Abstract
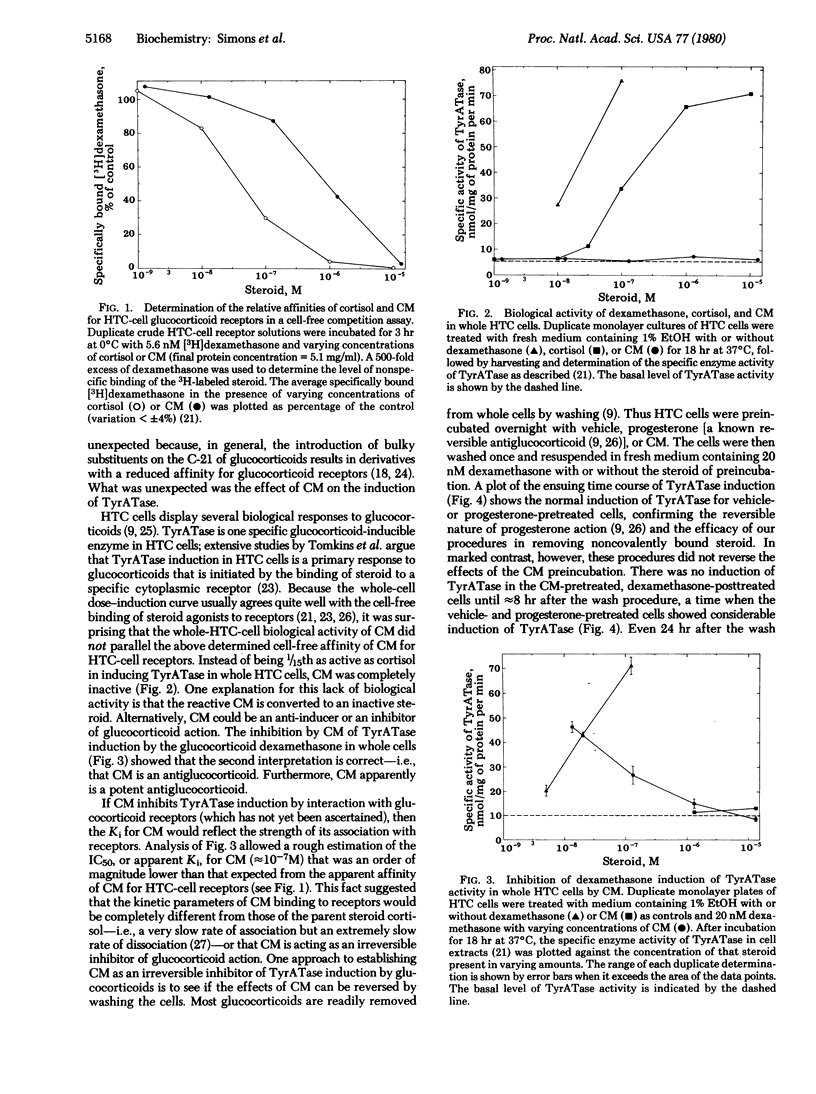
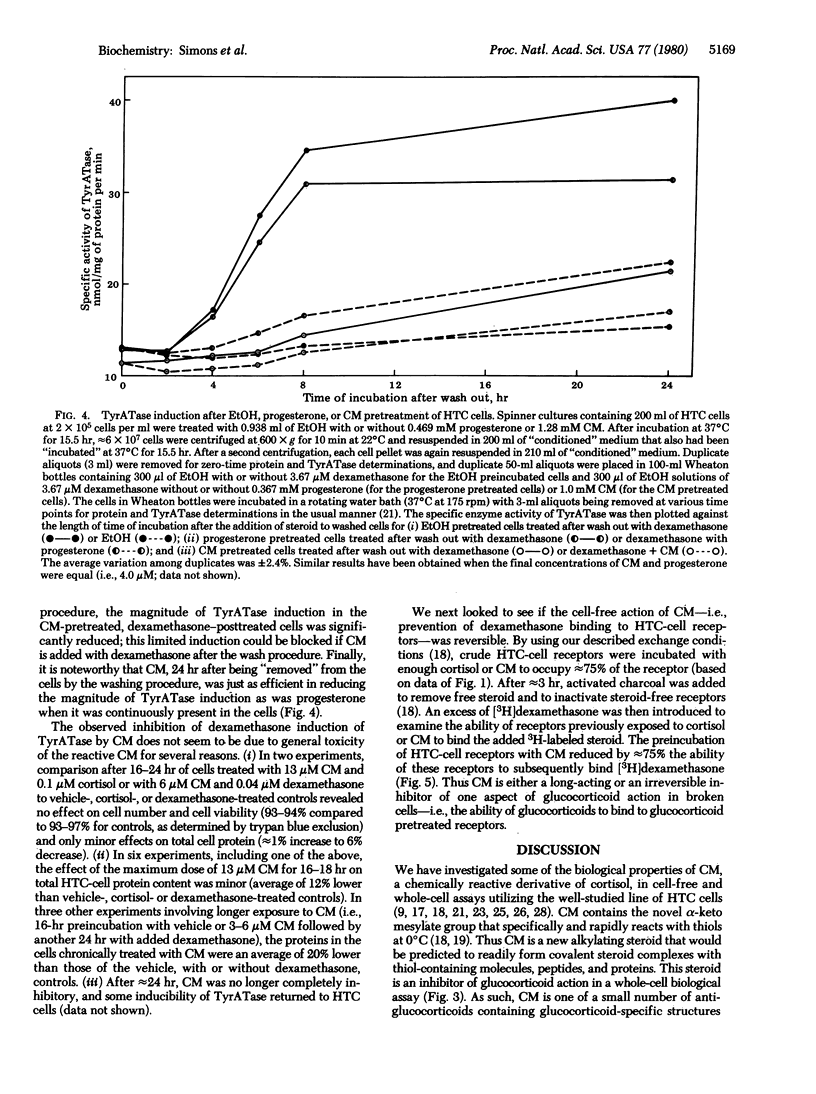
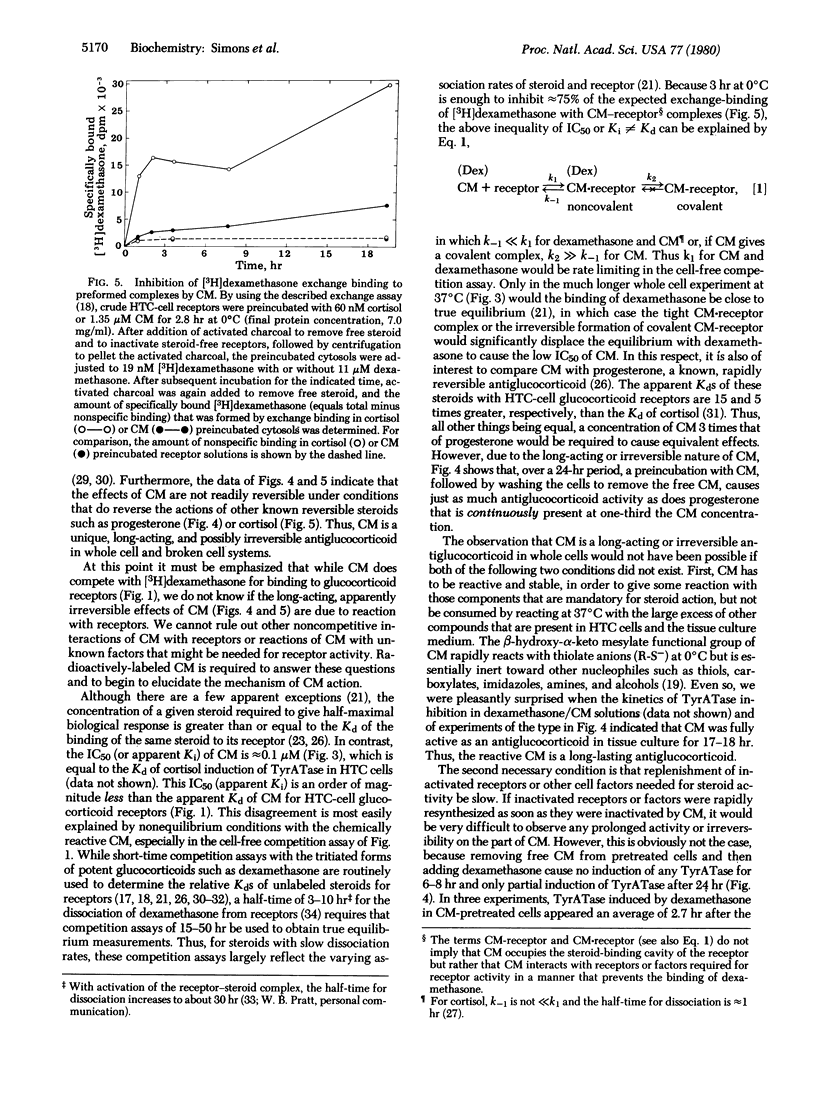
The biological properties of cortisol 21-mesylate (CM), an alkylating derivative of cortisol, were investigated in a line of rat hepatoma tissue culture (HTC) cells. CM appears to bind to glucocoticoid receptors in cell-free extracts because CM inhibits the specific binding of [3H]dexamethasone. However, in whole cells CM not only fails to induce the enzyme tyrosine aminotransferase (TyrATase) but also inhibits the induction of TyrATase by dexamethasone. Thus CM is an antiglucocorticoid. This is not caused by cell death, because CM is relatively nontoxic up to concentrations of 10 microM. The concentration of CM needed for half maximal inhibition of TyrATase induction is an order of magnitude lower than that predicted from the apparent cell-free affinity of CM for the glucocorticoid receptors of HTC cells, which suggests that the cell-free binding data does not reflect an equilibrium situation. In fact, the reactive alpha-keto mesylate group was intentionally incorporated into cortisol in hopes of obtaining a steroid capable of undergoing irreversible reactions. When HTC cells were preincubated with either CM or the reversible antiglucocorticoid progesterone and then washed to remove free steroid, only the CM-treated cells failed to show subsequent induction of TyrATase by dexamethasone. Furthermore, preincubation of HTC-cell cytosol with CM blocked approximately 75% of the subsequent exchange binding of [3H]dexamethasone to glucocorticoid receptor sites. Thus, the actions of CM in whole and broken cells either require an exceptionally long time for reversal or are not reversible. Together, these results indicate that CM is a unique antagonist and could be an irreversible antiglucocorticoid in vitro.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark J. H., Peck E. J., Jr, Anderson J. N. Oestrogen receptors and antagonism of steroid hormone action. Nature. 1974 Oct 4;251(5474):446–448. doi: 10.1038/251446a0. [DOI] [PubMed] [Google Scholar]
- Cutler G. B., Jr, Barnes K. M., Sauer M. A., Loriaux D. L. 11-Deoxycortisol: a glucocorticoid antagonist in vivo. Endocrinology. 1979 Jun;104(6):1839–1844. doi: 10.1210/endo-104-6-1839. [DOI] [PubMed] [Google Scholar]
- Failla D., Tomkins G. M., Santi D. V. Partial purification of a glucocorticoid receptor. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3849–3852. doi: 10.1073/pnas.72.10.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan T. V., Thompson E. B. Effect of concanavalin A on tyrosine aminotransferase in rat hepatoma tissue culture cells. Rapid reversible inactivation of soluble enzyme. J Biol Chem. 1977 Apr 25;252(8):2717–2725. [PubMed] [Google Scholar]
- Harper M. J., Walpole A. L. Contrasting endocrine activities of cis and trans isomers in a series of substituted triphenylethylenes. Nature. 1966 Oct 1;212(5057):87–87. doi: 10.1038/212087a0. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Nuclear mechanisms of estrogen action. Effects of estradiol and anti-estrogens on estrogen receptors and nuclear receptor processing. J Biol Chem. 1978 Nov 25;253(22):8185–8191. [PubMed] [Google Scholar]
- Ivarie R. D., O'Farrell P. H. The glucocorticoid domain: steroid-mediated changes in the rate of synthesis of rat hepatoma proteins. Cell. 1978 Jan;13(1):41–55. doi: 10.1016/0092-8674(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Kaine J. L., Nielsen C. J., Pratt W. B. The kinetics of specific glucocorticoid binding in rat thymus cytosol: evidence for the existence of multiple binding states. Mol Pharmacol. 1975 Sep;11(5):578–587. [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Ferguson E. R. Antiestrogen action in the uterus: biological ineffectiveness of nuclear bound estradiol after antiestrogen. Endocrinology. 1975 Jul;97(1):1–12. doi: 10.1210/endo-97-1-1. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Ferguson E. R., Lan N. C. Fundamental differences in the action of estrogens and antiestrogens on the uterus: comparison between compounds with similar duration of action. Endocrinology. 1977 May;100(5):1252–1259. doi: 10.1210/endo-100-5-1252. [DOI] [PubMed] [Google Scholar]
- Kiang D. T., Kennedy B. J. Tamoxifen (antiestrogen) therapy in advanced breast cancer. Ann Intern Med. 1977 Dec;87(6):687–690. doi: 10.7326/0003-4819-87-6-687. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legha S. S., Carter S. K. Antiestrogens in the treatment of breast cancer. Cancer Treat Rev. 1976 Dec;3(4):205–216. doi: 10.1016/s0305-7372(76)80010-2. [DOI] [PubMed] [Google Scholar]
- Munck A., Brinck-Johnsen T. Specific and nonspecific physicochemical interactions of glucocorticoids and related steroids with rat thymus cells in vitro. J Biol Chem. 1968 Nov 10;243(21):5556–5565. [PubMed] [Google Scholar]
- Pratt W. B., Kaine J. L., Pratt D. V. The kinetics of glucocorticoid binding to the soluble specific binding protein of mouse fibroblasts. J Biol Chem. 1975 Jun 25;250(12):4584–4591. [PubMed] [Google Scholar]
- Rochefort H., Capony F. Estradiol dependent decrease of binding inhibition by anti-estrogens (a possible test of receptor activation). Biochem Biophys Res Commun. 1977 Mar 21;75(2):277–285. doi: 10.1016/0006-291x(77)91040-3. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Baxter J. D., Tomkins G. M. Glucocorticoid receptors: relations between steroid binding and biological effects. J Mol Biol. 1972 Jun 14;67(1):99–115. doi: 10.1016/0022-2836(72)90389-0. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Kirchhoff J., Formstecher P., Lustenberger P. 17 beta-carboxamide steroids are a new class of glucocorticoid antagonists. Nature. 1979 May 10;279(5709):158–160. doi: 10.1038/279158a0. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Schmit J. P. Structure-activity relationships for glucocorticoids-I. Determination of receptor binding and biological activity. J Steroid Biochem. 1977 Sep;8(9):911–919. doi: 10.1016/0022-4731(77)90187-x. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tomkins G. M. Relation of steroid structure to enzyme induction in hepatoma tissue culture cells. J Mol Biol. 1970 Aug 28;52(1):57–74. doi: 10.1016/0022-2836(70)90177-4. [DOI] [PubMed] [Google Scholar]
- Simons S. S., Jr, Thompson E. B., Johnson D. F. Anti-inflammatory pyrazolo-steroids: potent glucocorticoids containing bulky A-ring substituents and no C3-carbonyl. Biochem Biophys Res Commun. 1979 Feb 14;86(3):792–800. doi: 10.1016/0006-291x(79)91782-0. [DOI] [PubMed] [Google Scholar]
- Simons S. S., Jr, Thompson E. B., Johnson D. F. Fluorescent chemoaffinity labeling. Potential application of a new affinity labeling technique to glucocorticoid receptors. Biochemistry. 1979 Oct 30;18(22):4915–4922. doi: 10.1021/bi00589a020. [DOI] [PubMed] [Google Scholar]
- Simons S. S., Jr, Thompson E. B., Merchlinsky M. J., Johnson D. F. Synthesis and biological activity of some novel, chemically reactive glucocorticoids. J Steroid Biochem. 1980 Mar;13(3):311–322. doi: 10.1016/0022-4731(80)90010-2. [DOI] [PubMed] [Google Scholar]
- Turnell R. W., Kaiser N., Milholland R. J., Rosen F. Glucocorticoid receptors in rat thymocytes. Interactions with the antiglucocorticoid cortexolone and mechanism of its action. J Biol Chem. 1974 Feb 25;249(4):1133–1138. [PubMed] [Google Scholar]



