Abstract
Particle separation is important to many chemical and biomedical applications. Magnetic field-induced particle separation is simple, cheap, and free of fluid heating issues that accompany electric, acoustic, and optical methods. We develop herein a novel microfluidic approach to continuous sheath-free magnetic separation of particles. This approach exploits the negative or positive magnetophoretic deflection to focus and separate particles in the two branches of a U-shaped microchannel, respectively. It is applicable to both magnetic and diamagnetic particle separations, and is demonstrated through the sorting of 5 μm and 15 μm polystyrene particles suspended in a dilute ferrofluid.
INTRODUCTION
Separating particles from a complex mixture is an important step in many chemical and biomedical applications. A variety of force fields have been demonstrated to implement particle separation in microfluidic devices, among which electric,1, 2 magnetic,3, 4 acoustic,5, 6 and optical7, 8 forces are the most often used.9, 10, 11, 12 While each of these methods has its own merit, magnetic field-induced particle manipulation is potentially the simplest and cheapest. Moreover, it is non-invasive and free of fluid heating issues (if permanent magnets are used) which accompany nearly all other methods, and is therefore well suited to handling biological particles.13, 14, 15, 16 However, in many of the reported continuous-flow magnetic separations the particle suspension needs to be first confined by a co-flowing buffer solution. Then, an external magnetic force acts on the suspended particles and deflects them to different flow paths in the sheath stream for a continuous sorting. This technique has been demonstrated for the separations of magnetic from diamagnetic (often called nonmagnetic by non-physicists) particles,17, 18, 19 magnetic from magnetic particles,20, 21, 22 and diamagnetic from diamagnetic particles.23, 24, 25 Its main drawback lies in the use of a sheath flow to focus particles, which not only complicates the flow control and device fabrication but also dilutes the separated particles at the cost of chemicals.
In this work, we develop a novel magnetic approach to continuously separate particles in a U-shaped microchannel without the use of sheath flow for particle focusing. This approach exploits the magnetophoretic deflection to focus and separate particles of dissimilar properties in the two branches of a U-shaped microchannel, respectively. It is applicable to both magnetic and diamagnetic particle separations. A proof-of-principle experiment is demonstrated using a binary mixture of polystyrene particles suspended in a dilute ferrofluid.
SEPARATION MECHANISM
We start with the explanation of the mechanism for magnetic separation of particles in a U-shaped microchannel. It is long known that a particle undergoes a magnetophoretic motion, Um, in a non-uniform magnetic field as long as its magnetization, Mp, is different from that of the suspending fluid, Mf26, 27
| (1) |
where μ0 is the free space permeability, d is the particle diameter, η is the fluid viscosity, and H is the magnetic field at the particle center. If the particle is more magnetizable than the fluid, i.e., Mp > Mf, one gets Um > 0 and the particle is pulled by positive magnetophoresis towards the magnetic source. This takes place when magnetic (or magnetically tagged) particles are suspended in diamagnetic solutions. In contrast, diamagnetic particles suspended in magnetic solutions (e.g., paramagnetic solutions and ferrofluids) are pushed away from the high magnetic field region by negative magnetophoresis. In either circumstance, Eq. 1 states that particles of dissimilar sizes, d, and/or magnetizations, Mp = χpH with χp being the magnetic susceptibility, migrate at unequal magnetophoretic velocities in the same suspending fluid. This relationship forms the basis of all the previous demonstrated magnetic field-induced particle separations and the present work as well.
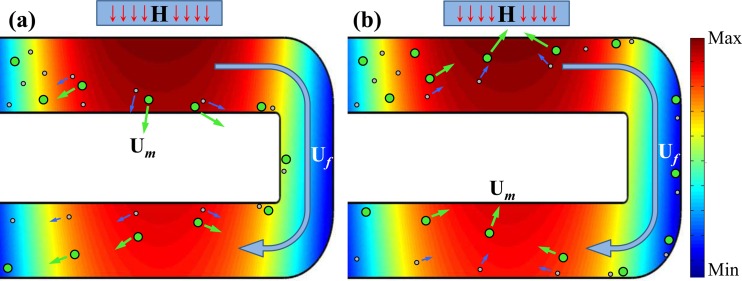
Let us first consider a binary mixture of diamagnetic particles suspended in a magnetic solution, which is pumped through a U-shaped microchannel with a nearby permanent magnet as schematically shown in Figure 1a. To simply the description below without loss of generality, the two types of particles are assumed to have similar magnetic properties (or more specifically an identical magnetic susceptibility, χp) and differ only in size. We admit this is not true as in practice even the particles made from the same batch can vary significantly in magnetic properties. The background of Figure 1a displays the contour of the magnetic field from the magnet within the microchannel, where magnetic field gradients occur throughout the U-turn. Therefore, the suspended diamagnetic particles experience negative magnetophoresis, Um, and should be repelled away from the magnet in each branch of the U-turn as indicated by the velocity arrows in Figure 1a. However, as Um in Eq. 1 is a function of particle size, the large particles can be deflected at a faster rate than the small ones. By adjusting the flow velocity, Uf, of the particle suspension, we are able to achieve a full-width deflection for both types of particles in the branch nearer to the magnet. Consequently, both the large and the small particles line the inner corner of the U-turn as illustrated in Figure 1a. Such a sheath-free magnetic focusing of particles is the advantage of the proposed separation technique over the existing ones.
Figure 1.
Schematic diagrams illustrating the mechanisms of sheathless size-based magnetic separations of diamagnetic particles in a magnetic solution (a) and magnetic particles in a diamagnetic solution (b) through a U-shaped microchannel. The long curving arrows indicate the direction of the fluid flow, Uf. The short arrows indicate the directions and magnitudes of the induced magnetophoretic motions, Um. The background colors show the contour of the magnetic field from the nearby permanent magnet (indicated by the box with an inside letter “H” on the top of each plot).
Subsequently in the other branch of the U-turn, the focused particle mixture is displaced from the inner corner to the outer corner at a size-dependent Um. However, as the magnetic field and field gradients are both smaller in this branch, the reduced Um is barely sufficient to deflect the large particles across the channel width and so the small particles are left half-way. The result is a split of the focused particle stream into two sub-streams flowing out of the U-turn along dissimilar flow paths as illustrated in Figure 1a. The displacement gap between the two particle streams can be maximized by optimizing the distance between the two branches and the distance between the magnet and the U-turn.
In contrast, if a suspension of two unequal-sized magnetic particles in a diamagnetic solution is pumped through the U-shaped microchannel, particles are dragged towards the magnet by positive magnetophoresis, Um, in each branch of the U-turn as shown in Figure 1b. Since Um in this circumstance still follows Eq. 1, the above description for diamagnetic particle separation in a magnetic solution should also be applicable to magnetic particles. In other words, the small and large magnetic particles are first focused in the entrance branch to a single stream flowing adjacent to the outer corner of the U-turn. Then, they are deflected across the channel width towards the inner corner in the other branch with the large particles being deflected farther due to their larger Um than that of the small particles.
EXPERIMENTAL TECHNIQUE
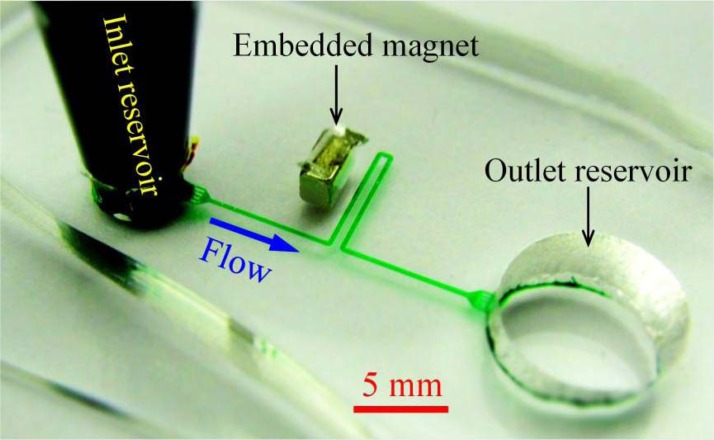
Figure 2 shows a picture of the microfluidic device used in our proof-of-principle separation experiment. The two branches of the U-shaped microchannel are each 6.5 mm long with a 400 μm gap in between. Each branch is connected to a reservoir through a 10 mm long straight section. Four identical blocks are evenly distributed at the junction of the microchannel and each reservoir. This design has two purposes: One is to filter the debris in the solution at the inlet for reducing particle clogging, and the other is to provide multiple passages at the outlet for separating sorted particles (cf. Figure 3). The microchannel is everywhere 200 μm wide and 40 μm deep. It was fabricated with polydimethylsiloxane (PDMS) using the standard soft lithography method as detailed elsewhere.28 One neodymium-iron-boron (NdFeB) permanent magnet (B221, 1/8″× 1/8″ × 1/16″, K&J Magnetics) was embedded in PDMS using the approach we developed earlier.29, 30 It is 600 μm away from the channel edge of the U turn and 1.5 mm from the straight section connecting to the inlet reservoir. Its magnetization direction (through the 1/16″ thickness) is normal to the branches of the U-turn.
Figure 2.
Picture of the U-shaped microchannel (filled with green food dye for clarity) used in the experiment. The block arrow indicates the flow direction.
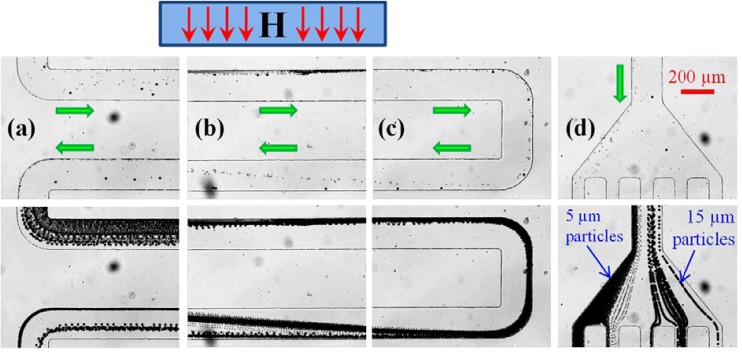
Figure 3.
Continuous sheath-free magnetic separation of 5 μm and 15 μm polystyrene particles in 0.01 × EMG 408 ferrofluid through a U-shaped microchannel: snapshot (top row) and streak (bottom row) images of particles motions at the entrance/exit of the U-turn (a), the magnet region (b), the U-turn (c), and the channel outlet (d). The average flow speed is 0.7 mm/s. The box with an inside letter “H” on the top of the figure indicates the location and magnetization direction of the permanent magnet (not drawn to scale). The block arrows indicate the particle moving directions.
To demonstrate the proposed magnetic separation method, 5 μm and 15 μm polystyrene (inherently a diamagnetic material31) particles from Sigma-Aldrich were mixed and re-suspended in 0.01 × EMG 408 ferrofluid (Ferrotec Corp.) to a final concentration of about 107 particles per milliliter. The dilute ferrofluid was prepared by mixing the original EMG 408 ferrofluid with pure water at a volume ratio of 1:99. The particle solution was introduced only to the inlet reservoir, which was formed by inserting a 1-ml pipette tip into the PDMS slab. The outlet reservoir was intentionally made large to minimize the back-flow effects due to fluid build-up and was emptied prior to experiment. Particle motion was visualized using an inverted microscope (Nikon Eclipse TE2000U, Nikon Instruments, Lewisville, TX) under a bright-field illumination. Digital videos (at a time rate of around 12 frames per second) and images were recorded through a CCD camera (Nikon DS-Qi1Mc), and post-processed using the Nikon imaging software (NIS-Elements AR 2.30).
RESULTS AND DISCUSSION
Figure 3 demonstrates the continuous sheath-free magnetic separation of 5 μm and 15 μm polystyrene particles in 0.01 × EMG 408 ferrofluid through the U-shaped microchannel. Both snapshot (top row) and streak (bottom row) images are presented in each of the four observation windows along the flow direction, which are (a) the entrance/exit of the U-turn, (b) the channel section closest to the magnet (a little shift to its back edge), (c) the U-turn, and (d) the channel outlet, respectively. The average flow speed of the particle suspension is 0.7 mm/s, which was estimated by tracking individual particles in the inlet section of the microchannel that is distant from the magnet. The particle streak images were obtained by superimposing a sequence of more than 200 snapshot images.
As seen from Figure 3a, the two sizes of particles enter into the top branch of the U-turn in a mixed state but tend to move towards the channel edge that is closer to the magnet (more obvious in the streak image, bottom row). This is a consequence of positive magnetophoresis, which is astonishing because polystyrene particles have been previously found to behave diamagnetic in ferrofluids by multiple research groups,32, 33, 34, 35, 36, 37 including ours.30, 38, 39 Such a counter-intuition phenomenon becomes much more obvious in Figure 3b, where the particle mixture migrates in a focused stream adjacent to the channel sidewall nearer to the magnet as a result of the positive magnetophoretic deflection. We speculate that these observed “pseudo-magnetic” polystyrene particles are formed due to the attachment of magnetic nanoparticles (the content of the suspending ferrofluid) onto their surfaces, which has been reported in a recent experiment.40
The magnetically focused 5 μm and 15 μm particles travel through the U-turn along its outer corner in a single stream as seen in Figure 3c. However, in the same figure one can see that 5 μm particles start being deflected by positive magnetophoresis towards the inner corner (where the magnetic field is higher) soon after the U-turn. In contrast, the trajectories of 15 μm particles remain nearly unaffected due to their slower magnetophoretic motion. The reason behind this inversely proportional dependence of magnetophoresis on particle size [which is against Eq. 1] is unclear at this moment. It might be a consequence of the mutual effects from both the particle magnetization and the particle size. As the particle mixture moves into the magnet region in Figure 3b, the displacement gap between the two particle streams grows almost linearly with the traveling distance. This separation achieves the maximum in Figure 3a before particles exit the U-turn, where 5 μm and 15 μm particles have been fully and half-way deflected across the channel width, respectively. As a result, the two sizes of particles flow into the outlet reservoir through distinct passages as demonstrated in Figure 3d.
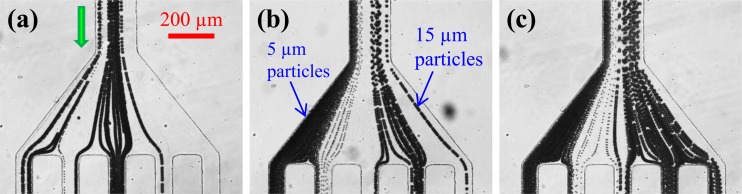
Figure 4 demonstrates the ferrofluid flow effects on the magnetic separation of 5 μm and 15 μm polystyrene particles in 0.01 × EMG 408 ferrofluid through the U-shaped microchannel. Illustrated are the particle streak images at the channel outlet under the average flow speed of 0.4 mm/s (a), 0.7 mm/s (b), and 1.0 mm/s (c). Note that the image in Figure 4b is identical to that shown in Figure 3d. At a slower flow than that in Figure 4b, 15 μm particles are exposed to the magnetic deflection for a longer time and can thus be displaced more than half of the channel width. This makes 15 μm particles partially overlap with 5 μm particles that are still fully deflected, leading to an incomplete separation of these two types of particles as seen in Figure 4a. It is important to note that 5 μm particles can get trapped in the first branch of the U-turn at low ferrofluid flow speeds. This turns out to be a practical concern for the separation of magnetic particles in a diamagnetic solution because they are expected to experience a stronger attractive magnetic force than do the observed “pseudo-magnetic” particles. Therefore, there is a risk that magnetic particles will be captured on the magnet rather than being focused in the flow. As a consequence, the design of the magnet-channel distance and the tuning of the suspension flow rate both become critical, leading to a potentially small window of parameters where the proposed magnetic separation approach can be put into use. However, we believe that this issue can be mitigated by the use of a long magnet that is placed further away from the U-turn. With this change, the magnetic force is reduced (and hence the particle trapping is diminished) while the particle residence time within the magnet region is increased. Similarly at a faster ferrofluid flow than that in Figure 4b, 5 μm particles also get mixed with 15 μm particles due to their decreased magnetic deflection, which again makes the separation incomplete as shown in Figure 4c.
Figure 4.
Streak images illustrating the ferrofluid flow effects on the magnetic separation of 5 μm and 15 μm polystyrene particles in 0.01 × EMG 408 ferrofluid through a U-shaped microchannel at the average flow speed of 0.4 mm/s (a), 0.7 mm/s (b), and 1.0 mm/s (c). The block arrow in (a) indicates the particle moving direction.
CONCLUSIONS
We have developed a new microfluidic approach to continuous-flow sheath-free magnetic separation of particles in a U-shaped microchannel. This approach is applicable to both magnetic and diamagnetic particles, and has been demonstrated through a continuous separation of unequal-sized polystyrene particles in a diluted ferrofluid. While the reasons behind the observed “pseudo-magnetic” phenomena of polystyrene particles are still unclear, the principle of the proposed magnetic separation approach has been verified. We are working on the optimization of the U-shaped microchannel and will use it to separate both real magnetic particles in diamagnetic solutions and real diamagnetic particles in magnetic solutions. As neither magnetic nor fluorescent labeling is needed in the demonstrated separation experiment, this approach is anticipated to find applications in label-free handling of biological particles.
ACKNOWLEDGMENTS
This work was supported by NSF under Grant CBET-1150670.
References
- Pethig R., Biomicrofluidics 4, 022811 (2010). 10.1063/1.3456626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin B. and Li D., Electrophoresis 32, 2410–2427 (2011). 10.1002/elps.201100167 [DOI] [PubMed] [Google Scholar]
- Pamme N., Lab Chip 6, 24–38 (2006). 10.1039/b513005k [DOI] [PubMed] [Google Scholar]
- Gijs M. A. M., Lacharme F., and Lehmann U., Chem. Rev. 110, 1518–1563 (2010). 10.1021/cr9001929 [DOI] [PubMed] [Google Scholar]
- Laurell T., Peterson F., and Nilsson A., Chem. Soc. Rev. 36, 492–506 (2007). 10.1039/b601326k [DOI] [PubMed] [Google Scholar]
- Lin S., Mao X., and Huang T., Lab Chip 12, 2766–2770 (2012). 10.1039/c2lc90076a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. H., Godin J. M., Chen C., Qiao W., Lee H., and Lo Y., Biomicrofluidics 4, 043001 (2010). 10.1063/1.3511706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayani A. A., Khoshmanesh K., Ward S. A., Mitchell A., and Kalantar-zadeh K., Biomicrofluidics 6, 031501 (2012). 10.1063/1.4736796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamme N., Lab Chip 7, 1644–1659 (2007). 10.1039/b712784g [DOI] [PubMed] [Google Scholar]
- Lenshof A. and Laurell T., Chem. Soc. Rev. 39, 1203–1217 (2010). 10.1039/b915999c [DOI] [PubMed] [Google Scholar]
- Bhagat A. A. S., Bow H., Hou H., Tan S., Han J., and Lim C., Med. Biol. Eng. Comput. 48, 999–1014 (2010). 10.1007/s11517-010-0611-4 [DOI] [PubMed] [Google Scholar]
- Gossett D. R., Weaver W. M., Mach A. J., Hur S. C., Tse H. T., Lee W., Amini H., and Di Carlo D., Anal. Bioanal. Chem. 397, 3249–3267 (2010). 10.1007/s00216-010-3721-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijs M. A. M., Microfluid. Nanofluid. 1, 22–40 (2004). 10.1007/s10404-004-0010-y [DOI] [Google Scholar]
- Liu C., Stakenborg T., Peeters S., and Lagae L., J. Appl. Phys. 105, 102014 (2009). 10.1063/1.3116091 [DOI] [Google Scholar]
- Suwa M. and Watarai H., Anal. Chim. Acta 690, 137–147 (2011). 10.1016/j.aca.2011.02.019 [DOI] [PubMed] [Google Scholar]
- Ramadan Q. and Gijs M. A. M., Microfluid. Nanofluid. (in press). 10.1007/s10404-012-1041-4 [DOI]
- Inglis D. W., Riehn R., Austin R. H., and Sturm J. C., Appl. Phys. Lett. 85, 5093–5095 (2004). 10.1063/1.1823015 [DOI] [Google Scholar]
- Adams J. D., Kim U., and Soh H. T., Proc. Natl. Acad. Sci. 105, 18165–18170 (2008). 10.1073/pnas.0809795105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. D., Thevoz P., Bruus H., and Soh H. T., Appl. Phys. Lett. 95, 254103 (2009). 10.1063/1.3275577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamme N. and Manz A., Anal. Chem. 76, 7250–7256 (2004). 10.1021/ac049183o [DOI] [PubMed] [Google Scholar]
- Tarn M. D., Peyman S. A., Robert D., Iles A., Wilhelm C., and Pamme N., J. Magn. Magn. Mater. 321, 4115–4122 (2009). 10.1016/j.jmmm.2009.08.016 [DOI] [Google Scholar]
- Robert D., Pamme N., Conjeaud H., Gazeau F., Iles A., and Wilhelm C., Lab Chip 11, 1902–1910 (2011). 10.1039/c0lc00656d [DOI] [PubMed] [Google Scholar]
- Peyman S. A., Kwan E. Y., Margarson O., Iles A., and Pamme N., J. Chromatogr. A 1216, 9055–9062 (2009). 10.1016/j.chroma.2009.06.039 [DOI] [PubMed] [Google Scholar]
- Zhu T., Marrero F., and Mao L., Microfluid. Nanofluid. 9, 1003–1009 (2010). 10.1007/s10404-010-0616-1 [DOI] [Google Scholar]
- Zhu T., Cheng R., Lee S. A., Rajaraman E., Eiteman M. A., Querec T. D., Unger E. R., and Mao L., Microfluid. Nanofluid. (in press). 10.1007/s10404-012-1004-9 [DOI] [PMC free article] [PubMed]
- Friedman G. and Yellen B., Curr. Opin. Colloid Interface Sci. 10, 158–166 (2005). 10.1016/j.cocis.2005.08.002 [DOI] [Google Scholar]
- Erb R. M. and Yellen B., in Nanoscale Magnetic Materials and Applications, edited by Liu J. P. (Springer, 2009), pp. 563–590. [Google Scholar]
- Liang L., Ai Y., Zhu J., Qian S., and Xuan X., J. Colloid Interface Sci. 347, 142–146 (2010). 10.1016/j.jcis.2010.03.039 [DOI] [PubMed] [Google Scholar]
- Zhu J., Liang L., and Xuan X., Microfluid. Nanofluid. 12, 65–73 (2012). 10.1007/s10404-011-0849-7 [DOI] [Google Scholar]
- Liang L. and Xuan X., Microfluid. Nanofluid. (in press). 10.1007/s10404-012-1003-x [DOI]
- Mirica K. A., Shevkoplyas S. S., Phillips S. T., Gupta M., and Whitesides G. M., J. Am. Chem. Soc. 131, 10049–10058 (2009). 10.1021/ja900920s [DOI] [PubMed] [Google Scholar]
- Erb R. M. and Yellen B. B., J. Appl. Phys. 103, 07A312 (2008). 10.1063/1.2831789 [DOI] [Google Scholar]
- Kose A., Fischer B., Mao L., and Koser H., Proc. Natl. Acad. Sci. 106, 21478–21483 (2009). 10.1073/pnas.0912138106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Cheng R., and Mao L., Microfluid. Nanofluid. 11, 695–701 (2011). 10.1007/s10404-011-0835-0 [DOI] [Google Scholar]
- Li K. H. and Yellen B. B., Appl. Phys. Lett. 97, 083105 (2010). 10.1063/1.3483137 [DOI] [Google Scholar]
- Zhu T., Lichlyter D. J., Haidekker M. A., and Mao L., Microfluid. Nanofluid. 10, 1233–1245 (2011). 10.1007/s10404-010-0754-5 [DOI] [Google Scholar]
- Kose A. R. and Koser A., Lab Chip 12, 190–196 (2012). 10.1039/c1lc20864k [DOI] [PubMed] [Google Scholar]
- Liang L., Zhu J., and Xuan X., Biomicrofluidics 5, 034110 (2011). 10.1063/1.3618737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., Chen C., Vedantam P., Brown V., Tzeng T., and Xuan X., J. Micromech. Microeng. 22, 105018 (2012). 10.1088/0960-1317/22/10/105018 [DOI] [Google Scholar]
- Benelmekki M., Martinez Ll. M., Andreu J. S., Camachod J., and Faraudo J., Soft Matter 8, 6039–6047 (2012). 10.1039/c2sm25243k [DOI] [Google Scholar]