Young female patients with localized breast cancer undergoing adjuvant or neoadjuvant anthracycline- or taxane-based chemotherapy were evaluated using transvaginal ultrasound and Doppler-flow velocity indices of the ovarian vasculature to assess chemotherapy-induced ovarian toxicity.
Keywords: Chemotherapy, Vascular toxicity, Ovary, Premenopausal, Breast cancer
Abstract
Background.
Chemotherapy-related amenorrhea is a frequent side effect observed in young breast cancer patients. Studies in mice revealed that chemotherapy-induced gonadal toxicity may result from vascular damage. We prospectively evaluated ovarian blood flow and function in young breast cancer patients following chemotherapy.
Methods.
Young female patients with localized breast cancer undergoing adjuvant or neoadjuvant anthracycline- or taxane-based chemotherapy were evaluated using transvaginal ultrasound prior to initiation of and immediately after cessation of chemotherapy. Doppler-flow velocity indices of the ovarian vasculature—resistance index (RI), pulsatility index (PI)—and size measurements were visualized. Hormonal profiles, anti-Müllerian hormone (AMH) levels, and menopausal symptoms were assessed at the same time points.
Results.
Twenty breast cancer patients were enrolled in the study. The median age was 34 ± 5.24 years. Ovarian blood flow was significantly reduced shortly following chemotherapy: RI decreased by 52.5% and PI decreased by 24.2%. The mean ovarian size declined by 19.08%. Patients who were treated with sequential chemotherapy experienced further reductions in ovarian blood flow and ovarian size after the second sequence. AMH levels dropped dramatically in all patients following treatment. Hormonal profiles after treatment depicted a postmenopausal profile for most patients, accompanied by related symptoms.
Conclusions.
Our results may imply a mechanism of chemotherapy-induced ovarian toxicity manifested by decreased ovarian blood flow accompanied by a reduction in ovarian size and diminished post-treatment AMH levels. Based upon our former preclinical studies, we assume that this may derive from an acute insult to the ovarian vasculature and may represent an initial event triggering a generalized phenomenon of end-organ toxicity.
Introduction
Seminal improvements in adjuvant systemic therapy have led to increased survival rates among women diagnosed with early breast cancer. It was recently shown that premenopausal women in whom amenorrhea developed as a consequence of adjuvant therapy had significantly better overall survival and disease-free survival outcomes than did women without amenorrhea, particularly when the tumor was estrogen receptor positive [1]. The risk for chemotherapy-related amenorrhea (CRA) is associated with patient age and the chemotherapeutic agents used, with age >40 years being the strongest predictor of ovarian failure [2]. Few prospective studies have been performed to assess the risk for amenorrhea from contemporary adjuvant therapy regimens [1, 3–9], implicating that treatment-induced amenorrhea by alkylating agents has been considered the prototype for biochemical oophorectomy. In a recent study in women with breast cancer receiving polychemotherapy regimens (including doxorubicin and taxanes), the incidence of CRA was substantial: 41% of women (median age, 39 years; range, 20–45 years) experienced an initial 6-month period of amenorrhea after chemotherapy [9]. However, most of the studies that addressed the incidence of CRA documented the lack of menses starting 6 months from the completion of chemotherapy and at later time points.
The mechanism that lies at the core of chemotherapy-induced ovarian failure, manifested by amenorrhea, remains to be elucidated. Moreover, no study has addressed the subacute effects of chemotherapy on ovarian function. In a former preclinical study, we established an in vivo platform in a mouse model of innovative high-resolution molecular imaging suitable for in vivo imaging of vessel characteristics, arterial blood flow, and organ blood volume that enables prolonged acute real-time detection of chemotherapy-induced effects in the same individuals. Following doxorubicin administration, we observed an acute reduction in ovarian blood flow and impairment in blood vessel walls [10]. Few studies have addressed the issue of chemotherapy-induced vascular toxicity as a precursor to acute and late organ toxicity and cardiovascular complications as well as a possible contributor to the initiation and progression of atherosclerosis [11–13]. In patients with disseminated testicular cancer treated with the bleomycin, etoposide, and cisplatin regimen, an increase in plasma von Willebrand factor (vWF) levels and common carotid intima media thickness (IMT) were noted [13]. In a pilot study assessing endothelial toxicity from anthracyclines in pediatric cancer patients, brachial artery diameters were measured. The brachial artery response to cuff occlusion was significantly lower than in controls who had not been exposed to anthracycline-based chemotherapy, suggesting that anthracyclines cause impaired endothelial function [14]. Our current protocol aimed to study the short-term effect of chemotherapy on ovarian architecture and function, and to characterize the correlation between morphological and vascular changes, ovarian function, and quality of life measures immediately after the administration of chemotherapy, prospectively, whereby each patient served as her own control.
Methods
Patients
Study participants were premenopausal women aged <43 years with regular spontaneous menstruation who were not previously exposed to chemotherapy and hormonal therapy and who intended to undergo neoadjuvant or adjuvant chemotherapy for nonmetastatic breast cancer. At the time of enrollment, information regarding demographics, reproductive and menstrual histories, and tumor characteristics was collected. The protocol was approved by the institutional review board of our institute and all patients signed an informed consent form.
Study Design and Measurements
Chemotherapy regimens were either anthracycline- or cyclophosphamide-based protocols followed by taxanes or taxane-based treatment plus trastuzumab for patients with human epidermal growth factor receptor –2 positive tumors in the neoadjuvant setting.
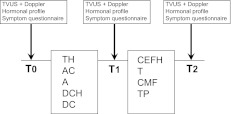
A gonadotropin-releasing hormone (GnRH) agonist was either administered or not based on the oncologist's preference prior to chemotherapy (3–7 days) and throughout the treatment period. Study measurements were performed within 1 week before the onset of chemotherapy (baseline) and within 3–4 days after the last course of chemotherapy (T1). In cases of GnRH agonist administration, baseline measurements were performed before GnRH agonist treatment. For patients who were treated with a sequential regimen, an additional measurement was performed within 3–4 days of the last dose of the second sequence (T2) (Fig. 1). If patients were to be treated for ovulation induction (for embryo or egg preservation), baseline measurements were performed before the administration of ovulation-inducing drugs. For all parameters, values after chemotherapy were compared with pretreatment values and every patient served as her own control.
Figure 1.
Time schedule of chemotherapy sequence and study measurements.
Abbreviations: A, doxorubicin; AC, doxorubicin and cyclophosphamide; CEFH, cyclophosphamide, epirubicin, 5-fluorouracil, and trastuzumab; CMF, cyclophosphamide, methotrexate, and 5-fluorouracil; DC, docetaxel, cyclophosphamide; DCH, docetaxell, carbplatinum, and trastuzumab; T, paclitaxel; TH, paclitaxel and trastuzumab; TP, paclitaxel, cisplatinum; TVUS, transvaginal ultrasound; T0, baseline; T1, after first chemotherapy sequence; T2, after second chemotherapy sequence.
Markers of Ovarian Function
Serum samples were collected at the aforementioned time points and stored in aliquots at −80°C until measurement. At each time point, serum anti-Müllerian hormone (AMH) and follicle-stimulating hormone (FSH) levels were measured. AMH was measured using an enzyme-linked immunoadsorbent assay (Diagnostic Systems Laboratories, Inc., Webster, TX) with sensitivity of 0.01 ng/mL.
Sonographic Measurements of the Ovaries and Data Acquisition of Blood Flow
Transvaginal sonography was performed in all patients by the same physician (I.M.) to avoid interobserver variability at the same time intervals selected for the serum collection and symptom survey. Measurements were performed in a blinded fashion (i.e., the observer had no data with regard to previous evaluations or type of treatment). A transvaginal ultrasound assessment of the ovaries was performed to determine ovarian volume (two measurements). Spectral Doppler sonographic examinations were performed at each time point. Doppler sonographic examinations were performed using an HDI 5000 ultrasound scanner (Vluson E8; GE Healthcare, Milwaukee, WI) with a 6- to 12-MHz vaginal transducer. After the ovarian vessels were identified, the resistance index (RI) and the pulsatility index (PI) were measured according to the following formula: PI = (S − D)/mean, RI = (S − D)/S, where S is the peak shifted Doppler frequency, D is the minimum Doppler shifted frequency, and mean is the mean maximum Doppler shifted frequency over the cardiac cycle.
Determining Menopausal Symptoms
A symptom survey (which included menstrual status determination) was performed on the schedule described for blood marker and sonographic evaluations. Pretreatment and post-treatment surveys of menopausal symptoms included questions regarding vaginal dryness, hot flashes, and sweating according to National Cancer Institute Common Toxicity Criteria, version 2 (NCI- CTC, v2) scales.
Statistical Evaluation
Quantitative measurements are presented as means ± standard deviations (SDs), medians, and maximums and minimums. Categorical measurements are presented using their percentages. Measurements were compared by treatment with a GnRH agonist.
Ovarian Measures
Because every patient served as her own control and no significant differences were noted between the axial measurements of the two ovaries in any patient (by performing a paired t-test), the data for the ovarian volume of the left and right ovaries were calculated together and the mean measurement of the two sides in an individual patient was used for statistical analyses. Changes in parameters over time were assessed using the paired t-test and nonparametric related-samples Wilcoxon test. For each parameter, a difference score was calculated: variable value before the onset of chemotherapy minus variable value after chemotherapy. Associations were estimated by means of the Spearman rank correlation coefficient.
Blood Markers and Menstrual Status
At each time point, the means and SDs of serum AMH and FSH levels were calculated for all patients. The paired t-test and Wilcoxon paired test were also used to test for differences in hormone levels at different time points before and after chemotherapy. Values of amenorrhea at all time points were tested with the Fisher's exact test. For determining differences in grades of symptoms, the Wilcoxon paired test was performed for pre- and post-treatment values. For all outcomes, p-values < .05 were considered significant. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) for Windows, release 18 (SPSS Inc, Chicago, IL).
Results
Patient Characteristics
Twenty patients were enrolled in the study from July 2009 to March 2011, signed an informed consent form, and completed the analysis (Table 1). The median age was 34 years (range, 26–43 years). Six patients were diagnosed with stage I disease, 10 had stage II disease, and four had stage III disease. Nine patients were treated with adjuvant chemotherapy and 11 were treated with neoadjuvant chemotherapy. The treatment was selected by the primary oncologist according to the tumor characteristics and the commonly used regimens in our institute. Seven patients were treated with trastuzumab-containing regimens. Four patients received regimens lacking cyclophosphamide. Three patients received regimens lacking anthracyclines. All patients were referred to a reproductive endocrinologist for fertility preservation. In 13 patients, embryo or egg preservation was performed. Ten patients were treated with a GnRH agonist concomitant with chemotherapy according to the physician's preference as an ancillary method for fertility preservation. All patients were menstruating at study enrollment; two of them experienced mild sweating, hot flashes, and vaginal dryness (NCI-CTC v2 grade 1) at baseline.
Table 1.
Patient characteristics
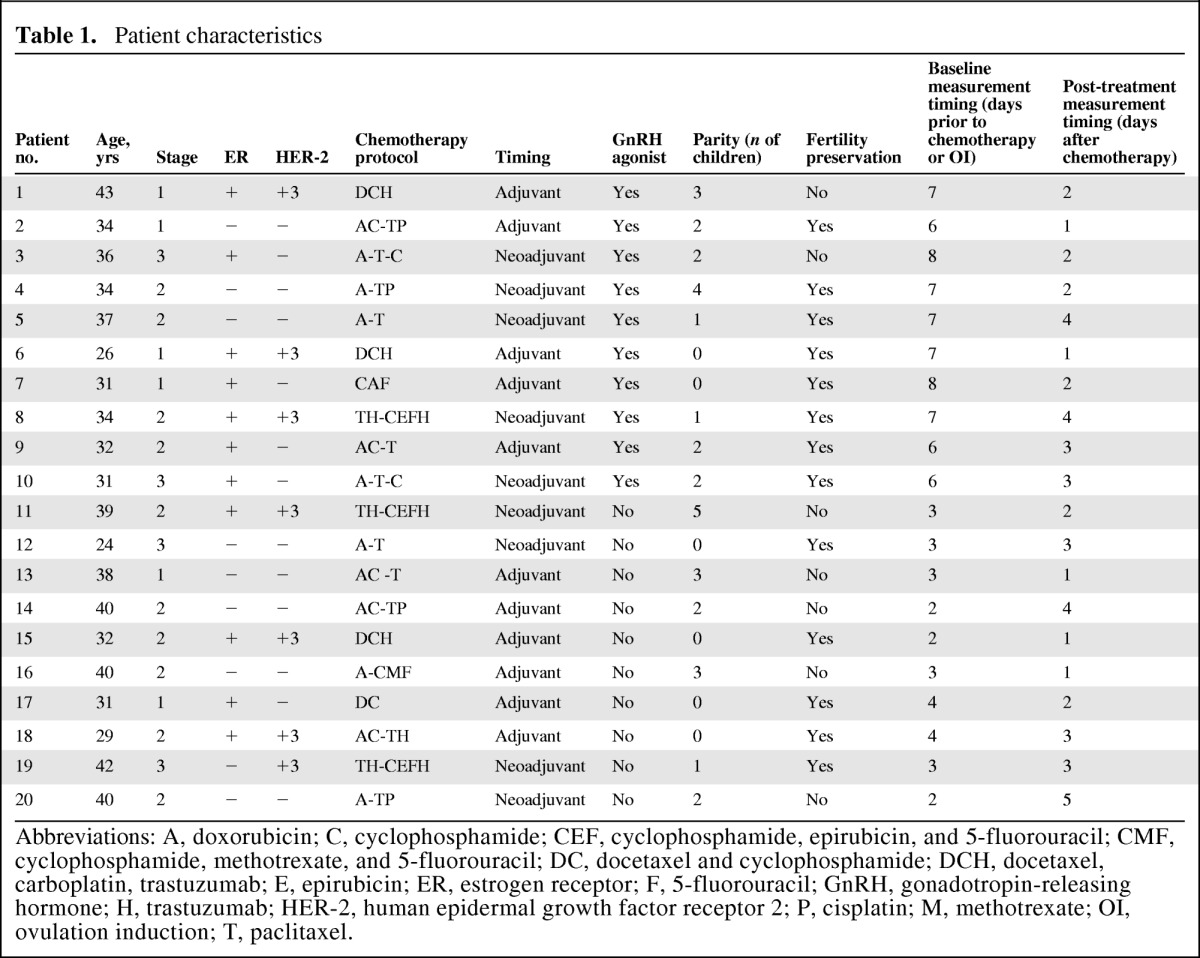
Abbreviations: A, doxorubicin; C, cyclophosphamide; CEF, cyclophosphamide, epirubicin, and 5-fluorouracil; CMF, cyclophosphamide, methotrexate, and 5-fluorouracil; DC, docetaxel and cyclophosphamide; DCH, docetaxel, carboplatin, trastuzumab; E, epirubicin; ER, estrogen receptor; F, 5-fluorouracil; GnRH, gonadotropin-releasing hormone; H, trastuzumab; HER-2, human epidermal growth factor receptor 2; P, cisplatin; M, methotrexate; OI, ovulation induction; T, paclitaxel.
Sonographic Measurement
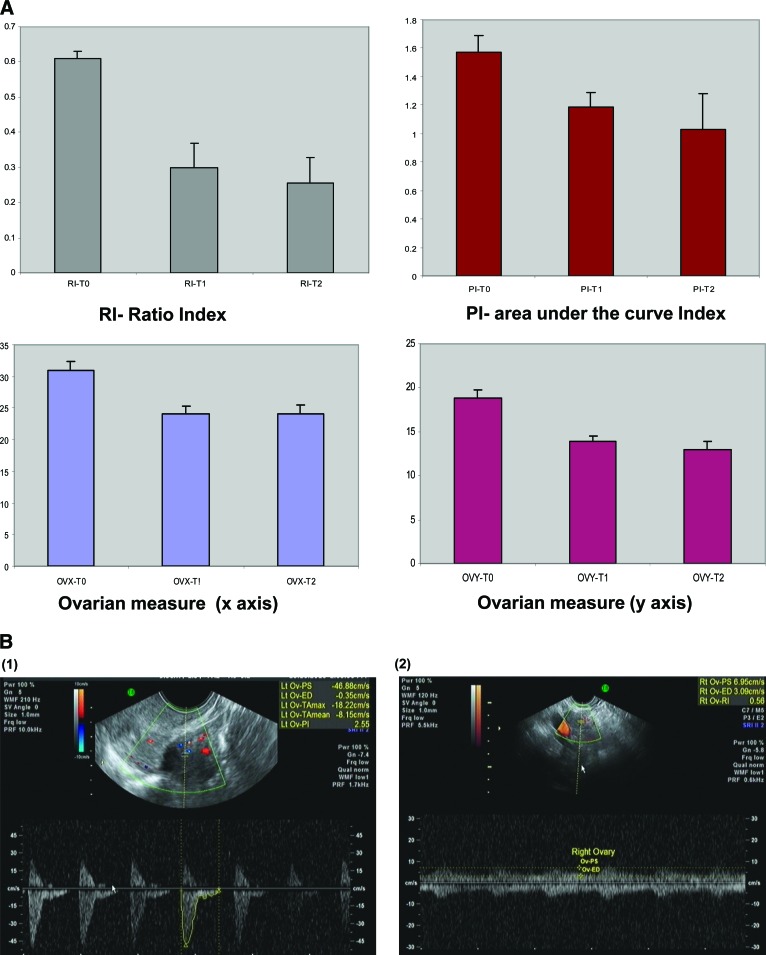
Data were collected for every ovary in each patient. Paired t-tests depicted a similar pattern for both ovaries of each patient for blood flow and volume. Because of the lack of statistical difference between the ovaries, mean values were calculated for each patient for the RI, PI, and ovarian bidimensional values (x, y). The RI and PI of the ovarian vessels decreased significantly after treatment, compared with baseline values, as depicted in Figure 1 and Table 2. The RI dropped by 52.5% at the end of the first treatment (T1) and remained low at the next measurement. The PI depicted the same pattern and dropped by 24.8% at the end of the first treatment and further declined at the second post-treatment measurement by 38.9% from the baseline value. Ovarian volume declined on both axes by 14.6% (x-axis) and 24.4% (y-axis) at the end of the first treatment; both values had declined further at the next post-treatment measurement (x-axis, −20.6%; y-axis, −33.26%). For all measurements, when compared with baseline, the p-value was <.05, as shown in Figure 2 and Table 2. Four patients had only a mild alteration in their ovarian blood flow. None of them received cyclophosphamide. Half of the cohort received a GnRH agonist. Patients treated with a GnRH agonist showed the same trend of ovarian toxicity pattern as patients who were not treated with a GnRH agonist (p < .05) (supplemental online Table 1). Stratification of results by patient age reveled that younger patients (five with a median age of 31 years and 15 with a median age of 36 years) had a smaller, yet significant, decrease in ovarian blood flow.
Table 2.
Mean change in ovarian sonographic measurements
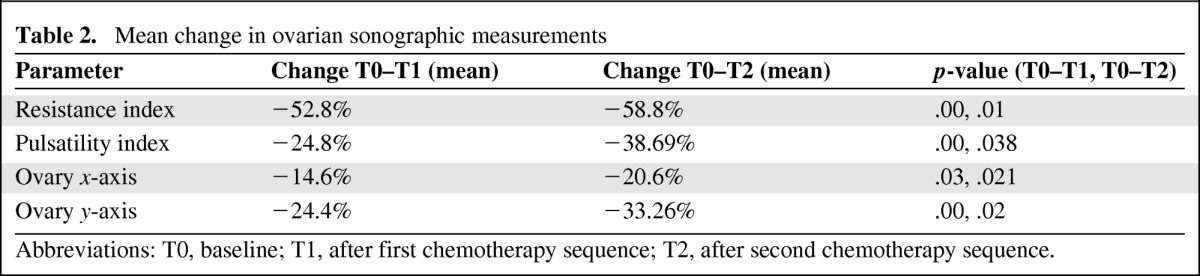
Abbreviations: T0, baseline; T1, after first chemotherapy sequence; T2, after second chemotherapy sequence.
Figure 2.
Changes in ovarian measurements using transvaginal ultrasound equipped with Doppler. (A): Graphic representation of change in ovarian measurements over time, indicating a decrease in ovarian blood flow following chemotherapy (p < .01). (B): Representative captured images of Doppler ultrasound performed at a fixed region of interest at T0 and T1.
Abbreviations: T0, baseline; T1, after first chemotherapy sequence; T2, after second chemotherapy sequence.
Hormonal Measurement
Pretreatment values for all patients were compatible with a premenopausal status (Table 3), whereby the mean FSH level was 5.882 IU/mL (SD, 0.99). Following treatment, the values depicted a postmenopausal pattern of rising FSH, to 35.9 IU/mL (SD, 8.89), that further increased by the second measurement (following the second phase of the protocol).
Table 3.
Hormonal measurements of ovarian function and reserve
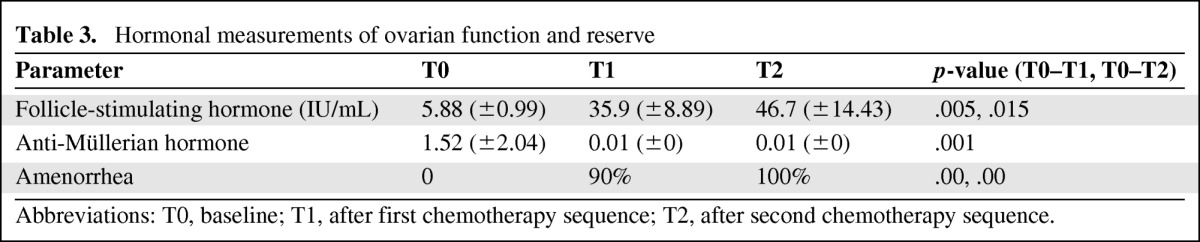
Abbreviations: T0, baseline; T1, after first chemotherapy sequence; T2, after second chemotherapy sequence.
In all patients, the post-treatment AMH level declined remarkably to an undetectable value, regardless of the pretreatment AMH level. For all measurements, the p-value was <.01. Patients treated with a GnRH agonist showed the same trend of hormonal alterations as patients who were not treated with a GnRH agonist (p < .05) (supplemental online Table 2).
Symptom Measurement
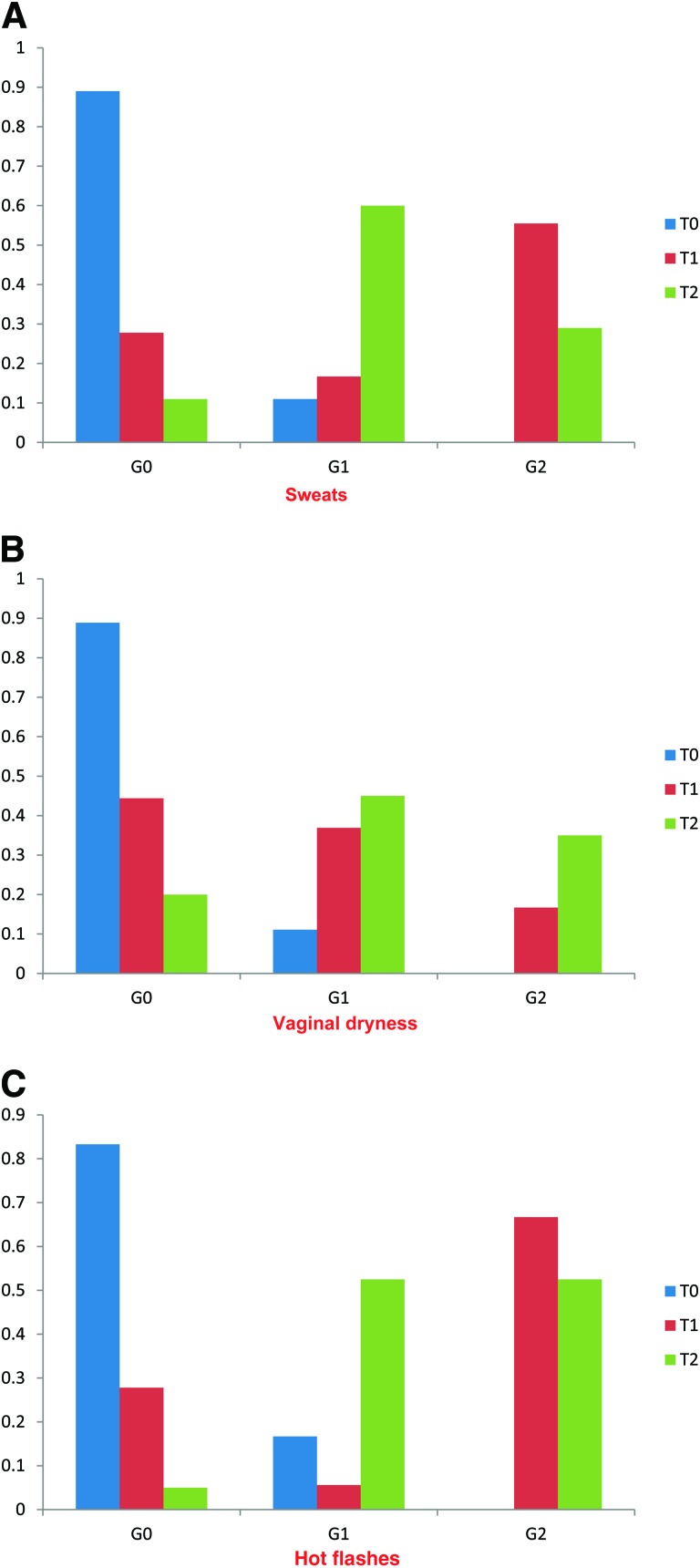
Symptoms such as vaginal dryness, hot flashes, and sweating were highly correlated with the postmenopausal pattern observed in hormonal markers. All patients experienced either new symptoms or worsening of existing symptoms (in two patients), as illustrated in Figure 3. The severity of hot flashes was higher (more grade 2 cases) than those of vaginal dryness and sweating. The differences between symptom grades at all time points were statistically significant. Multivariate repeated measures modeling demonstrated significant associations between symptom severity and hormonal levels at each time point. There was no difference in the pattern of symptom severity in patients treated with a GnRH agonist and patients who had not received this agent (p < .01).
Figure 3.
Change in degree of menopausal symptoms over time. Graphic representation of change in menopausal symptoms according to NCI-CTC v2 over time, indicating worsening of symptoms over time following chemotherapy (p < .01). (A): Vaginal dryness. (B): Sweating. (C): Hot flashes.
Abbreviations: G, grade; NCI-CTC v2, National Cancer Institute Common Toxicity Criteria, version 2; T0, baseline; T1, after first chemotherapy sequence; T2, after second chemotherapy sequence.
Discussion
In this pivotal prospective study, we characterized subacute chemotherapy-induced ovarian toxicity in a cohort of young breast cancer patients, which may be mediated via vascular insult. To our knowledge, this is the first documentation of an acute ovarian effect following chemotherapy and the first evidence for a correlation between vascular insult and alteration in ovarian blood flow, size, and function manifested by an abnormal hormonal profile and clinical symptoms. Our cohort consisted of 20 premenopausal patients with nonmetastatic breast cancer who were treated with neoadjuvant or adjuvant chemotherapy according to their tumor characteristics. Despite the variability in chemotherapeutic regimens and the age range of the patients, the observed pattern of acute ovarian failure was similar and homogeneous in all patients. At the post-treatment assessment, all patients had amenorrhea, but the most remarkable finding was a statistically significant decrease in ovarian blood flow. In patients who had been treated with sequential chemotherapy, blood flow decreased further after the second sequence of chemotherapy. Of note, four patients who had only a mild alteration in their ovarian blood flow had not received cyclophosphamide. The reduction in ovarian size was consistent with former studies in which patients were monitored at 6-month intervals after treatment [9, 15]. Furthermore, this pattern of vascular flow decline in the subacute phase following chemotherapy has not been described previously. There was a correlation between the morphological alterations in ovarian blood flow and volume, and the typical pattern of ovarian dysfunction observed in the serum hormonal profile (FSH). A striking elimination of serum AMH was observed in all patients. AMH has been acknowledged as a useful marker of ovarian dysfunction and predictor of chemotherapy-induced ovarian failure [16, 17]. Nevertheless, there are no data regarding the pattern of AMH alteration in the subacute phase following chemotherapy. Most studies were designed to assess fertility outcomes, and hence have portrayed the alteration in AMH levels at a minimum of 6 months postchemotherapy, because AMH is considered a reliable marker of ovarian reserve [18]. AMH is produced by early growing follicles at all stages up to the early antral stage [19], but it is unknown which fraction of follicle is the dominant AMH secretor. Former data suggest that chemotherapy is harmful mostly to the fraction of growing follicles [20]. The drastic decline in AMH observed in the current study may reflect the increasing apoptotic rate in this follicle class and the pronounced apoptosis of granulosa cells (which produce AMH in each follicle).
Most patients had developed the typical postmenopausal hormonal profile at the cessation of chemotherapeutic treatment. There also was a clear correlation between the hormonal profile and menopausal symptoms, such as hot flashes and vaginal dryness, experienced by the patients following treatment.
Half of the cohort received a GnRH agonist as an ancillary method for fertility preservation (in addition to embryo or egg preservation). There was no difference in any outcome between these patients and the patients who were not treated with a GnRH agonist. These findings are consistent with previous reports in which, in contrast to the effect of chemotherapy on ovarian volume and antral follicle count, gonadotrophin suppression by a GnRH agonist did not result in any significant change in either measure [21, 22].
The results of the current study correlate with our former preclinical study in mice, in which in vivo administration of doxorubicin resulted in a lower ovulation rate and smaller ovarian size, as observed by high-resolution magnetic resonance imaging [20]. An indication for acute insult to the ovary, reflected by the presence of periovarian edema, encouraged us to further investigate the potential acute vascular effect using a platform of live, high-resolution molecular mice imaging, suitable for capturing vessel characteristics, arterial blood flow, and organ blood volume. We followed the ovarian vasculature after doxorubicin administration and observed an acute reduction in ovarian blood flow (of 33% from baseline values) as well as impairment in blood vessel walls as visualized by fibered fluorescent endoscopic confocal microscopy [10].
Of all the chemotherapy-induced side effects, direct vascular injury is the least characterized. Few clinical studies have explored the incidence and characteristics of chemotherapy-induced vascular toxicity. Acute chemotherapy-induced vascular damage, reflected as increases in the plasma vWF level and IMT of the carotid artery, was studied in vivo in patients suffering from testicular cancer and treated with cisplatin-based chemotherapy [13]. This phenomenon also was described in 42 survivors of Hodgkin's lymphoma and in 51 survivors of nasopharyngeal carcinoma after radiation therapy to the neck, in whom the IMT was greater than in healthy controls [23, 24]. Brachial artery reactivity, a marker of endothelial vasodilatation, detected by high-resolution ultrasound was lower in pediatric patients who received ≥300 mg/m2 doxorubicin (or daunorubicin) than in control patients [14]. Furthermore, brachial artery flow–mediated dilation in patients undergoing doxorubicin-based chemotherapy was markedly attenuated after a single dose of doxorubicin [25]. Recently, it was shown, using phase-contrast cardiovascular magnetic resonance measurements of pulse wave velocity and aortic distensibility, that anthracyclines induce a significant thoracic aorta stiffening in patients receiving anthracyclines, compared with age- and sex-matched controls [26]. Our study links the paradigm of ovarian vascular toxicity as a possible contributor to the etiology of chemotherapy-induced ovarian dysfunction, manifested by amenorrhea.
Because each patient served as her own control and because the pattern of ovarian blood flow impairment was consistent and overt in all patients, the study reached statistical significance despite the modest sample size. There was a clear trend for a greater risk for ovarian failure in older patients, as expected, and in patients who were treated with a cyclophosphamide-based regimen.
Several studies in the past decade have appraised the rate of chemotherapy-related amenorrhea, and lately more studies also employed a quantitative facet in the form of biomarkers for ovarian reserve, such as AMH [27]. Nevertheless, the studies followed these parameters at later periods post-treatment, usually ≥6 months from the completion of chemotherapy.
The discrepancy in the well-established data regarding CRA rates >6 months post-treatment, which vary in the range of 20%–80% depending on patient age and the chemotherapeutic protocol, may derive from the differential recovery potential of ovarian blood flow (i.e., it may be reversible in younger patients and permanently compromised in older patients). The association between aging and vascular stiffness was established formerly in other organs [28].
A suggestion for future research is prospective continuous evaluation of ovarian measurements, including blood flow and biomarkers, over time. Acute ovarian failure resulting from vascular injury may represent acute universal chemotherapy-related vascular toxicity, an initial event in end-organ injury. Further studies to establish the incidence of acute ovarian failure are warranted as well as the evaluation of other end-organ toxicity as a reference tool. Chemotherapy-related amenorrhea plays a pivotal role in the nature of the disease in premenopausal breast cancer patients, and it is therefore prudent to explore this phenomenon. Moreover, the pathophysiology of developing CRA has a potential impact on our understanding of the practical issues of chemotherapy-induced ovarian toxicity with respect to future infertility.
Author Contributions
Conception/Design: Irit Ben-Aharon, Salomon M. Stemmer
Provision of study material or patients: Irit Ben-Aharon, Israel Meizner, Tal Granot, Salomon M. Stemmer, Shulamith Rizel
Collection and/or assembly of data: Irit Ben-Aharon, Israel Meizner, Tal Granot, Shiri Uri, Noa Hasky, Rinat Yerushalmi
Data analysis and interpretation: Irit Ben-Aharon, Israel Meizner, Tal Granot, Aaron Sulkes, Salomon M. Stemmer
Manuscript writing: Irit Ben-Aharon
Final approval of manuscript: Irit Ben-Aharon, Israel Meizner, Tal Granot, Shiri Uri, Noa Hasky, Shulamith Rizel, Rinat Yerushalmi, Aaron Sulkes, Salomon M. Stemmer
References
- 1.Swain SM, Jeong JH, Geyer CE, Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knobf MT. The influence of endocrine effects of adjuvant therapy on quality of life outcomes in younger breast cancer survivors. The Oncologist. 2006;11:96–110. doi: 10.1634/theoncologist.11-2-96. [DOI] [PubMed] [Google Scholar]
- 3.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 4.Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769–5779. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 5.Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: A prospective study. J Clin Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 6.Oktay K, Oktem O, Reh A, et al. Measuring the impact of chemotherapy on fertility in women with breast cancer. J Clin Oncol. 2006;24:4044–4046. doi: 10.1200/JCO.2006.06.9823. [DOI] [PubMed] [Google Scholar]
- 7.Fornier MN, Modi S, Panageas KS, et al. Incidence of chemotherapy-induced, long-term amenorrhea in patients with breast carcinoma age 40 years and younger after adjuvant anthracycline and taxane. Cancer. 2005;104:1575–1579. doi: 10.1002/cncr.21385. [DOI] [PubMed] [Google Scholar]
- 8.Abusief ME, Missmer SA, Ginsburg ES, et al. The effects of paclitaxel, dose density, and trastuzumab on treatment-related amenorrhea in premenopausal women with breast cancer. Cancer. 2010;116:791–798. doi: 10.1002/cncr.24835. [DOI] [PubMed] [Google Scholar]
- 9.Sukumvanich P, Case LD, Van Zee K, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: A prospective study. Cancer. 2010;116:3102–3111. doi: 10.1002/cncr.25106. [DOI] [PubMed] [Google Scholar]
- 10.Bar-Joseph H, Ben-Aharon I, Tzabari M, et al. In vivo bioimaging as a novel strategy to detect doxorubicin-induced damage to gonadal blood vessels. PLoS One. 2011;6:e23492. doi: 10.1371/journal.pone.0023492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bissett D, Kunkeler L, Zwanenburg L, et al. Long-term sequelae of treatment for testicular germ cell tumours. Br J Cancer. 1990;62:655–659. doi: 10.1038/bjc.1990.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger CC, Bokemeyer C, Schneider M, et al. Secondary Raynaud's phenomenon and other late vascular complications following chemotherapy for testicular cancer. Eur J Cancer. 1995;31A:2229–2238. doi: 10.1016/0959-8049(95)00460-2. [DOI] [PubMed] [Google Scholar]
- 13.Nuver J, Smit AJ, van der Meer J. Acute chemotherapy-induced cardiovascular changes in patients with testicular cancer. J Clin Oncol. 2005;23:9130–9137. doi: 10.1200/JCO.2005.01.4092. [DOI] [PubMed] [Google Scholar]
- 14.Chow AY, Chin C, Dahl G, et al. Anthracyclines cause endothelial injury in pediatric cancer patients: A pilot study. J Clin Oncol. 2006;24:925–928. doi: 10.1200/JCO.2005.03.5956. [DOI] [PubMed] [Google Scholar]
- 15.Nitzschke M, Raddatz J, Bohlmann MK, et al. GnRH analogs do not protect ovaries from chemotherapy-induced ultrastructural injury in Hodgkin's lymphoma patients. Arch Gynecol Obstet. 2010;282:83–88. doi: 10.1007/s00404-009-1308-5. [DOI] [PubMed] [Google Scholar]
- 16.Van Beek RD, van den Heuvel-Eibrink MM, Laven JS, et al. Anti-Müllerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin's lymphoma during childhood. J Clin Endocrinol Metab. 2007;92:3869–3874. doi: 10.1210/jc.2006-2374. [DOI] [PubMed] [Google Scholar]
- 17.Bath LE, Wallace WH, Shaw MP, et al. Depletion of ovarian reserve in young women after treatment for cancer in childhood: Detection by anti-Müllerian hormone, inhibin B and ovarian ultrasound. Hum Reprod. 2003;18:2368–2374. doi: 10.1093/humrep/deg473. [DOI] [PubMed] [Google Scholar]
- 18.Kelsey TW, Wright P, Nelson SM, et al. A validated model of serum anti-Müllerian hormone from conception to menopause. PLoS One. 2011;6:e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weenen C, Laven JS, Von Bergh AR, et al. Anti-Müllerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Aharon I, Bar-Joseph H, Tzarfaty G, et al. Doxorubicin-induced ovarian toxicity. Reprod Biol Endocrinol. 2010;8:20. doi: 10.1186/1477-7827-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson RA, Themmen AP, Al-Qahtani A, et al. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21:2583–2592. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- 22.Gerber B, von Minckwitz G, Stehle H, et al. German Breast Group Investigators. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: The GBG 37 ZORO study. J Clin Oncol. 2011;29:2334–2341. doi: 10.1200/JCO.2010.32.5704. [DOI] [PubMed] [Google Scholar]
- 23.King LJ, Hasnain SN, Webb JA, et al. Asymptomatic carotid arterial disease in young patients following neck radiation therapy for Hodgkin lymphoma. Radiology. 1999;213:167–172. doi: 10.1148/radiology.213.1.r99oc07167. [DOI] [PubMed] [Google Scholar]
- 24.So NM, Lam WW, Chook P, et al. Carotid intima-media thickness in patients with head and neck irradiation for the treatment of nasopharyngeal carcinoma. Clin Radiol. 2002;57:600–603. doi: 10.1053/crad.2001.0746. [DOI] [PubMed] [Google Scholar]
- 25.Duquaine D, Hirsch GA, Chakrabarti A, et al. Rapid-onset endothelial dysfunction with adriamycin: Evidence for a dysfunctional nitric oxide synthase. Vasc Med. 2003;8:101–107. doi: 10.1191/1358863x03vm476oa. [DOI] [PubMed] [Google Scholar]
- 26.Chaosuwannakit N, D'Agostino R, Jr, Hamilton CA, et al. Aortic stiffness increases upon receipt of anthracycline chemotherapy. J Clin Oncol. 2010;28:166–172. doi: 10.1200/JCO.2009.23.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Partridge AH, Ruddy KJ, Gelber S, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. 2010;94:638–644. doi: 10.1016/j.fertnstert.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 28.Ng EH, Chan CC, Yeung WS, et al. Effect of age on ovarian stromal flow measured by three-dimensional ultrasound with power Doppler in Chinese women with proven fertility. Hum Reprod. 2004;19:2132–2137. doi: 10.1093/humrep/deh387. [DOI] [PubMed] [Google Scholar]