Abstract
The nonsense mediated decay (NMD) pathway degrades mRNAs bearing premature translation termination codons. In mammals, SMG-8 has been implicated in the NMD pathway, in part by its association with SMG-1 kinase. Here we use four independent assays to show that C. elegans smg-8 is not required to degrade nonsense-containing mRNAs. We examine the genetic requirement for smg-8 to destabilize the endogenous, natural NMD targets produced by alternative splicing of rpl-7a and rpl-12. We test smg-8 for degradation of the endogenous, NMD target generated by unc-54(r293), which lacks a normal polyadenylation site. We probe the effect of smg-8 on the exogenous NMD target produced by myo-3::GFP, which carries a long 3′ untranslated region that destabilizes mRNAs. None of these known NMD targets is influenced by smg-8 mutations. In addition, smg-8 animals lack classical Smg mutant phenotypes such as a reduced brood size or abnormal vulva. We conclude that smg-8 is unlikely to encode a component critical for NMD.
Introduction
The nonsense mediated decay (NMD) pathway is an evolutionarily conserved mRNA surveillance mechanism that recognizes and degrades transcripts bearing premature translation termination codons [1], [2]. In C. elegans, mRNAs that have acquired a nonsense mutation or an extended 3′ untranslated region (UTR) are targeted for NMD [3]. In addition, physiological transcripts that carry an early stop codon are substrates for NMD [4], [5], [6]. The central players of the NMD pathway were discovered in S. cerevisiae [7] and C. elegans [1], [3], [8], [9] and comprise SMG-2/Upf1, SMG-3/Upf2 and SMG-4/Upf3. In worms, Drosophila and mammals, the NMD core components are modulated by additional SMG factors. Recent screens have also identified and confirmed new candidate NMD proteins in C. elegans, H. sapiens and D. rerio [10], [11], [12].
Elegant studies in several organisms have suggested a model in which NMD reflects a competition between SMG-2/UPF1 and Poly(A) Binding Protein (PABP) for ribosome-associated translation release factors. In mammals, the locations of mRNA splice junctions are marked by exon-junction complexes (EJC) during mRNA processing [1]. If the ribosome encounters a premature termination codon (PTC) during the pioneer round of translation, the SURF complex, (consisting of SMG-1, UPF1 and the release factors eRF1 and eRF3) interacts with the EJC, triggers UPF1 phosphorylation by SMG-1 and initiates NMD [13]. In C. elegans, EJC components are dispensable for NMD, and PTCs are instead distinguished from normal stop codons by the size of the 3′UTR [10], [14]. Once the PTC has been recognized, target mRNAs are destroyed by either the SMG-6 endonuclease (mammals, Drosophila and probably worms) [15], [16], and/or a SMG-5/SMG-7-dependent exonuclease (mammals, yeast and probably worms) [17], [18].
Recently, Yamashita and colleagues used immunoprecipation of HeLa cell lysates to identify proteins that interact with SMG-1, a phosphatidylinositol kinase-related protein kinase [19], [20]. SMG-1 bound two novel, conserved proteins, FLJ23205 and FJL12886, which were renamed SMG-8 and SMG-9 [21]. This pair bound strongly to each other, and modified SMG-1 kinase activity in vitro. Inactivation of SMG-8 and SMG-9 lead to a partial stabilization of ß-globin mRNAs in mammalian cell culture, suggesting they might play a role in NMD. The authors also used RNA interference (RNAi) to inactivate C. elegans smg-8 and smg-9, and concluded that smg-8, but not smg-9, contributed to NMD in worms [21].
Here we examine C. elegans smg-8 using a newly generated mutant allele. smg-8(tm2937) contains a 272 bp deletion and a 1 bp insertion within smg-8 (Figure 1A). This deletion encompasses 22 bp upstream of the start site, the initiator ATG and the first two exons. Using animals homozygous for this allele, we employed four approaches to investigate a possible role for smg-8 in the NMD pathway. Our findings suggest that smg-8 is unlikely to be a key component for NMD in C. elegans.
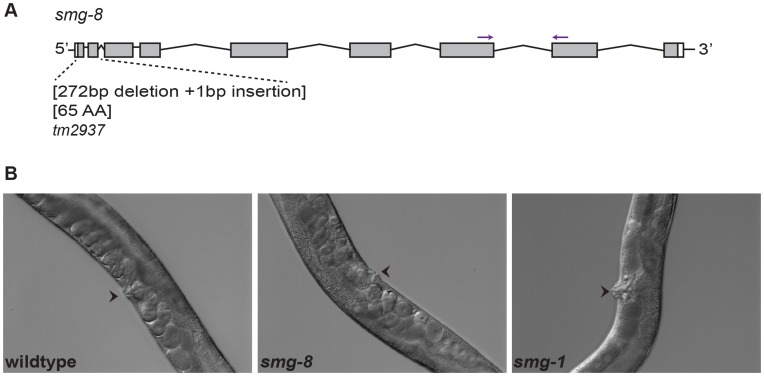
Figure 1. smg-8 lacks the vulva phenotype associated with mutations in other NMD genes.
(A) Schematic representation of the tm2937 allele, which contains a 272 bp deletion and a 1 bp insertion. This deletion encompasses 22 bp upstream of the start site and the first two exons. Arrows indicate primers used for RT-qPCR (B) Vulval protrusion is one of the phenotypes of canonical smg genes. Left panel shows a wildtype vulva, middle panel shows a smg-8(tm2937) mutant and right panel shows a smg-1(r861) mutant. smg-8 mutants are similar to wild-type and not to smg-1. Arrowheads denote the vulva.
Results and Discussion
smg-8 Mutants do not Exhibit Phenotypes Associated with NMD Mutants
For our first assay, we examined two of the classical phenotypes associated with smg genes. Hodgkin and colleagues reported a reduced brood size of 174 (range 147–211) for smg-1, which was lower than the mean brood size of wild-type animals (327, range 270–373) [9]. We observed a mean brood size of 301 (range 242–367, n = 10) for smg-8(2937), similar to the mean brood size of our wild-type strain (279, range 211–328, n = 10) (Table 1). In addition, animals bearing a mutation in a canonical smg gene have a protruding vulva due to morphological defects [9]. However, vulvae appeared normal in smg-8(tm2937) worms (Figure 1B). These data suggest that smg-8(tm2937) animals lack two overt phenotypes associated with canonical smg mutants.
Table 1. Brood Size Comparison of smg-1 and smg-8 vs wild-type.
| Strain | Mean Brood Size | Range |
| smg-1(r861) * | 174 | 174–211 |
| Wildtype* | 327 | 270–373 |
| smg-8(tm2937) | 301 | 242–367 |
| Wildtype | 279 | 211–328 |
Average progeny (n = 10 mothers) at 20°C. Progeny were counted every day until no more progeny were observed.
Data from [9].
smg-8 does not Show an NMD Phenotype for the Native NMD Target rpl-7a and rpl-12
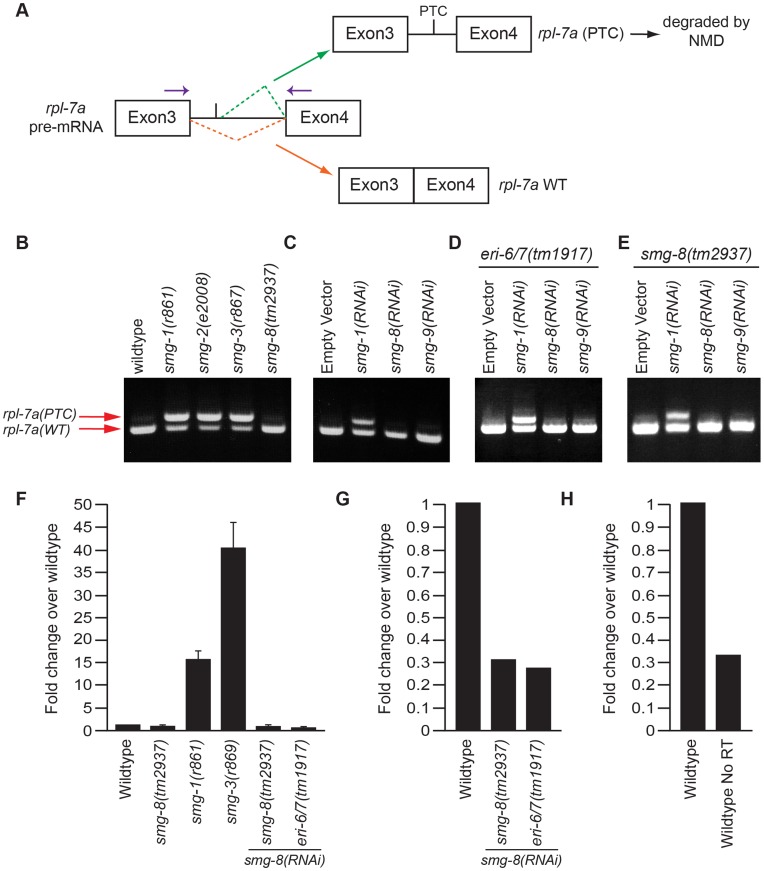
For our second assay, we examined transcripts for two ribosomal proteins rpl-7a and rpl-12, which are natural NMD targets [4]. These genes each generate two alternatively spliced mRNAs, one of which contains a premature termination codon (PTC; Figure 2A). When NMD is active, the longer isoform containing the PTC is degraded and only the shorter isoform accumulates. When the NMD pathway is compromised, the isoform containing the PTC is stabilized, and both mRNA isoforms accumulate (Figure 2A). Using RT-PCR primers that flank the PTC, it is possible to distinguish between the two transcripts [4]. smg-8 shows no NMD phenotype by this assay. When a known component of the NMD pathway, such as smg-1, smg-2 or smg-3, is mutated, the mRNA isoform containing the PTC is stabilized, generating a robust upper band (Figure 2B). In contrast, for smg-8(tm2937), a very faint upper band was observed, comparable to that of the wild-type strain (Figure 2B). To extend this result, we inactivated smg-8 and also smg-9 using RNAi, which reduced smg mRNA levels at least five-fold (Figure 2G) and similar to a No Reverse Transcriptase negative control (Figure 2H). The results were again negative for NMD (Figure 2C). RNAi is not always robust; therefore we repeated the assay using the strain eri-6/7(tm1917), which enhances RNAi [22], [23], and once more observed no NMD phenotype (Figure 2D). Finally, to exclude the possibility that the tm2937 allele was hypomorphic, we treated smg-8(tm2937) mutants with smg-8 RNAi or smg-9 RNAi, but again we observed no NMD phenotype (Figure 2E). Similar results were observed for rpl-12 (Figure 3).
Figure 2. smg-8 lacks an NMD phenotype for the native NMD target rpl-7a.
(A) Schematic representation of the two alternatively spliced isoforms of rpl-7a. The isoform containing the premature termination codon (PTC) is subject to degradation by NMD, whereas the shorter isoform is not. RT-PCR was performed using a pair of primers that distinguish the two spliced isoforms (purple arrows). (B) The upper, PTC band is visible only when the NMD pathway is compromised by smg-1, smg-2 or smg-3 mutations (lanes 2, 3 and 4). Only the lower WT band is observed in wild-type (lane 1) and smg-8 mutant (lane 5) animals. (C) Wild-type worms were fed bacteria expressing dsRNA targeting smg-1, smg-8 or smg-9 from the Ahringer dsRNA library [26]. RNA was analyzed as in (B). (D) An enhanced RNAi mutant strain eri-6/7 [22], [23] was used and RNAi conducted as in (C). RNA was analyzed as in (B). (E) As in D, using the smg-8(tm2937) mutant strain. (F) RT-qPCR using primers flanking the PTC-containing isoform of rpl-7a, mRNA levels were calculated using the delta-delta-CT method, relative to the control gene pmp-3 [27]. Fold enrichment of the PTC mRNA was normalized to 1 for wild-type. The smg-1 and smg-3 mutants show an enrichment of 15 and 38 fold, respectively. In contrast, in smg-8 mutants, the accumulation of the PTC containing isoform is similar to wild-type (0.7 fold enrichment). smg-8 and eri-6/7 mutant worms treated with smg-8 RNAi show 0.7 and 0.4 fold enrichment, respectively. (G) RT-qPCR to quantify smg-8 RNA. mRNA levels were calculated using the delta-delta-CT method, relative to the control gene pmp-3 [27]. Fold enrichment was normalized to 1 for wild-type. smg-8 and eri-6/7 worms treated with smg-8 RNAi show 0.3 and 0.26 fold enrichment, respectively. (H) As in (G) for wild-type animals and a negative control that lacked Reverse Transcriptase (No RT). Fold enrichment was normalized to 1 for wild-type. No RT control shows 0.3 fold enrichment.
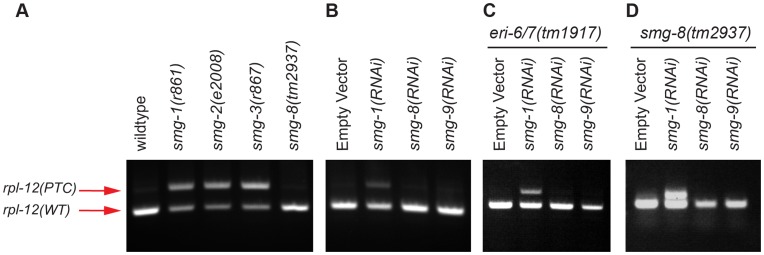
Figure 3. smg-8 lacks an NMD phenotype for the native NMD target rpl-12.
(A) RT-PCR was performed using a pair of primers that distinguish the two spliced isoforms of rpl-12; the upper, PTC band is visible only when the NMD pathway is compromised by smg-1, smg-2 or smg-3 mutations (lanes 2, 3 and 4). Only the lower, WT band is observed in wild-type (lane 1) and smg-8 mutant (lane 5) animals. (B) Wild-type worms were fed bacteria expressing dsRNA targeting smg-1, smg-8 or smg-9 from the Ahringer dsRNA library [26]. RNA was analyzed as in (A). (C) As in (B), using an enhanced RNAi mutant eri-6/7 [22], [23]. (D) As in (B), using the smg-8(tm2937) mutant strain.
To quantify these data, we used RT-qPCR to measure the increase of the PTC containing isoform. We observed a fold enrichment of 15 and 38 in smg-1 and smg-3 mutants respectively, compared to the wild-type (Figure 2F). The PTC containing isoform in smg-8 mutants remained similar to the wild-type (0.7 fold enrichment). A virtually identical result was obtained with smg-8 RNAi treatment of smg-8 or eri-6/7 mutant worms (0.7 and 0.4 fold enrichment respectively) (Figure 2F). Together, these data reveal that inactivation of smg-8 fails to stabilize two natural NMD targets, rpl-7a and rpl-12.
smg-8 is not Required for Endogenous NMD in C. elegans
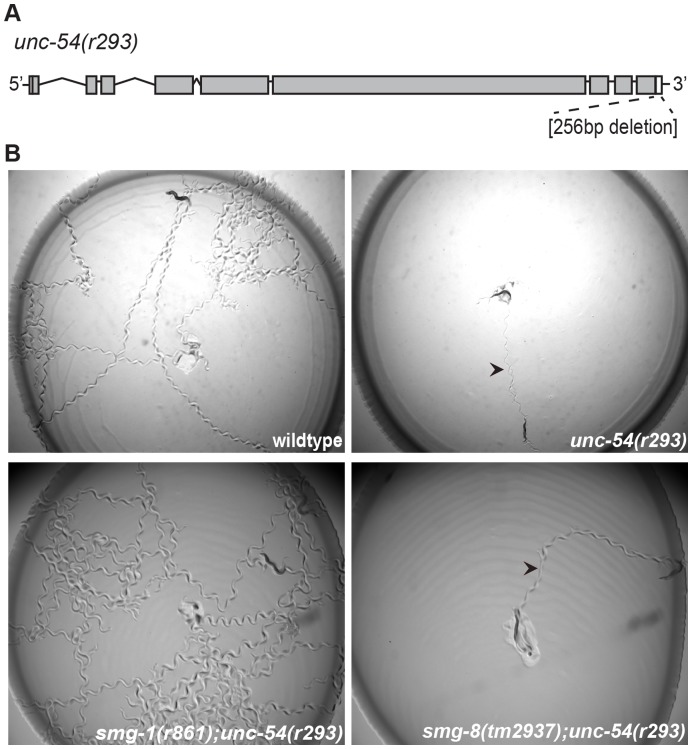
As a third test for NMD, we examined an endogenous target of the NMD pathway: unc-54(r293) [3], [9]. The unc-54 gene generates a muscle myosin heavy chain (MHC) in C. elegans, and the unc-54(r293) allele contains a 256 bp deletion that removes the normal 3′ cleavage/polyadenylation site and most of the 3′UTR [3] (Figure 4A). This deletion causes the production of a long unc-54 mRNA transcript that terminates at a cryptic poly(A) site and renders unc-54 an NMD target. Without the MHC, unc-54 mutant worms are paralyzed (Figure 4B). In the absence of NMD components such as smg-1, the unc-54(r293) mRNA is stabilized, wild-type protein is produced and the Unc phenotype is suppressed [3] (Figure 4B). We generated unc-54(r293); smg-8(tm2937) double mutants and observed no suppression of the paralysis phenotype (Figure 4B), consistent with our hypothesis that smg-8 is not required for NMD in C. elegans.
Figure 4. smg-8 is not required for endogenous NMD in C. elegans.
(A) unc-54 gene schematic. The r293 allele contains a 256 bp deletion within unc-54 that includes the 3′ cleavage and polyadenylation site. (B) smg-8(tm2937) or smg-1(r861) mutations were combined with unc-54(r293). Two worms were placed in the middle of the bacterial lawn and allowed to crawl for 45 minutes. Wild-type worms that crawl (top left) leave tracks in the lawn whereas unc-54(r293) mutants cannot move well (top right). smg-1(r861) suppresses unc-54(r293) mRNA degradation and restores movement [3] (bottom left). In contrast, smg-8 does not suppress the paralysis phenotype (bottom right). Arrowheads indicate the tracks left by paralyzed worms.
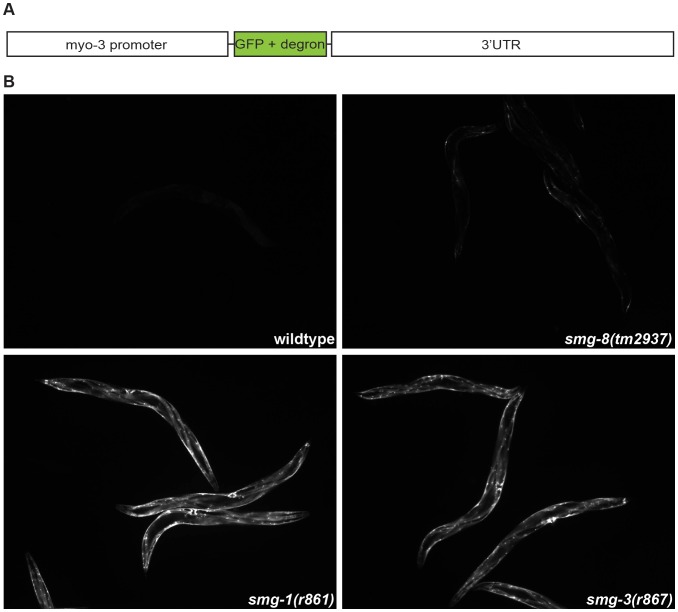
smg-8 does not Restore Expression the Exogenous NMD Target myo-3::GFP
As a fourth test for NMD, we examined an exogenous NMD target, myo-3::GFP. This strain carries a transgenic GFP reporter that is transcribed in body wall muscles and targeted for degradation by a long 3′UTR (Figure 5A) [24]. When the NMD pathway is inactive, GFP accumulates in muscle fibers, whereas wild-type worms accumulate almost no GFP (Figure 5B). We created double combinations of myo-3::GFP and smg-1, smg-3 or smg-8. The strain myo-3::GFP; smg-8(tm2937) accumulated very little GFP compared to the positive controls myo-3::GFP; smg-1(r861) or myo-3::GFP; smg-3(r867) (Figure 5B), indicating that smg-8 is not required for exogenous NMD in C. elegans.
Figure 5. smg-8 does not restore expression of myo-3::GFP, an exogenous NMD target.
(A) Schematic representation of the exogenous NMD GFP reporter, driven by the myo-3 promoter, which is destabilized by an amino acid sequence that marks a protein for degradation (degron), and a long 3′UTR [24]. (B) smg-8 and control mutations were introduced into CL724 (myo-3::GFP) worms. The double combinations were then inspected under a fluorescent microscope. smg-1 and smg-3 mutants express high levels of GFP. In contrast, smg-8 animals photographed under the same conditions show only a slight accumulation of GFP, similar to the wild type.
In summary, we tested smg-8 for a role in NMD using four different assays: i) anatomical phenotype and brood size, ii) accumulation of natural NMD targets rpl-7a and rpl-12, iii) rescue of the paralysis phenotype caused by the endogenous NMD target unc-54(r293) and iv) GFP accumulation of the exogenous NMD target reporter myo-3::GFP. The discrepancy between our study and the results presented by Yamashita and colleagues is due in part to the use of an allele (our study) vs. RNAi [21]. In addition, we note that the effect of smg-8 inactivation on NMD in C. elegans was not robust in the Yamashita study [21]. In all of our assays, smg-8 mutants resembled wild-type worms and differed from classical smg mutants. We detected no accumulation of mRNA or protein in smg-8 mutants, even when the smg-8 mutation was combined with RNAi. We suggest that smg-8 in C. elegans is a novel, conserved gene whose function remains to be elucidated.
Materials and Methods
Strains
Worm growth and maintenance were performed as described before [25]. Strains used: SM1618 unc-54(r293)I, SM456 smg-1(r861)I, SM436 smg-2(e2008)I, SM196 smg-3(r867)IV, smg-1(r861);unc-54(r293)I, smg-8(tm2937)II;unc-54(r293)I, CL724 dvIs38 [pCL60 (Pmyo-3::GFP::degron/long 3′ UTR)+pRF4], SM1944 smg-1(r861)I;myo-3::GFP, SM1929 smg-3(r867)IV;myo-3::GFP, SM1937 smg-8(tm2937)II;myo-3::GFP, SM1881 smg-8(tm2937)II eight times outcrossed.
RNA Interference
HT115 bacteria expressing double stranded RNA targeting smg-1, smg-8 or smg-9 grown for ∼8 hours at 37°C were plated using 1 mM IPTG (Sigma) and 50 mg/ml of Carbenicillin (Sigma). RNAi clones were derived from the Ahringer library [26] and verified by sequencing. Five wild-type, smg-8(tm2937) or eri-6/7(tm1917) worms were transferred at the L4 stage to RNAi plates and allowed to lay embryos for one day. The progeny was collected ∼48 hours later, when most worms had grown at least to the L4 stage, by rinsing with water and frozen at -80°C for subsequent RNA extraction. Nine 35 mm plates were used per strain per experiment.
RNA Extraction
For total RNA extraction, glass beads (Sigma) and 1 ml of Trizol Reagent (GibcoBRL) were added to frozen worm pellets. Pellets were lysed by vortex followed by chloroform extraction. RNA was precipitated with isopropanol and washed with 70% ethanol. Resuspended RNA was extracted with phenol:chloroform and precipitated with ethanol. A first-strand reaction kit (NEB) was used to perform the reverse transcriptase reaction, following the manufacturers protocol.
RT-PCR of rpl
Amplification of rpl-7a from the cDNA was performed as described in [4], and PCR product was analyzed in a 1% agarose gel. Primers used were rpl-7a-fw GACATCCAGCCAAAGAAGGA and rpl-7a-rv AACGGTGTTTGGTCTCTTGG.
Primers for rpl-12 are Rpl-12-F1 ACCCAAGACTGGAAGGGTCT and Rpl-12 R1 GCCATCGATCTTGGTCTCAT.
RT-qPCR
For smg-8 and rpl-7a, mRNA levels were calculated using the delta-delta-CT method, relative to the control gene pmp-3 [27]. Control mRNA was normalized to 1 and the mRNA levels are shown as relative fold change. Primers for smg-8 were smg8-fw-4348 GCTGCCAATATTTCCATCGT and smg8-rv-5165 TGACCACGGGAACATTCATA.
Brood Size
10 worms at the L4 stage were picked into individual plates at 20°C. Their progeny was counted everyday until no more progeny was generated. The number of progeny per plate was averaged (n = 10).
Acknowledgments
We thank C. Madubata for work on Figure 4, S. Mitani for providing the smg-8(tm2937) strain, and C. Link for providing the CL724 strain. We thank members of the Mango lab for helpful discussions, and John Calarco and Arneet Saltzman for critical reading of the manuscript.
Funding Statement
SEM received funding from the National Institutes of Health NIH (GM056264), the John D. and Catherine T. MacArthur Foundation and Harvard University. JR received funding from Harvard University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chang YF, Imam JS, Wilkinson MF (2007) The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem 76: 51–74. [DOI] [PubMed] [Google Scholar]
- 2. Nicholson P, Yepiskoposyan H, Metze S, Zamudio Orozco R, Kleinschmidt N, et al. (2010) Nonsense-mediated mRNA decay in human cells: mechanistic insights, functions beyond quality control and the double-life of NMD factors. Cell Mol Life Sci 67: 677–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pulak R, Anderson P (1993) mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev 7: 1885–1897. [DOI] [PubMed] [Google Scholar]
- 4. Mitrovich QM, Anderson P (2000) Unproductively spliced ribosomal protein mRNAs are natural targets of mRNA surveillance in C. elegans. Genes Dev 14: 2173–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramani AK, Nelson AC, Kapranov P, Bell I, Gingeras TR, et al. (2009) High resolution transcriptome maps for wild-type and nonsense-mediated decay-defective Caenorhabditis elegans. Genome Biol 10: R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barberan-Soler S, Lambert NJ, Zahler AM (2009) Global analysis of alternative splicing uncovers developmental regulation of nonsense-mediated decay in C. elegans. RNA 15: 1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leeds P, Wood JM, Lee BS, Culbertson MR (1992) Gene products that promote mRNA turnover in Saccharomyces cerevisiae. Mol Cell Biol 12: 2165–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cali BM, Kuchma SL, Latham J, Anderson P (1999) smg-7 is required for mRNA surveillance in Caenorhabditis elegans. Genetics 151: 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hodgkin J, Papp A, Pulak R, Ambros V, Anderson P (1989) A new kind of informational suppression in the nematode Caenorhabditis elegans. Genetics 123: 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Longman D, Plasterk RH, Johnstone IL, Caceres JF (2007) Mechanistic insights and identification of two novel factors in the C. elegans NMD pathway. Genes Dev 21: 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun Y, Yang P, Zhang Y, Bao X, Li J, et al. (2011) A genome-wide RNAi screen identifies genes regulating the formation of P bodies in C. elegans and their functions in NMD and RNAi. Protein Cell 2: 918–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anastasaki C, Longman D, Capper A, Patton EE, Caceres JF (2011) Dhx34 and Nbas function in the NMD pathway and are required for embryonic development in zebrafish. Nucleic Acids Res 39: 3686–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kashima I, Yamashita A, Izumi N, Kataoka N, Morishita R, et al. (2006) Binding of a novel SMG-1-Upf1-eRF1-eRF3 complex (SURF) to the exon junction complex triggers Upf1 phosphorylation and nonsense-mediated mRNA decay. Genes Dev 20: 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mango SE (2001) Stop making nonSense: the C. elegans smg genes. Trends Genet 17: 646–653. [DOI] [PubMed] [Google Scholar]
- 15. Eberle AB, Lykke-Andersen S, Muhlemann O, Jensen TH (2009) SMG6 promotes endonucleolytic cleavage of nonsense mRNA in human cells. Nat Struct Mol Biol 16: 49–55. [DOI] [PubMed] [Google Scholar]
- 16. Huntzinger E, Kashima I, Fauser M, Sauliere J, Izaurralde E (2008) SMG6 is the catalytic endonuclease that cleaves mRNAs containing nonsense codons in metazoan. RNA 14: 2609–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Unterholzner L, Izaurralde E (2004) SMG7 acts as a molecular link between mRNA surveillance and mRNA decay. Mol Cell 16: 587–596. [DOI] [PubMed] [Google Scholar]
- 18. Chiu SY, Serin G, Ohara O, Maquat LE (2003) Characterization of human Smg5/7a: a protein with similarities to Caenorhabditis elegans SMG5 and SMG7 that functions in the dephosphorylation of Upf1. RNA 9: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grimson A, O'Connor S, Newman CL, Anderson P (2004) SMG-1 is a phosphatidylinositol kinase-related protein kinase required for nonsense-mediated mRNA Decay in Caenorhabditis elegans. Mol Cell Biol 24: 7483–7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamashita A, Ohnishi T, Kashima I, Taya Y, Ohno S (2001) Human SMG-1, a novel phosphatidylinositol 3-kinase-related protein kinase, associates with components of the mRNA surveillance complex and is involved in the regulation of nonsense-mediated mRNA decay. Genes Dev 15: 2215–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamashita A, Izumi N, Kashima I, Ohnishi T, Saari B, et al. (2009) SMG-8 and SMG-9, two novel subunits of the SMG-1 complex, regulate remodeling of the mRNA surveillance complex during nonsense-mediated mRNA decay. Genes Dev 23: 1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhuang JJ, Hunter CP (2011) Tissue specificity of Caenorhabditis elegans enhanced RNA interference mutants. Genetics 188: 235–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fischer SE, Butler MD, Pan Q, Ruvkun G (2008) Trans-splicing in C. elegans generates the negative RNAi regulator ERI-6/7. Nature 455: 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Link CD, Taft A, Kapulkin V, Duke K, Kim S, et al. (2003) Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer's disease model. Neurobiol Aging 24: 397–413. [DOI] [PubMed] [Google Scholar]
- 25. Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kamath RS, Ahringer J (2003) Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30: 313–321. [DOI] [PubMed] [Google Scholar]
- 27. Hoogewijs D, Houthoofd K, Matthijssens F, Vandesompele J, Vanfleteren JR (2008) Selection and validation of a set of reliable reference genes for quantitative sod gene expression analysis in C. elegans. BMC Mol Biol 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]