Abstract
A mechanism that confers increased Al resistance in the Arabidopsis thaliana mutant alr-104 was investigated. A modified vibrating microelectrode system was used to measure H+ fluxes generated along the surface of small Arabidopsis roots. In the absence of Al, no differences in root H+ fluxes between wild type and alr-104 were detected. However, Al exposure induced a 2-fold increase in net H+ influx in alr-104 localized to the root tip. The increased flux raised the root surface pH of alr-104 by 0.15 unit. A root growth assay was used to assess the Al resistance of alr-104 and wild type in a strongly pH-buffered nutrient solution. Increasing the nutrient solution pH from 4.4 to 4.5 significantly increased Al resistance in wild type, which is consistent with the idea that the increased net H+ influx can account for greater Al resistance in alr-104. Differences in Al resistance between wild type and alr-104 disappeared when roots were grown in pH-buffered medium, suggesting that Al resistance in alr-104 is mediated only by pH changes in the rhizosphere. This mutant provides the first evidence, to our knowledge, for an Al-resistance mechanism based on an Al-induced increase in root surface pH.
Al is the most abundant metal in the earth's crust and occurs in a number of different forms in the soil. In neutral and basic soils, Al is mostly found as oxide or silicate precipitates that are not toxic to plants. However, in very acidic soils (pH < 5.0), Al speciates to a soluble octahedral hexahydrate form, commonly called Al3+, which is believed to be the primary phytotoxic Al species (Kochian, 1995). The initial and most dramatic symptom of Al toxicity is inhibition of root growth, which results in a reduced and damaged root system and can lead to mineral deficiencies and water stress. Al toxicity is a primary factor limiting agronomic production in acidic soils, which constitute more than 30% of the world's arable land (Von Uexkull and Mutert, 1995). The root apex is the primary target of Al toxicity, and the reduction in root growth is detectable within minutes after Al addition (Ryan et al., 1993; Jones and Kochian, 1995).
Cultivar or variety differences in Al resistance have been reported in a number of crop plants (for review, see Carver and Ownby, 1995). Two categories of Al-resistance mechanisms have been proposed: tolerance to higher concentrations of Al in the root symplast, and the ability to exclude Al from the root apex (Taylor, 1991; Delhaize and Ryan, 1995; Kochian, 1995). Whereas little is known about mechanisms of symplastic tolerance (Aniol, 1984), an Al-exclusion mechanism has recently been described. Delhaize et al. (1993a, 1993b) demonstrated in isogenic lines of wheat that the presence of Al induced the release of more malate from the root apex in the Al-tolerant genotypes. Like several other organic acids, malate chelates Al3+ in the rhizosphere and prevents Al uptake into the root. In these studies, it was shown that resistance segregated as a single dominant locus termed alt1. Malate release was subsequently shown to correlate with Al resistance in a number of other wheat cultivars (Ryan et al., 1995). A similar exclusion mechanism has been observed in maize, in which an Al-induced release of citrate at the root apex was reported (Pellet et al., 1994).
As early as the mid 1960s, Foy et al. (1965) proposed an Al-exclusion mechanism that involves increases in rhizosphere pH. Alkalinization of the rhizosphere would reduce the concentration of Al3+ in favor of less-toxic Al species such as Al hydroxides and Al phosphates (Martell and Motekaitis, 1989). There have been many reports of a general correlation between Al resistance and transient increases in growth solution pH for several species, including wheat (Foy et al., 1967, 1974; Mugwira et al., 1976, 1978; Mugwira and Elgawahry, 1979; Foy and Fleming, 1982; Fleming, 1983; Dodge and Hiatt, 1992), barley (Foy et al., 1967), pea (Klimashevsky and Bernadskaya, 1973), rye (Mugwira et al., 1976, 1978), and triticale (Mugwira et al., 1976, 1978; Mugwira and Patel, 1977), but to date, there have been no direct demonstrations of this Al-resistance mechanism. In most of these reports, it was not clear whether the pH differences were the cause of Al resistance or if they were the result of Al-induced inhibition of root function in the sensitive cultivars.
All of these studies were based on pH measurements of the bulk solution, which were shown to be problematic in two respects. First, the N source of the growth medium can have a significant impact on rhizosphere pH, since uptake of NO3− leads to an alkalinization of the medium, whereas NH4+ uptake can cause rhizosphere acidification (for review, see Taylor, 1988). Therefore, the ratio of NO3− to NH3 in the growth medium of each experiment can have a substantial effect on the pH of the growth solution (Taylor, 1991). Second, bulk-solution measurements reflect pH changes associated with the whole root and not specifically the root tip, which is the primary site of Al toxicity. For this reason, Miyasaka et al. (1989) used pH microelectrodes to map the surface pH along wheat roots and showed that the Al-resistant wheat cv Atlas maintained a slightly higher pH (approximately 0.15 pH unit) at the root tip, but not the mature parts of the root, compared with the Al-sensitive cv Scout. In these experiments, it was not determined whether the differences in rhizosphere pH at the apex led to Al resistance, or whether they merely reflected differences in root function after the onset of Al toxicity in cv Scout.
Recently, we have taken a molecular-genetic approach to gain a better understanding of Al toxicity and resistance by isolating Al-sensitive and Al-tolerant mutants in Arabidopsis thaliana. We previously reported on Al-sensitive Arabidopsis mutants. This trait was described by eight different complementation groups (Larsen et al., 1996). In the companion paper (Larsen et al., 1998), we describe a family of five Al-resistant mutants that map to two different loci on the Arabidopsis genome. All of these alr mutants exclude Al from the root apex to a greater degree than wild type. Four of the mutants mapped to the same location on chromosome 1 and used an Al-exclusion mechanism associated with the increased release of malate and citrate, whereas the other mutant mapped to chromosome 4.
We describe the Al-resistant chromosome 4 mutant alr-104, which does not exhibit enhanced root organic acid release. In this study, we investigated whether increased Al resistance in alr-104 was caused by an increased rhizosphere pH around the root apex. For these studies, we used a vibrating H+ microelectrode system that allowed us to measure root ion fluxes with a very high degree of spatial and temporal resolution (Kochian et al., 1992; Smith et al., 1994). We modified this technique to measure very small roots such as those found on Arabidopsis seedlings, and devised a method to quantify root H+ fluxes in a gel film containing a complex nutrient solution. alr-104 showed an Al-inducible increased H+ influx at the root tip, which resulted in a higher rhizosphere pH in this region (compared with wild type). The increase in apical rhizosphere pH in alr-104 accounts for greater Al tolerance in this mutant. This report provides the first direct evidence to our knowledge for an Al-tolerance mechanism based on a modification of apical rhizosphere pH.
MATERIALS AND METHODS
The genetic and general physiologic characteristics of Al resistance in wild type and alr-104 and alr-128 mutants of Arabidopsis thaliana Heyn. ecotype Columbia (Col-0) used in this study are described in the companion paper (Larsen et al., 1998). Arabidopsis seedlings were grown on a 2-mm layer of a gel (0.15% gellan gum; Gell-Gro, ICN) covering a microscope slide; the gel contained a nutrient solution as described by Larsen et al. (1996) (2 mm KNO3, 0.1 mm KH2PO4, 2 mm MgSO4, 0.25 mm [NH4]2SO4, 1 mm Ca[NO3]2, 1 mm CaSO4, 1 mm K2SO4, 1 μm MnSO4, 5 μm H3BO3, 0.05 μm CuSO4, 0.2 μm ZnSO4, 0.02 μm NaMoO4, 0.1 μm CaCl2, 0.001 μm CoCl2, and 1% Suc, pH adjusted to 4.2). The gel was bounded by a plastic support glued to the slide. Four to six seeds of wild type and alr mutants (alr-104 and alr-128) were sown onto the gel layer, and the slide was placed at a 30o angle in a growth chamber with a 16-h day/8-h night cycle for 4 to 5 d.
During incubation the lower end of the slides was submerged in nutrient solution of the same composition. The liquid medium was changed daily to prevent depletion of nutrients in the gel. Twelve hours before an experiment, the slides were oriented horizontally and the medium in the gel layer was equilibrated with 200 mL of nutrient solution. For the Al treatments the nutrient solution also contained 300 μm AlCl3. The pH of Al-containing solutions was adjusted before the addition of AlCl3, as described in detail by Larsen et al. (1996). When buffered nutrient solution was used, 10 mm Homo-Pipes (Research Organics, Cleveland, OH) was added before adjustment of pH and addition of AlCl3.
Root-Growth Measurements
Root growth was measured on an inverted microscope (model IM35, Zeiss) using a 40× long-working-distance objective (overall magnification, ×400). Root tips were aligned with the scale of an ocular micrometer, and root elongation was recorded 1, 2, and 4 min after alignment. The root growth rate was expressed in micrometers per minute as the average and se of 12 or more seedlings per group. To prevent mechanical disturbance of the roots while the slide was being handled, only roots fully embedded in the gel were chosen for measurement.
Measurement of Root H+ Fluxes with a Vibrating H+ Microelectrode
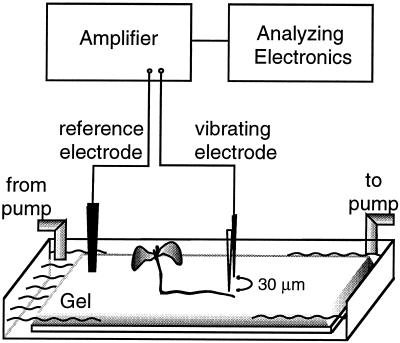
The slide with seedlings was placed in a 30 mm × 80 mm × 4 mm clear polycarbonate chamber. The chamber was filled with 5 mL of the appropriate nutrient solution, which was changed continuously at a rate of 1 mL/min. H+-selective microelectrodes (tip diameter, 1 μm) containing an H+-selective cocktail (catalog no. 95297, Fluka) were constructed as described previously (Lucas and Kochian, 1986). The vibrating microelectrode system has been described in detail elsewhere (Kochian et al., 1992; Smith et al., 1994) and was used with modifications. Unless noted otherwise, the microelectrode was oriented perpendicular to the root surface and vibrated along an axis perpendicular to the root. The electrode was vibrated within the gel between two positions, 5 and 35 μm from the root surface (Fig. 1). Again, only roots that were fully embedded in the gel were used for measurement.
Figure 1.
Experimental setup for measurement of H+ fluxes around Arabidopsis roots. The roots were growing within a thin layer of gel equilibrated with nutrient solution. The microelectrode was mounted vertically and perpendicular to the root and vibrated along its long axis between 5 and 35 μm from the root surface. The gel was held on a microscope slide within a chamber containing the appropriate nutrient solution. The solution was constantly exchanged using a peristaltic pump.
The efficiency of the vibrating H+ microelectrode to detect a H+ gradient within the gel was determined by following a procedure modified from Smith et al. (1994). An artificial H+ gradient was set up and measured with the vibrating H+ microelectrode, and the measured gradient was compared with the theoretical gradient values determined from diffusion equations. The following describes the modifications that were made to account for the buffering effect of the gel and the nutrient solution. A gel consisting of 0.15% gellan gum in nutrient solution was adjusted to pH 4.0 and introduced into a micropipette (tip diameter, approximately 5 μm). The pipette was mounted onto a microscope slide and embedded in a 2-mm layer of the same gel at pH 6.0. The ionic strength of the gel was adjusted by addition of 99 μm KOH to provide a cation for counterdiffusion and to minimize osmotic water flow between the two phases. After 4 h a stable H+-diffusion gradient developed around the micropipette tip, which served as an ion source (data not shown). The H+ gradient was measured with a pH microelectrode and compared with the expected diffusion gradient derived from the appropriate diffusion equation (Jaffe and Levy, 1987; Kühtreiber and Jaffe, 1990; Smith et al., 1994).
Measurement of Root Surface pH with a H+ Microelectrode
The rhizosphere pH along the root was measured with a stationary H+ microelectrode, using the same system described above for the flux measurements. The H+ concentration was determined in the unstirred layer adjacent to the root at a radial position 20 μm from the root surface. Together with the measurement of the H+ flux that is directed from this point toward the root surface, the pH at the root surface was calculated using Fick's law (Crank, 1975). This calculation assumes a radial diffusion of H+ into the cylindrical root:
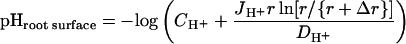 |
where CH+ is the H+ concentration 20 μm from the root surface, JH+ is the H+ flux at 20 μm from the root surface, r is the diameter of the root, Δr is the distance between the point of measurement and the root surface (20 μm), and DH+ is the diffusion coefficient for H+ (9.308 × 10−5 cm2 s−1). Measurements of the H+ concentration at various radial distances from the root surface followed an exponential function (data not shown) and thereby confirm the radial diffusion profile previously documented for maize roots by Kochian et al. (1992).
RESULTS
Efficiency of the H+-Selective Vibrating Microelectrode
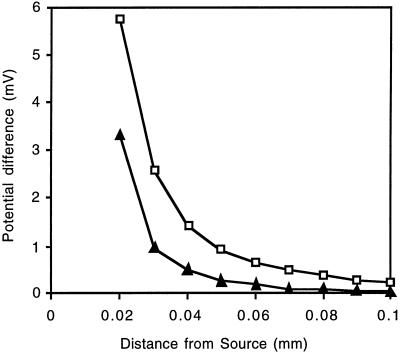
The vibrating electrode was calibrated to account for the buffering capacity of the complex medium and the time lag in measuring H+ gradients caused by the response time of the H+ ionophore (Smith et al., 1994). The efficiency of the microelectrode is the percentage of total ion flux that is detected by the electrode as a potential difference during the measurement. A defined H+ source was used to determine the efficiency (see Methods). The pH microelectrode was vibrated at varying distances from this source, and the potential differences in these positions were determined. These data were compared with the potential differences calculated according to Fick's law and assuming 100% efficiency of the electrode (Fig. 2). The efficiency of the system described here was 32%. It can be seen as the difference in the slopes of the graphs plotted in Figure 2. Repetitions of this calibration showed that only small differences exist between individual microelectrodes. The microelectrode efficiency was used to correct the measured flux values to account for the fact that the vibrating H+ electrode detected only 32% of the overall gradient.
Figure 2.
Efficiency of the vibrating H+-selective microelectrode. The electrode was vibrated at different positions from an H+ source (a micropipette containing gelled nutrient solution at pH 4.0) placed in a pH 6.0 nutrient solution. At each point, the potential difference (representing the H+ gradient between the end points of the vibration) was determined (▴). Comparison of these data with the theoretical values for the H+ gradient calculated according to Fick's law (□) allowed for determination of the efficiency of the system.
Al Effects on H+ Fluxes at the Arabidopsis Root Tip
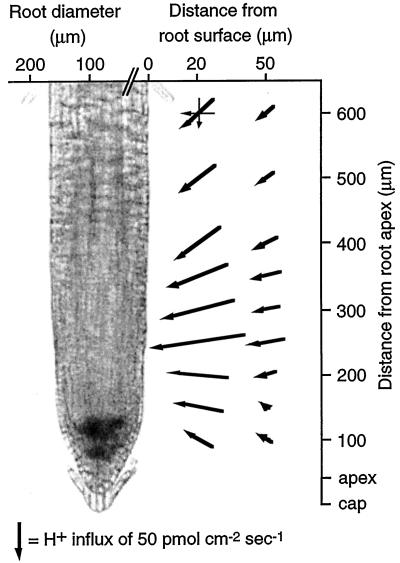
To determine the spatial pattern of H+ currents along the wild-type Arabidopsis root tip, two-dimensional flux measurements with a H+-selective microelectrode were made on 5-d-old seedlings. The measurements were taken at several positions along the root tip, at radial distances 20 and 50 μm from the root surface. At these points, the electrode was vibrated either parallel or perpendicular to the root surface to measure the H+ flux in each direction. The orthogonal flux vectors were summed at each position along the root to generate a two-dimensional map of net H+ fluxes along the root tip. As shown in Figure 3, there was a strong H+ influx into the root apical region. Most of the influx was localized to a region from 200 to 400 μm back from the root tip (within the elongation zone). The point of maximal flux was approximately 250 μm from the root tip, where the H+ current is oriented perpendicular to the root surface. All other measured currents along the root are oriented toward this specific root zone, suggesting that there is either a strong H+ influx or a localized efflux of an H+-binding solute in this region. The root cap and the more mature parts of the root maintain a smaller net H+ influx. Repetitions of this experiment showed that the position of maximum H+ influx varies somewhat among individual roots and lies between 250 and 400 μm behind the root tip.
Figure 3.
Vector diagram of net H+ fluxes along an Arabidopsis root tip. Orthogonal flux measurements (shown as thin vectors for one point 600 μm from the root tip) were taken at several positions at radial distances of 20 and 50 μm from the root surface. The length of the vector represents the flux magnitude. Addition of the orthogonal vector components determines the magnitude and direction of the net H+ current (bold vectors). The root thickness is not drawn to scale with the flux measurements.
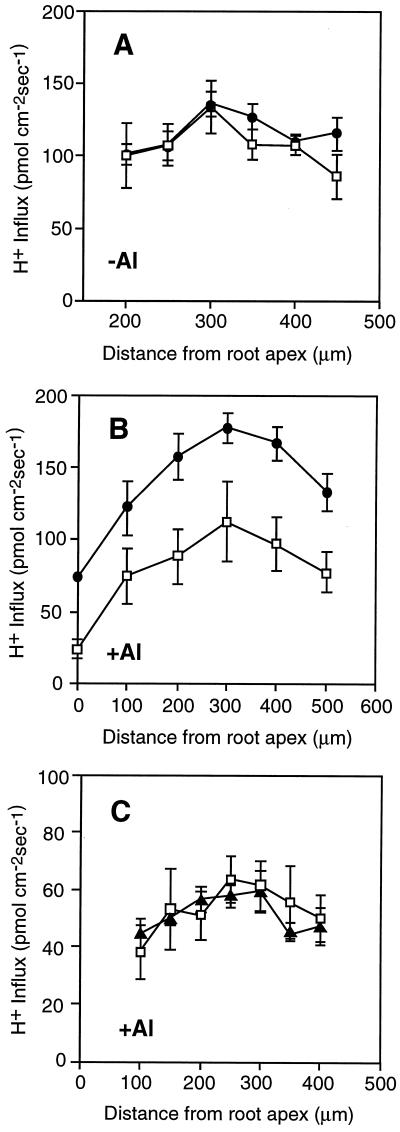
To compare root H+ fluxes between wild type and alr-104, we focused our flux measurements on the region of maximal influx (between 0 and 500 μm from the root tip). In this region the H+ current is primarily perpendicular to the root surface, which allowed us to measure the fluxes with a microelectrode vibrating at a 90o angle with respect to the root. Data from 8 to 12 roots were averaged to account for the differences between individual roots. In nutrient solution without Al, there was no difference in maximal H+ influx between alr-104 and wild type (approximately 120 pmol cm−2 s−1) (Fig. 4A). Also, at regions adjacent to the point of maximum H+ influx, no substantial flux differences were found between mutant and wild type.
Figure 4.
Influence of Al exposure on net H+ influx along Arabidopsis root tips. H+ influx was measured along wild-type (□) and alr-104 (•) roots in the absence (A) and presence (B) of 300 μm AlCl3. C, Root H+ influx along wild-type (□) and alr-128 (▴) roots in the presence of 300 μm AlCl3. Average net influx and se for 8 to 12 roots are shown.
H+-flux measurements were repeated in the presence of 300 μm AlCl3. Because the gel matrix binds Al3+, the Al3+ activity at 300 μm AlCl3 in the gel-layer system is comparable to the Al3+ activity in liquid nutrient solution containing 30 μm AlCl3, based on a bioassay of Al3+ activity (inhibition of root growth in wild-type seedlings; data not shown). After 12 h of incubation in Al-containing nutrient solution, the net H+ influx in alr-104 roots had increased to 180 pmol cm−2 s−1 at the point of maximal H+ flux, whereas the influx in wild-type roots remained at approximately 100 pmol cm−2 s−1 (Fig. 4B). This approximately 80% increase in H+ influx can be seen consistently along the first 500 μm of the root. Seedlings of the Al-resistant mutant alr-128, which is one of the four alr mutants mapping to the same locus on chromosome 1 (alr-104 maps to chromosome 4), were compared with wild-type seedlings in a separate experiment. These seedlings, which released increased amounts of Al-binding organic acids, did not have a detectable increase in H+ influx in the presence of Al (Fig. 4C).
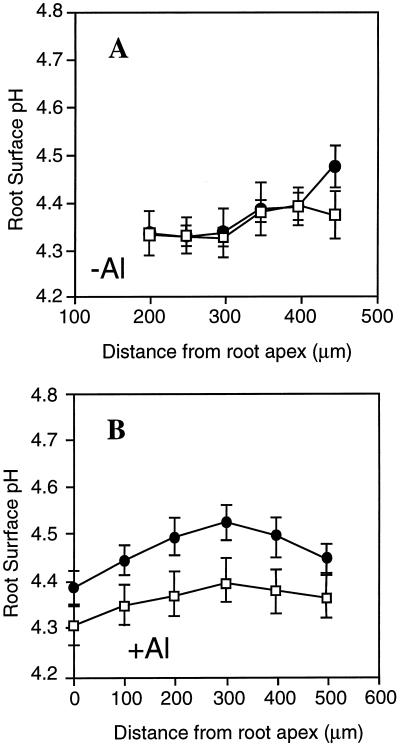
Al Effects on Rhizosphere pH at the Arabidopsis Root Tip
To test whether the altered H+ influx along the root tip of alr-104 had a significant effect on rhizosphere pH (and thereby on the speciation of Al within this region), surface pH along the root apex was determined with static and vibrating H+ microelectrodes. As shown in Figure 5A, the surface pH along alr-104 and wild-type root tips was between pH 4.3 and 4.4 in absence of Al (the pH of the bulk solution was 4.2). No significant pH difference could be found between the mutant and the wild type in the absence of Al. When the roots were exposed to 300 μm Al, the root surface pH of alr-104 increased to 4.53, whereas the root surface pH in wild-type seedlings remained at around 4.39 at the region of highest influx (Fig. 5B). Thus, in the presence of Al, alr-104 alkalinizes the rhizosphere at the root surface.
Figure 5.
Influence of Al exposure on rhizosphere pH along the surface of Arabidopsis root tips. The root surface pH was measured along roots of wild-type (□) and alr-104 (•) roots in the absence (A) and presence (B) of 300 μm AlCl3. Average root surface pH and se for 8 to 12 roots are shown.
Al Resistance in alr-104 Is Dependent on Rhizosphere pH Alteration
Experiments were conducted to determine whether the small Al-induced increase in rhizosphere pH in alr-104 was sufficient to confer increased Al resistance. Al resistance was determined by measuring the root growth rate in the presence of Al in alr-104 and wild type. Five-day-old Arabidopsis seedlings grown in a thin gel layer were incubated for 12 h in nutrient solution containing 300 μm AlCl3 in the presence and absence of 10 mm Homo-Pipes (pH 4.4 and 4.5, respectively). We have previously shown that Homo-Pipes buffers solutions in the pH range between 4.0 and 4.5 without complexing Al or disrupting normal root growth (Pellet et al., 1997). Using the vibrating H+ microelectrode, we found that inclusion of 10 mm Homo-Pipes in the root-bathing solution abolished any detectable pH gradient along the root tip of wild-type and alr-104 seedlings (data not shown).
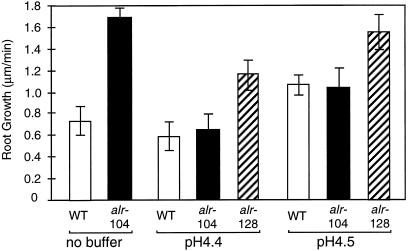
The root growth rate of the seedlings was determined in Al-containing medium that was either unbuffered or buffered at pH 4.4 or 4.5, respectively (Fig. 6). As previously demonstrated, the growth rate of alr-104 in unbuffered medium surpassed that of wild type in the presence of Al, which was consistent with an increased resistance to Al. When the pH of the medium was raised by 0.1 pH unit (from 4.4 to 4.5), the growth rate of both wild-type and alr-104 roots in the presence of Al was nearly doubled. Therefore, an increase in rhizosphere pH of 0.1 unit conferred a significant increase in Al resistance.
Figure 6.
Influence of Al and pH on the root growth rate of wild-type (WT), alr-104, and alr-128 seedlings grown in unbuffered and buffered medium. Seedlings were grown in a thin gel layer equilibrated with nutrient solution containing 300 μm AlCl3 with or without 10 mm Homo-Pipes adjusted to either pH 4.4 or 4.5. The average root growth rate and se of 12 seedlings after 12 h of incubation in the appropriate medium are shown.
Included in this experiment was another Al-resistant mutant, alr-128. This mutant displays a degree of Al resistance similar to alr-104 and was shown to release increased amounts of organic acids into the rhizosphere (Larsen et al., 1998). In buffered conditions that abolished Al resistance in alr-104, the Al resistance of alr-128 was maintained (Fig. 6). This suggests that Al resistance in alr-128 is independent of rhizosphere pH alteration, whereas Al resistance in alr-104 appears to involve a pH-mediated mechanism in which the roots of the mutant alkalinize the rhizosphere. The increased rhizosphere pH around the root apex of alr-104 should drive the speciation of Al toward less-toxic Al species, which would reduce Al toxicity. The similar growth rate of alr-104 and wild type in buffered conditions also demonstrates that the increased Al resistance of alr-104 is attributable solely to a mechanism based on rhizosphere pH alteration.
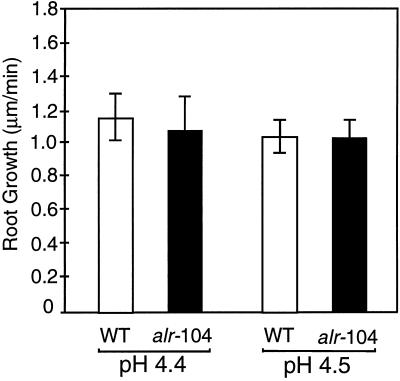
The root-growth studies were repeated without the addition of Al to determine whether the difference in acidic stress between pH 4.4 and 4.5 had an effect on the root growth rate. The results shown in Figure 7 suggest that this small pH difference in the nutrient solution does not have a significant influence on the root growth rate by itself.
Figure 7.
Influence of rhizosphere pH on Arabidopsis root growth in pH-buffered medium. Wild-type (WT) and alr-104 seedlings were grown in a thin gel layer equilibrated with medium containing 10 mm Homo-Pipes at either pH 4.4 or 4.5. The average root growth rate and se of 12 seedlings after 12 h of incubation in the appropriate medium are shown.
DISCUSSION
H+-Flux Measurements along the Arabidopsis Root Tip
In this study we investigated the mechanism that confers increased Al resistance in the Arabidopsis mutant alr-104. Because Al resistance in alr-104 is not associated with increased organic acid release (Larsen et al., 1998), we investigated the possibility that this mutant alters rhizosphere pH to exclude toxic Al3+ ions from uptake into the root. This pH-mediated Al-resistance mechanism has often been hypothesized in the literature, but has not been conclusively shown to exist in terrestrial plants.
It was necessary to modify the extracellular vibrating microelectrode technique to study ion fluxes in very small Arabidopsis roots embedded in a low-density gel matrix. To minimize disturbance of the gel, the electrode was vibrated perpendicular to the root and along the long axis of the microelectrode. H+ influx was measured along the root tip, which reached approximately 120 pmol cm−2 s−1 at the point of maximum influx. The two-dimensional mapping of the H+ influx indicated that most influx is localized within a rather small region in the root elongation zone, approximately four to eight epidermal root cells in length. The spatial pattern of net H+ current at the Arabidopsis root tip was similar to that of total ionic current (deduced from measurements of extracellular electric fields) measured in other species, including barley (Weisenseel et al., 1979), Lepidum sativum (Behrens et al., 1982), Trifolium repens (Miller et al., 1986), and tobacco (Miller et al., 1988).
In the Al-resistant Arabidopsis mutant alr-104, the increased H+ influx was induced by Al. Despite the change in flux intensity, no alteration in the spatial pattern of H+ influx was observed. Therefore, it is likely that the ion-transport processes in alr-104 are the same as those in the wild-type root tip, but are stronger in the presence of Al. However, the nature of the increased net H+ influx in alr-104 has yet to be determined. Because we are measuring a net H+ uptake into the root apex, a stimulation of this flux could be caused by a stimulation of unidirectional H+ influx or a decrease in H+ efflux. A decreased H+ efflux presumably would be caused by Al interaction with the plasma membrane H+-ATPase. Mutations in the H+-ATPase regulatory mechanism in alr-104 might allow a direct or indirect effect by Al.
The apparent increased H+ influx in alr-104 could also be attributable to the efflux of solutes that are protonated when they are released into the acidic rhizosphere. In terms of organic and inorganic acids, only pyruvate was found to be released at a higher rate in alr-104 than in wild type (see Larsen et al., 1998). However, increased release of pyruvate was constitutive and not induced by Al in alr-104. Furthermore, pyruvate is not protonated significantly when it is transported from a neutral cytoplasm to a rhizosphere with a pH around 4.0, and is therefore unlikely to have an effect on rhizosphere pH.
The increased net H+ influx in alr-104 could also be caused by an alteration in the H+-coupled transport system (H+ symport or antiport). One possible change is the uptake of N (as NO3− or NH4+), which is closely coupled to H+ transport. It has been shown from bulk-solution pH measurements that the uptake of NO3− and NH4+ is often associated with pH changes (for review, see Miller and Smith, 1996). In many of these studies, interpretation is often complicated because NO3− and/or NH4+ were eventually depleted in the hydroponic medium, causing dramatic changes in the pH of the bulk solution. In the experiments described here, roots of Arabidopsis plants were equilibrated with a large volume of nutrient solution, providing a constant NH4+/NO3− ratio. It is possible, however, that an Al-induced difference in NH4+ or NO3− uptake in alr-104 is the cause of the altered rhizosphere pH. That is, it is possible that in alr-104, Al exposure stimulates NO3− influx or inhibits NH4+ uptake, which in turn could increase rhizosphere pH.
Because of the complex nutrient requirements for maintaining Arabidopsis root growth, it will be difficult to identify an ion-transport process associated with Al resistance in alr-104. The mutation in alr-104 is a single mutation on chromosome 4 that was isolated from a population of ethyl methylsulfonate-mutagenized seeds. It is therefore likely to be a point mutation that confers a loss of function, although the inducibility of the H+ influx with Al suggests a gain of function. Another example of Al inducibility of resistance comes from the work of Delhaize and colleagues (1993a, 1993b) with wheat, in which the Al-resistance locus Alt1 was shown to confer an Al-induced Al resistance based on organic acid release. The cloning of the alr-104 gene in Arabidopsis is currently being pursued in our laboratories and, when successful, could shed some light on the mechanism of Al-induced rhizosphere pH increase.
pH Differences at the Root Surface Are Responsible for Al Resistance in alr-104
As the solution pH is increased from 4.0 to 5.0, Al speciation changes rapidly from the toxic Al3+ species to the less-toxic Al hydroxides and Al precipitates, so that small pH changes can result in significant changes in Al toxicity (Martell and Motekaitis, 1989). The pH measurements at the root surface of wild type and alr-104 revealed a difference of 0.1 to 0.15 pH unit along the root apex. This root region has been shown to be the primary site of Al toxicity in roots (Ryan et al., 1993). In previous studies, rhizosphere pH differences of similar magnitude were found along root tips of the wheat Al-resistant cv Atlas 66 and the Al-sensitive cv Scout grown in nutrient solution with Al (Miyasaka et al., 1989; Pellet et al., 1997).
Differences of 0.1 to 0.2 pH unit were also reported in bulk-solution measurements with several other wheat cultivars (Taylor and Foy, 1985a, 1985b). In all of these studies, it was not shown whether the small pH differences conferred increased Al resistance. It is important to note that the magnitude of the pH differences measured at the root surface might be even greater at the plasma membrane surface within the cell wall. Because the pH gradient is generated at the plasma membrane of root cells, the electrically charged cell wall might act as a barrier for ion release. Thus, it is possible that the pH difference between the plasma membrane surface and the external solution is greater than that measured in the unstirred layer adjacent to the root.
To determine whether Al resistance in alr-104 is indeed caused by a small (0.1–0.2 pH unit) rhizosphere alkalinization, we performed root-growth studies in nutrient solution in which the unstirred layer adjacent to the root was pH clamped with high concentrations of Homo-Pipes. The extent of buffering was sufficient to avoid the formation of pH gradients at the root surface, but we do not know how far the buffering extends into the root apoplast. Homo-Pipes is a biological buffer with a pK of 4.29 and we have found that it does not bind Al3+ (data not shown).
From these root-growth studies, two important pieces of information were obtained. First, when we abolished root-surface pH gradients with Homo-Pipes, Al resistance in alr-104 disappeared, suggesting that the increased Al resistance of alr-104 is solely the result of a mechanism based on rhizosphere pH alteration. Second, when rhizosphere pH was buffered at pH 4.5, a significant increase in Al resistance was observed compared with findings from Al-toxicity studies conducted in a solution buffered at pH 4.4. These results indicate that in alr-104, the small Al-induced increases in rhizosphere pH (0.1–0.2 unit) are sufficient to account for the observed Al resistance. In alr-128, in which we have demonstrated a correlation between Al resistance, Al exclusion from the root tip, and increased release of malate and citrate (Larsen et al., 1998), we were able to show that Al resistance does not involve changes in rhizosphere pH.
The increased Al resistance of the Arabidopsis mutant alr-104 appears to be caused by an Al-induced alkalinization of the rhizosphere. This increased alkalinization is localized to the root tip, which is the site of Al toxicity. Although this mechanism has often been proposed in the literature, these findings are the first strong evidence to our knowledge for an Al-resistance mechanism involving a rhizosphere pH barrier in higher plants. This mechanism of Al resistance in alr-104 is different from previously described Al-resistance mechanisms, which were based on the exudation of Al-chelating organic acids. In future studies we will investigate the role of ion-transport processes in the Al-induced alkalinization of the rhizosphere. We are also focusing on the isolation of the alr-104 gene by map-based cloning to better understand this mechanism of Al tolerance on a molecular level.
ACKNOWLEDGMENTS
The authors thank Jon E. Shaff for generous help with technical aspects of the vibrating probe. Dr. Peter J.S. Smith is acknowledged for advice regarding the efficiency measurements.
Footnotes
This work was supported in part by the U.S. Environmental Protection Agency, Office of Research and Development (project no. R82-0001-010 to S.H.H. and L.V.K.).
LITERATURE CITED
- Aniol A. Induction of aluminum tolerance in wheat seedlings by low doses of aluminum in the nutrient solution. Plant Physiol. 1984;123:223–227. doi: 10.1104/pp.76.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens HM, Weisenseel MH, Sievers A. Rapid changes in the pattern of electrical current around the root tip of Lepidum sativum L. following gravistimulation. Plant Physiol. 1982;70:1079–1083. doi: 10.1104/pp.70.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver BF, Ownby JD. Acid soil tolerance in wheat. Adv Agron. 1995;54:117–173. [Google Scholar]
- Crank J. The Mathematics of Diffusion. Oxford, UK: Clarendon Press; 1975. [Google Scholar]
- Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.). I. Uptake and distribution of aluminum in root apices. Plant Physiol. 1993a;103:685–693. doi: 10.1104/pp.103.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995;107:315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 1993b;103:695–702. doi: 10.1104/pp.103.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge CS, Hiatt AJ. Relationship of pH to ion uptake imbalance by varieties of wheat (Triticum vulgare) Agron J. 1992;64:476–481. [Google Scholar]
- Fleming AL. Ammonium uptake by wheat varieties differing in Al tolerance. Agron J. 1983;75:726–730. [Google Scholar]
- Foy CD, Burns GR, Brown JC, Fleming AL. Differential aluminum tolerance of two wheat varieties associated with plant-induced pH changes around their roots. Soil Sci Soc Am Proc. 1965;29:64–67. [Google Scholar]
- Foy CD, Fleming AL. Aluminum tolerances of two wheat genotypes related to nitrate reductase activities. J Plant Nutr. 1982;5:1313–1333. [Google Scholar]
- Foy CD, Fleming AL, Burns GR, Arninger WH. Characterization of differential aluminum tolerance among varieties of wheat and barley. Soil Sci Soc Am Proc. 1967;31:513–521. [Google Scholar]
- Foy CD, Lafever HN, Schwartz JW, Fleming AL. Aluminum tolerance of wheat cultivars related to region of origin. Agron J. 1974;66:751–758. [Google Scholar]
- Jaffe LF, Levy S. Calcium gradients measured with a vibrating calcium-selective electrode. Proc 9th Annu Conf IEEE. 1987;9:779–781. [Google Scholar]
- Jones DL, Kochian LV. Aluminum inhibition of the 1,4,5-trisphosphate signal transduction pathway in wheat roots: a role in aluminum toxicity? Plant Cell. 1995;7:1913–1922. doi: 10.1105/tpc.7.11.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimashevsky EL, Bernadskaya ML. The activity of ATPase and acid phosphatase in the root growth zones of two pea varieties with different tolerance to toxic Al ions. Sov Plant Physiol. 1973;20:257–263. [Google Scholar]
- Kochian LV . Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- Kochian LV, Shaff JE, Kühtreiber WM, Jaffe LF, Lucas WJ. Use of an extracellular, ion-selective vibrating microelectrode system for the quantification of K+, H+, and Ca2+ fluxes in maize roots and maize suspension cells. Planta. 1992;188:601–610. doi: 10.1007/BF00197055. [DOI] [PubMed] [Google Scholar]
- Kühtreiber WM, Jaffe LF. Detection of extracellular calcium gradients with a calcium-specific vibrating electrode. J Cell Biol. 1990;110:1565–1573. doi: 10.1083/jcb.110.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Degenhardt J, Tai C-Y, Stenzler LM, Howell SH, Kochian LV. Arabidopsis mutants with increased aluminum resistance exhibit altered patterns of aluminum accumulation and organic acid release from roots. Plant Physiol. 1998;117:9–17. doi: 10.1104/pp.117.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Tai C-Y, Kochian LV, Howell SH. Arabidopsis mutants with increased sensitivity to aluminum. Plant Physiol. 1996;110:743–751. doi: 10.1104/pp.110.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas WJ, Kochian LV. Ion transport processes in corn roots: an approach utilizing microelectrode techniques. In: Gensler WG, editor. Advanced Agricultural Instrumentation: Design and Use. Dordrecht, The Netherlands: Martinus Nijhoff; 1986. pp. 402–425. [Google Scholar]
- Martell AE, Motekaitis RJ (1989) Coordination chemistry and specification of Al(III) in aqueous solution. In TE Lewis, ed, Environmental Chemistry and Toxicology of Aluminum. Lewis Publishers, Chelsea, MI, pp 3–17
- Miller AL, Raven JA, Sprent JI, Weisenseel MH. Endogenous ion currents traverse growing roots and root hairs of Trifolium repens. Plant Cell Environ. 1986;9:79–83. [Google Scholar]
- Miller AL, Shand E, Gow NAR. Ion currents associated with root tips, emerging laterals and induced wound sites in Nicotiana tabacum: spatial relationship proposed between resulting electric fields and phytophthoran zoospore infection. Plant Cell Environ. 1988;11:21–25. [Google Scholar]
- Miller AJ, Smith SJ. Nitrate transport and compartmentation in cereal root cells. J Exp Bot. 1996;47:843–854. [Google Scholar]
- Miyasaka SC, Kochian LV, Shaff JE, Foy CD. Mechanisms of aluminum tolerance in wheat. An investigation of genotypic differences in rhizosphere pH, K+, and H+ transport, and root-cell membrane potentials. Plant Physiol. 1989;91:1188–1196. doi: 10.1104/pp.91.3.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugwira LM, Elgawahry SM. Aluminum accumulation and tolerance of triticale and wheat in relation to root cation exchange capacity. Soil Sci Soc Am J. 1979;43:736–740. [Google Scholar]
- Mugwira LM, Elgawahry SM, Patel SU. Differential tolerances of triticale, wheat, rye and barley to aluminum in nutrient solution. Agron J. 1976;68:782–786. [Google Scholar]
- Mugwira LM, Elgawahry SM, Patel SU. Aluminum tolerance in triticale, wheat and rye as measured by root growth characteristics and aluminum concentration. Plant Soil. 1978;50:681–690. [Google Scholar]
- Mugwira LM, Patel SU. Root zone pH changes and ion uptake imbalances by triticale, wheat, and rye. Agron J. 1977;69:719–722. [Google Scholar]
- Pellet DM, Grunes DL, Kochian LV. Organic acid exudation as an aluminum tolerance mechanism in maize (Zeamays L.) Planta. 1994;196:788–795. [Google Scholar]
- Pellet DM, Papernik LA, Jones DL, Darrah PR, Grunes DL, Kochian LV. Involvement of multiple aluminum exclusion mechanisms in aluminum resistance in wheat. Plant Soil. 1997;192:63–68. [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Malate efflux from root apices: evidence for a general mechanism of Al-tolerance in wheat. Aust J Plant Physiol. 1995;22:531–536. [Google Scholar]
- Ryan PR, DiTomaso JM, Kochian LV. Aluminum toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot. 1993;44:437–446. [Google Scholar]
- Smith PJS, Sanger RH, Jaffe LF. The vibrating Ca2+ electrode: a new technique for detecting plasma membrane regions of Ca2+ influx and efflux. Methods Cell Biol. 1994;40:115–134. doi: 10.1016/s0091-679x(08)61112-7. [DOI] [PubMed] [Google Scholar]
- Taylor GJ. Mechanisms of aluminum tolerance in Triticum aestivum (wheat). V. Nitrogen nutrition, plant-induced pH and tolerance to aluminum: correlation without causality? Can J Bot. 1988;66:694–699. [Google Scholar]
- Taylor GJ. Current views of the aluminum stress response: the physiological basis of tolerance. Curr Top Plant Biochem Physiol. 1991;10:57–93. [Google Scholar]
- Taylor GJ, Foy CD. Mechanisms of aluminum tolerance in Triticumaestivum L. (wheat). I. Differential pH induced by winter cultivars in nutrient solutions. Am J Bot. 1985a;72:695–701. [Google Scholar]
- Taylor GJ, Foy CD. Mechanisms of aluminum tolerance in Triticumaestivum L. (wheat). II. Differential pH induced by spring cultivars in nutrient solutions. Am J Bot. 1985b;72:702–706. [Google Scholar]
- Von Uexkull HR, Mutert E. Global extent, development and economic impact of acid soils. Plant Soil. 1995;171:1–15. [Google Scholar]
- Weisenseel MH, Dorn A, Jaffe LF. Natural H+ currents traverse growing roots and root hairs of barley (Hordeum vulgare L.) Plant Physiol. 1979;64:512–518. doi: 10.1104/pp.64.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]