Abstract
CC chemokine ligand 2 (CCL2) is the most potent monocyte chemoattractant and inter-individual differences in its expression level have been associated with genetic variants mapping to the cis-regulatory regions of the gene. An A to G polymorphism in the CCL2 enhancer region at position –2578 (rs1024611; A>G), was found in most studies to be associated with higher serum CCL2 levels and increased susceptibility to a variety of diseases such as HIV-1 associated neurological disorders, tuberculosis, and atherosclerosis. However, the precise mechanism by which rs1024611influences CCL2 expression is not known. To address this knowledge gap, we tested the hypothesis that rs1024611G polymorphism is associated with allelic expression imbalance (AEI) of CCL2. We used haplotype analysis and identified a transcribed SNP in the 3′UTR (rs13900; C>T) can serve as a proxy for the rs1024611 and demonstrated that the rs1024611G allele displayed a perfect linkage disequilibrium with rs13900T allele. Allele-specific transcript quantification in lipopolysaccharide treated PBMCs obtained from heterozygous donors showed that rs13900T allele were expressed at higher levels when compared to rs13900C allele in all the donors examined suggesting that CCL2 is subjected to AEI and that that the allele containing rs1024611G is preferentially transcribed. We also found that AEI of CCL2 is a stable trait and could be detected in newly synthesized RNA. In contrast to these in vivo findings, in vitro assays with haplotype-specific reporter constructs indicated that the haplotype bearing rs1024611G had a lower or similar transcriptional activity when compared to the haplotype containing rs1024611A. This discordance between the in vivo and in vitro expression studies suggests that the CCL2 regulatory region polymorphisms may be functioning in a complex and context-dependent manner. In summary, our studies provide strong functional evidence and a rational explanation for the phenotypic effects of the CCL2 rs1024611G allele.
Introduction
The CCL2(MCP-1)-CCR2 axis plays a pivotal role in monocyte-macrophage trafficking to sites of inflammation and has been implicated in the pathogenesis of various disease processes such as cardiovascular disease, diabetic nephropathy, rheumatoid arthritis, and several infectious diseases [1], [2], [3], [4], [5], [6], [7], [8], [9]. CCL2 expression levels among individuals are highly variable and this variability may contribute to differential susceptibility to various inflammatory disease states [10], [11]. Such variability in CCL2 expression levels have been ascribed to polymorphisms in the regulatory regions of the CCL2 gene [10], [11], [12], [13], [14], [15], [16], [17], [18]. In addition, polymorphisms in the TNF-LTA locus [19] and a non-synonymous polymorphism in the Duffy Antigen Receptor for Chemokines (DARC) [20] have also been shown to influence serum CCL2 levels.
Rovin et al. initially reported regulatory region polymorphism in the CCL2 and they found that a polymorphism annotated as rs1024611 (dbSNP database; originally designated as –2518G or –2578G) is associated with increased CCL2 expression [16]. It was subsequently demonstrated that the rs1024611G allele is associated with increased serum CCL2 levels and enhanced leukocyte recruitment to the tissues [10]. Studies emanating from several laboratories confirmed these earlier studies and rs1024611G allele was implicated in increased CCL2 expression levels in serum, plasma, urine and CSF in normal as well as in pathological conditions [11], [12], [13], [21], [22] and in tissues such as liver and skin [14], [18]. However, a number of studies failed to detect rs1024611G allele association with increased serum CCL2 levels [23], [24], [25]. A genome-wide association study (GWAS) that examined protein quantitative trait loci (pQTLs) in serum or plasma from 1200 individuals showed nominal evidence for association of rs1024611 with CCL2 expression levels, although this association did not reach genome-wide significance [26]. It has also been speculated that the increased CCL2 expression from the rs1024611G bearing allele is more pronounced under pro-inflammatory conditions [12].
Cohort-based association studies during the past few years have ascribed a deleterious role to rs1024611G allele with some exceptions. It has been implicated in a myriad of diseases including HIV-1 dementia [10], myocardial infarction and carotid atherosclerosis [11], [27], pulmonary tuberculosis [28] among others (Table 1). However, several other studies have indicated that rs1024611 polymorphism may not play a role in CCL2 expression levels and disease pathogenesis ([25] and Table 1) and it has been argued that most of the earlier studies that looked at the CCL2 expression levels have not taken multiple comparisons into account for their analyses [25]. Thus it is critically important to further dissect the effects of the rs1024611G allele on CCL2 expression and provide a mechanistic basis for its phenotypic effects.
Table 1. Disease associations of the CCL2 rs1024611 polymorphism.
| Disease | Association | Reference |
| Carotid Intima-Media Thickness (IMT) | Yes | [27], [66], [67], [68] |
| No | [17], [69] | |
| Atherosclerosis | Yes | [27], [70] |
| No | [44] | |
| Myocardial Infarction | Yes | [11] |
| No | [71] | |
| Coronary Artery Disease Risk | Yes | [72] |
| No | [25] | |
| Pulmonary Tuberculosis | Yes | [28], [54], [55] |
| No | [56], [57], [58] | |
| Acute Pancreatitis | Yes | [73] |
| Systemic Lupus Erythematosus | Yes | [74] |
| No | [75], [76], [77] | |
| Lupus Nephritis | Yes | [78], [79] |
| No | [80] | |
| Asthma | Yes | [81], [82] |
| No | [83] | |
| Hepatic Inflammation and fibrosis | Yes | [18] |
| Type II diabetes | Yes | [84] |
| No | [85] | |
| Metabolic Syndrome | Yes | [86] |
| Systemic Sclerosis | Yes | [14] |
| No | [87], [88] | |
| Crohn’s Disease | Yes | [89] |
| HIV-1 disease susceptibility | Yes | [10], [90] |
| HIV-1 Associated Neurological Disorders | Yes | [10] |
| Alzheimer’s Disease | Yes | [91] |
| No | [13], [92], [93] | |
| Breast Cancer | Yes | [94] |
| Atopic eczema and dermatitis | No | [95] |
| Hepatitis C infection | Yes | [18] |
| No | [96] | |
| Parkinson’s Disease | No | [92] |
Previous studies have suggested that the rs1024611 polymorphism mediated its effects via differential binding of various transcription factors and altered transcriptional activity [10], [16], [29], [30], [31]. Reporter assays conducted by various labs to determine differences in the transcriptional strength conferred by this polymorphism yielded variable results. Rovin et al. reported that the –2578G SNP increased transcriptional activity of the CCL2 distal enhancer [16]. By contrast, other studies suggested a reduced transcriptional activity associated with this SNP [23], [30]. One caveat of the aforementioned experiments is that these studies fail to take into consideration the potential role of the linked polymorphisms on transcription factor binding as well as transcriptional activity. Thus the functional basis of interindividual variation in CCL2 expression is complex and remains unresolved.
A powerful way to detect allelic differences in expression is to quantify the expression of transcripts derived from each individual allele in a heterozygous state [32], [33], [34]. This approach is based on the premise that in the absence of genetic or epigenetic variation there will be equal amount of expression from both the maternal and paternal alleles as they are exposed to identical intracellular environment. By contrast, individuals heterozygous for cis-acting polymorphisms that affect gene expression or mRNA processing or differences in their epigenetic signatures will show an altered level of mRNA expression originating from one allele compared with its partner allele. This phenomenon referred to as allelic expression imbalance (AEI) can serve as an integrative quantitative measure of effects of cis-acting and epigenetic variation [34]. We therefore hypothesized that the rs1024611G allele which is associated with strong phenotypic effects may exhibit increased expression in a heterozygous state. For this purpose, we performed extensive haplotype analysis of the CCL2 locus that identified a polymorphism in its transcribed region that showed complete linkage disequilibrium with rs1024611 that we successfully employed to elucidate AEI of CCL2. Our results conclusively demonstrate that haplotype containing rs1024611G allele is associated with increased expression in a heterozygous state providing a strong validation for its biological effects seen in cohort based studies.
Results
A Transcribed SNP can Serve as a Proxy for rs1024611
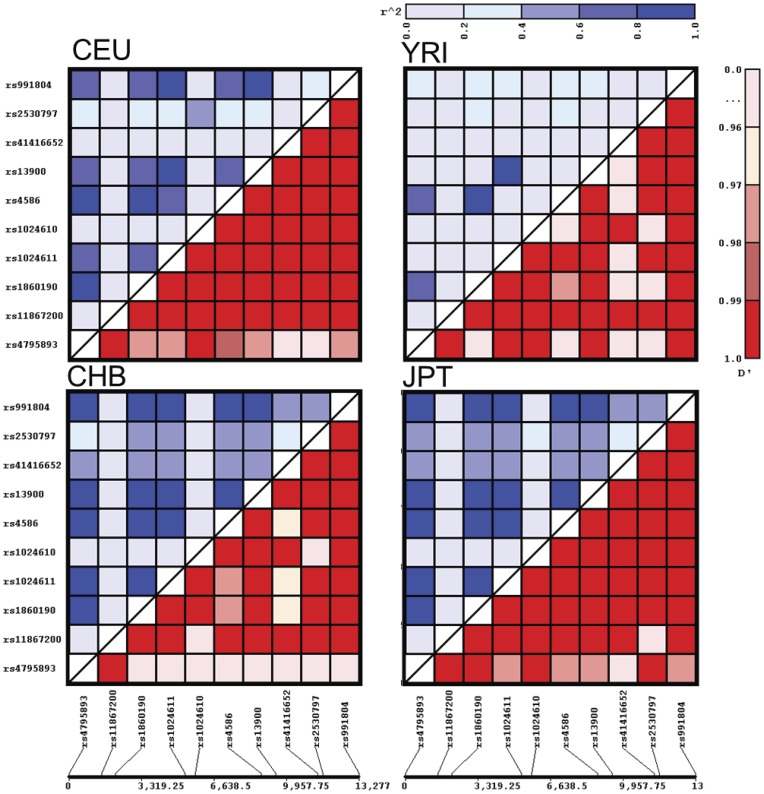
The rs1024611 polymorphism is located in the CCL2 enhancer region and thus cannot be used to assess differential expression of the transcribed alleles. To identify any possible SNPs in the transcribed region that can serve as a proxy for this polymorphism, we generated linkage disequilibrium (LD) maps of the CCL2 locus. For this, we used publicly available SNP information from a region that spanned a 25 kb region across the rs1024611 polymorphism (www.hapmap.org) [35]. Genotype data from Utah residents with Northern and Western European ancestry from the CEPH collection (CEU), Han Chinese from Beijing, China (CHB), Japanese from Tokyo (JPT), and Yoruban from Ibadan, Nigeria (YRI) was used for creating the LD map that included CCL2 far upstream region, enhancer, promoter, open reading frame, introns, 3′UTR and 3′-flanking region. Data was analyzed and visualized by using JLIN program and only polymorphisms that are shared by all the populations examined were included in the analysis (Fig. 1). The upper half of each LD plot for each denoted population that is depicted in various shades of blue represents the r2 (R-square) which is square of the correlation coefficient of a given marker pair. An r2 equal to 1 indicates complete pairwise LD between the markers in question. The lower-half of each LD plot represents the D′ (D-prime) which indicates normalized covariance for a given marker pair and values close to 1 indicate strong LD. Chromosomal position and location of the genetic variants used to generate the LD profiles for the Hapmap populations that are depicted in Fig. 1 are shown in Table 2. All the SNPs examined did not deviate from the expected Hardy-Weinberg proportions in each population that was analyzed (Table 3). The highest frequency of the rs1024611G allele was observed among Asian ancestry populations (CHB and JPT) followed by European ancestry CEU population. The African ancestry population showed the lowest frequency. We observed a strong high LD between rs1024611 and several other polymorphisms in CCL2 with r2 close to 1 (Table 4). The highest r2 values were observed for rs13900 and rs991804 (Table 4). Of note, the SNP at rs13900 maps to the transcribed region of the CCL2. Both the pairwise r2 and D′ values for rs1024611, rs13900 and rs991804 was equal to 1, suggesting that they can act as proxies for each other.
Figure 1. Linkage disequilibrium plots for the shared polymorphisms in the CCL2 locus.
In all, 9 different polymorphisms that are located within a 25 kb region that spans the rs1024611 polymorphism are shown. The heatmaps display pairwise r2 (above diagonal) or D’ (below diagonal) for the each pair of polymorphisms. The relative physical distance between the markers is shown in the bottom of the plots. Populations shown are Yoruba in Ibadan, Nigeria (YRI), Japanese in Tokyo, Japan (JPT), Han Chinese in Beijing, China (CHB) and CEPH (Utah residents with ancestry from northern and western Europe, CEU) populations (HapMap data release #28 August 2010 on Build 36). Only unrelated individuals were used for the analysis. LD maps were constructed using the JLIN program [61].
Table 2. Chromosomal positions and genomic location of the CCL2 locus HapMap SNPs.
| SNP ID | Chr. Position(hg18) | Location | Variation | AncestralAllele |
| rs4795893 | 29598561 | Inter-genic | G/A | A |
| rs11867200 | 29600082 | Enhancer | C/T | C |
| rs1860190 | 29600686 | Enhancer | T/A | T |
| rs1024611 | 29603901 | Enhancer | A/G | A |
| rs1024610 | 29604344 | Enhancer | A/T | T |
| rs4586 | 29607382 | cds-synonymous | C/T | C |
| rs13900 | 29608024 | 3′UTR | C/T | C |
| rs41416652 | 29609974 | Inter-genic | T/C | T |
| rs2530797 | 29610207 | Inter-genic | A/G | A |
| rs991804 | 29611838 | Inter-genic | A/G | G |
CCL2, CC Chemokine ligand 2; SNP, Single nucleotide polymorphism; cds-coding sequence; hg18 corresponds to NCBI build 36.1 (Release Date Mar. 2006). Ancestral allele shown is from the dBSNP database (www.ncbi.nlm.nih.gov/projects/SNP/ ).
Table 3. Relative minor allele frequencies of CCL2 HapMap SNPs in different populations.
| SNP ID | MAF(HWEp) | |||
| CEU(n = 112) | CHB(n = 84) | JPT(n = 86) | YRI(n = 116) | |
| rs4795893 | 0.6429(0.41) | 0.3735(0.82) | 0.3779(0.65) | 0.3147(0.195) |
| rs11867200 | 0.2232(0.79) | 0.0952(0.15) | 0.0756(1.0) | 0.0086(0.99) |
| rs1860190 | 0.6518(0.40) | 0.3750(1.0) | 0.3663(1.0) | 0.2414(0.46) |
| rs1024611 | 0.2991(0.04) | 0.6250(1.0) | 0.6279(0.49) | 0.1983(0.39) |
| rs1024610 | 0.1830(0.99) | 0.0536(1.0) | 0.0814(1.0) | 0.0517(1.0) |
| rs4586 | 0.6473(0.54) | 0.3750(1.0) | 0.3706(0.49) | 0.2414(0.46) |
| rs13900 | 0.2991(0.04) | 0.2991(1.0) | 0.6279(0.49) | 0.2026(0.56) |
| rs41416652 | 0.9777(0.99) | 0.5060(0.99) | 0.5174(0.28) | 0.9911(0.99) |
| rs2530797 | 0.6518(0.84) | 0.8012(0.99) | 0.7674(0.03) | 0.8922(0.36) |
| rs991804 | 0.2991(0.04) | 0.6310(0.99) | 0.6279(0.49) | 0.4267(0.13) |
The minor allele frequency as well as the Hardy-Weinberg equilibrium p-values (in parenthesis) for the different SNPs in each population are indicated.
CCL2, CC chemokine ligand 2; SNP, Single nucleotide polymorphism; CEU, Utah residents with Northern and Western European ancestry from the CEPH collection (CEPH, Centre du Etude Polymorphisme Humain); CHB, Han Chinese from Beijing, China; JPT, Japanese from Tokyo, Japan; YRI, Yoruban from Ibadan, Nigeria; the numbers in parenthesis next to the each population group show the genotyped individuals included in the analysis.
Table 4. Linkage disequilibrium between rs1024611 and other SNPs in the CCL2 locus.
| SNP ID | Distance (bp) | r2 |
| rs4795893 | −5340 | 0.73 |
| rs11867200 | −3819 | 0.124 |
| rs1860190 | −3215 | 0.792 |
| rs1024610 | +443 | 0.093 |
| rs4586 | +3481 | 0.778 |
| rs13900 | +4123 | 1.0 |
| rs41416652 | +6173 | 0.0535 |
| rs2530797 | +6306 | 0.231 |
| rs991804 | +7937 | 1.0 |
Single nucleotide polymorphism, SNP; bp, basepairs; CCL2, CC chemokine ligand 2; r2, square of the correlation coefficient between rs1024611 and the indicated SNP.
Haplotype analysis using these ten polymorphisms revealed haplotypes that are shown in Table 5. A total of 22 haplotypes were predicted in all the populations of which only 10 had a greater than 2% frequency in any given population. Haplotype 3 (h3), h4, and h11 contained the rs1024611G allele (Table 5). The frequency of the haplotype(s) bearing the rs1024611G allele in the East Asian population was greater than 60% whereas in CEU the frequency was 28%. Interestingly, h4 was more frequent in CHB and JPT whereas h3 was more common in CEU and YRI populations. h3, h4, h13, and h18 contained the rs13900, but as noted the frequency of both h13 and h18 is<1% (Table 5). Thus rs13900 is mainly restricted to the rs1024611G-bearing h3 and h4. In addition, both h3 and h4 contain the rs1860190T allele which is in the far upstream region which they share with both h16 and h17 that are restricted to the YRI population. Our results are in general agreement with previously reported haplotypes of the CCL2 locus [36] and their frequency [11].
Table 5. Inferred haplotypes in the CCL2 locus and their frequency.
| Haplotype | SNP ID | Hapmap population and haplotype frequencies | ||||||||||||
| rs4795893 | rs11867200 | rs1860190 | rs1024611 | rs1024610 | rs4586 | rs13900 | rs41416652 | rs2530797 | rs991804 | CEU(n = 224) | CHB(n = 164) | JPT(n = 170) | YRI(n = 222) | |
| h1 | G | T | A | A | A | T | C | C | G | G | 0.22 (50) | 0.01(16) | 0.07(12) | 0.01(2) |
| h2 | G | C | A | A | T | T | C | C | A | G | 0.18(41) | 0.04(7) | 0.08(14) | 0.02(4) |
| h3 | A | C | T | G | A | C | T | C | G | A | 0.28(62) | 0.13(22) | 0.15(25) | 0.19(43) |
| h4 | A | C | T | G | A | C | T | T | G | A | 0.02(4) | 0.48(78) | 0.47(80) | 0 (0) |
| h5 | A | C | T | A | A | C | C | C | G | G | 0.05(11) | 0 (0) | 0 (0) | 0.25(56) |
| h6 | G | C | A | A | A | C | C | C | A | G | 0.16(36) | 0.15(24) | 0.15(25) | 0.08(17) |
| h9 | G | C | A | A | A | T | C | C | G | G | 0.07(16) | 0.07(12) | 0.06(11) | 0.09(20) |
| h15 | G | C | A | A | A | T | C | C | G | G | 0 (0) | 0 (0) | 0 (0) | 0.03(7) |
| h16 | G | C | T | A | A | C | C | C | G | G | 0 (0) | 0 (0) | 0 (0) | 0.08(17) |
| h17 | A | C | T | A | A | C | C | C | G | A | 0 (0) | 0 (0) | 0 (0) | 0.22(49) |
| Anc. | A | C | T | A | T | C | C | T | A | G | ||||
Only haplotypes with frequency greater than 2% in any population are shown. Data were downloaded from the Hapmap web site (http://hapmap.ncbi.nlm.nih.gov/) and haplotypes inferred using the ARLEQUIN program. Only the reference polymorphisms that were shared by all the population groups examined were used to generate the haplotypes. Population description is as in Table 3. The numbers within the parenthesis indicate the number of individuals (n). The h18, h19, h20, h21, h22 were present exclusively in the YRI each with a frequency of 0.0045. h7 and h8 were present exclusively in CEU each with a frequency of 0.0045. h12 and h13 were present exclusively in CHB each with a frequency of 0.0061. h14 was exclusively present in JPT at a frequency of 0.0059. The haplotype frequency of h10 was 0.005 in CEU, 0.006 in CHB, and 0.009 in YRI. The haplotype frequency of h11 was 0.005 in CEU, 0.012 in CHB, and 0.012 in JPT. The ancestral state of the reference SNPs (Anc.) was obtained from the dBSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/).
Validation of Pyrosequencing Technique to Verify AEI of CCL2
Our LD analysis of CCL2 locus suggested that rs13900 C/T alleles can serve as a proxy for the rs1024611 A/G, respectively and could be targeted to assess allele-specific differences in expression (Fig. 2A). Pyrosequencing has been used extensively used to assess AEI for several genes [37] and we adapted this technique to assess the relative contribution of each allele to CCL2 transcript levels in heterozygous donors. The expression level of a specific allele as detected during pyrosequencing is reflected as the height of peak corresponding to that specific nucleotide in the pyrogram. We validated the pyrosequencing technique for the rs13900 to verify that the peaks in the pyrogram reflect the actual proportions of the C and T variants. For this we cloned CCL2 3′UTR from the donors who were homozygous for rs13900 C or T variants. We mixed the plasmids at specific concentrations and subjected the mixtures to PCR amplification and pyrosequencing (Fig. 2B). No or minimal amplification was detected when only one allele was used for amplification. The peaks of the pyrograms for the %C and %T obtained from the mixtures was in accord with the ratio of the plasmids in the template. To determine if there is a correlation over the range of concentrations tested, we plotted the expected and measured peak percentages and used linear regression analysis. The r2 was>0.99 suggesting that the pyrosequencing assay that we developed is highly quantitative and could be used for accurate assessment of the allelic differences in gene expression (Fig. 2C).
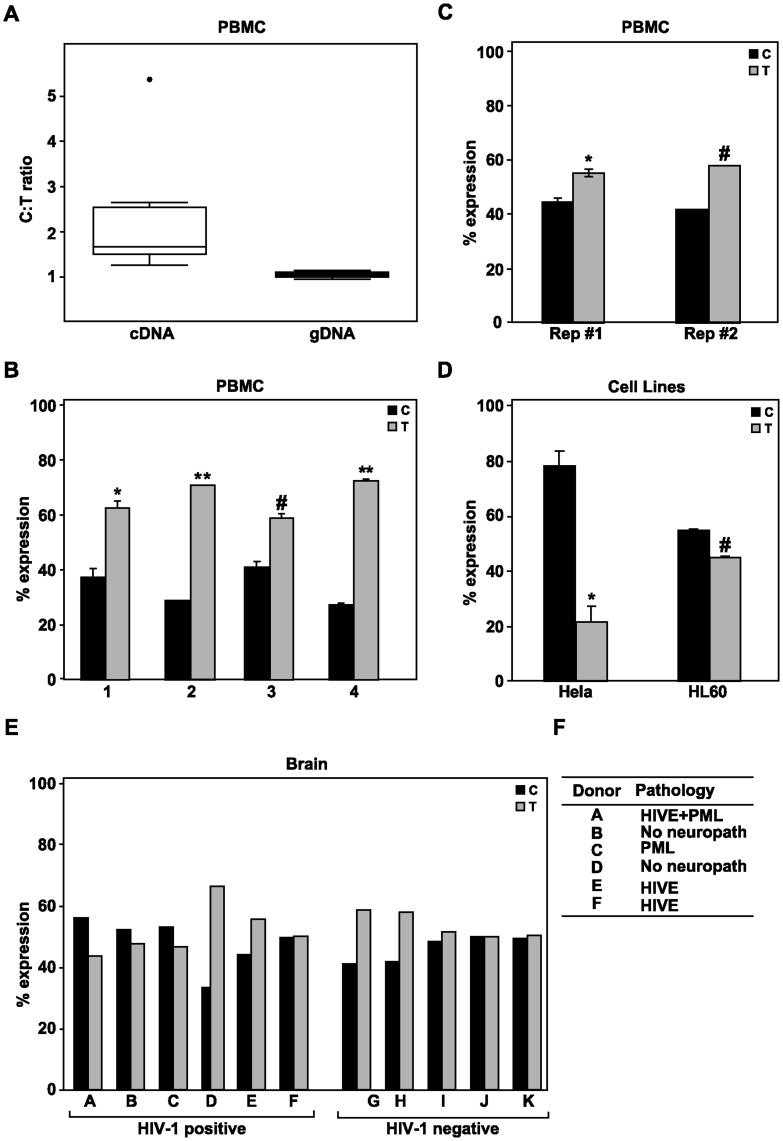
Figure 2. Validation of a pyrosequencing assay to measure AEI at rs13900 polymorphism.
A. Schematic of the CCL2 gene structure and the LD between regulatory region polymorphism rs1024611 and the transcribed polymorphism rs13900. Numbered boxes are exons and the dashed lines connecting the exons are the introns. rs1024611 is located 2578 bp upstream of the CCL2 translational start site and rs13900 is located in the 3′UTR. B. Stacked bar graph represents the levels of the C (black) and T (grey) alleles in PCR products as determined by pyrosequencing. The PCRs were performed on the plasmid mixtures containing rs13900C and rs 13900T allele combined at the indicated ratios to simulate homozygous (10∶0 and 0∶10) and heterozygous samples (5∶5) as well as different allelic levels (other indicated ratios). Data shown is from one representative experiment from three independent experiments which gave similar results. C. Regression analysis of amplification products obtained from the rs13900 C- and rs13900 T-bearing plasmids combined at different proportions. The measured levels of the C allele (y-axis) were plotted against the expected levels (x-axis). There was a near linear relationship between these values (R2 = 0.9969) suggesting that the pyrosequencing can serve as a sensitive assay to measure the levels of rs13900C and rs13900T alleles in heterozygous individuals. Similar results were obtained with using genomic DNA mixtures from homozygous and heterozygous individuals (data not shown).
Assessment of AEI in the CCL2 in Human Primary Cells, Brain, and Transformed Cell Lines
Having established that we can accurately quantify allele-specific expression in CCL2 gene we examined the degree of AEI in individuals who were heterozygous for the rs1024611 polymorphism. Total RNA and genomic DNA were isolated from PBMC from eight healthy human volunteer donors after they were treated with LPS for 3 h to induce CCL2 expression. The induction of CCL2 following lipopolysaccharide treatment was confirmed by qRT-PCR (Fig. S1). We then quantified the levels of the rs13900 C or T alleles in the transcript by subjecting the RT-PCR products to pyrosequencing. As a control, genomic DNA was also amplified from these donors and was subjected to pyrosequencing (Fig. 3A). All the donors who were heterozygous for rs1024611 were also heterozygous for rs13900. Boxplots illustrate striking differences in the detected levels of the C and T alleles in cDNA and gDNA suggesting that the CCL2 is subjected to AEI and that the expression level of the rs13900T allele (that is present in all the haplotypic phases containing rs1024611G) is relatively higher when compared to the allele bearing rs13900C allele (that is present in most haplotypic phases containing rs1024611A), respectively. We further confirmed that the allelic differences in CCL2 expression detected in LPS treated PBMC were also reproducible in similarly treated purified monocytes (Fig. S2).
Figure 3. AEI of CCL2 in PBMC, cell lines and brain.
A. Allelic ratios for cDNA and gDNA were determined by pyrosequencing in eight independent donors who were heterozygous for rs13900 and rs1024611 polymorphisms. RNA was extracted from PBMCs treated with LPS for 3 h. and cDNA was synthesized. Pyrosequencing was performed as described under Methods. The ratios of expression (C vs. T) are log2-transformed and are shown on the y-axis. Statistical significance was determined using a two-tailed Wilcoxon rank sum test (p = 0.0009). B. Stability of allele-specific differences in the CCL2 expression in LPS treated PBMC from heterozygous individuals. AEI was assessed at three different times in 4 independent donors over a period of 4–6 months with a gap of at least 2 weeks between experiments in a single donor. The y-axis indicates the percent level of expression of the C or the T allele. C. AEI in nascent RNA. LPS treated PBMC were cultured in presence of ethylene uridine (EU). The EU RNA was subjected to a click reaction that adds a biotin handle which is then captured by streptavidin beads. cDNA was synthesized from the captured nascent RNA and PCR amplified and subjected to pyrosequencing. Data shown are from two independent biological replicates from a single donor. D. rs13900C allele is expressed at higher levels in heterozygous cell lines. Differential expression of CCL2 alleles in heterozygous cell lines (HeLa- cervical cancer cell line; HL60-myeloid leukemia cell line). cDNA was synthesized using RNA extracted from LPS treated cell lines and PCR and pyrosequencing were performed. E. AEI in brain tissue. RNA was extracted from post-mortem brain tissues obtained from HIV-1 infected and normal donors and the extent of AEI in CCL2 was assessed in heterozygous donors by pyrosequencing. F. Clinical features and pathology associated with the HIV-1 positive donors. HIVE- HIV encephalitis; PML-Progressive Multifocal Leukoencephalopathy; Donors B & D did not exhibit any neuropathology. Statistical significance for differences in the levels of expression between the alleles was calculated using a paired t-test (*, p<0.05, **, p<0.001, #, p<0.0001).
We then asked if these differences in allele expression are stable within an individual over a period of time. To assess this we used three independent samples of RNA from four different donors and determined the relative expression of the rs13900C or rs13900T alleles. Our data suggested that all four donors consistently maintained higher expression of rs13900T allele relative to the C allele suggesting that AEI of CCL2 is highly stable (Fig. 3B). We reasoned that the differences in the expression levels between the two alleles in the heterozygous donors may be due to either transcriptional or post-transcriptional mechanisms. Therefore we tested AEI in newly transcribed RNA that was isolated from PBMC following LPS treatment. Nascent RNA was captured as described under Methods and pyrosequencing was performed to detect the relative levels of rs13900C or rs13900T alleles (Fig. 3C). We found that differences in the expression level of the rs13900T-bearing allele versus the rs13900C-bearing allele is similar to the levels seen in total RNA suggesting that the imbalance in the CCL2 allele expression most likely occurs at the transcriptional level although post-transcriptional mechanisms that alter transcript stability cannot be completely ruled out.
Genome-wide studies have provided rich data for linking polymorphisms with gene expression. We queried the Sanger gene expression database to assess the role of heterozygosity at rs1024611 polymorphism (rs13900 not used as it is not included in the database) in CEU (B-cells), T cells, fibroblasts, and fat tissue [38], [39]. Our analysis of these data indicated that there were no significant differences between the RNA expression levels of homozygous individuals carrying rs1024611A SNP and heterozygous individuals. We also examined the AEI in two heterozygous cell lines HeLa and HL60 and surprisingly we detected increased expression of the C allele relative to T allele (Fig. 3D). Taken together, our results suggest that AEI of CCL2 may be tissue-specific and cell-type specific and as noted before cannot be extrapolated between different studies and therefore need to be cautiously interpreted in the context of the disease pathogenesis.
CCL2 plays a key role in the recruitment of cells of monocyte/macrophage lineage to the brain and our group has previously shown that the presence of rs1024611G allele (–2578G) that leads to increased recruitment of mononuclear phagocytes in tissues is associated with increased risk to HIV-1 associated dementia [10]. Ragin et al. reported that CCL2 is the most prominent correlate of neurological injury in HIV-1 infected patients [40]. This prompted us to assess the extent of AEI in brain tissue obtained from HIV-1 positive and negative donors (Fig. 3E). We found that there was increased expression of rs13900 T in the post-mortem brain tissue obtained two HIV-1 positive donors (donors D & E) and two normal donors (G & H). In other samples the expression level of C allele was slightly higher or both alleles were present at similar levels. This lack of consistent AEI in brain from heterozygous individuals may be attributed to the cellular composition of sampled brain regions or other variables. Nevertheless, these findings suggest that CCL2 gene expression in brain may also be subjected to AEI and potentially could explain the increased CCL2 levels seen in CSF in individuals bearing rs1024611G allele and their increased susceptibility to HIV-1 related neurological disorders.
Mechanistic Basis of AEI in CCL2 Locus
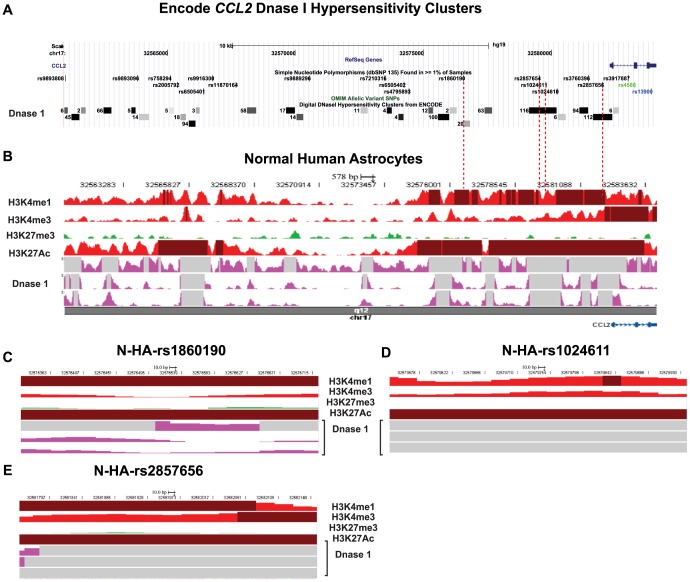
As previously discussed, most of the studies that examined the functional effects of rs1024611 allele have yielded conflicting results. This raised the possibility that other SNPs that are in LD with rs1024611G allele may modulate its expression. Our previous studies on CCL2 gene regulation support such a notion as there is a high degree of sequence as well as functional conservation in the CCL2 far upstream region. Haplotype analysis showed a high r2 between rs1860190 and rs1024611G alleles. rs1860190 is located ∼3.2 kb upstream of rs1024611 and potentially could influence transcriptional activity (Table 4). Two additional polymorphisms that are designated as rs2857654 and rs2857656 are located ∼250 bp upstream and ∼2.2 kb downstream of the rs1024611, respectively, could also be involved in AEI as they have r2>0.9 in CEU population. Please note that the latter two SNPs were not included in the haplotype maps shown in Fig. 1 and Table 5 due to their restricted population distribution.
We used two complementary approaches to determine the functional relevance of the regions that contain the SNPs that are linked to rs1024611. In the first approach, we took advantage of the publicly available whole genome ChIP-Seq and DNaseI hypersensitivity data to examine epigenetic profiles associated with these regions which might provide clues for their functional relevance. Cis-regulatory regions that either have a promoter-like or enhancer-like activity are often characterized by enrichment or depletion of specific post-translational histone modifications and increased DNase I hypersensitivity. For example, the enrichment of histone modification H3K4me3 marks the promoters whereas enrichment of the histone H3K4me1 and depletion of H3K4me3 marks the enhancers. Our analysis revealed the presence of multiple DNAse1 hypersensitivity regions in numerous cell lines as well as specific histone signature patterns in the CCL2 locus (Fig. 4). The polymorphisms rs2857654, rs1024611, and rs2857656 colocalize to regions that exhibit DNase 1 hypersensitivity in>100 cell types (Fig. 4A). The rs1860190 is located in a region that showed moderate level of hypersensitivity in 20 different cell types (Fig. 4A). There was strong enrichment of the H3K4me1 in the regions bearing rs1860190, rs1024611/rs2857654, and rs2857656 in normal human astrocytes (Fig. 4B). In addition, these regions also show strong enrichment of H3K27Ac that is thought to mark active enhancers and distinguish them from poised enhancers (Fig. 4B) [41]. As expected, there was a marked depletion of the H3K27me3 modification which is normally associated with repressive chromatin (Fig. 4). We also found that with a few exceptions the DNAse 1 hypersensitivity in the CCL2 correlates with the levels of H3K4me1 and H3K27Ac in normal human astrocytes (Fig. 4). These findings reaffirmed that several of the SNPs linked to rs1024611 are present in CCL2 regulatory regions that contain epigenetic marks associated with active transcription and could potentially modulate its regulation.
Figure 4. Epigenetic features associated with CCL2 locus in cell lines and normal human astrocytes.
(A–B) Relative location of the SNPs linked to rs13900 (highlighted by red dashed lines) to Encode DNase 1 hypersensitivity sites in the CCL2 locus as depicted in the UCSC genome browser (Panel A) and histone and DNase 1 tracks in the human epigenome browser (Panel B). Nucleotide numbering is according to hg19. The DNase 1 sites in panel A are depicted as boxes. The shade of the box is proportional to the signal strength detected with darker shaded boxes representing increased sensitivity to digestion. The numbers next to the boxes indicate the numbers of cell lines in which the region is hypersensitive. Panel B shows a wiggle plot depicting relative enrichment of the histone activation markers (H3K4me1, H3K4me3, H3K27Ac, indicated in red) and histone repressive marks (H3K27me3, indicated in green) across the CCL2 locus. The heatmap track was configured to set the threshold for the peaks at 20 and values higher than the threshold are shown in brown. Also shown are the tracks for DNAse 1 sensitivity (purple tracks). Regions in gray indicate the regions with higher peaks than the set threshold. (C–E) Heatmaps showing localized histone tracks in CCL2 5′-flanking regions that overlap with the linked polymorphisms. A 500 bp region that spans the indicated polymorphism is shown. No separate panel is shown for rs2857654 due to its proximity to rs1024611. Other details are as in Panel B. The source of the data used for the generation of the DNase 1 tracks is from the Geo accession number GSE29692 (DNaseI Hypersensitivity by Digital DNaseI from ENCODE/University of Washington; public release on June 03, 2011) and for the histone tracks is Geo Accession numbers GSM733763 (H3K27Ac), GSM733729 (H3K27Me), GSM733747 (H3K4me3), and GSM733710 (H3K4me1) deposited by the Bernstein Lab at the Broad Institute (Histone Modifications by ChIP-seq from ENCODE/Broad Institute; public release on Jun 2, 2011). The tracks were generated using ENCODE database and UCSC genome browser [63], [64] and Human epigenome browser [65].
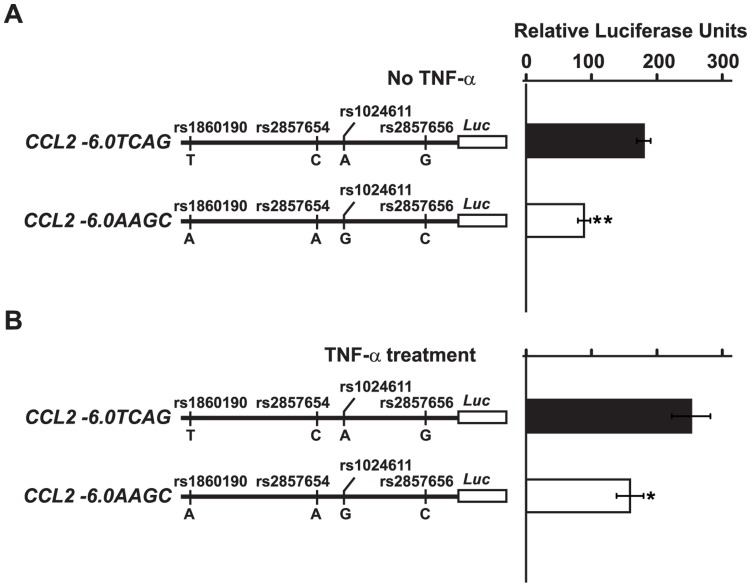
To directly test this hypothesis, we generated haplotype-specific constructs to determine the role of the linked polymorphisms on CCL2 transcriptional strength. Of note, these constructs were generated from a heterozygous donor who exhibited AEI. The reporter constructs spanned ∼ 6.0 kb upstream of the CCL2 transcriptional start site which included the SNPs rs1860190, rs2857654, rs1024611, rs2857656 under basal as well as under stimulatory conditions (Fig. 5). Haplotype-specific reporter constructs were transfected into U87MG which is an astroglioma cell line that expresses CCL2 and has been extensively used previously to study its regulation [42], [43]. Unexpectedly, we found that the reporter vector bearing the rs1024611G allele had a lower transcriptional activity when compared to the reporter vector containing the rs1024611A allele (Fig. 5). We also tested these constructs in normal human astrocytes and found that there was no significant difference in transcriptional strength between these constructs (Fig. S3). Our results suggested that there is discordance between the in vitro reporter assays and in vivo allelic differences in expression.
Figure 5. Transcriptional effects of the cis-regulatory region SNPs that are in LD with rs1024611.
(A–B). On the left is the schematic of the CCL2 haplotype-specific constructs that were examined for differences in transcriptional strength. The following pair of constructs bearing the indicated polymorphisms, CCL2 -6.0TCAG and CCL2 -6.0AAGC (rs1860190T, rs2857654C, rs1024611A, rs2857656G and rs1860190A, rs2857654A, rs1024611G, rs2857656C, respectively), were tested. The constructs were obtained from a single heterozygous donor exhibiting AEI. The constructs were transfected into U87MG astroglioma cells and were tested at basal level (panel A) and following TNF-α treatment (panel B). Luciferase activity was determined as described in the Methods. The relative luciferase units refer to the fold increase in activity obtained from the CCL2 -6.0 constructs relative to that obtained with the promoterless pGL4.16 vector. The data shown were obtained from 7 independent experiments and the error bars indicate the standard error of mean and statistical significance was calculated using two-tailed Student’s t test (**, p<0.0001, *, p = 0.02).
Discussion
Studies in knockout mice as well as overexpression studies have established the preeminent role of CCL2 as a monocyte chemoattractant as well as its non-redundant role during the inflammatory response [4]. The discovery of polymorphisms in CCL2 cis-regulatory region has provided a strong impetus to examine their role in association studies covering a wide spectrum of inflammatory diseases. Remarkably, in contrast to several widely studied polymorphisms, there is strong in vitro and in vivo experimental evidence linking the rs1024611G allele to CCL2 expression levels as well as leukocyte recruitment to the inflammatory sites [10], [14]. However, till date, no unifying molecular mechanism has been proposed that convincingly explains the phenotypic effects of this polymorphism. In this study, we provide a molecular explanation for the phenotypic effects and disease associations of rs1024611G allele and the significance of our findings is discussed further below.
Linkage Disequilibrium in the CCL2 Locus
We have developed an extensive linkage map spanning ∼25 kb region of the CCL2 locus including far upstream region, distal enhancer, and promoter as well as the transcribed region. Most studies till date have focused on a limited span of CCL2 locus for generating the haplotype maps [11], [25], [44]. The extended haplotype maps have allowed us to confirm that the rs1024611G SNP is in complete linkage disequilibrium with the transcribed rs13900 and help identify other potential SNPs in the locus that may be of functional relevance. Our results also agree with other reported haplotype maps that indicated strong LD between rs1024611 and rs13900 [36]. Of note, there was a high degree of linkage between rs1024611G and rs13900T alleles in all the populations examined, suggesting that rs13900C>T can be used to assess AEI in various population groups.
AEI of CCL2
This study provides a striking example of AEI as a powerful means to capture biologically meaningful variability and a functional assay to resolve conflicting phenotypic data. Our results from LPS stimulated PBMC from eight independent donors and repeated measurements from four donors consistently showed a higher expression level of transcript containing rs13900T. These studies also provide a cautionary note about inferring allele-specific differences using expression data from transformed cell lines as we could not detect the same in both HeLa and HL60 cell lines which also show robust CCL2 expression. Several of the genome-wide studies that used transformed lymphoblastoid cells (which have a very low expression levels of CCL2) and primary cells and tissues such as skin, fat, fibroblasts, and T cells also failed to detect significant differences between homozygous rs1024611A and heterozygous donors with respect to CCL2 expression levels [38], [39]. This can be attributed to different tissues sampled and/or the technology platforms used. Johnson et al. examined imbalance of CCL2 expression in heart and monocytes using rs4586 polymorphism in the coding region and detected AEI ratios of 0.67–1.87 [45]. They concluded that AEI observed with rs4586 is incompatible with the heterozygosity at rs1024611. This is not a surprising result because the r2 between rs4586 and rs1024611 is 0.778. While it could be argued that PBMC is a mixed population and cells belonging to multiple lineages could be contributing to CCL2 expression levels and thus to the AEI, monocytes are known to be the most predominant source of CCL2 from PBMC [1], [46]. In support of this, in qRT-PCR experiments to measure CCL2 in sorted cell populations, we found that the Ct values obtained from activated T-cells were>30, whereas the Ct values obtained with LPS treated monocytes were<22. As AEI detection needs robust expression levels [45], we did not further pursue it in fractionated cell populations.
Proinflammatory Conditions and their Contribution to CCL2 AEI
We have used mRNA isolated from LPS treated PBMC for assessing AEI as it is a potent inducer of CCL2 expression in cells of monocytic lineage. We have not evaluated other inducers of CCL2 expression such as IFN-γ and IL-1β in this study. However, studies from other laboratories have shown that increased CCL2 expression is associated with rs1024611G allele under a variety of stimulatory conditions such as IL-1β [12], [16], LPS [12], Mycobacterium tuberculosis antigens [28], and TNF-α [14]. Thus it is plausible that a common signal transduction pathway shared by several of the inflammatory cytokines/stimuli is responsible for differential expression of the allele linked to rs1024611G allele. On the other hand, we cannot completely rule out allele-specific differences in CCL2 expression at basal level in absence of stimulation. However, testing the latter hypothesis will be challenging as we could detect low levels of CCL2 transcripts immediately following PBMC isolation plausibly due to concurrent cellular stimulation (data not shown). Giving credence to the argument that basal conditions are adequate for higher CCL2 expression from the rs1024611G-bearing allele, several studies showed increased serum CCL2 levels in absence of stimulation [10], [12], [17]. Further supporting such a notion, Karrer et al. showed that under basal conditions the CCL2 mRNA expression was four-fold higher in rs1024611 G/G homozygous fibroblasts when compared to A/G heterozygous fibroblasts and ∼eight-fold higher expression levels when compared to A/A homozygous fibroblasts [14]. Nevertheless, our data showing that AEI could be readily detected in the nascent CCL2 transcripts suggests that the allele-specific differences in expression are most likely determined at the transcriptional level.
Transcriptional Basis for CCL2 AEI
Conflicting context-dependent results have been obtained with regard to transcriptional activities associated with reporter vectors containing rs1024611A and G polymorphisms. For example, testing the CCL2 distal enhancer in reporter assays alone led to three different conclusions with regard to the role of rs1024611G polymorphism in determining the transcriptional strength, namely, increase, decrease or no change [16], [23], [30]. Nyquist et al. analyzed the effect of rs2857656C polymorphism that is linked to rs1024611G in reporter assays and found that there was an increase in basal as well as activation induced transcription [47]. However, the construct spanned ∼1.1 kb and did not contain the distal enhancer region. By contrast, Intemann et al. found that a 14 bp intron 1 deletion that occurs in the context of rs1024611G or rs1024611A allele may reduce transcriptional activity [36]. To minimize such context-dependent confounding, we generated constructs that span a 6 kb cis-regulatory region which allowed us to directly compare the role of the linked SNPs on transcriptional strength. To the best of our knowledge, the haplotype-specific constructs tested in this study encompass the longest contiguous human CCL2 cis-regulatory region examined till date. Surprisingly, our results suggested that the rs1024611G-bearing haplotype-specific construct had a lower transcriptional strength when compared to the wild-type rs1024611A-containing haplotype-specific construct in U87MG cell line. In contrast, we could not find significant differences in transcription strength between these two constructs when transfected into normal human astrocytes. While it has been widely accepted that reporter assays provide functional evidence for transcriptional mechanisms, it is increasingly being recognized that reporter assays alone cannot be used as proof of functional polymorphism in absence of other in vivo evidence such as AEI [48].
The lack of concordance between the AEI and the in vitro reporter assays could be because of several reasons. Reporter based assays do not account for the chromatin context of the regulatory region in question and cannot address the three dimensional interactions (“looping”) between distal and proximal regulatory sequences that play an important role in CCL2 gene regulation [49], [50]. As AEI captures allele-specific differences in the context of the chromatin as well as genetic polymorphisms it provides a powerful alternative to the reporter based studies for highly polymorphic genes with complex and extended regulatory regions. Also we cannot rule out that additional polymorphic residues that are linked to the rs1024611G-bearing allele may lead to increased levels of expression. To evaluate such a possibility we assessed the role of two such polymorphisms, rs7210316 and rs9889296 which had an r2>0.9 with respect to rs1024611 in CEU population and were located ∼6.3 kb and ∼9.2 kb upstream of it (Fig. 4A and data not shown). While the region overlapping the rs9889296 was inactive in reporter assays, the region containing rs72110316 was marginally active (data not shown). In addition, both these regions show minimal epigenetic features associated with transcriptional activation and thus their contribution to CCL2 AEI may be negligible. We also cannot exclude the possibility of rs13900 influencing CCL2 transcription as our reporter constructs lacked the 3′UTR but experiments are underway to address this possibility. In summary, the results from this study and previously published reports suggest that identification of a single functional SNP that could explain AEI of CCL2 is going to be a challenging proposition because of an extended cis-regulatory region and extensive polymorphism.
Can Differentially Binding Transcription Factors be Linked to CCL2 AEI?
Several transcription factors have been demonstrated to bind differentially to CCL2 polymorphisms including IRF-1, PARP-1, STAT-1, Prep1/Pbx complexes [10], [29], [30], [51], in order to explain the increased CCL2 expression levels associated with the rs1024611G allele. While siRNA studies in conjunction with in vitro transcription assays suggest a role for Prep1/Pbx complexes in mediating haplotype specific activity of the CCL2 reporter vectors [30], [31], more direct evidence for their role on endogenous CCL2 transcription is still awaited. STAT-1 has been implicated in the CCL2 induction by IFN-γ and there are reported differences in STAT-1 binding between rs2857656G and rs2857656C alleles, with the former showing greater degree of binding [47]. However, the well characterized STAT-1 binding site that was previously reported to mediate the IFN-γ upregulation of CCL2 is at position –212 relative to the start codon [52] and does not overlap with the predicted –362 STAT binding site reported by Nyquist et al. The increased transcriptional activity associated with rs2857656C allele cannot be directly attributed to differences in STAT-1 binding because of the following two reasons. First, STAT-1 binding to the –362G site could not be confirmed by super-shift assays. Second, the rs2857656G site shows a greater affinity to STAT-1 which is an activating transcription factor but the reporter construct with this haplotype exhibited a lower transcription activity [47]. Furthermore, while there is a perfect LD between rs1024611G and rs2857656C polymorphism, it will be difficult to argue that most of the effects of the rs1024611G bearing allele are mediated through differential binding of STAT-1α to the –362 site because this transcription factor is not known to be a major player in either TNF-α or IL-1β mediated upregulation of CCL2 expression. Thus till date there is no conclusive evidence for the role of specific transcription factor(s) in haplotype-specific differences in the transcriptional activity and generating AEI in CCL2. It could be speculated that cumulative effect of multiple transcription factors with activation or repressive potential binding to various CCL2 cis-regulatory polymorphisms specifies haplotype-specific differences in transcriptional strength and ultimately determines allele-specific differences in expression.
CCL2 AEI and Disease Association Studies
Studies on rs1024611G have implicated it in a myriad of disease phenotypes but often the findings across various cohorts and disease models were not consistent. While a number of factors such as cohort size, racial composition of the cohort, and the phenotype tested among others can influence such outcomes, robust molecular evidence can help in developing accurate models for disease pathogenesis and disabuse some of the conflicts that are often encountered in cohort studies [53]. Our analysis of allele-specific differences in CCL2 gene expression in heterozygous individuals provides a powerful molecular link to the phenotypic effects that have been associated with rs1024611G allele and suggest that rs13900 SNP could serve as a reliable, potent, and functional marker for conducting future cohort-based studies.
A possible scenario where CCL2 AEI can be helpful is in resolving contradictory data obtained with the association of rs1024611G with pulmonary tuberculosis. The rs1024611G polymorphism showed strong association with tuberculosis susceptibility in cohorts from North-East Asia, Zambia, Central America, and South America [28], [54], [55] whereas such association was not detected in studies conducted in South-East Asian, South African Colored and Indian populations [56], [57], [58]. In contrast to the above mentioned studies, Thye et al. found that rs1024611G conferred strong protection to TB in a Ghanaian cohort [59]. Feng et al. speculated that such differences may be attributed to LD between rs1024611 and an adjacent causal SNP [60]. Thus it might be helpful to study CCL2 AEI in a population specific manner to assess whether it can explain the phenotypic differences seen in cohort-based studies thus providing a rational basis for such inconsistencies. However, other explanations for the observed race-specific differences in disease outcome are possible as polymorphisms in other gene loci have been shown to influence serum CCL2 levels [19], [20].
Conclusion
In summary, we demonstrated here that the rs1024611 regulatory region polymorphism is associated with allelic expression imbalance of CCL2. Our study provides a molecular explanation for higher CCL2 expression levels that have been associated with the rs1024611G polymorphism and increased susceptibility of persons carrying this allele to a multitude of diseases that are thought to be mediated by the cells of monocytic lineage. Our functional studies also support the growing notion that reporter-based transcriptional studies should not serve as the sole basis to evaluate the role of cis-regulatory region polymorphisms on gene expression and whenever possible should be supported by in vivo expression analysis in an appropriate cell type. Functional studies such as those described in this report will help in accurately assessing the role of pathogenic alleles that have been identified by genome-wide association studies or candidate gene association studies and provide a rational explanation for such associations.
Materials and Methods
CCL2 Genetic Analysis
Genotype data was downloaded from ∼25 kb that spanned the rs1024611 polymorphism from Yoruba in Ibadan, Nigeria (YRI), Japanese in Tokyo, Japan (JPT), Han Chinese in Beijing, China (CHB) and CEPH (Utah residents with ancestry from northern and western Europe) (CEU) populations (HapMap data release #28 August 2010 on Build 36). Only unrelated individuals were used for the analysis. LD maps were constructed using the JLIN (Java-based linkage disequilibrium plotter) program [61]. Exact tests were performed to identify departure from Hardy-Weinberg (HW) equilibrium. Haplotypes were obtained by using the best calculated genetic phases with the ELB algorithm, and pairwise LD was examined by calculation of the D’ and r2 coefficients estimated using the Arlequin software package (version 3.5.1.3; 2011) [62].
Cell and Tissue Sources
HeLa, U87MG, and HL60 cell lines were obtained from ATCC and were maintained under recommended conditions. Primary human astrocytes were obtained from a commercial source and were cultured according to the manufacturer’s recommendation (Lonza). PBMC were isolated from healthy volunteer donors using Ficoll-Hypaque and resuspended in RPMI containing 10% FBS at 1×106 per ml. PBMC were either left untreated or treated with LPS (Sigma; 1 µg/ml) for 3 h and total RNA (RNAqueous 4PCR kit; Ambion) and genomic DNA were isolated. Total RNA was treated with DNaseI to remove any genomic DNA contamination and was quantified using a Nanodrop spectrophotometer. The 260/280 ratios ranged between1.8–2.0. Monocytes were purified from freshly isolated PBMC using Pan Monocyte Isolation Kit (Miltenyi Biotec) according to manufacturer’s recommended protocol and their purity was>85%. Brain samples from anonymous HIV-1 infected and normal donors were obtained from National Neurological AIDS Bank, The Manhattan HIV Brain Bank, and Texas Repository for AIDS Neuropathogenesis Research that are components of the National NeuroAIDS tissue consortium (NNTC). RNA and DNA were extracted from the brain tissues by using the Trizol (Invitrogen) method according to manufacturer’s recommended protocol. Genotyping for rs1024611 polymorphism was performed as previously described [10].
Ethics Statement
All studies on human subjects were approved by the Institutional Review Board of the University of Texas Health Science Center, San Antonio and South Texas Veterans Health Care System. All participating volunteer donors signed a written consent form which was approved by the IRB.
cDNA Synthesis and Quantitative RT-PCR (qRT-PCR)
cDNA was synthesized (iScript cDNA synthesis kit, Biorad) using random primers according to manufacturer’s recommended protocol. Expression of CCL2 in the LPS or TNF-α stimulated cells was confirmed by qRT-PCR (Applied Biosystems assay numbers Hs00237140_m1 for CCL2 and Hs99999901_s1 for 18S). Data were normalized using the ΔΔCt method using 18S as a normalizer. The 18S rRNA levels were not significantly different between the untreated and LPS treated samples. No amplification was detected in control reactions with no added template and in reverse transcriptase negative samples.
Capture of Nascent RNA
Nascent RNA was isolated using Click-It Nascent RNA kit (Invitrogen) to capture newly synthesized RNA according to manufacturer’s recommended protocol. Briefly, PBMC from a heterozygous individual were stimulated with LPS and incubated with ethylene uridine (EU) which is an alkyne–modified nucleoside and a uridine analog. Total RNA was isolated using RNAqueous®-4PCR Kit (Ambion). The EU-labeled RNA is biotinylated with using a “click reaction” with an azide-modifed biotin. This reaction generates a biotin-based handle on RNA molecule that is used to capture the nascent RNA transcripts on the streptavidin magnetic beads. The captured RNA was used for cDNA synthesis (Superscript Vilo kit, Invitrogen), PCR amplification, and pyrosequencing.
Pyrosequencing
The region encompassing the rs13900 SNP was amplified by PCR in a 50 µl reaction mixture containing 2 µl cDNA, 1.5 µl 50 mM MgCl2, 5 µl 10X PCR Buffer II, 1 µl 10 mM dNTP, 0.25 µl AmpliTaq DNA polymerase (Applied Biosystems), and 8 µM each of forward and reverse primers. The nucleotide sequence of the forward primer used was 5′-CCCAAGAATCTGCAGCTAACTTAT-3′ and the biotinylated reverse primer was 5′-GGCATAATGTTTCACATCAACAA-3′. The following temperature conditions were used: 94°C for 2 min followed by 35 cycles of 94°C for 10 s, 58°C for 30 s, 72°C for 1 min, and 72°C for 7 min. The nucleotide sequence of the oligonucleotide used for pyrosequencing is 5′-CTTTCCCCAGACACC-3′. Pyrosequencing was performed by a commercial firm (EpigenDx, Hopkinton, MA).
Cloning, Transfections, and Reporter Assays
Haplotype specific constructs that contain the rs1024611A and rs1024611G polymorphisms were cloned from a heterozygous donor who exhibited AEI. For generating the haplotype-specific constructs, a 6 kb region was PCR amplified (Elongase, Invitrogen). The nucleotide sequences of the oligonucleotides used for amplification are 5′-AGCTGAGGCCCTGGTTGATTCT-3′ (forward) and 5′-GCTGGAGGCGAGAGTGCGAG-3′ (reverse). The conditions for the amplification are 94°C for 2 minutes, 35 cycles of 94°C for 10 seconds and 68°C for 6 min. The amplified fragment was cloned into pGL4.16 reporter vector (Promega) and their nucleotide sequence was verified by sequencing. Transfections into U87MG cell line, TNF-α treatment, and luciferase assays were performed as described previously [49]. Normal human astrocytes were transfected using Fugene HD (Roche) according to manufacturer’s recommended protocol. Luciferase values were normalized using the total protein content of the lysates as we found that use of the routinely used Renilla vector modulated the expression of the haplotype-specific reporter constructs that led to spurious results.
Supporting Information
LPS induction of CCL2 in PBMC. Freshly isolated PBMC were either left untreated or treated with LPS for 3 h and total RNA extracted as outlined in the Methods. qRT-PCR was used to assess CCL2 expression and fold increase following LPS treatment versus the untreated samples was calculated using the ΔΔCt method. 18S rRNA was used as a normalizer. Data shown are from two independent donors and statistical significance was calculated using Student’s t test (*, p = 0.01, **p = 0.03).
(EPS)
CCL2 AEI in purified monocytes. Monocytes were purified from freshly isolated PBMC using Pan Monocyte Isolation Kit (Miltenyi Biotec) according to manufacturer’s recommended protocol. Total RNA was extracted from LPS treated unfractionated PBMC or purified monocytes and allelic quantification was performed on the RT-PCR products by pyrosequencing. Data shown are from two independent donors. Statistical significance was calculated using a paired t-test (*, p<0.0001).
(EPS)
Transcriptional Activity of CCL2 reporter vectors in normal human astrocytes. Primary human astrocytes were transfected with the indicated reporter vectors and were left untreated (panel A) or treated with TNF-α (panel B). The cells were lysed after 24 h after transfection and luciferase values were measured as indicated in the Methods. Data is from three independent experiments each done in duplicate. Statistical significance was calculated using Student’s t test and was found not to be significant (p>0.3).
(EPS)
Acknowledgments
The authors thank Drs. Sunil K. Ahuja, Yogesh Kalkonde, and Robert A. Clark for discussion and advice. We also thank Dr. Mrunal Kalkonde and Lisa Adams for performing some of the early experiments, Kazi Begum, Roshani Patel, and Triparna Ghosh-Choudhury for providing excellent technical support and Fabio Jimenez for performing phlebotomy. We also thank all the volunteer donors who have provided blood and the NNTC for providing brain tissues for these studies. The contents are solely the responsibility of the authors and do not necessarily represent the official view or policy of the NNTC/NIH or the Department of Veterans Affairs or the United States government.
Funding Statement
This work is supported by the Veterans Affairs Office of Research and Development- Biomedical Laboratory Research and Development Service Award (I01BX000975) to SM. TL is supported by a Department of Veterans Affairs Career Development Award-2 (1IK2BX001276-01A1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The brain samples for examining the CCL2 AEI were obtained from National Neurological AIDS bank (5U01MH083500, NS38841), The Manhattan HIV Brain Bank (U01MH083501, R24MH59724), and the Texas Repository for AIDS Neuropathogenesis Research (U01MH083507, R24 NS 38841) that are part of the National NeuroAIDS Tissue Consortium that is funded by the National Institutes of Health (NIMH and NINDS).
References
- 1. Deshmane SL, Kremlev S, Amini S, Sawaya BE (2009) Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Melgarejo E, Medina MA, Sanchez-Jimenez F, Urdiales JL (2009) Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. Int J Biochem Cell Biol 41: 998–1001. [DOI] [PubMed] [Google Scholar]
- 3. Yadav A, Saini V, Arora S (2010) MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta 411: 1570–1579. [DOI] [PubMed] [Google Scholar]
- 4. Charo IF, Ransohoff RM (2006) The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354: 610–621. [DOI] [PubMed] [Google Scholar]
- 5. Dhillon NK, Williams R, Callen S, Zien C, Narayan O, et al. (2008) Roles of MCP-1 in development of HIV-dementia. Front Biosci 13: 3913–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iwamoto T, Okamoto H, Toyama Y, Momohara S (2008) Molecular aspects of rheumatoid arthritis: chemokines in the joints of patients. Febs J 275: 4448–4455. [DOI] [PubMed] [Google Scholar]
- 8. Donath MY, Shoelson SE (2011) Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11: 98–107. [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez-Scarano F, Martin-Garcia J (2005) The neuropathogenesis of AIDS. Nat Rev Immunol 5: 69–81. [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, et al. (2002) HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci U S A 99: 13795–13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McDermott DH, Yang Q, Kathiresan S, Cupples LA, Massaro JM, et al. (2005) CCL2 polymorphisms are associated with serum monocyte chemoattractant protein-1 levels and myocardial infarction in the Framingham Heart Study. Circulation 112: 1113–1120. [DOI] [PubMed] [Google Scholar]
- 12. Cho ML, Kim JY, Ko HJ, Kim YH, Kim WU, et al. (2004) The MCP-1 promoter -2518 polymorphism in Behcet’s disease: correlation between allele types, MCP-1 production and clinical symptoms among Korean patients. Autoimmunity 37: 77–80. [DOI] [PubMed] [Google Scholar]
- 13. Fenoglio C, Galimberti D, Lovati C, Guidi I, Gatti A, et al. (2004) MCP-1 in Alzheimer’s disease patients: A-2518G polymorphism and serum levels. Neurobiol Aging 25: 1169–1173. [DOI] [PubMed] [Google Scholar]
- 14. Karrer S, Bosserhoff AK, Weiderer P, Distler O, Landthaler M, et al. (2005) The -2518 promotor polymorphism in the MCP-1 gene is associated with systemic sclerosis. J Invest Dermatol 124: 92–98. [DOI] [PubMed] [Google Scholar]
- 15. Wang L, Yang L, Gao L, Gao TW, Li W, et al. (2008) A functional promoter polymorphism in monocyte chemoattractant protein-1 is associated with psoriasis. Int J Immunogenet 35: 45–49. [DOI] [PubMed] [Google Scholar]
- 16. Rovin BH, Lu L, Saxena R (1999) A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem Biophys Res Commun 259: 344–348. [DOI] [PubMed] [Google Scholar]
- 17. Tabara Y, Kohara K, Yamamoto Y, Igase M, Nakura J, et al. (2003) Polymorphism of the monocyte chemoattractant protein (MCP-1) gene is associated with the plasma level of MCP-1 but not with carotid intima-media thickness. Hypertens Res 26: 677–683. [DOI] [PubMed] [Google Scholar]
- 18. Muhlbauer M, Bosserhoff AK, Hartmann A, Thasler WE, Weiss TS, et al. (2003) A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology 125: 1085–1093. [DOI] [PubMed] [Google Scholar]
- 19. Berrahmoune H, Herbeth B, Lamont JV, Fitzgerald PS, Visvikis-Siest S (2007) Association between TNF and IL-1 bloc polymorphisms and plasma MCP-1 concentration. Atherosclerosis 192: 348–353. [DOI] [PubMed] [Google Scholar]
- 20. Schnabel RB, Baumert J, Barbalic M, Dupuis J, Ellinor PT, et al. (2010) Duffy antigen receptor for chemokines (Darc) polymorphism regulates circulating concentrations of monocyte chemoattractant protein-1 and other inflammatory mediators. Blood 115: 5289–5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Letendre S, Marquie-Beck J, Singh KK, de Almeida S, Zimmerman J, et al. (2004) The monocyte chemotactic protein-1–2578G allele is associated with elevated MCP-1 concentrations in cerebrospinal fluid. J Neuroimmunol 157: 193–196. [DOI] [PubMed] [Google Scholar]
- 22. Joven J, Coll B, Tous M, Ferre N, Alonso-Villaverde C, et al. (2006) The influence of HIV infection on the correlation between plasma concentrations of monocyte chemoattractant protein-1 and carotid atherosclerosis. Clin Chim Acta 368: 114–119. [DOI] [PubMed] [Google Scholar]
- 23. Kim HL, Lee DS, Yang SH, Lim CS, Chung JH, et al. (2002) The polymorphism of monocyte chemoattractant protein-1 is associated with the renal disease of SLE. Am J Kidney Dis 40: 1146–1152. [DOI] [PubMed] [Google Scholar]
- 24. Zietz B, Buchler C, Herfarth H, Muller-Ladner U, Spiegel D, et al. (2005) Caucasian patients with type 2 diabetes mellitus have elevated levels of monocyte chemoattractant protein-1 that are not influenced by the -2518 A–>G promoter polymorphism. Diabetes Obes Metab 7: 570–578. [DOI] [PubMed] [Google Scholar]
- 25. van Wijk DF, van Leuven SI, Sandhu MS, Tanck MW, Hutten BA, et al. (2010) Chemokine ligand 2 genetic variants, serum monocyte chemoattractant protein-1 levels, and the risk of coronary artery disease. Arterioscler Thromb Vasc Biol 30: 1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melzer D, Perry JR, Hernandez D, Corsi AM, Stevens K, et al. (2008) A genome-wide association study identifies protein quantitative trait loci (pQTLs). PLoS Genet 4: e1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alonso-Villaverde C, Coll B, Parra S, Montero M, Calvo N, et al. (2004) Atherosclerosis in patients infected with HIV is influenced by a mutant monocyte chemoattractant protein-1 allele. Circulation 110: 2204–2209. [DOI] [PubMed] [Google Scholar]
- 28. Flores-Villanueva PO, Ruiz-Morales JA, Song CH, Flores LM, Jo EK, et al. (2005) A functional promoter polymorphism in monocyte chemoattractant protein-1 is associated with increased susceptibility to pulmonary tuberculosis. J Exp Med 202: 1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mummidi S, Bonello GB, Ahuja SK (2009) Confirmation of differential binding of Interferon Regulatory Factor-1 (IRF-1) to the functional and HIV disease-influencing -2578 A/G polymorphism in CCL2. Genes Immun 10: 197–198; author reply 199. [DOI] [PubMed]
- 30. Wright EK Jr, Page SH, Barber SA, Clements JE (2008) Prep1/Pbx2 complexes regulate CCL2 expression through the -2578 guanine polymorphism. Genes Immun 9: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Page SH, Wright EK Jr, Gama L, Clements JE (2011) Regulation of CCL2 expression by an upstream TALE homeodomain protein-binding site that synergizes with the site created by the A-2578G SNP. PLoS One 6: e22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yan H, Yuan W, Velculescu VE, Vogelstein B, Kinzler KW (2002) Allelic variation in human gene expression. Science 297: 1143. [DOI] [PubMed] [Google Scholar]
- 33. Kurreeman FA, Schonkeren JJ, Heijmans BT, Toes RE, Huizinga TW (2004) Transcription of the IL10 gene reveals allele-specific regulation at the mRNA level. Hum Mol Genet 13: 1755–1762. [DOI] [PubMed] [Google Scholar]
- 34. Wang D, Sadee W (2006) Searching for polymorphisms that affect gene expression and mRNA processing: example ABCB1 (MDR1). Aaps J 8: E515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA, et al. (2010) Integrating common and rare genetic variation in diverse human populations. Nature 467: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Intemann CD, Thye T, Forster B, Owusu-Dabo E, Gyapong J, et al. (2011) MCP1 haplotypes associated with protection from pulmonary tuberculosis. BMC Genet 12: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang H, Elbein SC (2007) Detection of allelic imbalance in gene expression using pyrosequencing. Methods Mol Biol 373: 157–176. [DOI] [PubMed] [Google Scholar]
- 38. Dimas AS, Deutsch S, Stranger BE, Montgomery SB, Borel C, et al. (2009) Common regulatory variation impacts gene expression in a cell type-dependent manner. Science 325: 1246–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nica AC, Parts L, Glass D, Nisbet J, Barrett A, et al. (2011) The architecture of gene regulatory variation across multiple human tissues: the MuTHER study. PLoS Genet 7: e1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ragin AB, Wu Y, Ochs R, Scheidegger R, Cohen BA, et al. (2010) Biomarkers of neurological status in HIV infection: a 3-year study. Proteomics Clin Appl 4: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, et al. (2010) Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A 107: 21931–21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abraham S, Sweet T, Sawaya BE, Rappaport J, Khalili K, et al. (2005) Cooperative interaction of C/EBP beta and Tat modulates MCP-1 gene transcription in astrocytes. J Neuroimmunol 160: 219–227. [DOI] [PubMed] [Google Scholar]
- 43. Lim SP, Garzino-Demo A (2000) The human immunodeficiency virus type 1 Tat protein up-regulates the promoter activity of the beta-chemokine monocyte chemoattractant protein 1 in the human astrocytoma cell line U-87 MG: role of SP-1, AP-1, and NF-kappaB consensus sites. J Virol 74: 1632–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iwai N, Kajimoto K, Kokubo Y, Okayama A, Miyazaki S, et al. (2006) Assessment of genetic effects of polymorphisms in the MCP-1 gene on serum MCP-1 levels and myocardial infarction in Japanese. Circ J 70: 805–809. [DOI] [PubMed] [Google Scholar]
- 45. Johnson AD, Zhang Y, Papp AC, Pinsonneault JK, Lim JE, et al. (2008) Polymorphisms affecting gene transcription and mRNA processing in pharmacogenetic candidate genes: detection through allelic expression imbalance in human target tissues. Pharmacogenet Genomics 18: 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marsh CB, Wewers MD, Tan LC, Rovin BH (1997) Fc(gamma) receptor cross-linking induces peripheral blood mononuclear cell monocyte chemoattractant protein-1 expression: role of lymphocyte Fc(gamma)RIII. J Immunol 158: 1078–1084. [PubMed] [Google Scholar]
- 47. Nyquist PA, Winkler CA, McKenzie LM, Yanek LR, Becker LC, et al. (2009) Single nucleotide polymorphisms in monocyte chemoattractant protein-1 and its receptor act synergistically to increase the risk of carotid atherosclerosis. Cerebrovasc Dis 28: 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cirulli ET, Goldstein DB (2007) In vitro assays fail to predict in vivo effects of regulatory polymorphisms. Hum Mol Genet 16: 1931–1939. [DOI] [PubMed] [Google Scholar]
- 49. Bonello GB, Pham MH, Begum K, Sigala J, Sataranatarajan K, et al. (2011) An evolutionarily conserved TNF-alpha-responsive enhancer in the far upstream region of human CCL2 locus influences its gene expression. J Immunol 186: 7025–7038. [DOI] [PubMed] [Google Scholar]
- 50. Teferedegne B, Green MR, Guo Z, Boss JM (2006) Mechanism of action of a distal NF-kappaB-dependent enhancer. Mol Cell Biol 26: 5759–5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nyquist P, Zhang J, De Graba TJ (2010) The -928 G/C and -362 G/C single-nucleotide polymorphisms in the promoter of MCP-1: Increased transcriptional activity and novel binding sites. Cerebrovasc Dis 29: 242–247. [DOI] [PubMed] [Google Scholar]
- 52. Zhou ZH, Chaturvedi P, Han YL, Aras S, Li YS, et al. (1998) IFN-gamma induction of the human monocyte chemoattractant protein (hMCP)-1 gene in astrocytoma cells: functional interaction between an IFN-gamma-activated site and a GC-rich element. J Immunol 160: 3908–3916. [PubMed] [Google Scholar]
- 53. Buckland PR (2006) The importance and identification of regulatory polymorphisms and their mechanisms of action. Biochim Biophys Acta 1762: 17–28. [DOI] [PubMed] [Google Scholar]
- 54. Buijtels PC, van de Sande WW, Parkinson S, Petit PL, van der Sande MA, et al. (2008) Polymorphism in CC-chemokine ligand 2 associated with tuberculosis in Zambia. Int J Tuberc Lung Dis 12: 1485–1488. [PubMed] [Google Scholar]
- 55. Ganachari M, Ruiz-Morales JA, Gomez de la Torre Pretell JC, Dinh J, Granados J, et al. (2010) Joint effect of MCP-1 genotype GG and MMP-1 genotype 2G/2G increases the likelihood of developing pulmonary tuberculosis in BCG-vaccinated individuals. PLoS One 5: e8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alagarasu K, Selvaraj P, Swaminathan S, Raghavan S, Narendran G, et al. (2009) CCR2, MCP-1, SDF-1a & DC-SIGN gene polymorphisms in HIV-1 infected patients with & without tuberculosis. Indian J Med Res 130: 444–450. [PubMed] [Google Scholar]
- 57. Chu SF, Tam CM, Wong HS, Kam KM, Lau YL, et al. (2007) Association between RANTES functional polymorphisms and tuberculosis in Hong Kong Chinese. Genes Immun 8: 475–479. [DOI] [PubMed] [Google Scholar]
- 58. Moller M, Nebel A, Valentonyte R, van Helden PD, Schreiber S, et al. (2009) Investigation of chromosome 17 candidate genes in susceptibility to TB in a South African population. Tuberculosis (Edinb) 89: 189–194. [DOI] [PubMed] [Google Scholar]
- 59. Thye T, Nejentsev S, Intemann CD, Browne EN, Chinbuah MA, et al. (2009) MCP-1 promoter variant -362C associated with protection from pulmonary tuberculosis in Ghana, West Africa. Hum Mol Genet 18: 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Feng WX, Mokrousov I, Wang BB, Nelson H, Jiao WW, et al. Tag SNP polymorphism of CCL2 and its role in clinical tuberculosis in Han Chinese pediatric population. PLoS One 6: e14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carter KW, McCaskie PA, Palmer LJ (2006) JLIN: a java based linkage disequilibrium plotter. BMC Bioinformatics 7: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform Online 1: 47–50. [PMC free article] [PubMed] [Google Scholar]
- 63. Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, et al. (2002) The human genome browser at UCSC. Genome Res 12: 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rosenbloom KR, Dreszer TR, Pheasant M, Barber GP, Meyer LR, et al. (2010) ENCODE whole-genome data in the UCSC Genome Browser. Nucleic Acids Res 38: D620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou X, Maricque B, Xie M, Li D, Sundaram V, et al. (2011) The Human Epigenome Browser at Washington University. Nat Methods 8: 989–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brenner D, Labreuche J, Touboul PJ, Schmidt-Petersen K, Poirier O, et al. (2006) Cytokine polymorphisms associated with carotid intima-media thickness in stroke patients. Stroke 37: 1691–1696. [DOI] [PubMed] [Google Scholar]
- 67. Gonzalez-Enriquez GV, Rubio-Benitez MI, Garcia-Gallegos V, Portilla-de Buen E, Troyo-Sanroman R, et al. (2008) Contribution of TNF-308A and CCL2–2518A to carotid intima-media thickness in obese mexican children and adolescents. Arch Med Res 39: 753–759. [DOI] [PubMed] [Google Scholar]
- 68. Yuasa S, Maruyama T, Yamamoto Y, Hirose H, Kawai T, et al. (2009) MCP-1 gene A-2518G polymorphism and carotid artery atherosclerosis in patients with type 2 diabetes. Diabetes Res Clin Pract 86: 193–198. [DOI] [PubMed] [Google Scholar]
- 69. Alioglu E, Turk U, Cam S, Abbasaliyev A, Tengiz I, et al. (2009) Polymorphisms of the methylenetetrahydrofolate reductase, vascular endothelial growth factor, endothelial nitric oxide synthase, monocyte chemoattractant protein-1 and apolipoprotein E genes are not associated with carotid intima-media thickness. Can J Cardiol 25: e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kim MP, Wahl LM, Yanek LR, Becker DM, Becker LC (2007) A monocyte chemoattractant protein-1 gene polymorphism is associated with occult ischemia in a high-risk asymptomatic population. Atherosclerosis 193: 366–372. [DOI] [PubMed] [Google Scholar]
- 71. Bjarnadottir K, Eiriksdottir G, Aspelund T, Gudnason V (2006) Examination of genetic effects of polymorphisms in the MCP-1 and CCR2 genes on MI in the Icelandic population. Atherosclerosis 188: 341–346. [DOI] [PubMed] [Google Scholar]
- 72. Szalai C, Duba J, Prohaszka Z, Kalina A, Szabo T, et al. (2001) Involvement of polymorphisms in the chemokine system in the susceptibility for coronary artery disease (CAD). Coincidence of elevated Lp(a) and MCP-1–2518 G/G genotype in CAD patients. Atherosclerosis 158: 233–239. [DOI] [PubMed] [Google Scholar]
- 73. Cavestro GM, Zuppardo RA, Bertolini S, Sereni G, Frulloni L, et al. (2010) Connections between genetics and clinical data: Role of MCP-1, CFTR, and SPINK-1 in the setting of acute, acute recurrent, and chronic pancreatitis. Am J Gastroenterol 105: 199–206. [DOI] [PubMed] [Google Scholar]
- 74. Brown KS, Nackos E, Morthala S, Jensen LE, Whitehead AS, et al. (2007) Monocyte chemoattractant protein-1: plasma concentrations and A(-2518)G promoter polymorphism of its gene in systemic lupus erythematosus. J Rheumatol 34: 740–746. [PubMed] [Google Scholar]
- 75. Aguilar F, Gonzalez-Escribano MF, Sanchez-Roman J, Nunez-Roldan A (2001) MCP-1 promoter polymorphism in Spanish patients with systemic lupus erythematosus. Tissue Antigens 58: 335–338. [DOI] [PubMed] [Google Scholar]
- 76. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG (2010) Functional monocyte chemoattractant protein-1 promoter -2518 polymorphism and systemic lupus erythematosus: a meta-analysis. Mol Biol Rep 37: 3421–3426. [DOI] [PubMed] [Google Scholar]
- 77. Sanchez E, Sabio JM, Callejas JL, de Ramon E, Garcia-Portales R, et al. (2006) Association study of genetic variants of pro-inflammatory chemokine and cytokine genes in systemic lupus erythematosus. BMC Med Genet 7: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tucci M, Barnes EV, Sobel ES, Croker BP, Segal MS, et al. (2004) Strong association of a functional polymorphism in the monocyte chemoattractant protein 1 promoter gene with lupus nephritis. Arthritis Rheum 50: 1842–1849. [DOI] [PubMed] [Google Scholar]
- 79. Malafronte P, Vieira JM Jr, Pereira AC, Krieger JE, Barros RT, et al. (2010) Association of the MCP-1–2518 A/G polymorphism and no association of its receptor CCR2–64 V/I polymorphism with lupus nephritis. J Rheumatol 37: 776–782. [DOI] [PubMed] [Google Scholar]
- 80. Nakashima H, Akahoshi M, Shimizu S, Inoue Y, Miyake K, et al. (2004) Absence of association between the MCP-1 gene polymorphism and histological phenotype of lupus nephritis. Lupus 13: 165–167. [DOI] [PubMed] [Google Scholar]
- 81. Szalai C, Kozma GT, Nagy A, Bojszko A, Krikovszky D, et al. (2001) Polymorphism in the gene regulatory region of MCP-1 is associated with asthma susceptibility and severity. J Allergy Clin Immunol 108: 375–381. [DOI] [PubMed] [Google Scholar]
- 82. Chelbi H, Ghadiri A, Lacheb J, Ghandil P, Hamzaoui K, et al. (2008) A polymorphism in the CCL2 chemokine gene is associated with asthma risk: a case-control and a family study in Tunisia. Genes Immun 9: 575–581. [DOI] [PubMed] [Google Scholar]
- 83. Yao TC, Wu KC, Chung HT, Shaw CK, Kuo ML, et al. (2004) MCP-1 gene regulatory region polymorphism in Chinese children with mild, moderate and near-fatal asthma. Allergy 59: 436–441. [DOI] [PubMed] [Google Scholar]
- 84. Ahluwalia TS, Khullar M, Ahuja M, Kohli HS, Bhansali A, et al. (2009) Common variants of inflammatory cytokine genes are associated with risk of nephropathy in type 2 diabetes among Asian Indians. PLoS One 4: e5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Boger CA, Fischereder M, Deinzer M, Aslanidis C, Schmitz G, et al. (2005) RANTES gene polymorphisms predict all-cause and cardiac mortality in type 2 diabetes mellitus hemodialysis patients. Atherosclerosis 183: 121–129. [DOI] [PubMed] [Google Scholar]
- 86. Kaur S, Panicker SR, James T, Sarma PS, Thankappan KR, et al. (2009) Association of monocyte chemoattractant protein-1–2518 polymorphism with metabolic syndrome in a South Indian cohort. Metab Syndr Relat Disord 7: 193–198. [DOI] [PubMed] [Google Scholar]
- 87. Carulli MT, Spagnolo P, Fonseca C, Welsh KI, duBois RM, et al. (2008) Single-nucleotide polymorphisms in CCL2 gene are not associated with susceptibility to systemic sclerosis. J Rheumatol 35: 839–844. [PubMed] [Google Scholar]
- 88. Radstake TR, Vonk MC, Dekkers M, Schijvenaars MM, Treppichio WL, et al. (2009) The -2518A>G promoter polymorphism in the CCL2 gene is not associated with systemic sclerosis susceptibility or phenotype: results from a multicenter study of European Caucasian patients. Hum Immunol 70: 130–133. [DOI] [PubMed] [Google Scholar]
- 89. Palmieri O, Latiano A, Salvatori E, Valvano MR, Bossa F, et al. (2010) The -A2518G polymorphism of monocyte chemoattractant protein-1 is associated with Crohn’s disease. Am J Gastroenterol 105: 1586–1594. [DOI] [PubMed] [Google Scholar]
- 90. Vilades C, Broch M, Plana M, Domingo P, Alonso-Villaverde C, et al. (2007) Effect of genetic variants of CCR2 and CCL2 on the natural history of HIV-1 infection: CCL2–2518GG is overrepresented in a cohort of Spanish HIV-1-infected subjects. J Acquir Immune Defic Syndr 44: 132–138. [DOI] [PubMed] [Google Scholar]
- 91. Pola R, Flex A, Gaetani E, Proia AS, Papaleo P, et al. (2004) Monocyte chemoattractant protein-1 (MCP-1) gene polymorphism and risk of Alzheimer’s disease in Italians. Exp Gerontol 39: 1249–1252. [DOI] [PubMed] [Google Scholar]
- 92. Huerta C, Alvarez V, Mata IF, Coto E, Ribacoba R, et al. (2004) Chemokines (RANTES and MCP-1) and chemokine-receptors (CCR2 and CCR5) gene polymorphisms in Alzheimer’s and Parkinson’s disease. Neurosci Lett 370: 151–154. [DOI] [PubMed] [Google Scholar]
- 93. Combarros O, Infante J, Llorca J, Berciano J (2004) No evidence for association of the monocyte chemoattractant protein-1 (-2518) gene polymorphism and Alzheimer’s disease. Neurosci Lett 360: 25–28. [DOI] [PubMed] [Google Scholar]
- 94. Ghilardi G, Biondi ML, La Torre A, Battaglioli L, Scorza R (2005) Breast cancer progression and host polymorphisms in the chemokine system: role of the macrophage chemoattractant protein-1 (MCP-1) -2518 G allele. Clin Chem 51: 452–455. [DOI] [PubMed] [Google Scholar]
- 95. Kozma GT, Falus A, Bojszko A, Krikovszky D, Szabo T, et al. (2002) Lack of association between atopic eczema/dermatitis syndrome and polymorphisms in the promoter region of RANTES and regulatory region of MCP-1. Allergy 57: 160–163. [DOI] [PubMed] [Google Scholar]
- 96.Glas J, Torok HP, Tonenchi L, Schiemann U, Folwaczny C (2004) The -2518 promotor polymorphism in the MCP-1 gene is not associated with liver cirrhosis in chronic hepatitis C virus infection. Gastroenterology 126: 1930–1931; author reply 1931–1932. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LPS induction of CCL2 in PBMC. Freshly isolated PBMC were either left untreated or treated with LPS for 3 h and total RNA extracted as outlined in the Methods. qRT-PCR was used to assess CCL2 expression and fold increase following LPS treatment versus the untreated samples was calculated using the ΔΔCt method. 18S rRNA was used as a normalizer. Data shown are from two independent donors and statistical significance was calculated using Student’s t test (*, p = 0.01, **p = 0.03).
(EPS)
CCL2 AEI in purified monocytes. Monocytes were purified from freshly isolated PBMC using Pan Monocyte Isolation Kit (Miltenyi Biotec) according to manufacturer’s recommended protocol. Total RNA was extracted from LPS treated unfractionated PBMC or purified monocytes and allelic quantification was performed on the RT-PCR products by pyrosequencing. Data shown are from two independent donors. Statistical significance was calculated using a paired t-test (*, p<0.0001).
(EPS)
Transcriptional Activity of CCL2 reporter vectors in normal human astrocytes. Primary human astrocytes were transfected with the indicated reporter vectors and were left untreated (panel A) or treated with TNF-α (panel B). The cells were lysed after 24 h after transfection and luciferase values were measured as indicated in the Methods. Data is from three independent experiments each done in duplicate. Statistical significance was calculated using Student’s t test and was found not to be significant (p>0.3).
(EPS)