Abstract
In order to understand the physicochemical mechanisms that could explain the massive growth of Azolla arctica in the Eocene Arctic Ocean, we carried out a laboratory experiment in which we studied the interacting effects of rain and wind on the development of salinity stratification, both in the presence and in the absence of a dense Azolla cover. Additionally, we carried out a mesocosm experiment to get a better understanding of the nutrient cycling within and beneath a dense Azolla cover in both freshwater and brackish water environments. Here we show that Azolla is able to create a windproof, small-scale salinity gradient in brackish waters, which allows for efficient recycling of nutrients. We suggest that this mechanism ensures the maintenance of a large standing biomass in which additional input of nutrients ultimately result in a further expansion of an Azolla cover. As such, it may not only explain the extent of the Azolla event during the Eocene, but also the absence of intact vegetative Azolla remains and the relatively low burial efficiency of organic carbon during this interval.
Introduction
Azolla is a pleustonic freshwater fern known from temperate, tropical and subtropical regions all over the world. Distinctive to Azolla is that it encloses a permanent endosymbiotic prokaryotic community inside its leaf cavities [1]. The most notable member of this community is the nitrogen fixing cyanobacterium Anabaena azollae which is able to meet the entire nitrogen demand of the symbiosis, as long as Azolla provides it with a fixed source of carbon [2], [3].
Fossil Azolla species have been recognised in freshwater deposits from the mid to late Cretaceous onward. They can be found as complete plants and/or as megaspores and microspores, the latter often clumped together in so called massulae [4], [5]. More recently, high abundances of morphologically intact fossil microspores and megaspores of Azolla arctica [6] were found in mid Eocene (∼48.5 Ma) marine sediments [7]. These were recovered from the Lomonosov ridge in the central Arctic Ocean during the Arctic Coring Expedition of the Integrated Ocean Drilling Program. Many of the encountered mature megaspores in these marine sediments had microspore massulae attached indicating that Azolla arctica grew and reproduced in situ in the Arctic basin during the middle Eocene when this area had a relatively warm climate [6], [7].
The sediments representing the Eocene Azolla event, which lasted at least 800 000 years [7], were found to be laminated and the observed cyclicity of spore abundances appeared to be strongly related to changes in obliquity and precession cycles [8]. Such orbital processes are known to affect pole-ward atmospheric heat and moisture transport [9]. Indeed, it has been computed that the warm greenhouse conditions of the Eocene period induced an intensified hydrological cycle with precipitation exceeding evaporation at high latitudes [10], [11]. In combination with the relatively isolated geography of the Arctic Ocean during the Eocene [12], this enhanced freshwater input (by the precipitation itself and/ or via river discharge) frequently resulted in a highly stratified Arctic Ocean with a relatively freshwater layer (1–6 %) on top of a more saline (15–21%), occasionally anoxic deepwater layer [7], [13], [14].
Such variations in salinity are also well known from present day estuaries where the vertical salinity distribution, which may evolve as a consequence of freshwater input by rivers, is often characterized by a very steep salinity gradient at the interface of two water layers differing in density. When such a halocline is formed, it often hampers the mixing between water layers, although it cannot entirely prevent salinity from influencing the top water layers along the estuary, due to the transport of incoming deep saline water and the influence of wind and wave action on the circulation of the water column [15]. In the Eocene Arctic Ocean the halocline apparently was very strong during very long periods and the surface waters were frequently fresh or at least brackish enough to allow for the growth of Azolla arctica [7], [13], [14].
Beside relatively fresh water conditions there also must have been a sufficiently large influx and availability of nutrients in order to be able to explain the massive growth of Azolla in the Eocene Arctic Ocean. There is strong evidence that dinitrogen fixation was already a persistent feature in the Eocene Arctic ocean [16], [17]. Therefore, the growth of Azolla arctica was presumably not limited by nitrogen, but by phosphorus.
Present day Azolla species show a high phosphorus demand and typically occur in relatively shallow water bodies [18], [19]. It has been shown that high phosphate concentrations of over 75 µM may still stimulate Azolla growth [20], [21], [22]. Since Azolla is only able to utilize the dissolved inorganic phosphate in the surface water, its growth is often limited by the release of phosphate from the sediment [18]. In open waters this often is too slow to meet its requirements, but the presence of a dense cover of Azolla frequently renders the surface water anoxic [23], [24], which in turn may enhance the phosphate release from the sediment [25], [26]. In shallow water bodies, where there is little dilution, such a reductive solubility of phosphate can considerably increase its availability in the surface water.
In the Eocene Arctic Ocean dissolved inorganic phosphate in the surface water originated from the release from sediments within and adjoining the Arctic Ocean, and/or from the inflow of rivers. Given that the basin was relatively deep and highly stratified at the time of the extensive Azolla blooms, it seems unlikely that much of the sediment derived phosphate within the Arctic Ocean became available to Azolla arctica. In stratified lakes it is well known that the presence of a halocline often results in a hypolimnetic nutrient accumulation [15], [26]. If this were also the case in the Eocene Arctic Ocean, this would mean that the growth of Azolla arctica was largely dependent on the release of phosphate from shallow sediments adjoining the Arctic Ocean and especially on the phosphate input via rivers. Therefore, the fact that Azolla arctica nevertheless was able to disperse all the way to the central Arctic ocean, can only point to an enormous expansion of the Azolla from the coastal regions and a probable mechanism for efficient recycling of nutrients in the surface water layer of the open ocean.
Here, we studied the effect of the presence of Azolla on the development of a small-scale salinity gradient in slightly brackish waters in order to empirically test the hypothesis that the development of such a small-scale halocline facilitates nutrient recycling within a dense Azolla cover by trapping the nutrients that are lost from the decomposition of dead plant material within the top surface water layer. First, we carried out a laboratory experiment in which we studied the interacting effects of rain and wind on the development of salinity stratification, both in the presence and in the absence of a dense Azolla cover. Next we carried out a mesocosm experiment in order to get a better understanding of the nutrient cycling within and beneath a dense Azolla cover in both freshwater and brackish water environments.
Materials and Methods
Species
We used Azolla filiculoides, which is the northernmost occurring Azolla species. It is widely distributed, including the subtropical and tropical regions of the world [27]. In the Netherlands, it mostly grows in the western parts of the country where the maritime climate tempers winter periods, but it is increasingly expanding its distribution eastward.
Interacting effects of Azolla, rain and wind on salinity stratification
Sixteen 800 mL beakers were filled with 600 ml of a nutrient solution containing 1.5 mM NaHCO3, 0.5 mM KCl, 0.5 mM MgCl26H2O, 0.025 mM Fe-EDTA, 0.025 mM NaH2PO4H2O, 0.001 mM CuSO45H2O, 0.02 mM MnCl24H2O, 0.01 mM ZnSO47H2O, 0.003 mM Na2MoO42H2O, 0.02 mM H3BO3 and 0.004 mM CoCl26H2O (Sigma-Aldrich, Zwijndrecht, Netherlands). The composition of this growth medium was based on the results obtained in a field survey by de Lyon and Roelofs [28], who studied the distribution of A. filiculoides, among other aquatic plants in the Netherlands, in relation to water quality and other biogeochemical parameters. The final salinity of the nutrient solution was set to 3% by adding artificial sea salt (Tropic Marin, Wartenberg, Germany) and was determined by measuring the total dissolved salt concentration using a multi meter (18.52.SA multimeter, 18.56 Flow-through cell Compact, Eijkelkamp Agrisearch Equipment, Giesbeek, the Netherlands).
The surface waters of eight of the beakers were completely covered with 20 g fresh A. filiculoides, which had been cultivated in the laboratory prior to the experiment for approximately 8 weeks. Four of the covered and four of the non-covered beakers were subjected to wind influences. Wind was simulated by placing tubes diagonally above the Azolla or water surface from which air escaped at a speed of approximately 11.2 m s−1 (Windmaster 2, Kaindl Electronics, Rohrbach, Germany). Next, we subjected all sixteen beakers to a rain event which was simulated by sprinkling approximately 30 mm of demineralized water over the beakers from a distance of ∼1 meter using a watering can. The salinity of the nutrient solution was measured at the surface and at the bottom of the beakers at 0, 0.5, 4 and 20 hours after the rain event. At the end of the experiment (20 hours after the rain event) a complete salinity profile was determined by measuring the total dissolved salt concentration in the nutrient solution every 0.5 cm from the surface to the bottom of the beakers. The electrode was kept steady using a double column height dial gauge (Microscale measurements, The Hague, The Netherlands).
Mesocosm experiment
In the Netherlands A. filiculoides typically grows from late summer to early winter and the mesocosm experiment was therefore conducted from mid September to early December in basins outside the experimental greenhouse of the Radboud University Nijmegen (N51°82′29”; E5°87′16”). The plants used for inoculation had been collected from a ditch near an arable land in Elst, The Netherlands (N51°55′48”; E5°50′6”). No specific permits were required for this. At the time of collecting the Azolla the location was not privately owned or protected in any way.
Six semi-enclosed basins, with a depth of 92 cm and a radius of 185 cm (∼2500 liter), were filled with tap water and different chemicals were added to establish a nutrient solution with a composition similar to what was used in the laboratory experiment. In three of the six basins, however, artificial sea salt was not included. To avoid the influence of micro-climate differences a complete random design was used. The experiment started with the inoculation of ∼2.7 kg (1 kg m−2) fresh A. filiculoides to all of the basins. To prevent the depletion of phosphorus, iron and other micronutrients, their concentrations were restored at days 24 and 37. The experiment lasted 86 days. The amount of rainfall was monitored during the experiment, and from the moment that the basins were fully covered with A. filiculoides we measured dissolved oxygen concentrations and temperatures at the surface and the bottom of the basins using an YSI multiparameter probe (YSI Incorporated, Yellow Springs, USA).
Biomass sub-samples were taken at regular time intervals from the moment the basins were fully covered with A. filiculoides using a square sieve of 25 cm2. The harvested plants were washed with running demineralized water for half a minute and blotted dry with tissue paper, after which their fresh weight was determined. After drying at 70°C for 48 hours, dry weight was determined.
The nutrient solution in the basins was sampled weekly at the surface (top 5 cm), in the middle (45–50 cm at depth) and at the bottom (87–92 cm at depth). The sampling was carried out using 30 mL vacuum flasks connected to ceramic soil moisture samplers (Eijkelkamp Agrisearch Equipment, Giesbeek, the Netherlands). Concentrations of PO4 3− and Cl− were measured colorimetrically and concentrations of K+ and Na+ were measured flame photometrically using Auto Analyzer systems (Bran & Luebbe, Norderstedt, Germany). Total Ca, Mg and S concentrations were determined using an inductively-coupled plasma emission spectrophotometer (ICP-OES, model IRIS Intrepid II XDL, Thermo Electron Corporation, Franklin, MA).
Statistics
We used Mixed Linear Models in SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, U.S.A.) for statistical analyses. For the laboratory experiment we nested time as a repeated co-variable of the beakers and chose heterogeneous auto-regressive as a covariance type. We entered water layer (top or bottom), plant (with or without Azolla) and wind (wind or no wind) as fixed factors. For the analysis of the biomass data in the mesocosm experiment we used the same model except that we chose auto-regressive as a covariance type and entered treatment (fresh or brackish) as a fixed factor. The chloride concentrations in the brackish water basins were analyzed separately because salinity levels in the fresh and brackish water basins obviously were different. We only focussed on the concentrations in the different water layers that were measured from day 37 onward. We nested time as repeated co-variable of the basins and chose heterogeneous auto-regressive as a covariance type. The differences between the water layers in the brackish water basins were determined using Bonferroni post hoc analysis. We ran a univariate ANOVA on the chloride concentrations in the top water layers of the fresh and brackish water basins to see if they had become comparable at the end of the experiment. The dissolved oxygen concentrations and phosphorus concentrations were analyzed in a similar way as the chloride concentration, only now the water layers of the fresh water basins were included in the analyses, which voided the need to perform a separate univariate ANOVA on the data at the end of the experiment. In the data analysis of the phosphorus concentrations we used unstructured variations as a covariance type.
Results
Interacting effects of Azolla, rain and wind on salinity stratification
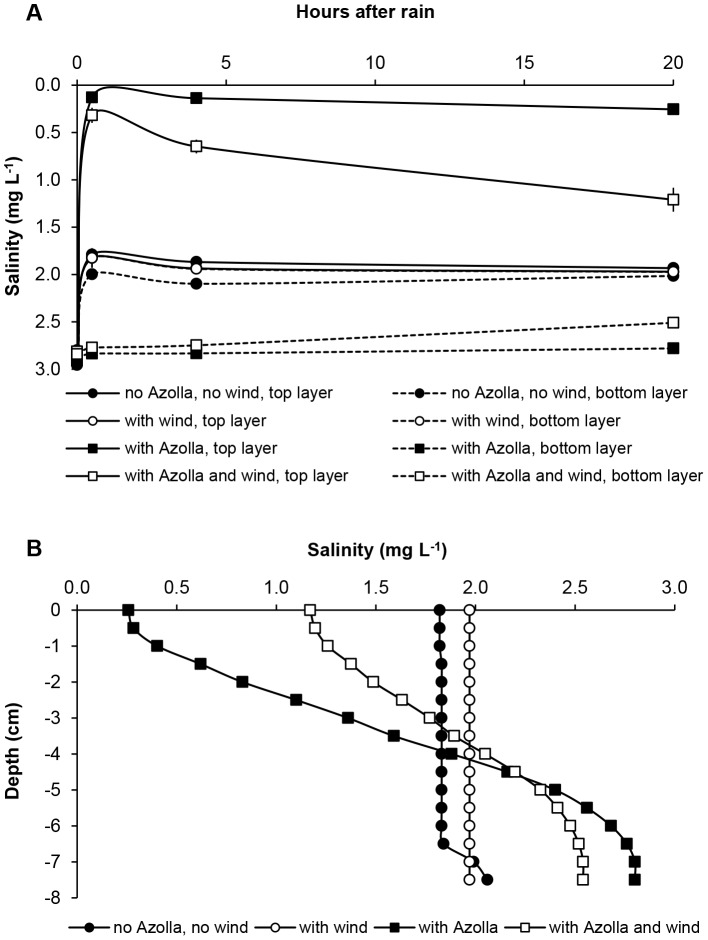
We found that the salinity of the top and bottom water layers became significantly different after the rain event (F1 = 3053.573, p<0.001) and that the presence or absence of Azolla (F1 = 262.963, p<0.001) or wind (F1 = 12.409, p<0.01) had a significant effect. The results of the laboratory experiment further showed that the presence of the Azolla cover significantly enhanced salinity stratification (F1 = 2500.946, p<0.001), whereas wind influences significantly decreased salinity stratification (F1 = 76.587, p<0.001). The presence of the Azolla cover significantly hampered the influence of wind mixing the water column (F1 = 30.795, p<0.001). In addition, we found a significant interaction between the salinity of the different water layers, the presence or absence of Azolla and the presence or absence of wind (F1 = 13.876, p<0.001) (Fig. 1A).
Figure 1. Interacting effects of Azolla, rain and wind on salinity stratification. A.
) Salinity (mg L−1 ± standard error) in the top water layers (solid lines) and in the bottom water layers (dotted lines) of the beakers in the absence of Azolla (rounds), in the presence of Azolla (squares), with no influence of wind (closed figures) or with influence of wind (open figures) hours after the rain event. B) Salinity (mg L−1) profiles in the beakers 20 hours after the rain event.
Also from the salinity profiles, which were taken 20 hours after the rain event, it became clear that the salinity gradient was steepest in the treatment with Azolla when there was no wind, followed by the treatment with Azolla and wind. In the treatment without Azolla and without wind, only a small gradient was left at the bottom of the beakers, whereas in the treatment without Azolla but with wind no gradient was found (Fig. 1B).
Mesocosm experiment
During the first ∼35 days of the mesocosm experiment the biomass in the basins increased. Biomass densities of Azolla in the freshwater and brackish water treatment did not differ (F1 = 2.817, p>0.05) (Fig. 2A). The Azolla cover, including roots, was approximately 5–7 cm thick and the roots were 3–5 cm long. At the end of the experiment part of the biomass started to decompose due to decreasing temperatures. The minimum air temperature at 10 cm above the ground occasionally fell below the freezing point, but the water temperature in the basins never became lower than 4.4°C (Fig. S1).
Figure 2. Mesocosm experiment.
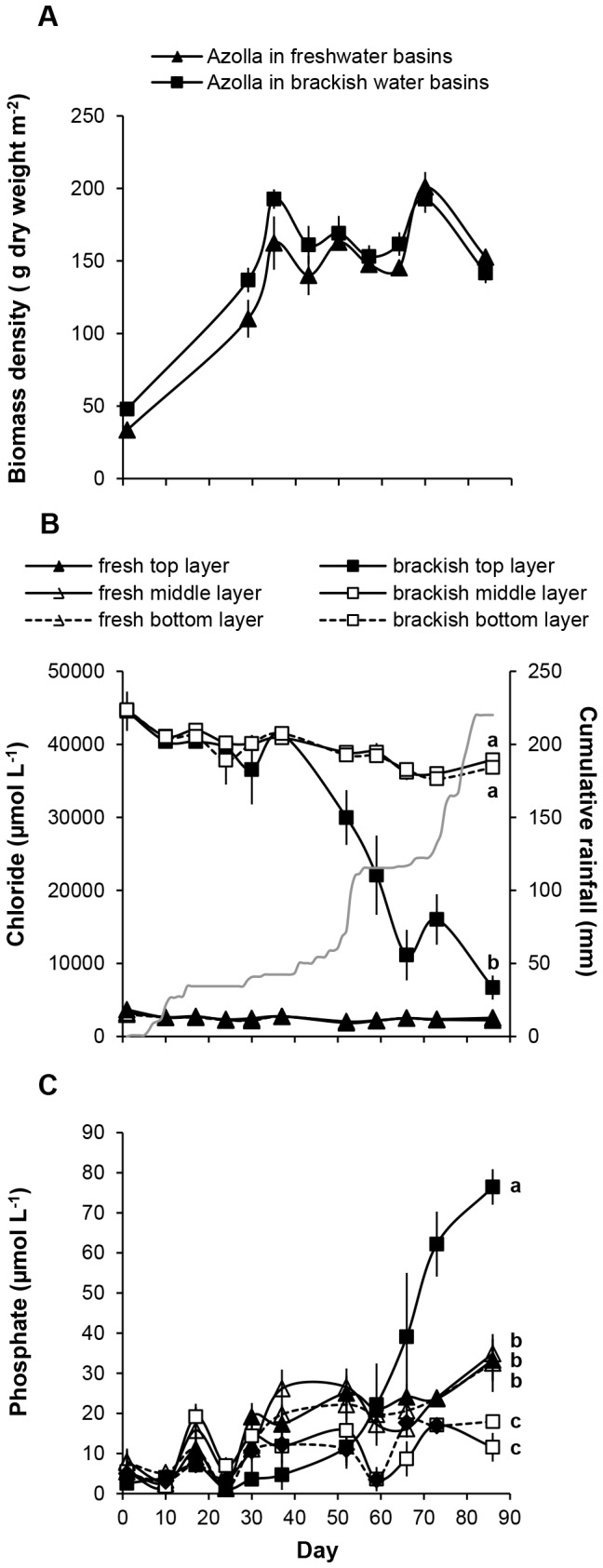
A) Development of the biomass density of Azolla filiculoides (g dry weight m−2 ± standard error) grown in freshwater or brackish water basins. 2B) Chloride concentrations and 2C) phosphate concentrations (µM ± standard error) in the top, middle and bottom water layers of the freshwater and brackish water basins during the mesocosm experiment. Significant differences between water layers are indicated by different letters. The cumulative amount of rainfall during the experiment (mm) is shown on the right axis in figure 2B .
In figure 2B the chloride concentration in the different water layers is shown as an indicator for the observed ionic salinity changes in the fresh and brackish water basins. The Ca2+, Mg2+, Na+, K+, SO4 − concentrations showed similar patterns (Fig. S2). At the start of the experiment, Cl- concentrations were evenly distributed throughout the water column, both in the fresh and brackish water basins. However, after ∼37 days the top water layer in the brackish water basins became significantly fresher than the middle and the bottom water layers (F2 = 12.662, p<0.01). The freshening of the top water layer in the brackish basins started at a day that had more than 25 mm of rain (Fig. 2B). At the end of the experiment the chloride concentration in the top water layer of the brackish water basins had become similar to the chloride concentration in the top water layer of the fresh water basins (F1 = 7.143, p>0.05) (Fig. 2B). This salinity stratification in the brackish water basins also became clear from the oxygen concentrations in the different water layers that, as opposed to the different water layers in the freshwater basins, showed a clear discontinuity between the top surface water layer and the bottom water layer (Fig. S3).
At the end of the experiment the dissolved inorganic phosphate concentrations in the basins started to increase due to the decomposition of dead biomass. The phosphate in the freshwater basins distributed equally throughout the water column, but in the brackish water basins the phosphate strongly accumulated in the top water layer (F5 = 356.132, p<0.001) (Fig. 2C).
Discussion
The results of the present research clearly show that when Azolla is growing in a brackish water environment it is able to generate small-scale salinity stratification (micro-halocline at approximately 5–7 cm) by muffling the force of the incoming rain and trapping the rainwater in between its biomass. For the Eocene Arctic basin it has been shown that salinity stratification was preserved during very long periods and that it was especially strong at times when spore abundances of Azolla arctica were very high [7], [8], [13]. The results of this research show that it is plausible that the presence of Azolla arctica in the Eocene Arctic basin contributed to the development of small-scale salinity gradients within the gradient that had already been established due to the enhanced freshwater input via rain and rivers [10], [11], not only by trapping rainwater, but also by diminishing the influence of wind action on the mixing of water layers.
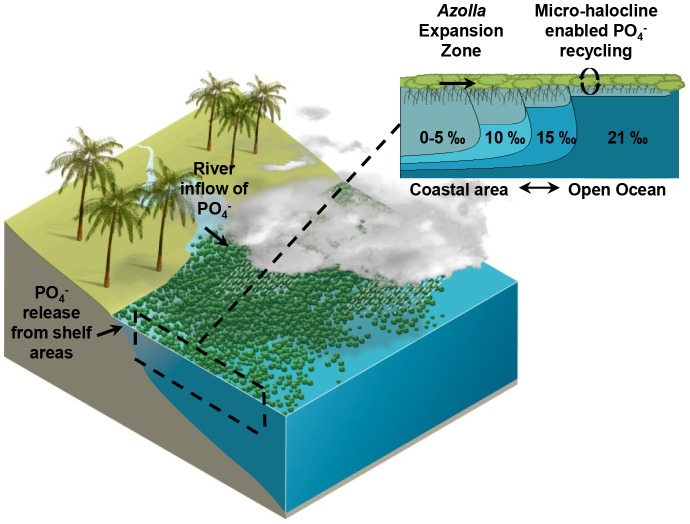
Present day Azolla species have been shown to be able to grow at salinities up to 7–10% [29], [30]. In addition, at even higher salt concentrations A. filiculoides may avoid salinity stress by shedding of roots as this temporarily prevents the development of ionic imbalances within the plant tissue (MML van Kempen, unpubl.). With regard to the Eocene era, such a protective mechanism, avoiding salinity stress at temporarily high salt concentrations, might have facilitated the expansion of Azolla arctica further away from the coast by helping it to survive sudden salinity changes and providing it with the opportunity to swiftly generate its own freshwater environment before regenerating its roots (Fig. 3).
Figure 3. Conceptual model showing how Azolla arctica may have colonized the Eocene Arctic Ocean using phosphate sources from coastal areas for expansion to the open ocean where small-scale salinity stratification allows for efficient recycling of nutrients to sustain the standing biomass.
In the mesocosm, decreasing temperatures resulted in a net mineralization of organic matter towards the end of the experiment. Evidently, the mineralization of the biomass mainly occurred in the surface water layer within the Azolla cover and, judging on the total dissolved phosphate concentration, was equally high in the fresh and brackish water basins. In the brackish water basins the nutrients that were released upon mineralization accumulated mostly in the top water layer, whereas in the freshwater basins these nutrients became distributed more equally throughout the water column. Most probably this was due to the salinity stratification in the brackish water basins, which can function as a chemical barrier [15]. Usually a halocline prevents phosphorus that is released from decomposing organic matter at the bottom of water bodies from reaching the water surface [15], [26]. In a floating Azolla mat, however, salinity stratification apparently works in a reverse way by preventing phosphorus that is released from biomass mineralization in the top surface water layer from becoming dispersed throughout the entire water body. Obviously, this strongly increases the potential recycling of nutrients within the system (Fig. 3).
Azolla is able to grow at low phosphate concentrations, but adding phosphate will almost always result in higher growth rates, even at very high concentrations [20], [22]. From the current research we conclude that Azolla acts as an ecosystem engineer being able to create a small scale salinity gradient within brackish waters in which potential salt stress is reduced and the efficient recycling of nutrients permits the maintenance of the standing biomass (Fig. 3). As such, we might presume that additional inputs of phosphate will ultimately result in a further expansion of an Azolla cover if other environmental factors are favourable and space is not limiting. With regard to the Eocene the release of phosphate from flooded shelf areas adjoining the Arctic basin and the entering of phosphate via river discharge, together with the micro-halocline enabled nutrient recycling at long distances from the coast, may have facilitated the enormous expansion of Azolla arctica from the coastal areas to the open ocean. Consequentially, our results may help to understand the extent of the Eocene Azolla event and may explain why no intact vegetative Azolla remains were found [7] and why the burial efficiency of organic carbon was found to be relatively low during this interval [31].
Supporting Information
Minimum water temperature ( C±SE) in the top water layers and the bottom water layers of the freshwater and brackish water basins and the minimum air temperature (°C) at 10 cm above ground level during the mesocosm experiment.
(TIF)
Nutrient concentrations (µM ± standard error) in the freshwater and brackish water basins during the mesocosm experiment. A) Calcium concentrations, B) Magnesium concentrations, C) Sodium concentrations, D) Potassium concentrations and E) Sulphate concentrations in the top, middle and bottom water layers of the basins.
(TIF)
Oxygen concentrations (mg L−1±SE) in the top water layers and the bottom water layers of the freshwater and brackish water basins during the mesocosm experiment.
(TIF)
Acknowledgments
We thank StatoilHydro for general support. Also, we thank Iris Bakker, Luuk Masselink and Martin Versteeg for their help with the mesocosm experiment and Jelle Eygensteyn, Germa Verheggen-Kleinheerenbrink and Roy Peters for their help in the chemical analyses. We thank Ronald Visser for designing the graphics in figure 3.
Funding Statement
Funding for this research was partially provided by the Darwin Centre for Biogeosciences (Program number 1070, project number 1071, http://www.darwincenter.nl/DarwinCenter.aspx?id=504&sub=proj&idsub=17). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
References
- 1. Carrapiço F (2002) The Azolla-Anabaena-Bacteria System as a Natural Microcosm. Proc. SPIE 4495: 261–265. [Google Scholar]
- 2. Peters GA, Mayne BC (1974a) Azolla, Anabaena azollae relationship. 1. Initial characterization of association. Plant Physiol. 53: 813–819 doi: 10.1104/.53.6.813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peters GA, Mayne BC (1974b) The Azolla, Anabaena azollae relationship: II. Localization of nitrogenase activity as assayed by acetylene reduction. Plant Physiol. 53: 820–824 doi: 10.1104/.53.6.820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collinson ME (2001) Cainozoic ferns and their distribution. Brittonia 53: 173–235 doi: 10.1007/BF02812700 [Google Scholar]
- 5. Collinson ME (2002) The ecology of Cainozoic ferns. Rev. Palaeobot. Palyno. 119: 51–68 doi: 10.1016/S0034–6667(01)00129–4 [Google Scholar]
- 6. Collinson ME, Barke J, van der Burgh J, van Konijnenburg-van Cittert JHA (2009) A new species of the freshwater fern Azolla (Azollaceae) from the Eocene Arctic Ocean. Rev. Palaeobot. Palyno. 155: 1–14 doi: 10.1016/j.revpalbo.2008.12.014 [Google Scholar]
- 7. Brinkhuis H, Schouten S, Collinson ME, Sluijs A, Sinninghe Damste JS, et al. (2006) Episodic fresh surface waters in the Eocene Arctic Ocean. Nature 441: 606–609 doi: 0.1038/nature04692 [DOI] [PubMed] [Google Scholar]
- 8. Barke J, van der Burgh J, van Konijnenburg-van Cittert JHA, Collinson ME, Pearce MA, et al. (2011) Orbitally forced Azolla blooms and Middle Eocene Arctic hydrology: Clues from palynology. Geology 39: 427–430 doi: 10.1130/G31640 [Google Scholar]
- 9. Milankovitch M (1948) Ausbau und gegenwartiger stand der astronomischen theorie der erdgeschichtelichen klimate. Experientia 4: 413–418 doi: 10.1007/BF021449 [DOI] [PubMed] [Google Scholar]
- 10. Huber M, Caballero R (2003) Eocene El Nino: Evidence for robust tropical dynamics in the “hothouse”. Science 299: 877–881 doi: 10.1126/science.1078766 [DOI] [PubMed] [Google Scholar]
- 11. Speelman EN, Sewall JO, Noone D, Huber M, von der Heydt A, et al. (2010) Modeling the influence of a reduced equator-to-pole sea surface temperature gradient on the distribution of water isotopes in the Early/Middle Eocene. Earth Planet. Sc. Lett. 298: 57–65 doi: 10.1016/j.epsl.2010.07.026 [Google Scholar]
- 12. Jakobsson M, Backman J, Rudels B, Nycander J, Frank M, et al. (2007) The early Miocene onset of a ventilated circulation regime in the Arctic Ocean. Nature 447: 986–990 doi: 10.1038/nature05924 [DOI] [PubMed] [Google Scholar]
- 13. Stickley CE, Koc N, Brumsack HJ, Jordan RW, Suto I (2008) A siliceous microfossil view of middle Eocene Arctic paleoenvironments: A window of biosilica production and preservation. Paleoceanography 23: 19 doi: 10.1029/2007PA001485 [Google Scholar]
- 14. Waddell LM, Moore TC (2008) Salinity of the Eocene Arctic Ocean from oxygen isotope analysis of fish bone carbonate. Paleoceanography 23: PA1S12 doi: 10.1029/2007PA001451 [Google Scholar]
- 15.Wetzel (2001) Limnology: Lake and River Ecosystems 3rd ed. San Diego Academic Press.
- 16. Knies J, Mann U, Popp BN, Stein R, Brumsack HJ (2008) Surface water productivity and paleoceanographic implications in the Cenozoic Arctic Ocean. Paleoceanography 23: 12 doi: 10.1029/2007PA001455 [Google Scholar]
- 17. Bauersachs T, Speelman EN, Hopmans EC, Reichart GJ, Schouten S, et al. (2010) Fossilized glycolipids reveal past oceanic N2 fixation by heterocystous cyanobacteria. Proc. Natl. Acad. Sci. U. S. A. 107: 19190–19194 doi: 10.1073/pnas.1007526107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yanni YG (1992) Contribution of inoculation with Azolla combined with nitrogen, phosphorus and zinc to rice in Nile Delta. World J. Microb. Biot. 8: 579–584 doi: 10.1007/BF01238792 [DOI] [PubMed] [Google Scholar]
- 19. Wagner GM (1997) Azolla: A review of its biology and utilization. Bot. Rev. 63: 1–26 doi: 10.1007/BF02857915 [Google Scholar]
- 20. Tung HF, Watanabe I (1983) Differential response of Azolla anabaena associations to high temperature and minus phosphorus treatments. New Phytol. 93: 423–431 doi: 10.1111/j.1469–8137.1983.tb03442.x [Google Scholar]
- 21. Sah RN, Goyal SS, Rains DW (1989) Interactive effects of exogenous combined nitrogen and phosphorus on growth and nitrogen-fixation by Azolla . Plant Soil 117: 1–8 doi: 10.1007/BF02206251 [Google Scholar]
- 22. Cary PR, Weerts PGJ (1992) Growth and nutrient composition of Azolla pinnata R. Brown and Azolla filiculoides Lamarck as affected by water temperature, nitrogen and phosphorus supply, light intensity and pH. Aquat. Bot. 43: 163–180 doi: 10.1016/0304–3770(92)90041-G [Google Scholar]
- 23. Pokorny J, Rejmankova E (1983) Oxygen regime in a fishpond with duckweeds (Lemnaceae) and Ceratophyllum. Aquat. Bot. 17: 125–137 doi: 10.1016/0304–3770(83)90109–2 [Google Scholar]
- 24. Janes R (1998) Growth and survival of Azolla filiculoides in Britain - I. Vegetative reproduction. New Phytol. 138: 367–375 doi: 10.1046/j.1469-8137.1998.00114.x [DOI] [PubMed] [Google Scholar]
- 25. Smolders AJP, Lamers LPM, Lucassen ECHET, van der Velde G, Roelofs JGM (2006) Internal eutrophication: How it works and what to do about it - a review. Chem. Ecol. 22: 93–111 doi: 10.1080/02757540600579730 [Google Scholar]
- 26. Slomp CP, Van Cappellen P (2007) The global marine phosphorus cycle: sensitivity to oceanic circulation. Biogeosciences 4: 155–171 doi:10.5194/bg-4-155–2007 [Google Scholar]
- 27. Lumpkin TA, Plucknett DL (1980) Azolla – Botany, physiology and use as a green manure. Econ. Bot. 34: 111–153 doi: 10.1007/BF02858627 [Google Scholar]
- 28.De Lyon MJH, Roelofs JGM (1986) Waterplanten in relatie tot waterkwaliteit en bodemgesteldheid. Katholieke Universiteit Nijmegen, Laboratorium voor Aquatische Oecologie.
- 29. Haller WT, Sutton DL, Barlowe WC (1974) Effects of salinity on growth of several aquatic macrophytes. Ecology 55: 891–894 doi: 10.2307/1934427 [Google Scholar]
- 30.Deng-Hui S, Ho W, Xi-Pan C (1985) The cultivation of Azolla filiculoides for the reclamation and utilization of heavy saline soils, p 274. In Azolla utilization: proceedings of the workshop on Azolla use. IRRI publications. ISBN 971-104–179–0.
- 31. Speelman EN, van Kempen MML, Barke J, Brinkhuis H, Reichart GJ, et al. (2009) The Eocene Arctic Azolla bloom: environmental conditions, productivity and carbon drawdown. Geobiology 7: 155–170 doi: 10.1111/j.1472-4669.2009.00195.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Minimum water temperature ( C±SE) in the top water layers and the bottom water layers of the freshwater and brackish water basins and the minimum air temperature (°C) at 10 cm above ground level during the mesocosm experiment.
(TIF)
Nutrient concentrations (µM ± standard error) in the freshwater and brackish water basins during the mesocosm experiment. A) Calcium concentrations, B) Magnesium concentrations, C) Sodium concentrations, D) Potassium concentrations and E) Sulphate concentrations in the top, middle and bottom water layers of the basins.
(TIF)
Oxygen concentrations (mg L−1±SE) in the top water layers and the bottom water layers of the freshwater and brackish water basins during the mesocosm experiment.
(TIF)