential oil from Felicia muricata leaves. Asian Journal of Plant Science. 2008;7:603–606. [Google Scholar]
- Askari F, Sefidkon F. Essential oil composition of Pimpinella affinis Ledeb. from two localities in Iran. Flavour and Fragrance Journal. 2006;21:754–756. [Google Scholar]
- Asllani U. Chemical composition of Albanian myrtle oil (Myrtus communis L.). Journal of Essential Oil Research. 2000;12:140–142. [Google Scholar]
- Asquith A, Burny DA. Honey bees attracted to the semiochemical methyl eugenol, used for male annihilation of the oriental fruit fly (Diptera: Tephritidae). Proceedings of the Hawaiian Entomological Society. 1998;33:57–66. [Google Scholar]
- Atal CK, Bradu BL. Search for aroma chemicals of industrial value from genus Cymbopogon. Part IV. Chandi and Kolar grasses as source of methyl eugenol. Indian Journal of Pharmacology. 1976;38:63–64. [Google Scholar]
- Atazhanova GA, Dembitskii AD, Yakovleva TD, Mikhailov VG, Adekenov SM. About composition of essential oil from Artemisia filatovae. . Chemistry of Natural Compounds. 1999;35:529–531. [Google Scholar]
- Avato P, Smitt UW. Composition of the essential oils from the roots of Thapsia maxima Miller and T. villosa L. Journal of Essential Oil Research. 2000;12:303–309. [Google Scholar]
- Avato P, Jacobsen N, Smitt UW. Chemotaxonomy of Thapsia maxima Miller. Constituents of the essential oil of the fruits. Journal of Essential Oil Research. 1992;4:467–473. [Google Scholar]
- Avato P, Trabace G, Smitt UW. Essential oils from fruits of three types of Thapsia villosa. . Phytochemistry. 1996a;43:609–612. doi: 10.1016/0031-9422(96)00300-7. [DOI] [PubMed] [Google Scholar]
- Avato P, Trabace G, Smitt UW. Composition of the essential oils of fruits from polyploid types of Thapsia villosa L.: Chemotaxonomic evaluation. Journal of Essential Oil Research. 1996b;8:123–128. [Google Scholar]
- Avato P, Fortunato IM, Ruta C, D'Elia R. Glandular hairs and essential oils in micropropagated plants of Salvia officinalis L. Plant Science. 2005;169:29–36. [Google Scholar]
- Azuma H, Kono M. Estragole (4-allylanisole) is the primary compound in volatiles emitted from the male and female cones of Cycas revoluta. . Journal of Plant Research. 2006;119:671–676. doi: 10.1007/s10265-006-0019-2. [DOI] [PubMed] [Google Scholar]
- Asuma H, Toyota M, Asakawa Y. Intraspecific variation of floral scent chemistry in Magnolia kobus DC. (Magnoliaceae). Journal of Plant Research. 2001;114:411–422. [Google Scholar]
- Azuma H, Toyota M, Asakawa Y, Takaso T, Tobe H. Floral scent chemistry of mangrove plants. Journal of Plant Research. 2002;115:47–53. doi: 10.1007/s102650200007. [DOI] [PubMed] [Google Scholar]
- Baggaley KH, Erdtman H, McLean NJ, Norin T, Eriksson G. Chemistry of the order Podocarpales. I. Heartwood constituents of the Huon pine (Dacrydium franklinii). Acta Chemica Scandinavica. 1967;21:2247–2253. [Google Scholar]
- Barkman TJ, Beaman JH, Gade DA. Floral fragrance variation in Cypripedium: implication for evolutionary and ecological studies. Phytochemistry. 1997;44:875–882. [Google Scholar]
- Barra A, Coroneo V, Dessi S, Cabras P, Angioni A. Characterization of the volatile constituents in the essential oil of Pistacia lentiscus L. from different origins and its antifungal and antioxidant activity. Journal of Agricultural and Food Chemistry. 2007;55:7093–7098. doi: 10.1021/jf071129w. [DOI] [PubMed] [Google Scholar]
- Baser KHC. Aromatic biodiversity among the flowering plant taxa of Turkey. Pure and Applied Chemistry. 2002;74:527–545. [Google Scholar]
- Baser KHC, Kurkcuoglu M. Composition of the essential oil of Morina persica L. flowers. Journal of Essential Oil Research. 1998;10:117–118. [Google Scholar]
- Baser KHC, Ozek T, Demirci B, Duman H. Composition of the essential oil of Prangos heyniae H. Duman et M.F. Watson, a new endemic from Turkey. Flavour and Fragrance Journal. 2000;15:47–49. [Google Scholar]
- Baser KHC, Demirci B, Duman H. Composition of the essential oils of two endemic species from Turkey: Achillea lycaonica and A. ketenoglui. . Chemistry of Natural Products. 2001a;37:245–252. [Google Scholar]
- Baser KHC, Demirci B, Duman H, Aytac Z, Adiguzel N. Composition of the essential oil of Achillea goniocephala Boiss. et Bal. from Turkey. Journal of Essential Oil Research. 2001b;13:219–220. [Google Scholar]
- Baser KHC, Demirci B, Tabanca N, Ozekn T, Goren N. Composition of the essential oils of Tanacetum armenum (DC.) Schultz Bip., T. balsamita L., T. chiliophyllum (Fisch. & Mey.) Schultz Bip. var. chiliophyllum and T. haradjani (Rech, fil.) Grierson and the enantiomeric distribution of camphor and carvone. Flavour and Fragrance Journal. 2001c;16:195–200. [Google Scholar]
- Basher KHC, Ozek T, Demirchakmak B, Nuriddinov KR, Abduganiev BY, Aripov KN, Khodzimatov KK, Nigmatullaev OA, Shamyanov ED. Essential oils of some Artemisia species from Central Asia. Chemistry of Natural Compounds. 1997;33:293–295. [Google Scholar]
- Baslas RK, Kumar P. Chemical examination of essential oil of Coleus aromaticus Benth. Journal of Indian Chemical Society. 1981;58:103–104. [Google Scholar]
- Baydar H, Schulz H, Kruger H, Erbas S, Kineci S. Influences of fermentation time, hydro-distillation time and fractions on essential oil composition of Damask rose (Rosa damascena Mill.). Journal of Essential Oil Bearing Plants. 2008;11:224–232. [Google Scholar]
- Beauchamp PS, Chea E, Dimaano JG, Dev V, Ly B, Miranda AE. Essential oil composition of six Lomatium species attractive to Indra swallowtail butterfly (Papilio indra): Principal component analysis against essential oil composition of Lomatium dissectum var. multifidum. . Journal of Essential Oil Research. 2009;21:535–542. [Google Scholar]
- Benitez NP, Leon EMM, Stashenko EE. Eugenol and methyl eugenol chemotypes of essential oil of species Ocimum grastissimum L. and Ocimum campechianum Mill, from Colombia. Journal of Chromatographic Science. 2009;47:800–803. doi: 10.1093/chromsci/47.9.800. [DOI] [PubMed] [Google Scholar]
- Berger RG, Akkan Z, Drawert F. The essential oil of Coleonema album (Rutaceae) and of a photomixotrophic cell culture derived thereof. Zeitschrift für Naturforschung Section C, Biosciences. 1990;45:187–195. [Google Scholar]
- Bertrand C, Comte G, Piola F. Solidphase microextraction of volatile compounds from flowers of two Brunfelsia species. Biochemical Systematics and Ecology. 2006;34:371–375. [Google Scholar]
- Bettaieb I, Bourgou S, Wannes WA, Hamrouni I, Limam F, Marzouk B. Essential oils, phenolics, and antioxidant activities of different parts of Cumin (Cuminum cyminum L.). Journal of Agricultural and Food Chemistry. 2010;58:10410–10418. doi: 10.1021/jf102248j. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Tewary DK, Kumar R, Kumar V, Sinha AK, Shanker A. Larvicidal and structure—activity studies of natural phenylpropanoids and their semisynthetic derivatives against the tobacco armyworm Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Chemistry and Biodiversity. 2010;7:168–177. doi: 10.1002/cbdv.200800345. [DOI] [PubMed] [Google Scholar]
- Bhuiyan MNI, Chowdhury JU, Begum J, Azim MA. Aromatic plants of Bangladesh: constituents of leaf and rhizome oil of Etlingera linguiforme. . Dhaka University Journal of Science. 2010;58:13–15. [Google Scholar]
- Bicchi C, Frattini C, Sacco T. Essential oils of three asiatic Artemisia species. Phytochemistry. 1985;24:2440–2442. [Google Scholar]
- Billet D, Favre-Bonvin J. Constituants de l'huile essentielle de Vepris madagascarica (Essential oil constituents of Vepris madagasscarica). Phytochemistry. 1973;12:1194. [Google Scholar]
- Bohannon WB, Kleiman R. Myristicin. The major volatile component in Maere seed of Portenschlagia ramosissima. . Lipids. 1977;12:321–323. [Google Scholar]
- Borejsza-Wysocki W, Hrazdina G. Aromatic polyketide synthases (purification, characterization, and antibody development to benzalacetone synthase from raspberry fruits). Plant Physiology. 1996;110:791–799. doi: 10.1104/pp.110.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaada O, Ammar S, Saidana D, Chriaa J, Chraif I, Daami M, Helal AN, Mighri Z. Chemical composition and antimicrobial activity of volatile components from captula and aerial parts of Rhaponticum acaule DC growing wild in Tunisia. Microbiological Research. 2007;163:87–95. doi: 10.1016/j.micres.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Boutaghane N, Nacer A, Kabouche Z, Ait-Kaki B. Comparative antibacterial activities of the essential oils of stems and seeds of Pituranthos scoparius from Algerian septentrional Shara. Chemistry of Natural Compounds. 2004;40:606–607. [Google Scholar]
- Bracho R, Crowley KJ. The essential oils of some Venezuelan Croton species. Phytochemistry. 1966;5:921–926. [Google Scholar]
- Brandt JJ, Schultze W. Composition of the essential oils of Ligusticum mutellina (L.) Crantz (Apiaceae). Journal of Essential Oil Research. 1995;7:231–235. [Google Scholar]
- Brophy JJ, Jogia MK. Essential oils from Fijian Ocimum basilicum L. Flavour and Fragrance Journal. 1986;1:53–55. [Google Scholar]
- Brophy JJ, Lassak EV. Melaleuca leucadendra L. leaf oil: two phenylpropanoid chemotypes. Flavour and Fragrance Journal. 1988;3:43–46. [Google Scholar]
- Brophy JJ, Goldsack RJ. The essential oils of Choricarpia leptoptala (F. Muell.) Domin and C. subargentea (C.T. White) L.A.S. Johnson (Myrtaceae). Flavour and Fragrance Journal. 1994;9:7–10. [Google Scholar]
- Brophy JJ, Davies N, Southwell I, Stiff I, Williams L. Gas chromatographic quality control for oil of Melaleuca alternifolia terpinen-4-ol type (Australian Tea Tree). Journal of Agricultural and Food Chemistry. 1989;37:1330–1335. [Google Scholar]
- Brophy JJ, Goldsack RJ, House APN, Lassak EV. Essential oils of the genus Doryphora. . Journal of Essential Oil Research. 1993;5:581–586. [Google Scholar]
- Brophy JJ, Goldsack RJ, Forster PI. The essential oils of Choricarpia leptopetala (F. Muell) Domin and C. subargentea (C.T. White) L.A.S. Johnson (Myrtaceae). Flavour and Fragrance Journal. 1994;9:7–10. [Google Scholar]
- Brophy JJ, Goldsack RJ, Fookes CJR, Forster PI. Leaf oils of the genus Backhousia (Myrtaceae). Journal of Essential Oil Research. 1995;7:237–254. [Google Scholar]
- Brophy JJ, Goldsack RJ, Forster PI. Chemotype variation in the leaf essential oils of Melicope melanophloia C.T. White (Rutaceae). Journal of Essential Oil Research. 1997;9:279–282. [Google Scholar]
- Brophy JJ, Goldsack RJ, Rozefelds AC. Chemistry of the Australian gymnosperms-part 5: Leaf essential oils of some endemic Tasmanian gymnosperms: Diselma archeri, Lagarostrobos franklinii, Microcachrys tetragona and Phyllocladus aspleniifolius. . Journal of Essential Oil Research. 2003;15:139–142. [Google Scholar]
- Brophy JJ, Goldsack RJ, Hook JM, Fooke CJR, Forster PI. The leaf essential oils of the Australian species of Pseuduvaria (Annonaceae). Journal of Essential Oil Research. 2004;16:362–366. [Google Scholar]
- Brophy JJ, Goldsack RJ, Forster PI. What is the smell of the “fruit salad plant”?: The leaf oil of Leionema ambiens (Rutaceae). Journal of Essential Oil Research. 2006;18:131–133. [Google Scholar]
- Brophy JJ, Buchanan AM, Copeland LM, Dimitriadia E, Goldsack RJ, Hibbert DB. Differentiation between the two subspecies of Antherosperma moschatum Labill. (Antherospermataceae) from their leaf oils. Biochemical Systematics and Ecology. 2009;37:479–483. [Google Scholar]
- Brunke E-J, Hammerschmidt F-J, Schmaus G. Headspace analysis of Hyacinth flowers. Flavour and Fragrance Journal. 1994;9:59–69. [Google Scholar]
- Bueno-Sanchez JG, Martinez-Morales JR, Stashenko EE, Ribon W. Antitubercular activity of eleven aromatic and medicinal plants occurring in Colombia. Biomedica. 2009;29:51–60. [PubMed] [Google Scholar]
- Bunralhep S, Palanuvej C, Ruangungsi N. Chemical compositions and antioxidative activities of essential oils from four Ocimum species endemic to Thailand. Journal Health Research. 2007;21:201–206. [Google Scholar]
- Buttery RG, Black DR, Guadagni DG, Ling LC, Connolly G, Teranishi R. California bay oil. I. Constiuents, odor properties. Journal of Agricultural and Food Chemistry. 1997;22:773–777. doi: 10.1021/jf60195a025. [DOI] [PubMed] [Google Scholar]
- Bylaite E, Venskutonis RP, Roozen JP. Influence of harvesting time on the composition of volatile components in different anatomical parts of Lovage (Levisticum officinale Koch.). Journal ofAgricultural and Food Chemistry. 1998;46:3735–3740. doi: 10.1021/jf990679u. [DOI] [PubMed] [Google Scholar]
- Caredda A, Marongiu B, Porcedda S, Soro C. Supercritical carbondioxide extraction and characterization of Laurus nobilis essential oil. Journal of Agricultural and Food Chemistry. 2002;50:1492–1496. doi: 10.1021/jf0108563. [DOI] [PubMed] [Google Scholar]
- Carlini EA, De Oliveira AB, Oliveira GG. Psychopharmacological effects of the essential oil fraction and of the hydrolate obtained from the seeds of Licaria puchurymajor. . Journal of Ethnopharmacology. 1983;8:225–236. doi: 10.1016/0378-8741(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Carovic-Stanko K, Orlic S, Politeo O, Strikic F, Kolak I, Milos M, Satovic Z. Composition and antibacterial activities of essential oils of seven Ocimum taxa. Food Chemistry. 2010;119:196–201. [Google Scholar]
- Chalchat JC, Chabard JL, Gorunovic MS, Djermanovic V, Bulatovic V. Chemical composition of Eucalyptus globulus oils from the Montenegro coast and east coast of Spain. Journal of Essential Oil Research. 1995;7:147–152. [Google Scholar]
- Chang CL, Cho IK, Li QX. Insecticidal activity of basil oil, trans-anethole, estragole, and linalool to adult fruit flies of Ceratitis capitata, Bactrocera dorsalis, and Bactrocera Cucurbitae. . Journal of Economic Entomology. 2009;102:203–209. doi: 10.1603/029.102.0129. [DOI] [PubMed] [Google Scholar]
- Charles DJ, Simon JE, Widrlechner MP. Characterization of essential oil of Agastache species. Journal of Agricultural and Food Chemistry. 1991;39:1946–1949. [Google Scholar]
- Chaumont PJP, Mandin D, Sanda K, Koba K, De Souza CA. Activités antimicrobiennes in vitro de cinq huiles essentielles de Lamiacées togolaises vis-à-vis de germes representatives de la microflore cutanée. Acta Botanica Gallica. 2001;148:93–101. English Abstract. [Google Scholar]
- Chen Y, Li Z, Li H. Analysis of fragrance volatiles of fresh flowers by precolumn absorption and GC/MS. Chromatographia. 1987a;23:502–506. [Google Scholar]
- Chen Y, Wang R, Xue D, Li Z, Han H. Analysis of volatile constituents of Hedysarum polybotrys Hand, by capillary gas chromatography-mass spectrometry. Gaodeng Xuexiao Huaxue Xuebao. 1987b;8:538–541. in Chinese. [Google Scholar]
- Cheng B-Q, Xu Y, Zheng G, Yu XJ, Ding JK. Ways of propagating Cinnamomum tenuipilum and variations in its leaf essential oil components. Yunnan Zhiwu Yanjiu. 1993;15:78–82. (in Chinese) Database: CAPLUS. [Google Scholar]
- Chialva F, Doglia G. Essential oil constituents of chinotto (Citrus aurantium L. var. myrtifolia Guill.). Journal of Essential Oil Research. 1990;2:33–35. [Google Scholar]
- Chialva F, Monguzzi F, Manitto P, Akgul A. Essential oil constituents of Trachyspermum copticum (L.) Link fruits. Journal of Essential Oil Research. 1993;5:105–106. [Google Scholar]
- Chin KW, Sun HL. Analysis of the phenolic compound in betel quid. Journal of Chinese Agricultural and Chemical Society. 1990;31:623–632. [Google Scholar]
- Cho YS, Cho IH, Park HJ, Chun HK. Analysis of fragrance volatiles of Korean Rosa hybrida using gas chromatography-mass spectrometry. Agricultural Chemistry and Biotechnology. 2006;49:180–185. [Google Scholar]
- Choi YK, Cho G-S, Hwang S, Kim BW, Lim JH, Lee J-C, Kim HC, Kim W-K, Kim YS. Methyleugenol reduces cerebral ischemic injury by suppression of oxidative injury and inflammation. Free Radical Research. 2010;44:925–935. doi: 10.3109/10715762.2010.490837. [DOI] [PubMed] [Google Scholar]
- Chowdhury JU, Mobarok MH, Bhuiyan MNI, Nandi NC. Constituents of essential oils from leaves and seeds of Foeniculum vulgare Mill, cultivated in Bangladesh. Bangladesh Journal of Botany. 2009;38:181–183. [Google Scholar]
- Christensen LP, Jakobsen HB, Paulsen E, Hodal L, Andersen KE. Airborne Compositae dermatitis: monoterpenes and no parthenolide are released from flowering Tanacetum parthenium (feverfew) plants. Archives of Dermatological Research. 1999;291:425–431. doi: 10.1007/s004030050433. [DOI] [PubMed] [Google Scholar]
- Chu SS, Liu SL, Jiang GH, Liu ZL. Composition and toxicity of essential oil of Illicium simonsii Maxim (Illiciaceae) fruit against the maize weevils. Records of Natural Products. 2010;4:205–210. [Google Scholar]
- Chuah CH, Yong HS, Goh SH. Methyl eugenol, a fruit-fly attractant, from the browning leaves of Proiphys amboinensis (Amaryllidaceae). Biochemical Systematics and Ecology. 1997;25:391–393. [Google Scholar]
- Ciccio JF, Segnini M. Composition of the essential oil from leaves of Croton jimenezil from Costa Rica. Journal of Essential Oil Research. 2002;14:357–360. [Google Scholar]
- Close DC, McArthur C. Rethinking the role of many plant phenolics-protection from photodamage not herbivores? Oikos. 2002;99:166–172. [Google Scholar]
- Comai S, Dall' Acqua S, Grillo A, Castagliuolo I, Gurung K, Innocenti G. Essential oil Lindera neesiana fruit: Chemical analysis and its potential use in topical applications. Fitoterapia. 2010;81:11–16. doi: 10.1016/j.fitote.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Compagnone RS, Chavez K, Mateu E, Orsini G, Arvelo F, Suarez AI. Composition and cytotoxic activity of essential oils from Croton matourensis and Croton micans from Venezuela. Records of Natural Products. 2010;4:101–108. [Google Scholar]
- Cook WB, Howard AS. The essential oil Illicium anisatum Linn. Canadian Journal of Chemistry. 1966;44:2461–2464. [Google Scholar]
- Costa LCB, Pinto JEBP, Castro EM, Alves E, Rosal LF, Bertolucci SKV, Alves PB, Evanglino TS. Yield and composition of the essential oil of Ocimum selloi Benth. cultivated under colored netting. Journal of Essential Oil Research. 2010;22:34–39. [Google Scholar]
- Crabas N, Marongiu B, Piras A, Pivetta T, Procedda S. Extraction, separation and isolation of volatiles and dyes from Calendula officinalis L. and Aloysia triphylla (L'Her.) Britton by supercritical CO2. Journal of Essential Oil Research. 2003;15:350–355. [Google Scholar]
- Craveiro AA, Andrade CHS, Matos FJA, De Alencar JW. Anise-like flavor of Croton aff. zehntneri Pa. et Hoffm. Journal of Agricultural and Food Chemistry. 1978;26:772–773. [Google Scholar]
- Craveiro AA, Andrade CHS, Matos FJA, Alencar JW, Machado MIL. Volatile constituents of Mangifera indica Linn. Revista Latinoamericana de Química. 1980;11:129. [Google Scholar]
- Crockett SL, Demirci B, Baser KHC, Khan IA. Analysis of the volatile constituents of five African and Mediterranean Hypericum L. (Clusiaceae, Hypericoideae) species. Journal of Essential Oil Research. 2007;19:302–306. [Google Scholar]
- Custodio L, Serra H, Nogueira JMF, Goncalves S, Romano A. Analysis of the volatiles emitted by whole flowers and isolated flower organs of the Carob tree using HS-SPME-GC/MS. Journal of Chemical Ecology. 2006;32:929–942. doi: 10.1007/s10886-006-9044-9. [DOI] [PubMed] [Google Scholar]
- De Etcheves MDC-P, Gros EG, Retamar JA. Chemical study of Crotonparvifolius. . Essenze Derivati Agrumari. 1981;51:253–261. in French. [Google Scholar]
- De Fenik J, Ines S, Retamar JA. Essential oil of Pseudocaryophyllus guili. . Anais da Academia Brasileira de Ciências. 1972;44:175–180. Suppl. in Spanish. [Google Scholar]
- De Feo V, Soria EU, Soria RU, Pizza C. Composition and in vitro toxicity of the essential oil of Tagetes terniflora HBK. (Asteraceae). Flavour and Fragrance Journal. 2005;20:89–92. [Google Scholar]
- De Pooter HL, Omar MN, Coolsaet BA, Schamp NM. The essential oil of greater galangal (Alpinia galanga) from Malaysia. Phytochemistry. 1985;245:93–96. [Google Scholar]
- De Vincenzi M, Silano M, Stacchini P, Scazzocchio B. Constituents of aromatic plants: I. Methyl eugenol. Fitoterapia. 2000;71:216–221. doi: 10.1016/s0367-326x(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Degen T. Ph.D. thesis, Universitat Basel; Basel, Switzerland: 1998. Host-plant acceptance by the carrot fly: some underlying mechanisms and the relationship to host-plant suitability. . [Google Scholar]
- Demetzos C, Angelopoulou D, Perdetzoglou D. A comparative study of the essential oils Cistus salviifolius in several populations of Crete (Greece). Biochemical Systematics and Ecology. 2002;30:651–665. doi: 10.1016/s0305-1978(00)00071-5. [DOI] [PubMed] [Google Scholar]
- Dey BB, Choudhuri MA. Characterization of a few morphological and biochemical parameters to serve as indicators of maximum oil yielding stage of Ocimum sanctum. . PAFAI Journal. 1985;7:11–16. [Google Scholar]
- Diaz D, Pedro P, Dorado VJ. Chemical constituents of the leaves of Piper lenticellosum C.D.C. Anais da Academia Brasileira de Ciências. 1986;17:58–60. [Google Scholar]
- Dicke M, Hilker M. Induced plant defences: from molecular biology to evolutionary ecology. Basic Applied Ecology. 2003;4:3–14. [Google Scholar]
- Dighe VV, Gursale AA, Sane RT, Menon S, Patel PH. Quantitative determination of eugenol from Cinnamomum tamala Nees and Eberm. leaf powder and polyherbal formulation using reverse phase liquid chromatography. Chromatographia. 2005;61:442–446. [Google Scholar]
- Dimitriev LB, Mumladze MG, Klyuev NA, Kobakhidze SK, Grandberg II. Dynamics of accumulation and composition of Agastache foeniculum during plant growth and storage. Izvestiya Timiryazeuvsk S-kh Akademii. 1981;6:86–91. (in Russian) [Google Scholar]
- Dixon RA, Palva NL. Stress-induced phenylpropanoid metabolism. The Plant Cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson HEM, Bergstrom G. The ecology and evolution of pollen odors. Plant Systematics and Evolution. 2000;222:63–87. [Google Scholar]
- Dobson HEM, Bergström J, Bergström G, Groth I. Pollen and flower volatiles in two Rosa species. Phytochemistry. 1987;26:3171–3173. [Google Scholar]
- Dobson HEM, Arroyo J, Bergstrom G, Groth I. Interspecific variation in floral fragrances within the genus Narcissus (Amaryllidaceae). Biochemical Systematics and Ecology. 1997;25:685–706. [Google Scholar]
- Dominguez XA, Canales A, Garza JA, Gomez E, Garza L. Medicinal plants of Mexico. XVIII. Constituents of leaves and branches of Helietta parvifolia. . Phytochemistry. 1971;10:1966. [Google Scholar]
- Dotted S, Wolfe LM, Jurgens A. Qualitative and quantitative analyses of flower scent in Silene latifolia. . Phytochemistry. 2005;66:203–213. doi: 10.1016/j.phytochem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Dregus M, Engel K-H. Volatile constituents of uncooked rhubarb (Rheum rhabarbarum L.) stalks. Journal of Agricultural and Food Chemistry. 2003;51:6530–6536. doi: 10.1021/jf030399l. [DOI] [PubMed] [Google Scholar]
- Duke JA. 1981. Handbook of legumes of world economic importance. Plenum Press.
- Duke JA. CRC Press; 1985. Acorus calamus L. In: CRC Handbook of Medicinal Herbs ; pp. 14–15. [Google Scholar]
- Dung NX, Tham NT, Van Khien P, Quang NT, Thi Le H, Leclercq PA. Characterization of the oils from various parts of Talauma gioi Aug. Chev. (Magnoliaceae) from Vietnam. Journal of Essential Oil Research. 1997;9:119–121. [Google Scholar]
- Dzamic A, Sokovic M, Ristic MS, Grijic-Jovanovid S, Vukojevic J, Marin PD. Chemical composition and antifungal activity of Illicium verum and Eugenia caryophyllata. . Chemistry of Natural Compounds. 2009;45:259–261. [Google Scholar]
- Ehret C, Maupetit P, Petrzilka M. Proceedings of the 11th Essential Oil Congress. Oxford and IBH Publishing; 1989. New organoleptically important components from Narcissus absolute (Narcissuspoeticus LJ). [Google Scholar]
- Ekundaya O, Laakso I, Adegbola R-M, Oguntimein B, Sofowora A, Hiltunen R. Essential oil constituents of Ashanti pepper (Piper guineense) fruits (berries). Journal of Agricultural and Food Chemistry. 1988;36:880–882. [Google Scholar]
- Elamrani A, Zrira S, Benjilali B. A study of Moroccan rosemary oils. Journal of Essential Oil Research. 2000;12:487–495. [Google Scholar]
- EMEA. Public statement on the use of herbal medicinal products containing methyl eugenol. European Medicines Agency, Evaluation of medicines for human use. 2005. Available online, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/04/WC500089961.pdf.
- Engle KH, Heidlas J, Tressl R. The flavour of tropical fruits (banana, melon, pineapple). In: Morton ID, MacLeod JD, editors. Food Flavors: Part C, The Flavor of Fruits, Elsevier Scientific Publications; 1990. pp. 195–219. [Google Scholar]
- Esmaeili A, Nematollahi F, Rustaiyan A, Moazami N, Masoudi S, Bamasian S. Volatile constituents of Achillea pachycephala, A. oxyodonta and A. biebersteinii from Iran. Flavour Fragrance Journal. 2006;21:253–256. [Google Scholar]
- Fakhari AR, Sonboli A. Essential oil composition of Pimpinella barbata (DC.) Boiss. from Iran. Journal of Essential Oil Research. 2006;18:679–681. [Google Scholar]
- Farag RS, Shalaby AS, El-Baroty GA, Ibrahim NA, Ali MA, Hassan EM. Chemical and biological evaluation of the essential oils of different Melaleuca species. Phytotherapy Research. 2004;18:30–35. doi: 10.1002/ptr.1348. [DOI] [PubMed] [Google Scholar]
- Ferraz ABF, Balbino JM, Zini CA, Ribeiro VLS, Bordignon SAL, von Poser G. Acaricidal activity and chemical composition of the essential oil from three Piper species. Parasitology Research. 2010;107:243–248. doi: 10.1007/s00436-010-1878-y. [DOI] [PubMed] [Google Scholar]
- Ferrer J-L, Austin MB, Stewart C, Jr, Noel JP. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiology and Biochemistry. 2008;46:356–370. doi: 10.1016/j.plaphy.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamini G, Cioni PL, Morelli I, Maccioni S, Baldini R. Phytochemical typologies in some populations of Myrtus communis L. on Caprione Promontory (East Liguria, Italy). Food Chemistry. 2004;85:599–604. [Google Scholar]
- Fiorini C, Fouraste I, David B, Bessiere JM. Composition of the flower, leaf and stem essential oils from Laurus nobilis L. Flavour and Fragrance Journal. 1997;12:91–93. [Google Scholar]
- Firuzi O, Asadollahi M, Gholami M, Javidnia K. Composition and biological activities of essential oils from four Heracleum species. Food Chemistry. 2010;122:117–122. [Google Scholar]
- Flamini G, Cioni PL, Morelli I. Differences in the fragraces of pollen and different floral parts of male and female flowers of Laurus nobilis. . Journal of Agricultural and Food Chemistry. 2002;50:4647–4662. doi: 10.1021/jf020269x. [DOI] [PubMed] [Google Scholar]
- Flamini G, Cioni PL, Morelli I. Differences in the fragrances of pollen and different floral parts of male and female flowers of Laurus nobilis. . Journal of Agricultural and Food Chemistry. 2002;50:4647–4652. doi: 10.1021/jf020269x. [DOI] [PubMed] [Google Scholar]
- Flamini G, Tebano M, Cioni PL. Composition of the essential oils from leafy parts of the shoots, flowers and fruits of Eryngium amthystinum from Amiata Mount (Tuscany, Italy). Journal of Agricultural and Food Chemistry. 2008;107:671–674. [Google Scholar]
- Flamini G, Cioni PL, Morelli I, Ceccarini L, Andolfi L, Macchia M. Composition of the essential oil of Medicago marina L. from the coastal dunes of Tuscany, Italy. Flavour and Fragrance Journal. 2003;18:460–462. [Google Scholar]
- Fleisher A, Fleisher Z. The fragrance of biblical Mandrake. Economic Botany. 1994;48:243–251. [Google Scholar]
- Fleisher Z, Fleisher A. Volatiles of the Mastic tree — Pistacia lentiscus L. Aromatic plants of the holy land and the Sinai. Part X. Journal of Essential Oil Research. 1992;4:663–665. [Google Scholar]
- Fletcher BS, Bateman MA, Hart NK, Lamberton JA. Identification of a fruit fly attractant in an Australian plant, Zieria smithii as O-methyl eugenol. Journal of Economic Entomology. 1975;68:815–816. [Google Scholar]
- Fokialakis N, Magiatis P, Mitaku S. Essential oil constituents of Valeriana italica and Valeriana tuberosa. Stereochemical and conformational study of 15acetoxyvaleranone. Zeitschrift für Naturforschung. 2002;57c:791–796. doi: 10.1515/znc-2002-9-1006. [DOI] [PubMed] [Google Scholar]
- Fournier G, Hadjiakhoondi A, Leboeuf M, Cave A, Charles B. Essential oils of Annonaceae. Part VIII. Volatile constituents of the essential oils from three Guatteria species. Journal of Essential Oil Research. 1997;9:275–278. [Google Scholar]
- Franceschi VR, Krokene P, Christiansen E, Krekling T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytology. 2005;167:353–376. doi: 10.1111/j.1469-8137.2005.01436.x. [DOI] [PubMed] [Google Scholar]
- Franchomme P, Peneol D. L'Aromatherapie Exactement. 1995. Jollois R. (in French)
- Fujita H, Yamashita M. Constituents of the essential oil from Alpinia speciosa. . Yakugaku Zasshi. 1973;93:1635–1638. doi: 10.1248/yakushi1947.93.12_1635. in Japanese. [DOI] [PubMed] [Google Scholar]
- Fujita S, Fujita Y. Essential oils of the plants from various territories. XXXIII. Essential oil of Agastache rugosa. . Yakugaku Zasshi. 1973;93:1679–1681. doi: 10.1248/yakushi1947.93.12_1679. in Japanese. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Fujita S, Yoshikawa H. Biogenesis of essential oils in camphor trees. XXXIII. Components of essential oils of Cinnamomum doederleinii. I. Nippon Nogei Kagaku Kaishi. 1974;48:633–636. In Japanese. [Google Scholar]
- Fun CE, Svendsen AB. The essential oil of Lippia alba (Mill.) N.E. Br. Journal of Essential Oil Research. 1990;2:265–267. [Google Scholar]
- Fun CE, Svendsen AB. The essential oil of Hyptis suaveolens grown in Aruba. Flavour and Fragrance Journal. 1990;5:161–163. [Google Scholar]
- Găinar MZI, Bala D. Influence of the process parameters on supercritical CO2 extraction of fennel essential oil. AnaleleUniversitătii din Bucureşti — Chimie, Anul XV. 2006;1:107–111. [Google Scholar]
- Garcia-Fajardo J, Martinez-Sosa M, Estarron-Espinosa M, Vilarem G, Gaset A, De Santos JM. Comparative study of the oil and supercritical CO2 extract of Mexican pimento (Pimenta dioica Merrill). Journal of Essential Oil Research. 1997;9:181–185. [Google Scholar]
- Georgiou C, Koutsaviti A, Bazos I, Tzakou O. Chemical composition of Echinophora tenuifolia subsp. sibthorpiana essential oil from Greece. Records of Natural Products. 2010;4:167–170. [Google Scholar]
- Ghani A, Saharkhiz MJ, Hassanzadch M, Msaada K. Changes in the essential oil content and chemical compositions of Echinophora platyloba DC. during three different growth and developmental stages. Journal of Essential Oil Bearing Plants. 2009;12:162–171. [Google Scholar]
- Gnavi G, Bertea CM, Usai M, Maffei ME. Comparative characterization of Santolina insularis chemotypes by essential oil composition, 5S-rRNA-NTS sequencing and EcoRV RFLP-PCR. Phytochemistry. 2010;71:930–936. doi: 10.1016/j.phytochem.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Goel D, Mallavarupu GR, Kumar S, Singh V, Ali M. Volatile metabolite compositions of the essential oil from aerial parts of ornamental and artemisinin rich cultivars of Artemisis annua. . Journal of Essential Oil Research. 2008;20:147–152. [Google Scholar]
- Gogus F, Mustafa ZO, Lewis AC. Extraction of essential oils of leaves and flowers of Achillea monocephala by superheated water. Flavour and Fragrance Journal. 2006;21:122–128. [Google Scholar]
- Gottlieb OR, Da Rocha AI. Chemistry of Brazilian Lauraceae. XIX. Constituents of Aniba hostmanniana. . Phytochemistry. 1972;11:1861–1863. [Google Scholar]
- Gracza L. HPLC determination of phenylpropane derivatives in drugs and drug preparations. Part 3. Analysis of phenylpropane derivatives. Deutsche Apotheker Zeitung. 1980;120:1859–1863. [Google Scholar]
- Gregonis DE, Portwood RD, Davidson WH, Durfee DA, Levinson AS. Volatile oils from foliage of coast redwood and big tree. Phytochemistry. 1968;7:975–981. [Google Scholar]
- Griffiths DW, Robertson GW, Shepherd T, Ramsay G. Epicuticular waxes and volatiles from faba bean (Vicia faba) flowers. Phytochemistry. 1999;52:607–612. [Google Scholar]
- Grosso C, Teixeira G, Gomes I, Martins ES, Barroso JG, Pedro LG, Figueiredo AC. Assessment of the essential oil composition of Tornabenea annua, Tornabenea insularis and Tornabenea tenuissima fruits from Cape Verde Islands. Biochemical Systematics and Ecology. 2009;37:474–478. [Google Scholar]
- Gu J, Liu L, Zhang Y. Study on the essential oil from the fruits of Cinnamomum camphora (L.) Presl var. linaloolifera Fujita. Linchan Huaxue Yu Gongye. 1990;10:77–82. Chinese with English summary. [Google Scholar]
- Guchu E, Diaz-Maroto MC, Diaz-Maroto IJ, Vila-Lameiro P, Perez-Coello MS. Influence of the species and geographical location on volatile composition of Spanish oak wood (Quercus petraea Lieb, and Quercus robur L.). Journal of Agricultural and Food Chemistry. 2006;54:3062–3066. doi: 10.1021/jf053055z. [DOI] [PubMed] [Google Scholar]
- Gudaityte O, Venskutonis PR. Chemotypes of Achillea millefolium transferred from 14 different locations in Lithuania to the controlled environment. Biochemical Systematics and Ecology. 2007;35:582–592. [Google Scholar]
- Guerrini A, Sacchetti G, Muzzoli M, Rueda GM, Medici A, Besco E, Bruni R. Composition of the volatile fraction of Ocotea bofo Kunth (Lauraceae) calyces by GC-MS and NMR fingerprinting and its antimicrobial and antioxidant activity. Journal of Agricultural and Food Chemistry. 2006;54:7778–7788. doi: 10.1021/jf0605493. [DOI] [PubMed] [Google Scholar]
- Gulati BC, Khan MN. Essential oil of Artemesia pallens Wall (Davana) - as study of minor constituents. 7th International Congress of Essential Oils. 1979;7:357–359. [Google Scholar]
- Gutirrez RMP, Luna HH, Garrido SH. Antioxidant activity of Tagetes erecta essential oil. Journal of the Chilean Chemical Society. 2010;51:882–886. [Google Scholar]
- Hadad M, Zygadlo JA, Lima B, Derita M, Feresin GE, Zacchino SA, Tapia A. Chemical composition and antimicrobial activity of essential oil from Baccharis grisebachii Heiron (Asteraceae). Journal of Chilean Chemical Soceity. 2007;52:1186–1189. [Google Scholar]
- Halabi S, Battah AA, Aburjai T, Hudaib M. Phytochemical and platelet investigation of Gundelia tournifortil. . Pharmaceutical Biology. 2005;43:496–500. [Google Scholar]
- Hamm S, Bleton J, Connan J, Tchapla A. A chemical investigation by headspace of SPME and GC-MS of volatile and semivolatile terpenes in various olibanum samples. Phytochemistry. 2005;66:1499–1514. doi: 10.1016/j.phytochem.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Hammami S, Khoja I, Jannet HB, Halima MB, Highri Z. Flowers essential oil composition of Tunisian Matthiola longipetala and its bioactivity against Tribolium confusum insect. Journal of Essential Oil Bearing Plants. 2007;10:162–167. [Google Scholar]
- Han A-R, Kim HJ, Shin M, Hong M, Kim YS, Bae H. Constituents of Asarum sieboldii with inhibitory activity on lipopolysaccharide (LPS)-induced NO production in BV-2 microglial cells. Chemical Biodiversity. 2008;5:346–351. doi: 10.1002/cbdv.200890033. [DOI] [PubMed] [Google Scholar]
- Hassanpouraghdam MB, Shalamzari MS, Sepehri N. GC/MS analysis of Echinophora platyloba DC. Essential oil from Northwest Iran: a potential source of (Z)-βocimene and α-phellandrene. Chemija. 2009;20:120–123. [Google Scholar]
- Hayashi N, Maeshima K, Komae H. Phenol ethers of three north american Hexastylis species. Phytochemistry. 22:299. [Google Scholar]
- Hazzit M, Baaliouiamer Z, Faleiro ML, Miquel MG. Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities. Journal of Agricultural and Food Chemistry. 2006;54:6314–6321. doi: 10.1021/jf0606104. [DOI] [PubMed] [Google Scholar]
- Hee AKW, Tan KH. Male sex pheromonal components derived from methyl eugenol in the haemolymph of fruit fly Bactrocera papayae. . Journal of Chemical Ecology. 2004;30:2127–2138. doi: 10.1023/b:joec.0000048778.02561.70. [DOI] [PubMed] [Google Scholar]
- Herrmann KM, Weaver LM. The shikimate pathway. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:473–503. doi: 10.1146/annurev.arplant.50.1.473. [DOI] [PubMed] [Google Scholar]
- Hethelyi E, Danos B, Tetnyl P, Juhasz G. Phytochemical studies on Tagetes species; infraspecific differences of the essential oil in T. minuta and T. tenuifolia. . Herba Hungarica. 1987;26:145–158. [Google Scholar]
- Ho C, Wang EIC, Wei X, Lu S, Su Y. Composition and bioactivities of the leaf essential oils of Cinnamomum subavenium Miq. from Taiwan. Journal of Essential Oil Research. 2008;20:328–334. [Google Scholar]
- Horvat RJ, Senter SD. Identification of the volatile constituents from scuppernong berries (Vitis rotundifolia). Journal of Food Science. 1984;49:64–66. [Google Scholar]
- Horvat RJ, Chapman GW, Robertson JA, Meredith FI, Scorza R, Callihan AM, Morgan P. Comparison of the volatile compounds from several commercial peach cultivars. Journal of Agricultural and Food Chemistry. 1990;38:234–237. [Google Scholar]
- Hosni K, Msaada K, Taarit MB, Ouchikh O, Kallel M, Marzouk B. Essential oil composition of Hypericum perfoliatum L. and Hypericum tomentosum L. growing wild in Tunisia. Industrial Crops and Products. 2008;27:308–314. [Google Scholar]
- Howes M-JR, Kite GC, Simmonds MSJ. Distinguishing Chinese star anise from Japanese star anise using thermal desorptiongas chromatography-mass spectrometry. Journal of Agricultural and Food Chemistry. 2009;57:5783–5789. doi: 10.1021/jf9009153. [DOI] [PubMed] [Google Scholar]
- Howlett FM. VII. The effect of oil of Citronella on two species of Dacus. . Transactions of the Entomological Society of London. 1912;60:412–418. [Google Scholar]
- Howlett FM. Chemical reactions of fruit flies. Bulletin of Entomological Research. 1915;6:297–305. [Google Scholar]
- Hsiao Y-Y, Tsai W-C, Kuoh C-S, Huang T-H, Wang H-C, Wu T-S, Leu Y-L, Chen W-H, Chen H-H. Comparison of transcripts in Phalaenopsis bellina and Phalaenopsis equestris (Orchidaceae) flowers to deduce monoterpene biosynthesis pathway. BMC Plant Biology. 2006;6:1–14. doi: 10.1186/1471-2229-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber FK, Kaiser R, Sauter W, Schiestl FP. Floral Scent Emission and Pollinator Attraction in two species of Gymnadenia (Orchidaceae). Oecologia. 2005;142:564–575. doi: 10.1007/s00442-004-1750-9. [DOI] [PubMed] [Google Scholar]
- Hudaib MM, Aburjai TA. Composition of the essential oil from Artemisis herba-alba grown in Jordan. Journal of Essential Oil Research. 2006;18:301–304. [Google Scholar]
- Hussain RA, Poveda LJ, Pezzuta JM, Soejarto DD, Kinghorn AD. Sweetening agents of plant origin: Phenylpropanoid constituents of seven sweet-tasting plants. Economic Botany. 1990;44:174–182. [Google Scholar]
- Hussein MS, El-Sherbeny SE, Khalil MY, Naguib NY, Aly SM. Growth characters and chemical constituents of Dracocephalum moldavica L. plants in relation to compost fertilizer and planting distance. Scientia Horticultae. 2006;108:322–331. [Google Scholar]
- IAEA. Trapping guidelines for areawide fruit fly programmes. International Atomic Energy Agency; 2003. Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture. [Google Scholar]
- Ibanez E, de Murga AOG, Lopez-Sebastian S, Tabera J, Reglero G. Supercritical fluid extraction and fractionation of different preprocessed Rosemary plants. Journal of Agricultural and Food Chemistry. 1999;47:1400–1404. doi: 10.1021/jf980982f. [DOI] [PubMed] [Google Scholar]
- Iscan G, Kirimer N, Kurkuoglu M, Arabach T, Kupeli E, Baser KHC. Biological activity and composition of the essential oils of Achillea schischkinii Sosn. and Achillea aleppica Dc. Subsp. aleppica. . Journal of Agricultural and Food Chemistry. 2006;54:170–173. doi: 10.1021/jf051644z. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Tani K. Odor of flower of Koufuku-no-Ki (Dracaena fragrans ‘Massangeana’). Japanese with English Abstract Koryo. 2007;236:89–98. [Google Scholar]
- Jakobson HB, Olsen CE. Influence of climatic factors on emission of flower volatiles in situ. Planta. 1994;192:365–371. [Google Scholar]
- Jantan I, Ayop N, Au BH, Ahmad AS. Chemical composition of the essential oils of Cinnamomum cordatum Kosterm. Flavour and Fragrance Journal. 2002;17:212–214. [Google Scholar]
- Jantan I, Ayob N, Ali NAM, Ahmad AS, Yalvema MF, Muhammad K, Azizi AR. The essential oils of Cinnamom rhycophyllum Miq. as natural sources of benzyl benzoate, safrole and methyl (E)-cinnamate. Flavour and Fragrance Journal. 2004;19:260–262. [Google Scholar]
- Jarikassem S, Brophy JJ, Rerk-am U, Supattanakul W. Essential oil constituents from two uncommon Zingiber rhizomes. 47th Annual Meeting of Society for Economic Botany - Folk Botanical Wisdom: Towards Global Markets. 2006.
- Javidnia K, Banani A, Miri R, Kamalinejad M, Javidnia A. Constituents of the volatile oil of Inula oculus-christl L. from Iran. Journal Essential Oil Research. 2006;18:676–678. [Google Scholar]
- Jean F-I, Deslauriers H, Collin GJ, Gagnon M, Hachey J-M, Pare JR, Belanger A. The essential oil of Ligusticum scoticum L. Journal of Essential Oil Research. 1990;2:37–44. [Google Scholar]
- Jerkovic I, Mastelic J, Milos M, Juteau F, Masotti V, Viano J. Chemical variability of Artemisia vulgaris L. essential oils originated from the Mediterranean area of France and Croatia. Flavour and Fragrance Journal. 2003;18:436–440. [Google Scholar]
- Jeong ES, Choi KY, Kim SC, Son IS, Cho HC, Ahn SY, Woo MH, Hong JT, Moon DC. Pattern recognition of the herbal drug, Magnoliae flos according to their essential oil components. Bulletin of Korean Chemical Society. 2009;30:1211–1126. [Google Scholar]
- Jerkovic I, Mastelic J. Volatile compounds from leaf-buds of Populus nigra L. (Salicaceae) Phytochemistry. 2003;63:109–113. doi: 10.1016/s0031-9422(02)00706-9. [DOI] [PubMed] [Google Scholar]
- Jeya Johti G, Benniamin A, Maridass M, Raju G. Identification of essential oils composition and antifungal activity of Eugenia singampattiana fruits. Pharmacology online. 2009;2:727–733. [Google Scholar]
- Jirovetz L, Smith D, Buchbauer G. Aroma compound analysis of Eruca sativa (Brassicaceae) SPME headspace leaf samples using GC, GC-MS, and olfactometry. Journal of Agricultural and Food Chemistry. 2002a;50:4643–4646. doi: 10.1021/jf020129n. [DOI] [PubMed] [Google Scholar]
- Jirovetz L, Buchbauer G, Ngassoum MB, Geissler M. Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction—gas chromatography, solid- phase microextraction—gas chromatography— mass spectrometry and olfactometry. Journal of Chromatography A. 2002b;976:265–275. doi: 10.1016/s0021-9673(02)00376-x. [DOI] [PubMed] [Google Scholar]
- Jirovetz L, Buchbauer G, Stoyanova AS, Georgiev EV, Damianova ST. Composition, quality control, and antimicrobial activity of the essential oil of long-time stored dill (Anethum graveolens L.) seeds from Bulgaria. Journal of Agricultural and Food Chemistry. 2003;51:3854–3857. doi: 10.1021/jf030004y. [DOI] [PubMed] [Google Scholar]
- Jirovetz L, Buchbauer G, Stoyanova A, Balinova A, Guangjiun Z, Xihan M. Solid phase microextraction/gas chromatogaphic and olfactory analysis of the scent and fixative properties of the essential oil of Rosa damascena L. from China. Flavour and Fragrance Journal. 2004;20:7–12. [Google Scholar]
- Jirovetz L, Buchbauer G, Ngassoum MB, Parmentier M. Chemical composition and olfactory characterization of essential oils of fruits and seeds of African pear (Dacryodes edulis (G. Don) H.J. Lam) from Cameroon. Flavour and Fragrance Journal. 2005;20:215–218. [Google Scholar]
- Jirovetz L., Buchbauer G, Stoilova I, Stoyanova A, Krastanov A, Schmidt E. Chemical composition and antioxidant properties of clove leaf essential oil. Journal of Agricultural and Food Chemistry. 2006;54:6303–6307. doi: 10.1021/jf060608c. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Ellis A, Dotterl S. Specialization for pollination by beetles and wasps: the role of lollipop hairs and fragrance in Satyrium microrrhynchum (Orchidaceae). American Journal of Botany. 2007;94:47–55. doi: 10.3732/ajb.94.1.47. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Griffiths ME, Peter CI, Lawes MJ. Pollinators, “mustard oil” volatiles, and fruit production in flowers if the diecious tree Drypetes natalensis (Putranjivaceae). American Journal of Botany. 2009;96:2080–2086. doi: 10.3732/ajb.0800362. [DOI] [PubMed] [Google Scholar]
- Joichi A, Yomogida K, Awano K, Ueda Y. Volatile components of tea-scented modern roses and ancient Chinese roses. Flavour and Fragrance Journal. 2005;20:152–157. [Google Scholar]
- Jordan MJ, Martnez RM, Goodner KL, Baldwin EA, Sotomayor JA. Seasonal variation of Thymus hyemails Lange and Spanish Thymus vulgaris L. essential oils composition. Industrial Crops and Products. 2006;24:253–263. [Google Scholar]
- Joy B, Rao JM. Essential oil of the leaves of Annona squamosa L. Journal of Essential Oil Research. 1997;9:349–350. [Google Scholar]
- Jurgens A, Dotted S. Chemical composition of anther volatiles in Ranunculaceae: Genera-specific profiles in Anemone, Aquilegia, Caltha, Pulsatilla, Ranunculus, and Trollius species. Journal of Botany. 2004;91(12):1969–1980. doi: 10.3732/ajb.91.12.1969. [DOI] [PubMed] [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. Flower scent composition in Dianthus and Saponaria species (Caryophyllaceae) and its relevance for pollination biology and taxonomy. Biochemical Systematics and Ecology. 2003;31:345–357. [Google Scholar]
- Juteau F, Masotti V, Bessière JM, Viano J. Compositional characteristics of the essential oil of Artemisia campestris var. glutinosa. . Biochemical Systematics and Ecology. 2002;30:1065–1070. [Google Scholar]
- Kainulainen P, Tarhanen J, Tiilikkala K, Holopainen JK. Foliar and emission composition of essential oil in two carrot varieties. Journal of Agricultural and Food Chemistry. 1998;46:3780–3784. [Google Scholar]
- Kaiser R. New volatile constituents of the flower concrete of Michelia champaca L. Journal of Essential Oil Research. 1991;3:129–146. [Google Scholar]
- Kaiser R. Elsevier The Scent of OrchidsOlfactory and Chemical Investigations. 1993.
- Kaluzna-Czaolinska J. GC-MS analysis of biologically active compounds in cosmopolitan grasses. ACTA Chromographica. 2007;19:279–282. [Google Scholar]
- Kamdem DP, Gage DA. Chemical composition of essential oil from the root bark of Sassafras albidum. . Planta Medica. 1995;61:574–575. doi: 10.1055/s-2006-959379. [DOI] [PubMed] [Google Scholar]
- Kameoka H. The Essential Oil Constituents of Some Useful Plants from China. Recent Developments in Flavour and Fragrance Chemistry — Proceedings of the 3rd International Haarman and Reimer Symposium Pub. 1993. . VCH NY.
- Kameoka H, Wang CP. The constituents of the essential oils from Trachycarpus excelsa Wendl and T. fortunei W. Nippon Nogei Kagaku Kaishi. 1980;54:111–115. in Japanese. [Google Scholar]
- Kameoka H, Sagara K, Miyazawa M. Components of essential oils of Kakushitsu (Daucus carota L. and Carpesium abrotanoides L.) Nippon Nogei Kagaku Kaishi. 1989;63:185–188. in Japanese. [Google Scholar]
- Kapetamos C, Karrioti A, Bovic S, Marin P, Vejic M, Skaltsa H. Chemical and principal-component analyses of the essential oils of Apiodeae taxa (Apiaceae) from Ventral Balkan. Chemical Biodiversity. 2008;5:101–118. doi: 10.1002/cbdv.200890000. [DOI] [PubMed] [Google Scholar]
- Kapteyn J, Qualley AV, Xie ZZ, Fridman E, Dudareva N, Gang DR. Evolution of cinnamate/p-coumarate carboxyl methyltransferases and their role in the biosynthesis of methylcinnamate. The Plant Cell. 2007;19:3212–3229. doi: 10.1105/tpc.107.054155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamenderes C, Demirci , Baser KHC. Composition of essential oils of ten Centaurea L. taxa from Turkey. Journal of Essential Oil Research. 2008;20:342–349. [Google Scholar]
- Katsouri E, Demetzos C, Perdetzoglou D, Loukis A. An interpopulation study of the essential oils of various parts of Crithmum maritimum L. growing in Amorgos Island, Greece. Journal of Essential Oil Research. 2001;13:303–308. [Google Scholar]
- Katsumi Y. Minor components from growing buds of Artemisia capillaris that act as insect antifeedants. Journal of Agricultural and Food Chemistry. 1987;35(6):889–891. [Google Scholar]
- Kawano T, Saito T. Study on attractant of the rice stem borer, Chilo suppressalis Walker. Botyu-Kagaku. 1968;33:122–130. in Japanese with English summary. [Google Scholar]
- Kawano Y, Mitchell WC, Matshmoto H. Identification of the male oriental fruit fly attractant in the golden shower blossom. Journal of Economic Entomology. 1968;61:986–988. [Google Scholar]
- Kawata J, Kameda M, Miyazawa M. Constituents of essential oil from the dried fruits and stems of Akebia quinata (Thunb.) Decne. Journal of Oleo Science. 2007;56:59–63. doi: 10.5650/jos.56.59. [DOI] [PubMed] [Google Scholar]
- Kaya A, Demirci B, Baser KHC. Study of the essential oils from the flowers and fruits of Scandix iberica Bieb. growing in Turkey. Journal of Essential Oil Research. 2007;19:155–156. [Google Scholar]
- Kayser O, Latte K, Kolodziej H, Hammerschmidt F-J. Composition of the essential oils of Pelargonium sidoides DC. and Pelargonium reniforme Curt. Flavour and Fragrance Journal. 1998;13:209–212. [Google Scholar]
- Kelm MA, Nair MG, Schutzki RA. Mosquitocidal compounds from Magnolia salicifolia. . International Journal of Pharmacognosy. 1997;35:84–90. [Google Scholar]
- Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- Khan VA, Dubovenko ZV, Pentegova VA. Oxygen-containing mono- and sesquiterpenoids of the oleoresin of Picea koraiensis. . Kihmiya Prirodnykh Soedinenii. 1983;1:109–110. in Russian. [Google Scholar]
- Khan VA, Bol'hakova VL, Grigorovich ML, Shmidt EN, Dubovenko ZV, Pentegova VA. Terpenoids of Pinus funebris. . Kihmiya Prirodnykh Soedinenii. 1984;4:444–448. in Russian. [Google Scholar]
- Khodakov GV, Kotikov IV, Pankovetskii VN. Component composition of essential oil from Artemisia abrotanum and A. dracunculus. . Chemistry of Natural Compounds. 2009;45:905–908. [Google Scholar]
- Khosla MK, Pushpangadan P, Thappa RK, Sobti SN. Search for new aroma chemicals from Ocimum species. III. Studies on genetic variability for essential oil and other allied characters of South American species O. micranthum Willd. Indian Perfumer. 1980;24:148–152. [Google Scholar]
- Khosla MK, Bradu BL, Thapa RK. Biogenetic studies on the inheritance of different essential oil constituents of Ocimum species, their F1 hybrids and synthesized allopolyploids. Herba Hungaria. 1989;28:13–19. [Google Scholar]
- Kilic A, Hafizoglu H, Kollmannsberger H, Nitz S. Volatile constituents and key odorants in leaves, buds, flowers, and fruits of Laurus nobilis L. Journal of Agricultural and Food Chemistry. 2004;52:1601–1606. doi: 10.1021/jf0306237. [DOI] [PubMed] [Google Scholar]
- Kim H-J, Kim K, Kim N-S, Lee D-S. Determination of floral fragrances of Rosa hybrida using solid-phase trapping-solvent extraction and gas chromatography-mass spectrometry. Journal of Chromatography A. 2000;902:389–404. doi: 10.1016/s0021-9673(00)00863-3. [DOI] [PubMed] [Google Scholar]
- Kim J-Y, Kim S-S, Oh T-H, Baik JS, Song G, Lee NH, Hyun C-G. Chemical composition, antioxidant, anti-elastase, and anti-inflammatory activities oilllicium anisatum esstential oil. ACTA Pharmaceutica. 2009;59:289–300. doi: 10.2478/v10007-009-0022-y. [DOI] [PubMed] [Google Scholar]
- Kiran SR, Devi PS, Reddy KJ. Bioactivity of essential oils and sesquiterpenes of Chloroxylon swietenia DC against Helicoverpa armigera. . Current Science. 2007;93:544–548. [Google Scholar]
- Kittibarmruangsook P. Effects of volatile oils extracted from some plants to the Oriental fruit fly (Dacus dorsalis Hendel). M. Sc. Thesis in Agriculture. Kasetsart University; Bangkok, Thailand: 1980. [Google Scholar]
- Kivanc M. Antimicrobial activity of “Cortuk” (Echinophora sibthorpiana Guss.) spice, its essential oil and methyleugenol. Nahrung. 1988;332:635–637. doi: 10.1002/food.19880320631. [DOI] [PubMed] [Google Scholar]
- Kivanc M, Akgul A. Mould growth on black table olives and prevention by sorbic acid, methyl-eugenol and spice essential oil. Nahrung. 1990;34:369–373. doi: 10.1002/food.19900340419. [DOI] [PubMed] [Google Scholar]
- Klischies M, Stockigt j, Zenk MH. Biosynthesis of the allylphenols eugenol and methyleugenol in Ocimum basilicum L. Journal of the Chemical Society: Chemical Communications. 1975;1975:879–880. [Google Scholar]
- Knudsen JT, Mori SA. Floral scents and pollination in neotropical Lecythidaceae. Biotropica. 1996;28:42–60. [Google Scholar]
- Knudsen JT, Tollsten T, Bergstrom LG. Floral scents — a checklist of volatile compounds isolated by head-space techniques. Phytochemistry. 1993;33:253–280. [Google Scholar]
- Knudsen JT, Andersson S, Bergman P. Flroal scent attraction in Geonoma macrostachys, an understorey palm of the Amazonian rain forest. Oikos. 1999;85:409–418. [Google Scholar]
- Knudsen JT, Tollsten L, Ervik F. Flower scent and pollination in selected neotropical palms. Plant Biology. 2001;3:642–653. [Google Scholar]
- Knudsen JT, Eriksson R, Stahl B. Diversity and distribution of floral scent. Botanical Review. 2006;72:1–120. [Google Scholar]
- Knudsen JT, Tollsten L, Grothe I, Bergstrom G, Raguso RA. Trends in floral scent chemistry in pollination syndromes: floral scent composition in hummingbird-pollinated taxa. Botanical Journal of the Linnean Society. 2004;146:191–199. [Google Scholar]
- Koezuka Y, Honda G, Tabata M. Genetic control of phenylpropanoids in Perilla frutescens. . Phytochemistry. 1986;25:2085–2087. [Google Scholar]
- Koo BS, Park KS, Ha JH, Park JH, Lim JC, Lee DU. Inhibitory effects of the fragrance inhalation of essential oil from Acorus gramineus on central nervous system. Biological and Pharmaceutical Bulletin. 2003;26:978–982. doi: 10.1248/bpb.26.978. [DOI] [PubMed] [Google Scholar]
- Kordali S, Kotan R, Mavi A, Cakir A, Ala A, Yilsdrim A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia abshinthium, A. dracunculus, Artemisia santonicum and Artemisia spicigera essential oils. Journal of Agricultural and Food Chemistry. 2005;53:9452–9458. doi: 10.1021/jf0516538. [DOI] [PubMed] [Google Scholar]
- Kosuge T, Yokota M, Nukaya H, Gotoh Y, Nagasawa M. Studies on antitussive principles of Asiasari radix. . Chemical and Pharmaceutical Bulletin. 1978;26:2284–2285. in Japanese. [Google Scholar]
- Kothari SK, Bhattacharya AK, Ramesh S, Garg SN, Khanuja SPS. Volatile constituents in oil from different plant parts of methyl eugenol-rich Ocimum tenuiflorum L.f. (syn. O. sanctum L.) grown in South India. Journal of Essential Oil Research. 2005;17:656–658. [Google Scholar]
- Kovacevic NN, Simic MD, Ristic MS. Essential oil of Laurus nobilis from Montenegro. Chemistry of Natural Compounds. 2007;43:408–411. [Google Scholar]
- Krauze-Baranowska M, Mardarowicz M, Wiwart M, Poblocka L, Dynowska M. Antifungal activity of the essential oils from some species of the genus Pinus. . Zeitschrift für Naturforschung. 2002;57c:478–482. doi: 10.1515/znc-2002-5-613. [DOI] [PubMed] [Google Scholar]
- Kristiawan M, Sobolik V, Allaf K. Isolation of Indonesian cananga oil using multi-cycle pressure drop process. Journal of Chromatography A. 2008;1192:306–318. doi: 10.1016/j.chroma.2008.03.068. [DOI] [PubMed] [Google Scholar]
- Krüger H, Hammer K, Hammerschmidt F-J, Hennig L. Methyl Eugenol and gasarone two new main components in fennel. 2005 Abstract (unpublished). [Google Scholar]
- Kubo I, Himejima M, Muroi H. Antimicrobial activity of flavor components of cardamom Elattaria cardamomum (Zingiberaceae) seed. Journal of Agricultural and Food Chemistry. 1991;39:1984–1986. [Google Scholar]
- Kumar N, Motto MG. Volatile constituents of Peony flowers. Phytochemistry. 1986;25:250–253. [Google Scholar]
- Kundakovic T, Fokialakis N, Kovacevic N, Chinou I. Essential oil composition of Achillea lingulata and A. umbellata. . Flavour and Fragrance Journal. 2007;22:184–187. [Google Scholar]
- Kurita N, Miyaji M, Kurane R, Takahara Y. Antifungal activity of components of essential oils. Agricultural Biology and Chemistry. 1981;45:945–952. [Google Scholar]
- Kuo YH, Lin YT, Lin YT. Studies on the extractive constituents of the nutmeg of Myristica fragrans Houtt. Journal of Chinese Chemical Society. 1983;30:63–67. [Google Scholar]
- Kurkcuoglu M, Baser KHC. Studies on Turkish rose concrete, absolute and hydrosol. Chemistry of Natural Compounds. 2003;39:457–464. [Google Scholar]
- Lamarque AL, Maestri DM, Zygadlo JA, Grosso NR. Volatile constituents from flowers of Acacia caven (Mol.) Mol. var. caven, Acacia aroma Gill, ex Hook., Erythrina crista-galli L. and Calliandra tweedii Benth. Flavour and Fragrance Journal. 1998;13:266–268. [Google Scholar]
- Lane AL, Kubanek J. Structure—activity relationship of chemical defenses from the freshwater plant Micranthemum umbrosum. . Phytochemistry. 2006;67:1224–1231. doi: 10.1016/j.phytochem.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Lassaak EV, Pinhey JT. Constituents of Eriostemom trachyphyllus. Structure of trachtyphyllin, a new couomarin. Australian Journal of Chemistry. 1969;22:2175–2185. [Google Scholar]
- Lavid N, Wang J, Shalit M, Guterman I, Bar E, Beuerle T, Menda N, Shafir S, Zamir D, Adam Z, Vainstein A, Weiss D, Pichersky E, Lewinsohn E. O-Methyltransferases involved in the biosynthesis of volatile phenolic derivatives in rose petals. Plant Physiology. 2002;129:1899–1907. doi: 10.1104/pp.005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechat-Vahirua I, Francois P, Menut C, Lamaty G, Bessiere JM. Aromatic plants of French Polynesia. I. Constituents of the essential oils of rhizomes of three Zingiberaceae: Zingiber zerumbet Smith, Hedychium coronariium Koenig and Etlingera cevuga Smith. Journal of Essential Oil Research. 1993;5:55–59. [Google Scholar]
- Lee S-E, Lee B-H, Choi W-S, Park B-S, Kim J-G, Campbell BC. Fumigant toxicity of volatile natural products from Korean spices and medicinal plants towards the rice weevil, Sitophilus oryzae (L). Pest Management Science. 2001;57:548–553. doi: 10.1002/ps.322. [DOI] [PubMed] [Google Scholar]
- Lewinsohn E, Ziv-Raz I, Dudai N, Tadmor Y, Lastochkin E, Larkov O, Chaimovitsh D, Ravid U, Putievsky E, Pichersky E, Shoham Y. Biosynthesis of estragole and methyl-eugenol in sweet basil (Ocimum basilicium L). Development and chemotypic association of allylphenol O-methyltransferase activities. Plant Science. 2000;160:27–35. doi: 10.1016/s0168-9452(00)00357-5. [DOI] [PubMed] [Google Scholar]
- Lewis JA, Moore CJ, Fletcher MT, Drew RAI, Kitching W. Volatile compounds from the flowers of Spathiphyllum cannaefolium. . Phytochemistry. 1988;27:2755–2757. [Google Scholar]
- Lim GK. Chemical constituents and biological activity of Clausena excavata (Rutaceae). M.Sc. Thesis. School of Graduate Studies; Universiti Putra Malaysia: 2005. [Google Scholar]
- Limberger RP, Scopel M, Sobral M, Henriques AT. Comparative analysis of volatiles from Drimys brasiliensis Miers and D. angustifolia Miers (Winteraceae) from Southern Brazil. Biochemical Systematics and Ecology. 2007;35:130–137. [Google Scholar]
- Lin Z, Hua Y. Chemical constituents of the essential oil from Mosla scabra (Thunb.) C.Y. Wu et H.W. Li. Zhiwu Xuebao. 1989;31:320–322. in Chinese. [Google Scholar]
- Lis-Balchin M. The essential oils of Pelargonium grossularioides and Erodium cicutarium (Geraniaceae). Journal of Essential Oil Research. 1993;5:317–318. [Google Scholar]
- Lis-Balchin M, Roth G. Composition of the essential oils of P. odoratissimum, P. exstipulatum and P. fragrans (Geraniaceae). Flavour and Fragrance Journal. 2000;15:391. [Google Scholar]
- Liu X, Zhao M, Luo W, Yang B, Jiang Y. Identification of volatile components in Phyllanthus emblica L. and their antimicrobial activity. Journal of Medicinal Food. 2009;12:423–428. doi: 10.1089/jmf.2007.0679. [DOI] [PubMed] [Google Scholar]
- Liu C-T, Zhang J-X, Yiao R-R, Gan L-X. Chemical studies on the essential oils of Cymbopogon genus. Acta Chimica Sinica. 1981;39:241–247. [Google Scholar]
- Lopes NF, Kato MJ, de Aguiar FH, Andrade JG, Maia S, Yoshida M. Circadian and seasonal variation in the essential oil from Virola surinamensis leaves. Phytochemistry. 1997;46:689–693. [Google Scholar]
- Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczky PP. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 2008;69:1732–1738. doi: 10.1016/j.phytochem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Lu B, Li Y, Mai L, Sun B, Zhu L. Chemical constituents of essential oil from Cinamomum rigidissimum, a new natural resource of safrole. Linchan Juaxue Yu Gongye. 1986;6:39–44. in Chinese. [Google Scholar]
- Macini B. Pharmacognostic study of leaves and stems of Wedelia paludosa DC var. vialis DC., Compositae. Analysis of the essential oil. Revista de Ciencias Farmaceuticas. 1980;2:61–76. in Portugese. [Google Scholar]
- MacLeod AJ, de Troconis NG. Volatile flavor components of Sapodilla fruit (Achras sapota L.). Journal of Agricultural and Food Chemistry. 1982;30:515–517. [Google Scholar]
- Magalhães PJC, Criddle DN, Tavares RA, Melo EM, Mota TL, Leal-Cardoso JH. Intestinal myorelaxant and antispasmodic effects of the essential oil of Croton nepetaefolius and its constituents cineole, methyl-eugenol and terpineol. Phytotherapy Research. 1998;12:172–177. [Google Scholar]
- Mhamdi B, Wannes WA, Dhiffi W, Marzouk B. Volatiles from leaves and flowers of Borage (Borgo officinalis L.). Journal of Essential Oil Research. 2009;21:504–507. [Google Scholar]
- Maia JGS, Andrade EHA. Database of the Amazon aromatic plants and their essential oils. Quimica Nova. 2009;32:595–622. [Google Scholar]
- Maia JGS, Ramos LS, Luz AIR. Studies on the essential oil from Licaria puchurymajor by gas chromatography/mass spectrometry (GC/MS). ACTA Amazonica. 1985;15:179–183. in Portugese. [Google Scholar]
- Maia JGS, da Silva MHL, Andrade EHA, Carreira LMM. Essential oil variation in Lippia glandulosa Schauer. Journal of Essential Oil Research. 2005;17:676–680. [Google Scholar]
- Malagon O, Vila R, Iglesias J, Zaragoza T, Canigueral S. Composition of the essential oils of four medicinal plants from Ecuador. Flavour and Fragrance Journal. 2003;18:527–531. [Google Scholar]
- Mansour ES, Maatooq GT, Khalil AT, Marwan EM, Sallam AA. Essential oil of Daucus glaber Forssk. Zeitschrift für Naturforschung. 2004;59c:373–378. doi: 10.1515/znc-2004-5-615. [DOI] [PubMed] [Google Scholar]
- Manzoor-i-Khuda A, Rahman M, Yusuf M, Chaowdhury J. Studies on essential oil bearing plants of Bangladesh. Part II. Five species of Cymbopogon from Bangladesh and the chemical constituents of their essential oils. Bangladesh Journal of Scientific and Industrial Research. 1986;21:70–82. [Google Scholar]
- Marotti M, Piccaglia R, Biavati B, Marotti I. Characterization and yield evaluation of essential oils from different Tagetes species. Journal of Essential Oil Research. 2004;16:440–444. [Google Scholar]
- Martins ER, Casali VWD, Barbosa LCA, Carazza F. Essential oil in the taxonomy of Ocimum selloi Benth. Journal of Brazilian Chemical Society. 1997;8:29–32. [Google Scholar]
- Martins AP, Salgueiro L, Vila R, Tomi F, Canigural S, Casanova J, Da Cunha AP, Adzet T. Essential oils from four Piper species. Phytochemistry. 1998;49:2019–2023. [Google Scholar]
- Marzouki H, Piras A, Marongiu B, Rosa A, Dessì MA. Extraction and separation of volatile and fixed oils from berries of Laurus nobilis L. by supercritical CO2. Molecules. 2008;13:1702–1711. doi: 10.3390/molecules13081702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzouki H, Piras A, Haj Salah KB, Medini H, Pivetta T, Bouzid S, Marongiu B, Falconieri D. Essential oil composition and variability of Laurus nobilis L. growing in Tunisia, comparison and chemometri investigation of different plant organs. Natural Product Research. 2009;23:343–354. doi: 10.1080/14786410802076200. [DOI] [PubMed] [Google Scholar]
- Masoudi S, Rustaiyan A, Ameri N, Monfared A, Komeilizadeh H, Kamalinejad M, Jami-Roodi J. Volatile oils of Carum copticum (L.) CB. Clarke in Benth. et Hook. and Semenovia tragioides (Boiss.) Manden. from Iran. Journal of Essential Oil Research. 2002;14:288–289. [Google Scholar]
- Massumi MA, Fazeli MR, Alavi SHR, Ajani Y. Chemical constituents and antibacterial activity of essential oil of Prangos ferulacea (L.) Lindl. fruits. Iranian Journal of Pharmaceutical Sciences. 2007;3:171–176. [Google Scholar]
- Matasyoh LG, Matasyoh JC, Wachira FN, Kinyua MG, Muigai AWT, Mukiama TK. Chemical composition and antimicrobial activity of the essential oil of Ocimum gratissimum L. growing in Eastern Kenya. African Journal of Biotechnology. 2007;6:760–765. [Google Scholar]
- Mathela CS, Pande C, Pant AK, Singh AK. Phenylpropanoids constituents of Cymbopogon microstachys. . Journal of Indian Chemical Society. 1990;67:526–528. [Google Scholar]
- Matsuda H, Morikawa T, Ishiwada T, Managi H, Kagawa M, Higashi Y, Yoshikawa M. Medicinal Flowers. VIII. Radical scavenging constituents from the flowers of Prunus mume: Structure of prunose III. Chemical and Pharmaceutical Bulletin. 2003;51:440–443. doi: 10.1248/cpb.51.440. [DOI] [PubMed] [Google Scholar]
- Matsumoto F, Idtsuki H, Harada K. Volatile components of Hedychium coronarium Koenig flowers. Journal of Essential Oil Research. 1993;5:123–133. [Google Scholar]
- Mazza G. GCMS investigation of volatile components of myrtle berries. Journal of Chromatography. 1983;264:304–311. [Google Scholar]
- Medina AL, Lucero ME, Holguin FO, Estell RE, Posakony JJ, Simon J, O'Connell MA. Composition and antimicrobial activity of Anemopsis californica leaf oil. Journal of Agricultural and Food Chemistry. 2005;53:8694–8698. doi: 10.1021/jf0511244. [DOI] [PubMed] [Google Scholar]
- Medina AL, Holguin FO, Micheletto S, Goehle S, Simon JA, O'Connell MA. Chemotypic variation of essential oils in the medicinal plant, Anemopsis californica. . Phytochemistry. 2008;69:919–927. doi: 10.1016/j.phytochem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle H, Ferriol M, Boira H, Blazquez A. Composition of the essential oil of Dictamnus hispanicus from Spain. Journal of Essential Oil Research. 2006;18:483–485. [Google Scholar]
- Metcalf RL. Chemical ecology of Dacinae fruit flies (Diptera: Tephritidae). Annals of Entomological Society of America. 1990;83:1017–1030. [Google Scholar]
- Metcalf RL, Mitchell WC, Fukuto TR, Metcalf ER. Attraction of the oriental fruit fly, Dacus dorsalis, by methyl eugenol and related olfactory stimulants. Proceedings of the National Academy of Sciences USA. 1975;72:2501–2505. doi: 10.1073/pnas.72.7.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf RL, Metcalf ER. Fruit flies of the family Tephritidae. In: Metcalf RL, Metcalf ER, editors. Plant Kairomones in Insect Ecology and Control. Chapman Hall; 1992. pp. 109–152. [Google Scholar]
- Mhamdi B, Wannes WA, Dhiffi W, Marzouk B. Volatiles from leaves and flowers of Borage (Borago officinalis L.). Journal of Essential Oil Research. 2009;21:504–506. [Google Scholar]
- Mihara S, Tateba H, Nishimura O, Machii Y, Kishino K. Volatile components of Chinese quince (Pseudocydonia sinensis Schneid). Journal of Agricultural and Food Chemistry. 1987;35:532–537. [Google Scholar]
- Miles DH, Kokpol U, Mody NV, Hedin PA. Volatiles in Sarracenia flava. . Phytochemistry. 1975;14:845–846. [Google Scholar]
- Minott DA, Brown HA. Differentiation of fruiting and non-fruiting Pimenta dioica (L.) Merr. trees based on composition of leaf volatiles. Journal of Essential Oil Research. 2007;19:354–357. [Google Scholar]
- Mishra RK, Chaudhary , Pandey R, Gupta S, Mallavarpu GR, Kumar S. Analysis of linalool content in the inflorescence (flower) essential oil and leaf oil of Lippia alba cultivar ‘kavach’. Journal of Essential Oil Research. 2010;22:3–7. [Google Scholar]
- Mitić V, Đorđević S. Essential oil composition of Hyssopus officinalis L. cultivated in Serbia. Facta Universitatis Series: Physics, Chemistry and Technology. 2000;2:105–108. [Google Scholar]
- Miyazawa M, Kameoka H. Volatile flavor components of crude drugs. Part VII. Volatile flavor components of corni fructus (Cornus officinalis Sieb. et Zuec). Agricultural and Biological Chemistry. 1989;53:3337–3340. [Google Scholar]
- Miyazawa M, Kawata J. Identification of the key aroma compounds in dried roots of Rubia cordifolia. Journal of Oleo Science. 2006;55:37–39. [Google Scholar]
- Miyazawa M, Minamino Y, Kameoka H. Volatile components of the rhizomes of Rheum palmatum L. Flavour and Fragrance Journal. 1996;11:57–60. [Google Scholar]
- Miyazawa M, Kurose K, Itoh A, Hiraoka N. Comparison of the essential oils of Glehnia littoralis from northern and southern Japan. Journal of Agricultural and Food Chemistry. 2001;49:5433–5436. doi: 10.1021/jf010219c. [DOI] [PubMed] [Google Scholar]
- Miyazawa M, Fujita T, Yamafuji C, Matsui M, Kasahara N, Takagi Y, Ishikawa Y. Chemical composition of volatile oil from the roots of Periploca sepium. Journal of Oleo Science. 2004;53:511–513. [Google Scholar]
- Moraes LAS, Facanall R, Marques MOM, Ming LC, Meireles MAA. Phytochemical characterization of essential oil from Ocimum selloi. . Anais da Academia Brasileira de Ciências. 2002;74:183–186. doi: 10.1590/s0001-37652002000100014. [DOI] [PubMed] [Google Scholar]
- Moreira DL, Souza PO, Kaplan MAC, Pereira NA, Cardoso GL, Guimaraes EF. Effect of leaf essential oil from Piper solmsianum C. Dc. in mice behaviour. Anais da Academia Brasileira de Ciencias. 2001;73:33–37. doi: 10.1590/s0001-37652001000100004. [DOI] [PubMed] [Google Scholar]
- Moshonas MG, Shaw PE. Compounds new to essential orange oil from fruit treated with abscission chemicals. Journal of Agricultural and Food Chemistry. 1978;26:1288–1290. doi: 10.1021/jf60220a020. [DOI] [PubMed] [Google Scholar]
- Mostafavi A, Afzali D. Chemical composition of the essential oils of Rosa damascena from two different locations in Iran. Chemistry of Natural Compounds. 2009;45:110–113. [Google Scholar]
- Moraes LAS, Facanall R, Marques MOM, Ming LC, Meireles MAA. Phytochemical characterization of essential oil from Ocimum selloi. Anais da Academia Brasileira de Ciências. 2002;74(1):183–186. doi: 10.1590/s0001-37652002000100014. [DOI] [PubMed] [Google Scholar]
- Moreira DL, Souza PO, Kaplan MAC, Pereira NA, Cardoso GL, Guimaraes EF. Effect of leaf essential oil from Piper solmsianum C. Dc. In mice behaviour. Anais da Academia Brasileira de Ciências. 2001;73:33–37. doi: 10.1590/s0001-37652001000100004. [DOI] [PubMed] [Google Scholar]
- Mostafavi A, Afzali D. Chemical composition of thate essential oils of Rosa damascena from two different locations in Iran. Chemistry of Natural Compounds. 2009;45:110–113. [Google Scholar]
- Muckensturm B, Duplay D, Muhammadi F, Moradi A, Robert PC, Simonis MT, Kienlen JC. Role of natural phenylpropanoids as antifeeding agents for insects. Colloques de l'INRA. 1982;7:131–135. in French. [Google Scholar]
- Naeole CKM, Haymer DS. Use of oligonucleotide arrays for molecular taxonomic studies of closely related species in the oriental fruit fly (Bactrocera dor salis) complex. Molecular Ecology Notes. 2003;3:662–665. [Google Scholar]
- Nagasawa M, Murakami T, Ikada K, Hisada Y. Geographical variation of essential oils of Flos magnoliae. Yakugaku Zasshi. 1969;89:454–459. doi: 10.1248/yakushi1947.89.4_454. [DOI] [PubMed] [Google Scholar]
- Najjaa H, Neffati M, Zouari S, Ammar E. Essential oil composition and antibacterial activity of different extracts of Allium roseum L., a North African endemic species. Comptes Rendus Chimie. 2007;10:820–826. [Google Scholar]
- Nath SC, Sarma KK, Vajezikova I, Leclercq PA. Comparison of volatile infrorescence oils and taxonomy of certain Cymbopogon taxa described as Cymbopogon flexuosus (Nees ex Steud.) Wats. Biochemical Systematics and Ecology. 2002;30:151–162. [Google Scholar]
- Newell IM, Haramoto FH. Biotic factors influencing populations of Dacus dorsalis in Hawaii. Proceedings of the Hawaiian Entomological Society. 1968;20:81–139. [Google Scholar]
- Ngamo TSL, Noudjou WF, Ngassoum MB, Mapongmestsem PM, Aminatou Boubakaary AB, Malaisse F, Haubruge E, Lognsy G, Hance T. Investigations on both chemical composition and insecticidal activities of essential oils of Vepris heterophylla (Rutaceae) from two localities of northern Cameroon towards Tribolium castaneum (Heerbst) (Coleoptera: Tenebrionidae). Research Journal of Biological Sciences. 2007;2:57–61. [Google Scholar]
- Ngassoum MB, Ngamo LT, Maponmetsem PM, Jirovetz L, Buchbaluer G. Investigations of medicinal aromatic plants from Cameroon: GD/Fid, GC/MS and olfactoric analyses of essential oils of Ocimum suave Willd. (Lamiaceae). ACTA Pharmaceutica Turica. 2003;45:69–75. [Google Scholar]
- Ngoh SP, Choo LEW, Pang FY, Huang Y, Kini MR, Ho SH. Insecticidal and repellent properties of nine volatile constituents of essential oils against the American cockroach, Periplaneta americana (L.). Pesticide Science. 1998;54:261–268. [Google Scholar]
- Niemeyer HM. Composition of essential oils from five aromatic species of Asteraceae. Journal of Essential Oil Research. 2009;21:350–353. [Google Scholar]
- Nishda R, Tan KH, Serit M, Lajis NH, Sukari AM, Takahashi S, Fukami H. Accumulation of phenylpropanoids in the rectal glands of male Oriental fruit fly, Dacus dorsalis. . Experientia. 1988;44:534–536. [Google Scholar]
- Nishida R, Tan KH, Takahashi S, Fukami H. Volatile components of male rectal glands of the melon fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae). Applied Entomology and Zoology. 1990;25:105–112. [Google Scholar]
- Nishida R, Iwahashi I, Tan KH. Accumulation of Dendrobium (Orchidaceae) flower fragrance in the rectal glands by males of the melon fly, Dacus Cucurbitae (Tephritidae). Journal of Chemical Ecology. 1993;19:713–722. doi: 10.1007/BF00985003. [DOI] [PubMed] [Google Scholar]
- Nishida R, Tan KH, Wee SL, Hee AKW, Toong YC. Phenylpropanoids in the fragrance of the fruit fly orchid, Bulbophyllum cheiri, and their relationship to the pollinator, Bactrocera papayae. Biochemical Systematics and Ecology. 2004;32:245–252. [Google Scholar]
- Nivinskienė O, Butkienė R, Gudalevič A, Mockutė D, Meškauskienė V, Grigaliūnaitė B. Influence of urban environment on chemical composition of Tilia cordata essential oil. Chemija. 2007;18:44–49. [Google Scholar]
- Nogueira. PCL. Bittrich V, Shepherd GJ, Lopes AV, Marsaiolia AJ. The ecological and taxonomic importance of flower volatiles of Clusia species (Guttiferae). Phytochemistry. 2001;56:443–452. doi: 10.1016/s0031-9422(00)00213-2. [DOI] [PubMed] [Google Scholar]
- Nor Azah MA, Zaridah MZ, Majid JA, Said AA, Faridz ZM, Rohani A. 2006. Chemical composition of essential oils and their related biological activities. In: Highlights of FRIM's IRPA Projects 2005 156 160 [Google Scholar]
- Ntezurubanza L, Scheffer JJC, Svendsen AB. Composition of the essential oils of Ocimum urticifolium (Lamiaceae) chemotypes grown in Rwanda. Botanical Journal of the Linnean Society. 1988;96:97–104. [Google Scholar]
- Nurdijati S, Tan KH, Toong YC. Basil plants (Ocimum spp.) and their prospects in the management of fruit flies. In: Chua TH, Khoo SG, editors. Problems and Management of Tropical Fruit Flies. Kai Wah Press; 1996. pp. 47–51. [Google Scholar]
- Oger JM, Rucginne P, Guinaudeau H. Aniba canelilla (H.B.K.) Mez essential oil: Analysis of chemical constituents, fungistatic properties. Journal of Essential Oil Research. 1994;6:493–497. [Google Scholar]
- Ohloff G. Importance of minor components in flavors and fragrances. Perfume Flavor. 1978;3:10–22. [Google Scholar]
- Orankanok W, Chinvinijkul S, Sawatwangkhoungm A, Pinkaew S, Orankanok S. Application of chemical supplements to enhance Bactrocera dorsalis and B. correcta sterile males performance in Thailand. 2009. Fourth FAO/IAEA Research Co-ordination meetings on “Improving Sterile Male Performance in Fruit Fly SIT Programmes”.
- Ozean MM, Chalchat J-C. The flavor profile of young shoots, flowerbuds, and unripe fruits of capers growing wild in Turkey. Chemistry of Natural Compounds. 2007;43:336–338. [Google Scholar]
- Ozcan M, Bagci Y, Akgul A, Dural H, Novak J. Chemical composition of the essential oil of Prangos uechtritzii Boiss. et Hausskn. fruits from Turkey. Journal of Essential Oil Research. 2000;12:183–185. [Google Scholar]
- Ozcan M, Bagci Y, Ertugrul K, Novak J. Comparison of the leaf, root and fruit oils of Diplotaenia cachrydifolia from Turkey. Journal of Essential Oil Research. 2004;16:211–213. [Google Scholar]
- Ozek G, Ozek T, Baser KHC, Hamzaoglu E, Duran A. Composition of the essential oil of Hippomarathrum cristatum (DC.) Boiss. Journal of Essential Oil Research. 2007;19:540–542. [Google Scholar]
- Ozman M, Chalchar J-C. Aroma profile of Thymus vulgaris L. growing wild in Turkey. Bulgarian Journal of Plant Physiology. 2004;30:68–73. [Google Scholar]
- Pai KF, Chen CC, Yang JT, Chen CJ. Green lacewing Ankylopteryx exquisite attracted to methyl eugenol. Plant Protection Bulletin. 2004;46:93–97. [Google Scholar]
- Paniandy JC, Chane-Ming J, Pieribattesti JC. Chemical composition of the essential oil and headspace solid-phase microextraction of the guava fruit (Psidium guajava L.). Journal of Essential Oil Research. 2000;12:153–158. [Google Scholar]
- Paramonov EA, Khalilova AZ, Odinokov VN, Khalilov LM. Identification and biological activity of volatile organic compounds isolated from plants and insects. III. Chromatography-mass spectrometry of volatile compounds of Aegopodium podagraria. . Chemistry of Natural Compounds. 2000;36:584–586. [Google Scholar]
- Park BS, Lee KG, Shibamoto T, Lee SE, Takeoka GR. Antioxidant activity and characterization of volatile constituents of taheebo (Tabebuia impetiginosa Martius ex DC). Journal of Agricultural and Food Chemistry. 2003;51:295–300. doi: 10.1021/jf020811h. [DOI] [PubMed] [Google Scholar]
- Park I-K, Kim J, Lee S-G, Shin S-C. Nematicidal activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioic) and litsea (Litsea cubeba) essential oils against pine wood nematode (Bursaphelenchus xylophilus). Journal of Nematology. 2007;39:275–279. [PMC free article] [PubMed] [Google Scholar]
- Pavela R, Sajfrtova M, Sovova H, Barnet M, Karban J. The insecticidal activity of Tanacetum parthenium (1.) Schultz Bip. extracts obtained by supercritical fluid extraction and hydrodistillation. Industrial Crops and Products. 2010;31:449–454. [Google Scholar]
- Pedro LG, Santos AG, Da Silva JA, Figueiredo AC, Barroso JG, Deans SG, Looman A, Scheffer JJC. Essential oils from Azorean Laurus azorica. . Phytochemistry. 2001;57:245–250. doi: 10.1016/s0031-9422(00)00497-0. [DOI] [PubMed] [Google Scholar]
- Perez AG, Cert A, Rios JJ, Olias JM. Free and glycosidically bound volatile compounds from two banana cultivars: Valery and Pequena Enana. Journal of Agricultural and Food Chemistry. 1997;45:4393–4397. [Google Scholar]
- Perfumi M, Valentini G, Bellomaria B. Chemical constituents and spasmolytic activity in guinea-pig ileum of essential oil of Artemisia alba from two geographically and ecologically different localities. Journal of Essential Oil Research. 1999;11:223–228. [Google Scholar]
- Piccaglla R, Pace P, Tammaro F. Characterization of essential oils from three Italian ecotypes of Hyssop [Hyssopus officinalis L. subsp. aristatus (Gordon) Briq.]. Journal of Essential Oil Research. 1999;11:693–699. [Google Scholar]
- Pichersky E, Gang DR. Genetics and biochemistry of secondary metabolites in plants: an evolutionary perspective. Trends in Plant Science. 2000;5:439–445. doi: 10.1016/s1360-1385(00)01741-6. [DOI] [PubMed] [Google Scholar]
- Picone JM, Clery RA, Watanabe N, MacTavish HS, Turnbull CGN. Rhythmic emission of floral volatiles from Rosa damascena semperflorens cv. ‘Quatre Saisons’. Planta. 2004;219:468–478. doi: 10.1007/s00425-004-1250-5. [DOI] [PubMed] [Google Scholar]
- Pino JA, Quijano CE. Volatile compounds of Pourouma cecropiifolia Mart. fruits from Colombia. Journal of Essential Oil Research. 2008;20:242–244. [Google Scholar]
- Pino JA, Marbot R, Aguero J. Volatile components of baga (Annona glabra L.) fruit. Journal of Essential Oil Research. 2002;14:252–253. [Google Scholar]
- Pina JA, Mesa J, Munoz Y, Marti MP, Marbot R. Volatile components from Mango (Mangifera indica L.) cultivars. Journal of Agricultural and Food Chemistry. 2005;53:2213–2223. doi: 10.1021/jf0402633. [DOI] [PubMed] [Google Scholar]
- Pripdeevech P, Machan T. Fingerprint of volatile flavor constituents andantioxidant activities of teas from Thailand. Food Chemistry. 2010;125:797–802. DOI: 10.1016/j.foodchem.2010.09.074. [Google Scholar]
- Prudent D, Perineau F, Bessiere JM, Michel G, Bravo R. Chemical analysis, bacteriostatic and fungistatic properties of the essential oil of the Atoumau from Martinique (Alpinia speciosa K. Schum.). Journal of Essential Oil Research. 1993;5:255–264. [Google Scholar]
- Quilici S, Duyck PF, Franck A. Preliminary experiments on the influence of exposure to methyleugenol on mating success of males in the peach fruit fly, Bactrocera zonata. 1st RCM on improving sterile male performance in fruit fly SIT; Antigua, Guatemala: 2004. [Google Scholar]
- Rabha LC, Hazarika AK, Bordoloi DN. Cymbogon khasianus a new rich source of methyl eugenol. Indian Perfumer. 1986;30:339–344. [Google Scholar]
- Radriamiharisoa R, Gaydou EM, Bianchini JP, Ravelojaonha G, Vernin G. Study of the variation in the chemical composition and classification of basil essential oils from Madagascar. Sciences des Aliments. 1986;6:221–231. in French. [Google Scholar]
- Radulescu V, Oprea E. Analysis of volatile compounds of officinal Tiliae flos by gas-chromatography coupled with mass spectrometry. Farmacia. 2008;56:129–138. [Google Scholar]
- Radulovic N, Dekic M, Stojanovic-Radic Z, Palic R. Volatile constituents of Erodium cicutarium (L.) L'Herit. (Geraniaceae). Central European Journal of Biology. 2009;4:404–410. [Google Scholar]
- Raguso RA, Pichersky E. Floral volatiles from Clarkia breweri and C. concinna (Onagraceae): recent evolution of floral scent and moth pollination. Plant Systematics and Evolution. 1995;194:55–57. [Google Scholar]
- Raina VK, Kumar A, Srivastava SK, Syamsundar KV, Kahol AP. Essential oil composition of ‘kewda’ (Pandanus odoratissimus) from India. Flavour and Fragrance Journal. 2004;19:434–436. [Google Scholar]
- Raina VK, Verma SC, Dhawan S, Khan M, Ramesh S, Singh SC, Yadav A, Srivastava SK. Essential oil composition of Murraya exotica from the plains of northern India. Flavour and Fragrance Journal. 2006;21:140–142. [Google Scholar]
- Rajeswara Rao BT, Kaul PN, Bhattacharya AK, Rajput DK. Comparative chemical composition of stearm-distilled and watersoluble essential oils of South American marigold (Tagetes minuta L.). Journal of Essential Oil Research. 2006;18:622–626. [Google Scholar]
- Ramanoelina PA, Viano J, Bianchini J-P, Gaydou EM. Occurrence of various chemotypes in Niaouli (Melaleuca quinquenervid) essential oils from Madagascar using multivariate statistical analysis. Journal of Agricultural and Food Chemistry. 1994;42:1177. [Google Scholar]
- Ramanoelina PAJ, Rasoarahona JRE, Gaydou EM. Chemical composition of Ravensara aromatica Soon, leaf essential oils from Madagascar. Journal of Essential Oil Research. 2006;18:215–217. [Google Scholar]
- Rao JT, Nigam SS. Chemical investigation of essential oil recovered from Ageratum conyzoides. Koerperpflegem. 1973;23:209–210. in German. [Google Scholar]
- Ravindran PN, Shulaja M, Babu KN, Krishnamoorthy B. Botany and crop improvement of cinnamon and cassia. In: Ravindran PN, Babu KN, Shylaja M, editors. Cinnamomum and Cassia: The genus Cinnamomum. CRC Press; 2003. pp. 80–120. [Google Scholar]
- Reinaldo S, Chavez K, Mateu E, Orsini G, Arvelo F, Suarez AI. Composition and cytotoxic activity of essential oils from Croton matourensis and Croton micans from Venezuela. Records of Natural Products. 2010;4:101–108. [Google Scholar]
- Reish JD, Bergenthal SK, Adesina D, Akinwusi D, Olatunji AO. Consitutents of Fagara macrophylla and Zanthoxylum rigidifollum pericarps. Journal of Natural Products. 1986;49:1169–1171. [Google Scholar]
- Ribnicky DM, Poulev A, O'Neal J, Wnorowski G, Malek DE, Jager D, Raskin I. Toxicological evaluation of the ethanolic extract of Artemisia dracunculus L. for use as a dietary supplement and in functional foods. Food and Chemical Technology. 2004;42:585–598. doi: 10.1016/j.fct.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Robison SH, Barr DB. Use of biomonitoring data to evaluate methyl eugenol exposure. Environmental Health Perspectives. 2006;114:1797–1801. doi: 10.1289/ehp.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodilla JM, Tinoco MT, Morais JC, Gimenez C, Cabrera R, Martin-Benito D, Castillo L, Gonzalez-Coloma A. Laurus novocanariensis essential oil: Seasonal variation and valorization. Biochemical Systematics and Ecology. 2008;36:167–176. [Google Scholar]
- Rossi P, Bao L, Luciani A, Panighi J, Desjobert J, Costa J, Casanova J, Bolla J, Berti L. (E)methylisoeugenol and elemicin: Antibaterial components of Daucus carota L. essential oil against Campylobacter jujuni. . Journal of Agricultural and Food Chemistry. 2007;55:7332–7336. doi: 10.1021/jf070674u. [DOI] [PubMed] [Google Scholar]
- Ruff C, Hor K, Weckerle B, Konig T, Schreier P. Authencity assessment of estragole and methyl eugenol by on-line gas chromtography-isotope ratio mass spectrometry. Journal of Agricultural and Food Chemistry. 2002;50:1028–1031. doi: 10.1021/jf011204h. [DOI] [PubMed] [Google Scholar]
- Russell GF, Jennings WG. Constituents of Black Pepper — Some oxygenated compounds. Journal of Agricultural and Food Chemistry. 1969;17:1107–1112. doi: 10.1021/jf60165a026. [DOI] [PubMed] [Google Scholar]
- Ryan D. Aromatherapy - the Encyclopedia of Plants and Oils and How They Help You. Judy Piatkus Ltd; 1991. [Google Scholar]
- Saad H-EA, El-Sharkawy SH, Halim AF. Essential oils of Daucus carota ssp. maximus. . Pharmaceutics Acta Helvetiae. 1995;70:79–84. [Google Scholar]
- Sadeghpour O, Asghari G, Ardekani MR. Composition of essential oil of Artemisia persica Boiss. from Iran. Iran Journal of Pharmaceutical Research. 2004;3:65–67. [Google Scholar]
- Saeidnia S, Gohari AR, Yassa N, Shafiee A. Composition of the volatile oil of Achillea conferta DC. from Iran. DARU Journal of Pharmaceutical Science. 2005;13:34–36. [Google Scholar]
- Safaei-Ghomi J, Bamoniri A, Hafami A, Batooli H. Determination of volatile components in Iranian Rosa hemisphaerica. . Chemistry of Natural Compounds. 2007;43:738–740. [Google Scholar]
- Saglam H, Gozler T, Kivcak B, Demirci B, Baser KHC. Composition of the essential oil of Haplophyllum myrtifolium. . Chemistry of Natural Compounds. 2001;37:439–441. [Google Scholar]
- Sagrero Nieves L, Bartley JP. Volatile components of avocado leaves (Persea americana Mill) from the Mexican race. Journal of the Science of Food and Agriculture. 1995;67:49–51. [Google Scholar]
- Saidana D, Mahjoub S, Boussaada O, Chriaa J, Mahjoub MA, Cheraif I, Daami M, Mighri Z, Helal AN. Antibacterial and antifungal activities of the essential oils of two Saltcedar species from Tunisia. Journal of American Oil Chemical Society. 2008a;85:817–826. [Google Scholar]
- Saidana D, Mahjoub MA, Boussaada O, Chriaa J, Cheraif I, Daami M, Mighri Z, Helal AN. Chemical composition and antimivrobial activity of volatile compounds of Tamarix boveana (Tamaricaceae). Microbiological Research. 2008b;63:445–455. doi: 10.1016/j.micres.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Saiki Y, Saito T, Sasaki H, Fukushima S. Gas chromatography of natural volatile oils. I. On the volatile oil of Chinese drup “Xixin”. Yakugaku Zasshi. 1967a;87:1524–1528. doi: 10.1248/yakushi1947.87.12_1524. [DOI] [PubMed] [Google Scholar]
- Saiki Y, Akakori Y, Noro T, Morinaga K, Taira T, Fukushima S, Harada T. Gas chromatography of natural volatile oils. II. Asiasarum and Asarum genera. Yakugaku Zasshi. 1967b;87:1529–1534. doi: 10.1248/yakushi1947.87.12_1529. [DOI] [PubMed] [Google Scholar]
- Saiki Y, Akakori Y, Morinaga K, Taira T, Noro T, Fudushima S, Harada T. Gas chromatography of natural volatile oils. III. Gas chromatograpy of the volatile oils of plants belonging to the Heteroptera genus. 1. Yakugaku Zasshi. 1967c;87:1535–1538. doi: 10.1248/yakushi1947.87.12_1535. [DOI] [PubMed] [Google Scholar]
- Saiki Y, Akakori Y, Morinaga K, Taira T, Noro T, Fudushima S, Harada T. Gas chromatography of natural volatile oils. IV. Gas chromatograpy of the volatile oils of plants belonging to the Heteroptera genus. 2. Yakugaku Zasshi. 1967d;87:1539–1543. doi: 10.1248/yakushi1947.87.12_1539. [DOI] [PubMed] [Google Scholar]
- Saiki Y, Akakori Y, Morinaga K, Taira T, Noro T, Fudushima S, Harada T. Gas chromatography of natural volatile oils. V. Gas chromatograpy of the volatile oils of plants belnging to the Heteroptera genus. 3. Yakugaku Zasshi. 1967e;87:1544–1547. doi: 10.1248/yakushi1947.87.12_1544. [DOI] [PubMed] [Google Scholar]
- Sainsbury M, Sofowora EA. Essential oil from the leaves and inflorescence of Ocimum gratissimum. . Phytochemistry. 1971;10:3309–3310. [Google Scholar]
- Sajjadi SE, Ghannadi A. Essential oil of the Persian sage, Salvia rhytidea Benth. ACTA Pharmaceutica. 2005;55:321–326. [PubMed] [Google Scholar]
- Sajjadi SE, Mehregan I. Chemical composition of the essential oil of Prangos asperula Boiss. subsp. haussknechtii (Boiss.) Herrnst et Heyn fruits. DARU Journal of Pharmaceutical Science. 2003;11:79–81. [Google Scholar]
- Sammy GM, Nawar WW. Identification of the major components of nutmeg oil by gas chromatography and mass spectrometry. Chemistry and Industry. 1968;38:1279–1280. [Google Scholar]
- Sanford KJ, Heinz DE. Effects of storage on the volatile composition of nutmeg. Phytochemistry. 1971;10:1245–1250. [Google Scholar]
- Sangun MK, Aydin F, Timur M, Karadeniz H, Caliskan M, Ozkan A. Comparison of chemical composition of the essential oil of Laurus nobilis L. leaves and fruits from different regions of Hatay, Turkey. Journal of Environmental Biology. 2007;28:731–733. [PubMed] [Google Scholar]
- Sarker SD, Armstrong JA, Waterman PG. (-)-1,12-oxaguai-10(15)-ene: A sesquiterpene from Eriostemon fitzgeraldii. . Phytochemistry. 1995;40:1159–1162. [Google Scholar]
- Saroglou V, Dorizas N, Kypriotakis Z, Skaltsa HD. Analysis of the essential oil composition of eight Anthemis species from Greece. Journal of Chromatography. 2006;1104:313–322. doi: 10.1016/j.chroma.2005.11.087. [DOI] [PubMed] [Google Scholar]
- Saxena BP, Koul O, Tikku K, Atal CK. A new insect chemosterilant isolated from Acorus calamus L. Nature. 1977;270:512–513. [Google Scholar]
- Scheuer PJ. The constituents of mokihana (Pelea anisata Mann). Chemistry and Industry. 1955;33:1257–1258. [Google Scholar]
- Schecter A, Lucier GW, Cunningham ML, Abdo KM, Blumenthal G, Silver AG, Melnick R, Portier C, Barr DB, Barr JR, Stanfill SB, Patterson DG, Jr., Needham LL, Stopford W, Masten S, Mignogna J, Tung KC. Human consumption of methyleugenol and its elimination from serum. Environmental Health Perspectives. 2004;112:678–683. doi: 10.1289/ehp.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl FP. The evolution of floral scent and insect chemical communication. Ecology Letters. 2010;13:643–656. doi: 10.1111/j.1461-0248.2010.01451.x. [DOI] [PubMed] [Google Scholar]
- Schwerdtfeger M, Gerlach G, Kaiser R. Anthecology in the Neotropical genus Anthurium (Araceae): A preliminary report. Selbyana. 2002;23:258–267. [Google Scholar]
- Scrivanti LR, Anton AM, Zygadlo JA. Essential oil composition of Bothriochloa Kuntze (Poaceae) from South America and their chemotaxonomy. Biochemical Systematics and Ecology. 2009;37:206–213. [Google Scholar]
- Sellami IH, Maamouri E, Chahed T, Wannes WA, Kchouk ME, Marzouk B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.). Industrial Crops and Products. 2009;30:395–402. [Google Scholar]
- Senanayake UM, Lee TH, Wills RBH. Volatile constituents of Cinnamon (Cinnamomum zeylanicum) oils. Journal of Agricultural and Food Chemistry. 1978;26:822–824. [Google Scholar]
- Senatore F, Sorta EU, Soria RU, Delia Porta G, Taddeo R, De Feo V. Essential oil of Eremocharis triradiata (Wolff.) Johnston (Apiaceae) growing wild in Peru. Flavour and Fragrance Journal. 1997;12:257–259. [Google Scholar]
- Senatore F, De Feo V. Chemical composition of the essential oil from Tagetes mandonii Sch. Bip. (Asteraceae). Flavour and Fragrance Journal. 1999;14:32–34. [Google Scholar]
- Sezik E, Basaran A. Phytochemical investigations on the plants used as folk medicine and herval tea in Turkey. II. Essential oil of Stachys lavandulfolia Vahl var. lavandulifolia. Istanbul Universitesi Eczacilik Fakültesi Mecmuasi. 1985;21:98–107. [Google Scholar]
- Sezik E, Usun O, Demirci B, Baser KHC. Composition of the oils of Pinus nigra Arnold from Turkey. Turkey Journal of Chemistry. 2010;34:313–325. [Google Scholar]
- Shatar S, Altantsetseq S, Dung NX, Ngoc PH, Klinkby N, Leclercq PA. The essential oil of Artemisia subdigitata Mattf. from Mongolian the desrt-Gobi. Journal of Essential Oil Research. 2002;14:99–100. [Google Scholar]
- Sharma ML, Rawat AKS, Blalasubrhmanyam VR, Singh A. Studies on essential oil of betel vine leaf (Piper betle Linn.). Indian Perfumer. 1983;27:91–93. [Google Scholar]
- Shatar S, Altantsetseg S. Essential oil composition of some plants cultivated in Mongolian climate. Journal of Essential Oil Research. 2000;12:745–750. [Google Scholar]
- Shaver TN, Bull DL. Environmental fate of methyl eugenol. Bulletin of Environmental Contamination and Toxicology. 1980;24:619–626. doi: 10.1007/BF01608164. [DOI] [PubMed] [Google Scholar]
- Shelly TE. Effects of methyl eugenol and raspberry ketone/cue lure on the sexual behavior of Bactrocera species (Diptera: Tephritidae). Applied Entomology and Zoology. 2010;45:349–361. [Google Scholar]
- Shelly TE, Dewire A-LM. Chemically mediated mating success in male Oriental fruit flies (Diptera: Tephritidae). Annals of the Entomological Society of America. 1994;87:375–382. [Google Scholar]
- Shelly TE, Dewire A-LM. Flowerfeeding affects mating performance in male Oriental fruit flies (Diptera: Tephritidae). Ecological Entomology. 2000;25:109–114. [Google Scholar]
- Shelly TE, Edu J, McInnis D. Prerelease consumption of methyl eugenol increases the mating competitiveness of sterile males of the oriental fruit fly, Bactrocera dorsalis, in large field enclosures. Journal of Insect Science. 2010;10:8. doi: 10.1673/031.010.0801. Available online, insectscience.org/10.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum ACS, Smadja J. Volatile constituents of Faham (Jumellea fragrans (Thou.) Schltr.). Journal of Agricultural and Food Chemistry. 1992;40:642–646. [Google Scholar]
- Siderhurst MS, Jang EB. Female-biased attraction of Oriental fruit fly, Bactrocera dorsalis (Hendel), to a blend of host fruit volatiles from Terminalia catappa L. Journal of Chemical Ecology. 2006;32:2513–2524. doi: 10.1007/s10886-006-9160-6. [DOI] [PubMed] [Google Scholar]
- Silou T, Loubak L, Figueredo G, Chalchat JC. Study of essential oil composition of Elyonurus hensii Schum from Congo. Journal of Essential Oil Research. 2006;18:518–520. [Google Scholar]
- Silva CGV, Zago HB, Junior HJGS, Da Camara CAG, De Oliverira JV, Barros R, Schwartz MOE, Lucena MFA. Composition and insecticidal activity of the essential oil of Croton grewloides Baill. against Mexican bean weevil (Zabrotes subfasciatus Boheman). Journal of Essential Oil Research. 2008;20:179–182. [Google Scholar]
- Simon JE, Morales MR, Phippen WB, Vieira RF, Hao Z. Basil: A source of aroma compounds and a popular culinary and ornamental herb. In: Janick J, editor. Perspectives on New Crops and New Uses. ASHS Press; 1999. pp. 499–505. [Google Scholar]
- Simonsen HT, Adsersen A, Bremner P, Heinrich M, Wagner SU, Jaroszewski JW. Antifungal constituents of Melicope borbonica. . Phytotherapy Research. 2004;18:542–545. doi: 10.1002/ptr.1482. [DOI] [PubMed] [Google Scholar]
- Sinchaisri P, Areekul S. Natural attractants in Colocasia exculenta blossom for the Oriental fruit fly, Dacus dorsalis Hendel. Thai Agricultural Research Journal. 1985;3:182–190. in Thai with English Abstract. [Google Scholar]
- Singh AK, Yadaw A. Composition of essential oils from different parts of Thuja orientalis (L.) syn. Biota orientalis Endl. Indian Perfumer. 2005;49:173–177. [Google Scholar]
- Singh G, Kapoor IPS, Singh P, de Heluani CS, de Lampasona MP, Catalan DAN. Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale. . Food Chemistry and Toxicology. 2008;46:3295–3302. doi: 10.1016/j.fct.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Smith RL, Adamsb TB, Doullc J, Ferond VJ, Goodmane JJ, Marnettf LJ, Portogheseg PS, Waddellh WJ, Wagneri BM, Rogers AE, Caldwellk J, Sipesl IG. Safety assessment of allylalkoxybenzene derivatives used as flavouring substances - methyl eugenol and estragole. Food and Chemical Toxicology. 2002;40:851–870. doi: 10.1016/s0278-6915(02)00012-1. [DOI] [PubMed] [Google Scholar]
- Smitt UW. A chemotaxonomic investigation of Thapsia villosa L., Apiaceae (Umbelliferae). Botanical Journal of the Linnean Society. 1995;119:367–377. [Google Scholar]
- Sobti SN, Khosla MK, Pushpangadan P, Thapa RK. Search for new aroma chemicals from Ocimum species. II. Essential oil of American species of O. carnosum Lk. et Otto. Herba Hungaria. 1981;20:49–56. [Google Scholar]
- Sookar P, Alleck M, Ahseek N, Khayrattee FB, Permalloo S. Improving male reproductive performance of Bactrocera zonata and Bactrocera cucurbitae. . Fourth FAO/IAEA Research Co-ordination meetings on “Improving Sterile Male Performance in Fruit Fly SIT Programmes”. 2009.
- Srivastava AK, Srivastava SK, Syamsundar KV. Volatile composition of Curcuma angustifolia Roxb. Rhizome from central and southern India. Flavour and Fragrance Journal. 2006;21:423–426. [Google Scholar]
- Staniszewska M, Kula J. Composition of the essential oil from wild carrot umbels (Daucus carota L. ssp. Carota) growing in Poland. Journal of Essential Oil Research. 2001;13:439–441. [Google Scholar]
- Stashenko EE, Martinez JR, Ruız CA, Arias G, Duran C, Salgar W, Cala M. Lippia origanoides chemotype differentiation based on essential oil GC-MS and principal component analysis. Journal of Separation Science. 2010;33:93–103. doi: 10.1002/jssc.200900452. [DOI] [PubMed] [Google Scholar]
- Steiner LF. A method of estimating the size of native populations of Oriental, Melon and Mediterranean fruit flies, to establish the overflooding ratios required for sterile-male releases. Journal of Economic Entomology. 1969;62:4–7. [Google Scholar]
- Steiner LF, Mitchell WC, Harris EJ, Kozuma TT, Fujimoto MS. Oriental fruit fly eradication by male annihilation. Journal of Economic Entomology. 1965;58:961–964. [Google Scholar]
- Suanarunsawat T, Ayutthaya WDN, Songsak T, Thirawarapan S, Poungshompoo S. Antioxidant activity and lipid-lowering effect of essential oils extracted from Ocimum sanctum Linn, leaves in rats fed with a high cholesterol diet. Journal of Clincal Biochemistry and Nutrition. 2010;46:52–59. doi: 10.3164/jcbn.09-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C, Enrique L. Hydrosyotobain and reticuline from Ocotea caparrapi. . Revista Latinoamericana de Quimica. 1980;11:110–111. in Spanish. [Google Scholar]
- Suarez M, Bonilla J, De Diaz AMP, Achenbach H. Studies of Colobian Lauraceae. Dehydrodieugenols from Nectandra polita. . Phytochemistry. 1983;22:609–610. [Google Scholar]
- Suárez AI, Vásquez LJ, Manzano MA, Compagnone RS. Essential oil composition of Croton cuneatus and Croton malambo growing in Venezuela. Flavour and Fragrance Journal. 2005;20:607–610. [Google Scholar]
- Suarez AI, Vasquez LJ, Taddei A, Arvelo F, Compagnone RS. Antibacterial and cytoxic activity of leaf essential oil of Croton malambo. . Journal of Essential Oil-Bearing Plants. 2008;11:208–213. [Google Scholar]
- Suda DY, Cunningham RT. Chrysopa basalis captured in plastic traps containing methyl eugenol. Journal of Economic Entomology. 1970;63:1706. [Google Scholar]
- Sudhakar P, Latha P, Sreenivasulu Y, Reddy BV, Hemalaatha TM, Balakrishna M, Reddy KR. Inhibition of Aspergillus flavus colonization and aflatoxin (AfB1) in peanut by methyleugenol. Indian Journal of Experimental Biology. 2009;47:63–67. [PubMed] [Google Scholar]
- Sukari MA, Takahashi S. Biological activity of some Malaysian plant extracts. Pertanika. 1988;11:249–253. [Google Scholar]
- Suliman FEO, Fatope MO, Al-Saidi SH, Al-Kindy SMZ. Composition and antimicrobial activity of he essential oil of Pluchea arabica from Oman. Flavour and Fragrance Journal. 2006;21:469–471. [Google Scholar]
- Svendsen CE, Baerheim A. The essential oil of Lippia alba (Mill.) N.E. Br. Journal of Essential Oil Research. 1990;2:265–267. [Google Scholar]
- Taarit MB, Msaada K, Hosni K, Hammanmi M, Kchouk ME, Marzouk B. Plant growth, essential oil yield and composition of sage (Salvia officinalis L.) fruits cultivated under salt stress conditions. Industrial Crops and Products. 2009;30:333–337. [Google Scholar]
- Taarit MB, Msaada K, Hosni K, Chahed T, Marzouk B. Essential oil composition of Salvia verbenaca L. growing wild in Tunisia. Journal of Food Biochemistry. 2010;34:142–151. [Google Scholar]
- Tabanca N, Kirimer N, Demira B, Demirci F, Baser KHC. Composition and antimicrobial activity of the essential oils of Micromeria cristata subsp. phrygia and the enantiomeric distribution of borneol. Journal of Agricultural and Food Chemistry. 2001;49:4300–4303. doi: 10.1021/jf0105034. [DOI] [PubMed] [Google Scholar]
- Tabanca N, Demirci B, Kirimer N, Baser KHC, Bedir E, Khan IA, Wedge DE. Gas chromatographic-mass spectrometric analysis of essential oils from Pimpinella aurea, Pimpinella corymbosa, Pimpinella peregina and Pimpinella puberula gathered from eastern and southern Turkey. Journal of Chromatography A. 2005a;109:192–198. doi: 10.1016/j.chroma.2005.10.047. [DOI] [PubMed] [Google Scholar]
- Tabanca N, Douglas AW, Bedir E, Dayan FE, Kirimer N, Baser KHC, Aytac Z, Khan IA, Scheffler BE. Patterns of essential oil relationships in Pimpinella (Umbelliferae) based on phylogenetic relationships using nuclear and chloroplast sequences. Plant Genetic Resources. 2005b;3:149–169. [Google Scholar]
- Tabanca N, Demirci B, Baser KHC, Aytac Z, Ekici M, Khan SI, Jacob MR, Wedge DE. Chemical composition and antifungal activity of Salvia macrochlamys and Salvia recognita essential oils. Journal of Agricultural and Food Chemistry. 2006;54:6593–6597. doi: 10.1021/jf0608773. [DOI] [PubMed] [Google Scholar]
- Talenti ECJ. Study of the essential oil of Pluchea sagittalis (Lamarck) Cabrera. 9. Essenze Derivati Agrumari. 1982;52:21–51. in Spanish. [Google Scholar]
- Talenti ECJ, Mancini PME, Retamar JA. Study of the essential oil of Acacia caven. . Essenze Derivati Agrumari. 1981;51:98–100. in Spanish. [Google Scholar]
- Tan KH. Estimation of native populations of male Dacus spp. by Jolly's stochastic method using a new designed attractant trap in a village ecosystem. Journal of Plant Protection in the Tropics. 1985;2:87–95. [Google Scholar]
- Tan KH. Recent Trends on Sterile Insect Technique and Area-Wide Integrated Pest Management - Economic Feasibility, Control Projects, Farmer Organization and Bactrocera dorsalis Complex Control Study. Research Institute for the Subtropics; 2003. Interbreeding and DNA analysis of Bactrocera dorsalis sibling species. pp. 113–122. [Google Scholar]
- Tan KH. Fruit fly pests as pollinators of wild orchids. Orchid Digest. 2009;73:180–187. [Google Scholar]
- Tan KH, Jaal Z. Comparison of male adult population densities of the Oriental and Artocarpus fruit flies, Dacus spp. (Diptera: Tephritidae), in two nearby villages in Penang, Malaysia. Researches on Population Ecology. 1986;25:85–89. [Google Scholar]
- Tan KH, Lee SL. Species diversity and abundance of Dacus (Diptera: Tephritidae) in five ecosystems of Penang, Malaysia. Bulletin of Entomological Research. 1982;72:709–716. [Google Scholar]
- Tan KH, Nishida R. Sex pheromone and mating competition after methyl eugenol consumption in Bactrocera dorsalis complex. In: McPheron BA, Steck GJ, editors. Fruit Fly Pests — A World Assessment of their Biology and Management. St. Lucie Press; 1996. pp. 147–153. [Google Scholar]
- Tan KH, Nishida R. Ecological significance of a male attractant in the defence and mating strategies of the fruit fly pest, Bactrocera papayae. . Entomologia Experimentalis et Applicata. 1998;89:155–158. [Google Scholar]
- Tan KH, Nishida R. Mutual reproductive benefits between a wild orchid, Bulbophyllum patens, and Bactrocera fruit flies via a floral synomone. Journal of Chemical Ecology. 2000;26:533–546. doi: 10.1023/a:1016277500007. [DOI] [PubMed] [Google Scholar]
- Tan KH, Nishida R. Synomone or Kairomone? - Bulbophyllum apertum (Orchidaceae) flower releases raspberry ketone to attract Bactrocera fruit flies. Journal of Chemical Ecology. 2005;31:509–519. doi: 10.1007/s10886-005-2023-8. [DOI] [PubMed] [Google Scholar]
- Tan KH, Nishida R. Zingerone in the floral synomone of Bulbophyllum baileyi (Orchidaceae) attracts Bactrocera fruit flies during pollination. Biochemical Systmatics and Ecology. 2007;35:334–341. [Google Scholar]
- Tan KH, Serit M. Movements and population density comparisons of native male adult Dacus dorsalis and Dacus umbrosus (Diptera: Tephritidae) between three ecosystems in Tanjong Bungah, Penang. Journal of Plant Protection in the Tropics . 1988;5:17–21. [Google Scholar]
- Tan KH, Serit M. Adult population dynamics of Bactrocera dorsalis (Diptera: Tephritidae) in relation to host phenology and weather in two villages of Penang Island, Malaysia. Environmental Entomology. 1994;23:267–275. [Google Scholar]
- Tan KH, Nishida R, Toong YC. Bulbophyllum cheiri's floral synomone lures fruit flies to perform pollination. Journal of Chemical Ecology. 2002;28:1161–1172. doi: 10.1023/a:1016277500007. [DOI] [PubMed] [Google Scholar]
- Tan KH, Tan LT, Nishida R. Floral phenylpropanoid cocktail and architecture of Bulbophyllum vinaceum orchid in attracting fruit flies for pollination. Journal of Chemical Ecology. 2006;32:2429–2441. doi: 10.1007/s10886-006-9154-4. [DOI] [PubMed] [Google Scholar]
- Tan KH, Tokushima I, Ono H, Nishida R. Comparison of phenylpropanoid volatiles in male rectal pheromone gland after methyl eugenol consumption, and molecular phylogenetic relationship of four global pest fruit fly species - Bactrocera invadens, B. dorsalis, B. correcta and B. zonata. . Chemoecology. 2011a;21:25–33. [Google Scholar]
- Tan K.H., Wee S.L., Hee A.K.W., Ono H., Tokushima I., Nishida R. Comparison of male sex pheromonal components after methyl eugenol consumption in seven highly invasive Bactrocera species. 6th Asia-Pacific Conference on Chemical Ecology, October 11–15, 2011, Beijing, China. 2011b. Available online, http://apacenews.org/archives/APACE2011_program_and_abstracts.pdf.
- Tanker N, Tanker M, Sener B, Svendsen AB. Gas-liquid chromatographic research on the volatile oil of Echinophora tenuifolia subsp. sibthorpiana (Umbelliferae). Ankara Universitesi Eczacilik Fakültesi Mecmuasi. 1976;6:161–180. in Turkish. [Google Scholar]
- Tellez MR, Estell RE, Fredrickson EL, Havstad KM. Essential oil of Dyssodia acerosa DC. Journal of Agricultural and Food Chemistry. 1997;45:3276–3278. [Google Scholar]
- Thongdon-A J, Inprakhon P. Composition and biological activities of essential oils from Limnophila geoffrayi Bonati. World Journal of Microbiology and Biotechnology. 2009;25:1313–1320. [Google Scholar]
- Thoppil JE. Essential oil compositon of Moschosma polystachya (L). Benth. Indian Journal of Pharmceutical Sciences. 1997;59:191–192. [Google Scholar]
- Tian Z, Dong SN, Wang BR, Lou ZC. Identification of the constituents of volatile oils from Chinese Asarum species. I. Volatile oil from Liao xixin, Asarum hertropoides var. mandshuricum. . Pei-ching I Hsueh Yuan Hsueh Pao. 1981a;13:179–182. in Chinese. [Google Scholar]
- Tian Z, Dong SN, Wang BR, Lou ZC. Identification of the constituents of volatile oils from Chinese Asarum species. II. Volatile oil from huaxixin, Asarum sieboldii. . Beijing Yixueyuan Xuebao. 1981b;13:282–284. in Chinese. [Google Scholar]
- Torrado A, Saurez M, Duque C, Krajewski D, Neugebauer W, Schreier P. Volatile constituents from Tamarillo (Cyphomandra betacea Sendtn.) fruit. Flavour and Fragrance Journal. 1995;10:349–354. [Google Scholar]
- Tram Ngoc LY, Yamauchi R. Kato K. Volatile components of the essential oils in Galanga (Alpinia officinarum Hance) from Vietnam. Food Science and Technology Research. 2001;7:303–306. [Google Scholar]
- Treutter D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biology. 2005;7:581–591. doi: 10.1055/s-2005-873009. [DOI] [PubMed] [Google Scholar]
- Trusheva B, Popova M, Bankova V, Simova S, Marcucci MC, Miorin PL, Pasin FR, Tsvetkova I. Bioactive constituents of Brazilian red propolis. Evidence-based Complementary and Alternative Medicine. 2006;3:249–254. doi: 10.1093/ecam/nel006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AO, Maciarello MJ. Volatile oils of Illicium floridanum and I.parviflorum (Illiciaceae) of the southeastern United States and their potential economic utilization. Economic Botany. 1997;53:435–436. [Google Scholar]
- Tucker AO, Maciarello MJ, Clancy K. Sweet goldenros (Solidago odora, Asteraceae): A medicine, tea and state herb. Economic Botany. 1999;53:281–284. [Google Scholar]
- Tucker AO, Maciarello MJ, Adams RP, Landrynm LR, Zanoni TA. Volatile leaf oils of Caribbean Myrtaceae. I. Three varities of Pimenta racemosa (Miller) J. Moore of the Dominican Republic and the commercial bay oil. Journal of Essential Oil Research. 1991;3:323–329. [Google Scholar]
- Tutupalli LV, Brown JK, Chaubal MG. Essential oil of Saururus cernuus. . Phytochemistry. 1975;14:595–596. [Google Scholar]
- Tutupalli LV, Chaubal MG, Malone MH. Saururaceae. VI. Hippocratic screening of Anemopsis californica. . Lloydia. 1975;38:352–354. [PubMed] [Google Scholar]
- Tzakou O, Loukis A, Argyriadou N. Volatile constituents of Achillea crithmifolia flowers from Greece. Journal of Essential Oil Research. 1993;5:345–346. [Google Scholar]
- Tzakou O, Loukis A, Said A. Essential oil from the flowers and leaves of Cassia fistula L. Journal of Essential Oil Research. 2007;19:360–361. [Google Scholar]
- Ueyama Y, Hashimoto S, Nii H. The chemical composition of the essential oil of Daphne genkwa Sieb. et Zucc. Journal of Essential Oil Research. 1990;2:247–250. [Google Scholar]
- Vagionas K, Ngassaoa O, Runyoro D, Graikou K, Gortzi O, Chinou I. Chemical analysis of edible aromatic plants growing in Tanzania. Food Chemistry. 2007;105:1711–1717. [Google Scholar]
- van Dort HM, Jiigers PP, ter Heide R, van der Weerdt AJA. Narcissus trevithian and Narcissus geranium. Analysis and synthesis of compounds. Journal of Agricultural and Food Chemistry. 1993;41:2063–2075. [Google Scholar]
- Vanhaelen M, Vanhaelen-Fastre R. Constituents of essential oil of Myrtus communis. . Planta Medica. 1980;39:164–167. [Google Scholar]
- Vargas RI, Shelly TE, Leblanc L, Pinero JC. Recent advances in methyl eugenol and cue-lure technologies for fruit fly detection, monitoring, and control in Hawaii. Vitamins and Hormones: 2010;83:575–596. doi: 10.1016/S0083-6729(10)83023-7. [DOI] [PubMed] [Google Scholar]
- Verghese A. Methyl eugenol attracts female mango fruit fly, Bactrocera dorsalis Hendel. Insect Environment. 1998;4:101. [Google Scholar]
- Verite P, Nacer A, Kabouche Z, Seguin E. Composition of seeds and stems essential oils of Pituranthos scoparius (Cross. & Dur.) Schinz. Flavour and Fragrance Journal. 2004;19:562–564. [Google Scholar]
- Vernin G, Vernin C, Pieribattesti JC, Roque C. Analysis of the volatile compounds of Psidium cattleianum Sabine fruit from Reunion Island. Journal of Essential Oil Research. 1998;10:353–362. [Google Scholar]
- Vilegas JHY, Lancas FM, Vilegas W. Composition of the volatile compounds from Aniba canelilla (H.B.K.) Mez. Extracted by CO2 in the supercritical state. Revista Brasileira de Farmacognosia. 1998;8:7. 13–19. [Google Scholar]
- Viljoen AM, Moolla AF, van Vuuren SL, van Zyl R, Baser KHC, Demici B, Ozek T, Trinder-Smith T. The biological activity and essential oil composition of 17 Agathosma (Rutaceae) species. Journal of Essential Oil Research. 2006;18:2–16. [Google Scholar]
- Viña A, Murillo E. Essential oil composition from twelve varieties of basil (Ocimum spp) grown in Colombia. Journal of Brazilian Chemical Society. 2003;14:744–774. [Google Scholar]
- Vinutha AR, Von Rudloff E. Gas-liquid chromatography of terpenes. XVII. Volatile oil of the leaves of Juniperus virginiana. . Canadian Journal of Chemistry. 1968;46:3743–3750. [Google Scholar]
- Viswanathan MB, Maridass M, Thangadurai D, Ramesh N. Chemical constituents of the fruit essential oil of Diospyros malabarica (Desr.) Kostel (Ebenaceae). ACTA Pharmaceutica. 2002;52:207–211. [Google Scholar]
- Von Bulow MV, Franca NC, Gottlieb OR, Suarez AMP. Guianan: A neolignan from Aniba guianensis. . Phytochemistry. 1973;12:1805–1808. [Google Scholar]
- Von Guelow MV, Franca NC, Gottlieb OR, Saurez AMP. Chemistry of Brazilian Lauraceae. XXIV. Guianin. Neolignan from Aniba guinensis. . Phytochemistry. 1973;12:1805–1808. [Google Scholar]
- Vuts J, Imrei Z, Toth M. New coattractants synergizing attraction of Cetonia aurata aurata and Potosia cuprea to the known floral attractant. Journal of Applied Entomology. 2010;134:9–15. [Google Scholar]
- Wakayama S, Namba S, Ohno M. Odorous constituents of lilac flower oil. Nippon Kagaku Zasshi. 1970;92:256–259. in Japanese. [Google Scholar]
- Wang DJ. Studies on the constituents of the essential oils of four aromatic flowers. K'o Hsssueh Fa Chan Yueh K' an. 1979;7:1036–1048. in Chinese. [Google Scholar]
- Wang J, Dudareva N, Bhakta S, Raguso RA, Pichersky E. Floral scent production in Clarkia breweri (Onagraceae). II. Localization and developmental modulation of the enzyme SAM: (Iso)Eugenol Omethyltransferase and phenylpropanoid emission. Plant Physiology. 1997;114:213–221. doi: 10.1104/pp.114.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wang X, Xia X. Analysis of season variation of methyleugenol and safrole in Asarum heterotropoides by gas chromatography. Chinese Journal of Chromatography. 1997;15:85–86. in Chinese with English summary. [PubMed] [Google Scholar]
- Wannes WA, Mhamdi B, Marzouk B. Variations in essential oil and fatty acid composition during Myrtus communis var. italica fruit maturation. Food Chemistry. 2009;112:621–626. [Google Scholar]
- Wannes WA, Mhamdi B, Sriti J, Marzouk B. Changes in essential oil composition of Tunisian Myrtus communis var. italica L. during its vegetative cycle. Journal of Essential Oil Research. 2010;22:13–18. [Google Scholar]
- Wannes WA, Mhamdi B, Sriti J, Jemia MB, Ouchikh O, Hamdaoui G, Kchouk ME, Marzouk B. Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food and Chemical Toxicology. 2010;48:1362–1370. doi: 10.1016/j.fct.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Weyerstahl P, Marschall H. Constituents of the leaf extract of Amomyrtus meli (R. A. Philippi) Legrand et Kausel, Amomyrtus luma (Molina) Legrand et Kausel and of Amomyrtella guili (Speg.) Kausel. Flavour and Fragrance Journal. 1992;7:247–251. [Google Scholar]
- Weyerstahl P, Marschall-Weyerstahl H, Manteuffel E, Kaul VK. Constituents of the essential oil of Strobilanthes callosus Nees. Journal of Essential Oil Research. 1992;4:281–285. [Google Scholar]
- Wee SL, Tan KH, Nishida R. Pharmacophagy of methyl eugenol by males enhances sexual selection of Bactrocera carambolae (Diptera: Tephritidae). Journal of Chemical Ecology. 2007;33:1272–1282. doi: 10.1007/s10886-007-9295-0. [DOI] [PubMed] [Google Scholar]
- Williams NH, Whitten WM. Orchid floral fragrances and male euglossine bees: methods and advances in the sesquidecade. Biological Bulletin. 1983;164:355–395. [Google Scholar]
- Wilson LA, Senechal NP, Widriechner MP. Headspace analysis of the volatile oils of Agastache. . Journal of Agricultural and Food Chemistry. 1992;40:1362–1366. [Google Scholar]
- Wu CS, Wang Y, Zhan DW, Sun SW, Ma YP, Chen J. The main components of the essential oil from Rosa rugosa Thunb. Zhiwu Xuebao. 1985;27:510–515. in Chinese. [Google Scholar]
- Wu SJ, Kao YH, Li TY, Yang CC. Study on the chemical constituents in the volatile oil of Michelia hedyosperma Law. Chung Ts'ao Yao. 1981;12:8–10. [Google Scholar]
- Wu S, Watanabe N, Mita S, Ueda Y, Shibuya M, Ebizuka Y. Two Omethyltransferases isolated from flower petals of Rosa chinensis var. spontanea involved in scent biosynthesis. Journal of Bioscience and Bioengineering. 2003;96:119–128. [PubMed] [Google Scholar]
- Yaacob KB, Zakaria Z, Ramli Z. Major constituents of Cinnamomum parthenoxylon wood oil. Journal of Essential Oil Research. 1990;2:51. [Google Scholar]
- Yano K. Minor components from growing buds of Artemisia capillaris that act as insect antifeedants. Journal of Agricultural and Food Chemistry. 1987;35:889–891. [Google Scholar]
- Yassa N, Akhani H, Aqaahmadi M, Salimian M. Essential oils from two endemic species of Apiaceae from Iran. Zeitschrift Naturforschung. 2003;58:459–463. doi: 10.1515/znc-2003-7-802. [DOI] [PubMed] [Google Scholar]
- Yomogida K. Scent of modern roses. Koryo. 1992;175:65–89. in Japanese. [Google Scholar]
- Yu J, Lei J, Yu H, Cai X, Zou G. Chemical composition and antimicrobial activity of the essential oil Scutellaria barbata. . Phytochemistry. 2004;65:881–884. doi: 10.1016/j.phytochem.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Zabaras D, Wyllie SG. The effect of mechanical wounding on the composition of essential oil from Ocimum minimum L. leaves. Molecules. 2001;6:79–86. [Google Scholar]
- Zabka M, Pavela R, Slezakova L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Industrial Crops and Products. 2009;30:250–253. [Google Scholar]
- Zanoni TA, Adams RP. Essential oils of plants from Hispaniola: 5. The volatile leaf oil of Lepechinia urbanii (Briq.) Epling (Lamiaceae). Flavour and Fragrance Journal. 1991;6:75–77. [Google Scholar]
- Zhang F, Xu Q, Fu S, Ma X, Xiao H, Liang X. Chemical constituents of the essential oil of Asarum forbesii Maxim (Aristolochiaceae). Flavour and Fragrance Journal. 2005;20:318–320. [Google Scholar]
- Zhao C-X, Li X-N, Liang Y-Z, Fang H-Z, Huang L-F, Guo F-Q. Comparative analysis of chemical components of essential oils from different samples of Rhododendron with the help of chemometrics methods. Chemometrics and Intelligent Laboratory Systems. 2006;82:218–228. [Google Scholar]
- Zhao C, Zeng Y, Wen M, Li R, Liang Y, Li C, Zeng Z, Chau F-T. Comparative analysis of essential oils from eight herbal medicines with pungent flavor and cool nature by GC-MS and chemometric resolution methods. Journal of Separation Science. 2009;32:660–670. doi: 10.1002/jssc.200800484. [DOI] [PubMed] [Google Scholar]
- Zhao M-M, Liu XL, Cul C, Luo W. Composition and antimicrobial activity of essential oil from Phyllanthus emblica L., by supercritical CO2 extraction. Journal of South China University of Technology. 2007;35:116–120. [Google Scholar]
- Zhou G-D, Moorthy B, Bi J, Donnelly KC, Randerath K. DNA adducts from alkoxyallybenzene herb and spice constituents in cultured human (HepG2) cells. Environmental and Molecular Mutagenesis. 2007;48:715–712. doi: 10.1002/em.20348. [DOI] [PubMed] [Google Scholar]
- Zhou H, Huang K, Pan Z, Huang G, Guan S, Su T, Lin C. Analysis of essential oil from the fruit peel of Clausena indica (Datz.) Oliv by gas chromatography-mass spectrometry. Jingxi Huagong. 2008;25:65–67. in Chinese with English summary. [Google Scholar]
- Zhu L, Lu B, Xu D. Preliminary study on the chemical constituents of the essential oil of Michelia alba DC. Zhiwu Xuebao. 1982;24:355–359. [Google Scholar]
- Zimowska GJ, Handler AM. PiggyBac transposons in Bactrocera dorsalis and their use for species identification. 2005. Abstract in 8th Exotic Fruit Fly Symposium.
- Zoghbi MDGB, Andrade EHA. Essential oil composition of Bacopa axillaris (Benth.) Standi. Journal of Essential Oil Research. 2006;18:142–143. [Google Scholar]