The role of regional ultrasound in providing clinically relevant information was evaluated in the assessment of regional lymphatics for accurate staging and treatment of breast cancer patients. Regional ultrasound was found to be beneficial in initial staging evaluations.
Keywords: Regional ultrasound, Breast cancer, Staging, Locally advanced
Learning Objectives
After completing this course, the reader will be able to:
Describe the ways in which regional ultrasound has contributed to more accurate staging in a population of locally advanced breast cancer patients.
Explain how regional nodal information leads to changes in radiation therapy portals and total doses.
Discuss the role of regional ultrasound in reflecting a truer level of disease burden in locally advanced breast cancer patients before therapies, including neoadjuvant chemotherapy, may limit knowledge of disease extent and consequently affect radiation treatment planning.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Assessment of the regional lymphatics is important for accurate staging and treatment of breast cancer patients. We sought to determine the role of regional ultrasound in providing clinically relevant information. We retrospectively analyzed data from patients who were treated curatively in 1996–2006 at The University of Texas MD Anderson Cancer Center for clinical stage III breast cancer. We compared differences in regional lymph node staging based on ultrasound versus mammography and physical examination in the 865 of 1,200 patients who had external-beam radiation as part of their treatment and regional ultrasound studies as part of their initial evaluation. Ultrasound uniquely identified additional lymph node involvement beyond the level I or II axilla in 37% of the patients (325 of 865), leading to a change in clinical nodal stage. Ninety-one percent of these abnormalities that could be biopsied (266 or 293) were confirmed to contain disease. The sites of additional regional nodal disease were: infraclavicular disease, 32% (275 of 865); supraclavicular disease, 16% (140 of 865); and internal mammary disease, 11% (98 of 865). All patients with involvement in the extra-axillary regional nodal basins received a radiation boost to the involved areas ≥10 Gy. Thus, over one third of patients with advanced breast cancer had their radiation plan altered by the ultrasound findings. Regional ultrasound evaluation in patients with advanced breast cancer commonly revealed abnormalities within and beyond the axilla, which changed the clinical stage of disease and the radiation treatment strategy. Therefore, regional ultrasound is beneficial in the initial staging evaluation for such patients.
Introduction
For patients diagnosed with locally advanced breast cancer, the National Comprehensive Cancer Network guidelines recommend a history and physical examination, CBC, liver function tests, chest x-ray, bilateral diagnostic mammogram, ultrasound of the breast if necessary, pathology review with expression analysis for receptor status (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2/Neu), possible breast magnetic resonance imaging for aid in assessing occult lesions, bone scan if indicated by alkaline phosphatase levels, and possible abdominal computed tomography for staging [1]. Based on these evaluations, the clinical staging of the regional lymph nodes is predominately determined on the basis of physical examination. Initial surgery and the resultant pathologic evaluation commonly provide additional nodal assessment in patients with early breast cancer. But, because preoperative chemotherapy has become the standard initial management of patients with locally advanced cancer, the need for imaging assessment of the disease burden within the extra-axillary lymph nodes has become of greater clinical importance. Specifically, the extent of initial regional lymph node disease can influence the prognosis of the patient and subsequent radiation treatment decisions [2, 3]. Without accurately identifying the presence of disease in the axillary, supraclavicular, internal mammary, and infraclavicular nodal basins, these areas may not be adequately covered with primary radiation fields or boost treatments [4].
To date, there are only relatively small studies available evaluating the value of regional ultrasound to accurately quantitate regional lymph node disease. In general, these limited data have suggested that ultrasound is more accurate in detecting disease in the axilla, supraclavicular, infraclavicular, and internal mammary regions than mammography and clinical examination [2–15]. At the University of Texas MD Anderson Cancer Center (UTMDACC), the use of ultrasound to assess potential disease in the regional nodal basins (axillary, infraclavicular, supraclavicular, and internal mammary regions) is part of our practice pattern for newly diagnosed breast cancer patients. We undertook this study to evaluate the clinical benefits of regional ultrasound evaluation for patients with locally advanced breast cancer. We sought to evaluate the utility of ultrasound with respect to determination of an accurate clinical stage and evaluate how often the results of the ultrasound influenced radiation treatment decisions.
Materials and Methods
We conducted a retrospective review of data obtained from individual patient charts. The study evaluated patients who received radiation therapy in 1996–2006 at UTMDACC as a component of their treatment for locally advanced breast cancer (defined as tumor [T] stage T3 and nodal [N] stage N1–N3, stage T4, or stage N2–N3). All patients received multimodality treatment that included chemotherapy, surgery, and radiation and, if indicated, hormonal therapy. From this group, we then only included patients who had regional ultrasounds at the time of initial diagnosis. The UTMDACC institutional review board approved a protocol to perform this study.
Mammographic data were obtained from the initial UTMDACC mammography report. Data from the patient's clinical examination findings were obtained through a review of the physical examinations performed by each patient's medical oncologist, radiation oncologist, and surgical oncologist. When there was a discrepancy in physical examination findings between pretreatment examinations done by different physicians, the more advanced clinical stage was recorded. Data regarding radiation therapy and total radiation dose, target, boost treatments, technique, fractionation, and energy were recorded from treatment records. Information on the chemotherapy treatments used and the use of other imaging modalities was also recorded. To assess the potential benefit of, specifically, regional ultrasound to clinical staging, every evaluable patient was assigned a clinical N stage using the 2009 American Joint Committee on Cancer (AJCC) system [16] twice—once with only the physical examination and mammography information and once with the addition of the regional nodal ultrasound data. All references in the paper to changes in staging were based on the 2009 AJCC staging criteria for breast cancer [16].
Extended regional ultrasound studies with fine-needle aspiration (FNA) and appropriate biopsies were performed according to UTMDACC practice standards. The targeted areas included the ipsilateral axillary, infraclavicular, supraclavicular, and internal mammary regions. Scanning of the lymph nodes was performed using an Elegra unit (Siemens Medical Solutions, Malvern, PA) with a 10- to 5-MHz linear array transducer or an ATL UltraMark 9 unit (Philips, Andover, MA) with a 10- to 5-MHz linear array transducer. Transverse and sagittal ultrasound images were obtained in the axillary, infraclavicular, internal mammary, and supraclavicular regions. Scanning was initially performed by sonographers. Targeted repeat sonography was then performed by 1 of 12 dedicated breast imagers, who reported the findings. Suspicious lymph nodes, as determined by morphology, size, and ultrasound imaging criteria, underwent ultrasound-guided FNA with a 20- or 21-gauge needle. A normal lymph node included oval shaped structures with an echogeneic hilum. An abnormal lymph node included those with a thickened cortex, cortical bulge, compressed or displaced hilum, or nonvisualized hilum. Abnormal lymph nodes underwent FNA evaluation. The criteria for considering a lymph node abnormal were the same within all extra-axillary sites.
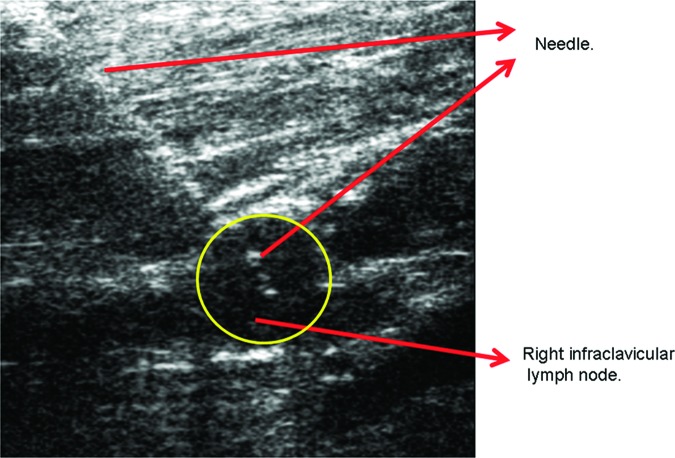
Immediately following the FNAs, cytology technologists prepared the slides with the aspirated material. The slides were reviewed by staff cytologists before the patient was discharged from the breast imaging suite. Most of the suspicious lesions were sampled using FNA and verified to contain disease (Fig. 1). Because of the location of potential disease near critical vascular structures, however, only 10% of suspicious internal mammary chain nodes were pathologically sampled by FNA. All internal mammary nodes evaluated pathologically were positive for disease.
Figure 1.
Right infraclavicular lymph node ultrasound with fine-needle aspiration. Highlighted in the yellow circle is the location of the right infraclavicular lymph node and demonstration of adequate targeting of the fine-needle aspiration attempt to the specific node by ultrasound.
Results
Patient and Tumor Characteristics
From January 1996 through June 2006, ≥1,200 patients were treated with a multimodality, curative approach at UTMDACC for clinical stage III breast cancer. Of this group, 865 (72%) had external-beam radiation as part of their treatment and a comprehensive extended regional ultrasound study as part of their initial diagnostic workup. All these ultrasounds were performed and reviewed by a UTMDACC diagnostic radiologist. This group of 865 patients is the study group for our analysis.
Tumor and patient characteristics were collected to offer a comparison with other locally advanced breast cancer patient populations. All 865 patients selected for the study were female. The mean age for all patients was 50 years, with 385 (45%) aged <45 years and 480 (55%) aged ≥45 years. The histologic type was primarily infiltrating ductal carcinoma, in 626 patients (72%), and was invasive lobular carcinoma in 117 patients (13%). The other 15% of patients had mixed histologies. Ninety-one patients (11%) had inflammatory breast cancer by a clinical and pathologic diagnosis. By location, 482 (56%) primary breast lesions were found in the upper outer quadrant, 144 (17%) were found in the upper inner quadrant, 73 (8%) were found in the lower outer quadrant, and 54 (6%) were found in the lower inner quadrant. The remaining primary lesions, 112 (13%), were considered to be located in overlapping quadrants. Forty-eight percent (n = 415) of the lesions were designated as poorly differentiated, 23% (n = 199) were moderately differentiated, 4.3% (n = 37) were well differentiated, and the remainder were either of mixed differentiation or this was unstated in the medical record.
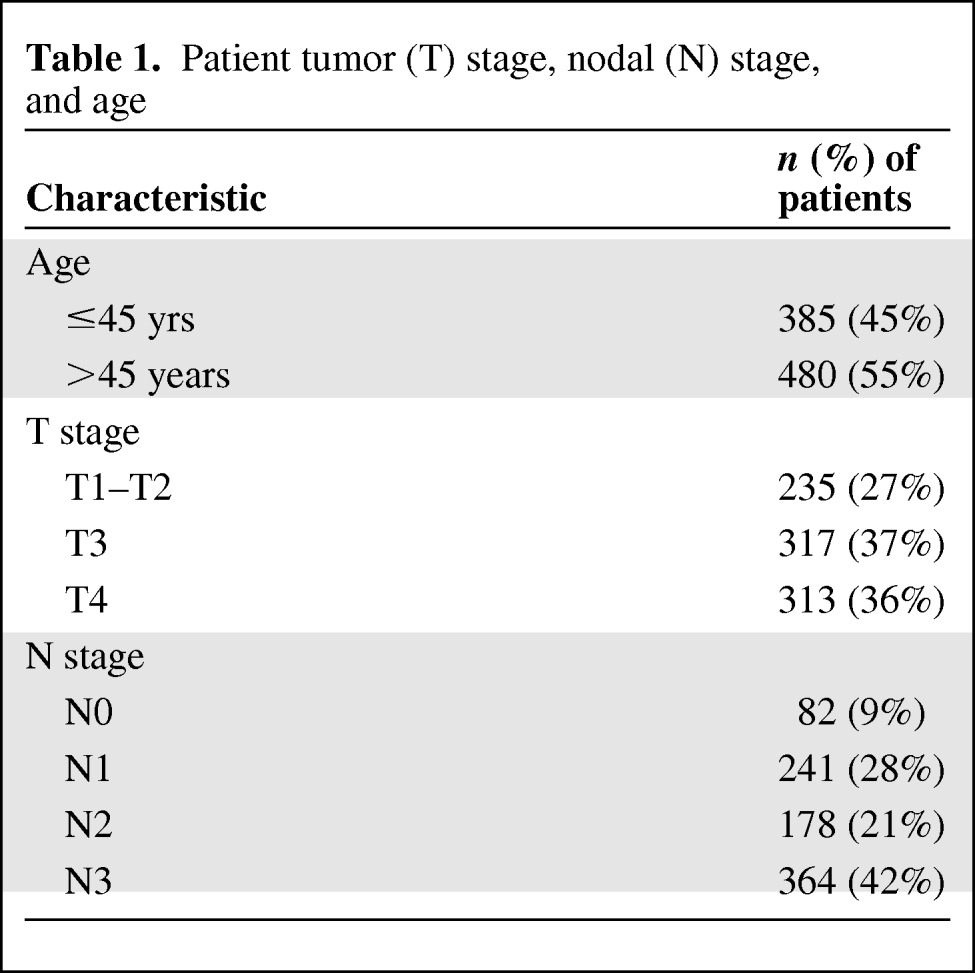
By clinical staging (after mammography, physical examination, and extended regional ultrasound) on initial evaluation, 235 (27%) primary breast lesions were T1 or T2, 317 (37%) were T3, and 313 (36%) were T4 tumors. The lymph node clinical stage was N0 in 82 (9%) patients, N1 in 241 (28%) patients, N2 in 178 (21%) patients, and N3 in 364 (42%) patients (Table 1).
Table 1.
Patient tumor (T) stage, nodal (N) stage, and age
Of the 865 patients, nearly all (n = 800, 92%) completed neoadjuvant chemotherapy prior to evaluation for potential surgery. The other 8% of patients did not complete neoadjuvant chemotherapy because of toxicity, disease progression, and other unspecified reasons. Most often, the neoadjuvant chemotherapy regimen included both a taxane and an anthracycline. All patients completed the external-beam radiation treatments as scheduled in the adjuvant setting. Depending on the type of surgery planned, postoperative radiation therapy was targeted to the intact breast or the chest wall. All radiation treatments included the ipsilateral infraclavicular region and the supraclavicular fossa to a median dose of 50 Gy in 25 fractions. A majority of patients also received radiation to the upper three interspaces of the internal mammary lymph nodes. Radiation was delivered after surgery in all patients, with a very limited cohort receiving preoperative radiation for reasons of borderline resectability.
Regional Ultrasound Accuracy in Detecting Disease
Figure 2 summarizes the findings of the regional ultrasounds. Of the 865 cases, 325 (37%) patients had disease in the lymphatics identified on ultrasound but not identified on mammography or clinical examination. Of these 325 patients, 293 (90%) had the most easily accessible suspicious node (supraclavicular, infraclavicular, axillary, or very rarely internal mammary chain) biopsied with FNA. Two hundred sixty-six of 293 (91%) lymph nodes sampled with FNA were positive for metastatic carcinoma, leading to a final pathologic confirmation rate of 82% (266 of 325).
Figure 2.
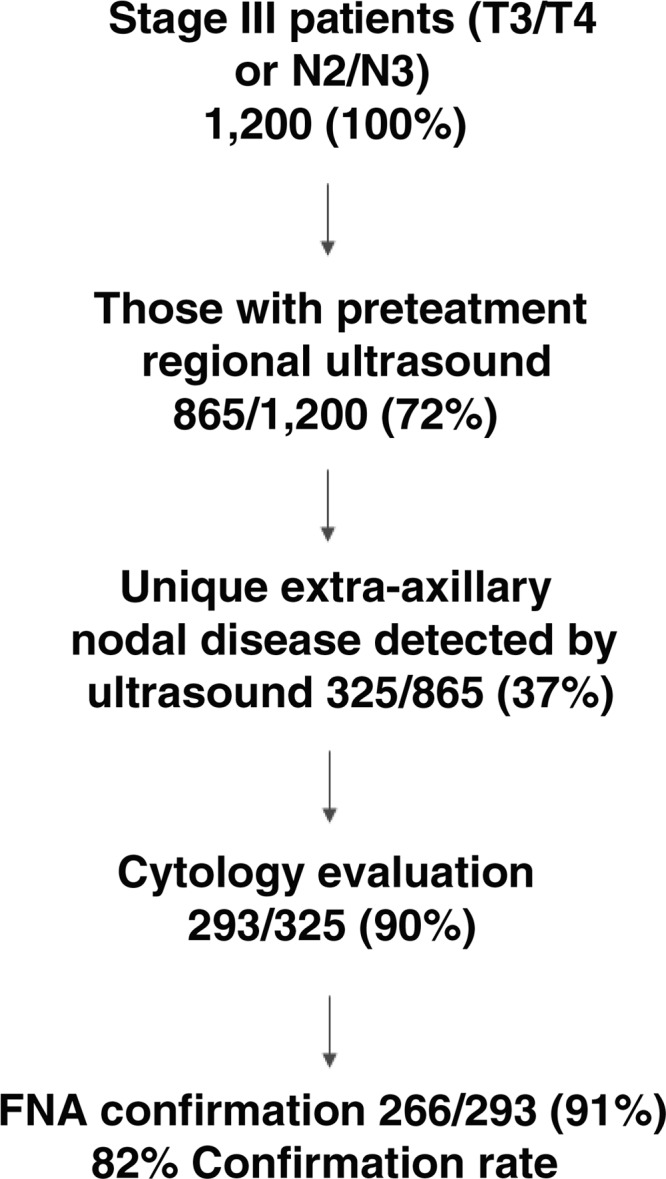
Schematic of regional ultrasound findings and relevant cytology results by fine-needle aspiration (FNA).
Abbreviations: N, node stage; T, tumor stage.
No FNA sampling was performed in 90% (88 of 98) of all cases with suspicious internal mammary chain disease identified by ultrasound because of concern for the safety of the patient. In particular, there was significant potential for damaging nearby vascular structures. This was the most common reason why a patient with any suspicious node did not undergo ultrasound-guided FNA. All 10% of cases with suspicious internal mammary chain nodes that were sampled with FNA were positive for metastatic disease on cytology. Internal mammary chain nodes are not usually pathologically evaluated by UTMDACC radiologists because of the proximity to the internal mammary artery. Of the 10% (32 of 325) of nodes not examined pathologically in our full cohort, a third represented unevaluated internal mammary chain nodes. Other nodes were not sampled because of their location, failure to obtain tissue despite apparent access, and situations not addressed in the clinical record.
Regional Ultrasound Influence on Staging
There was commonly congruence in the findings from mammography, physical examination, and ultrasound with regard to identifying extramammary nodal disease. Indeed, 540 patients (63%) had similar clinical N-stage findings on all three studies. For patients with a difference in findings, this was typically a consequence of regional ultrasound adding new information to the mammography and physical examination data. Specifically, ultrasound detected new or additional infraclavicular, supraclavicular, and/or internal mammary chain lymph node disease in 325 of 865 patients (37%), which led to a change in the clinical N stage. The rates of new disease detected only by regional ultrasound were 32% (275 of 865) for infraclavicular disease, 16% (140 of 865) for supraclavicular disease, and 11% (98 or 865) for internal mammary chain disease. Furthermore, 13% of the 865 patients (n = 115) had suspicious findings in more than one nodal basin. Eighty-five percent of all patients diagnosed with extended nodal disease (275 of 325) had suspicious findings in the infraclavicular lymph nodes. Only 15% of the patients with changes (50 of 325) in staging had disease found by ultrasound in the supraclavicular or the internal mammary regions without disease in the infraclavicular region. For all patients, 90 of 325 (28%) new extra-axillary sites of nodal disease were identified in those with T1–T2 lesions, 94 of 325 (29%) were identified in patients with T3 lesions, and 141 of 325 (43%) were identified in patients with T4 lesions.
The most common change in clinical N stage was a change from N1 to N3 disease, followed by N2 to N3 disease. In the T1–T2 cohort, there was a shift from N1 to N3 disease in 22% of patients, with an equal shift from N2 to N3 disease. The N1 to N3 nodal designation shift resulted in all of these T1–T2 patients going from either stage IIA or IIB to IIIC disease. The N2 to N3 shift led to changes within stage III for patients with T1–T2 tumors, from stage IIIA to IIIC disease. For T3 patients, 13% had a change from N1 to N3 disease, and 7% had a change from N2 to N3 disease. Thirteen percent of T4 patients went from N1 to N3 disease and 11% of T4 patients went from N2 to N3 disease (Table 2).
Table 2.
Evaluation of change in nodal (N) stage from N1 or N2 to N3 as a function of tumor (T) stage based on unique regional ultrasound findings
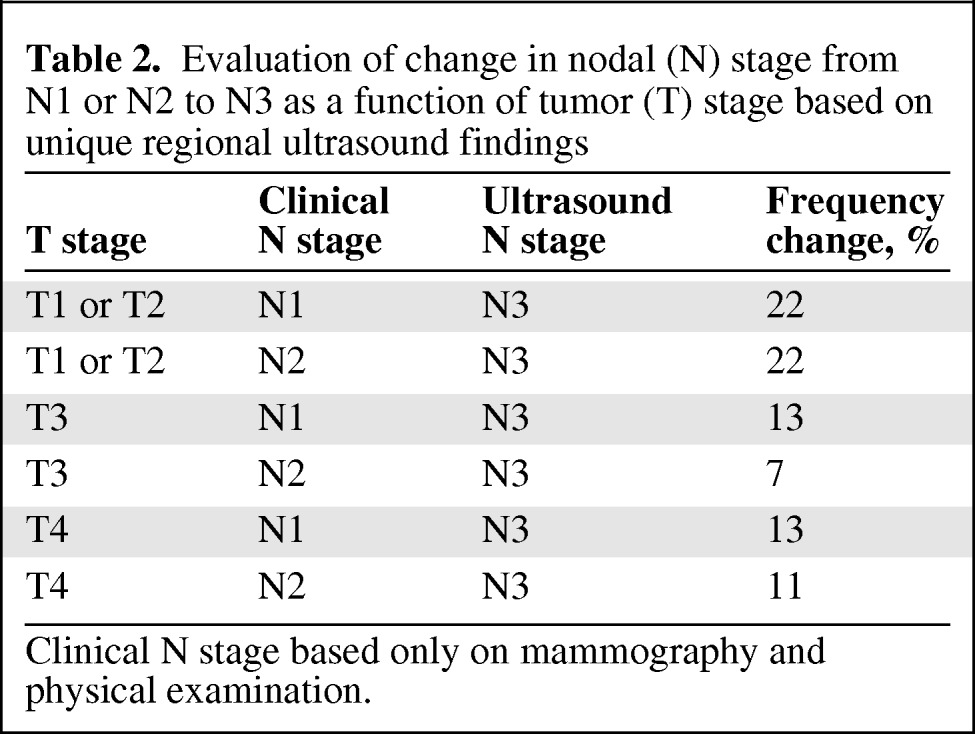
Clinical N stage based only on mammography and physical examination.
Patients were also evaluated for nodal shifts from N1 to N3B disease, representing individuals who only had new internal mammary chain lymphadenopathy identified from a previous diagnosis of axillary spread. Of T1–T2 patients, 1.2% (3 of 235) had a shift specifically from N1 to N3B disease. No T3 patients had a shift from N1 to N3B disease and 1% of T4 patients (3 of 313) had the described shift. A majority of patients with regional ultrasound–determined internal mammary lymphadenopathy had either infraclavicular or supraclavicular disease identified as well, demonstrating why so few patients had this unique stage change.
Finally, a cohort of patients was incorrectly classified as having node-negative breast disease without regional ultrasound. With ultrasound, those patients were correctly diagnosed as having stage III disease (Table 3). The methods of this examination (study inclusion criteria for stage) did not permit us to assess what percentage of patients with T1N0–T2N0 disease by clinical examination and mammography actually had N1 disease determined by regional ultrasound. However, 9% (21 of 235) of T1–T2N0 patients ended up having clinical N2 disease after ultrasound and 7.6% (18 of 235) had N3 disease after ultrasound. By stage, these changes resulted in shifts from stage I and stage IIA to stage IIIA and stage IIIC, respectively. For T3 tumors, 10% (32 of 317) of all patients in this group had newly diagnosed N1 disease, 1.3% (4 of 317) had new N2 disease, and 2% (6 of 317) had new N3 disease. These changes resulted in stage shifts from IIB to various stage III designations. For T4 tumors, none of the 313 patients went from N0 to N1 disease, 2% (6 of 313) went from N0 to N2 disease, and 5% (16 of 313) went from N0 to N3 disease (Table 3). For the T4 population, only shifts from N0 to N3 led to a staging difference from stage IIIB to stage IIIC.
Table 3.
Evaluation of change in nodal (N) stage from N0 to N1, N2, or N3 as a function of tumor (T) stage based on unique regional ultrasound findings
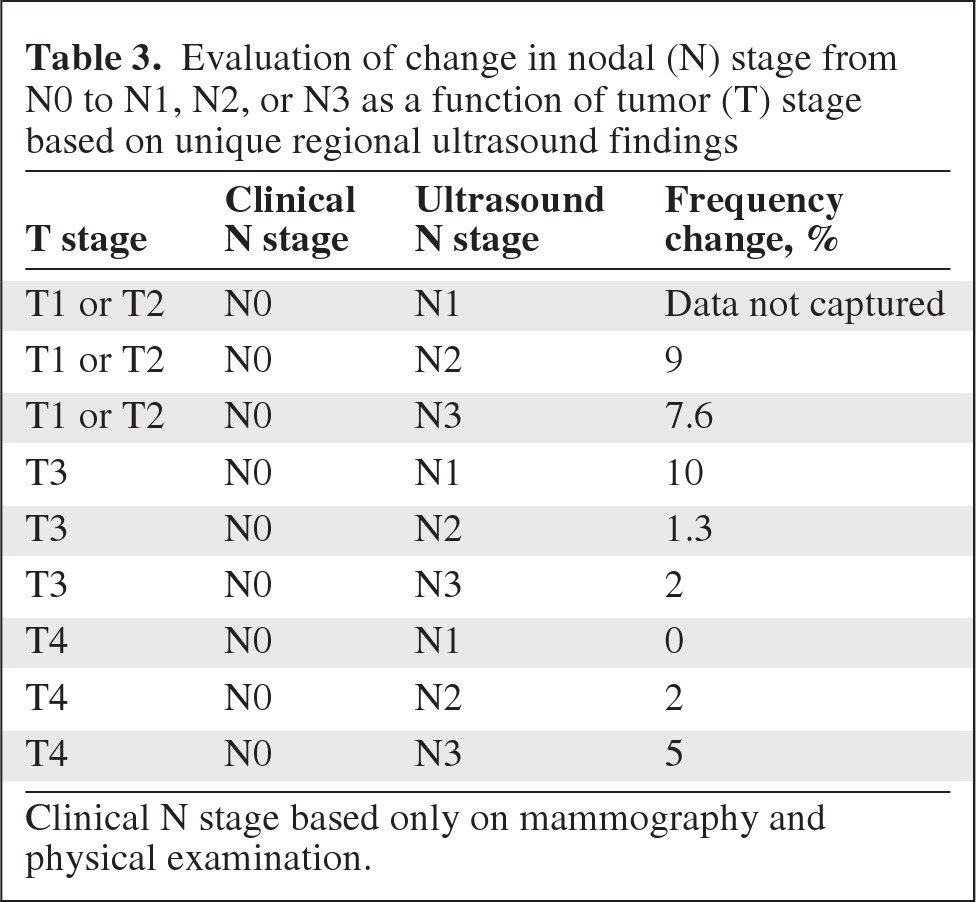
Clinical N stage based only on mammography and physical examination.
Overall, the rates of change in N stage according to tumor size were as follows: 34% (111 of 235 patients) for T1–T2 primary tumors, 29% (93 of 317 patients) for T3 primary tumors, and 31% (121 of 313 patients) for T4 primary tumors. Whereas 66% of the patients with ultrasound-identified regional nodal involvement had T3 (93 of 325) or T4 (121 of 325) primary tumors, 34% of those patients had T1 or T2 lesions (111 of 325).
All patients diagnosed with infraclavicular, supraclavicular, and internal mammary chain lymphadenopathy by regional ultrasound in our cohort had a radiation treatment boost to that area of disease ≥10 Gy over five fractions. Thirty-seven percent of our study population had a change in their treatment plan based on findings of the ultrasound examinations.
Discussion
This study demonstrates that regional ultrasound changed the clinical stage and thus altered the treatment of more than one third of patients with locally advanced breast cancer. Despite this demonstrated clinical utility, routine sonographic evaluation of all regional lymph node regions has not been widely used in the initial evaluation of individuals with invasive breast cancers. Occasionally, targeted ultrasound has been used to evaluate patients who already have a clinically suspicious lymph node based on mammography and physical examination. Furthermore, ultrasound as a staging modality for breast cancer has often been reserved for premenopausal women with dense breasts, for whom mammography may demonstrate low sensitivity. A possible explanation for the lack of regional ultrasound use may be the lack of high-quality data showing its utility. To date, there have been several small studies that have suggested the potential of regional ultrasound to detect disease in the infraclavicular, supraclavicular, and internal mammary chain regions [2–15]. However, none of those studies quantitatively evaluated the importance of ultrasound in terms of accurate staging and subsequent locoregional treatment management.
The UTMDACC has been using extended regional ultrasounds along with mammography and physical examination as part of breast cancer staging evaluations for >10 years, starting in 1996. The use of regional lymph node ultrasound at UTMDACC coincided with the routine use of neoadjuvant chemotherapy for patients with advanced disease. Using chemotherapy prior to surgery further increases the importance of accurate clinical staging, because many locoregional treatment decisions are based on the pretreatment disease extent [2, 3]. Particularly for radiation oncologists, an understanding of the true extent of disease is necessary when planning treatment fields, primary treatment doses, and, ultimately, radiation treatment boosts. Local radiation therapy treatments may be important in eliminating microscopic breast disease that is resistant to chemotherapy or not surgically excised [4]. Disease in the supraclavicular, infraclavicular, and internal mammary nodal basins is particularly important in that these regions are not usually resected at the time of definitive breast cancer surgery.
Ultrasound was able to delineate disease to a far better extent in the infraclavicular, supraclavicular, and internal mammary chain nodal basins than mammography and physical examination. Thirty-seven percent of our entire cohort had a shift in N stage secondary to information provided only by regional ultrasound. The shift in staging as a result of ultrasound evaluation was not limited to T3 or T4 disease, because nearly 35% of all patients found to have new nodal disease had T1–T2 primary tumors. It may be safe to assume that, although a higher T stage may portend the potential for more nodal disease, patients with T1 or T2 lesions also may have extended nodal disease, perhaps as a function of the biologic characteristics of the primary tumors. In our study, patients with T1 or T2 primary disease had the highest percentage of shifts from N1 or N2 to N3 disease (22% in both cases). Whereas clinicians may wish to preferentially examine the nodal basins with ultrasound in patients with T3 or T4 disease, we believe that staging nodal ultrasound may also be of benefit in patients with T1 and T2 disease with lymph node–positive disease. Most patients with T1 or T2 disease will not have regional adenopathy. Our study does demonstrate, however, that a significant percentage of patients eventually diagnosed with clinical stage III disease originally had T1 or T2 primary tumor diagnoses. Therefore, in the future, if we are able to better predict biologically, or by other means, which T1 or T2 patients may have more aggressive tumors, predisposing them to nodal metastases, we could target that group for regional ultrasound studies.
Our study also noted that some patients with apparent early-stage, node-negative disease can be found to have clinical stage III disease with regional ultrasound. For this subgroup, locoregional treatment plans were also altered by the ultrasound findings.
There are important limitations to recognize in this study. First, the study was a retrospective analysis. Second, the study represents the experiences of a single institution. Third, our study only evaluated patients with an ultimate clinical stage III designation. As a result of the selection of patients, our study did use a cohort with naturally higher rates of disseminated disease.
Finally, there has been some question regarding our treatment survival outcomes with this cohort of patients using the ultrasound information as a rationale for radiation boosts to extra-axillary nodal basins. Because this was a retrospective, nonrandomized evaluation, we do not have data comparing treatment outcomes with and without the use of the ultrasound data. We could compare UTMDACC outcomes with those from another institution that does not use regional ultrasound for all their patients, but there would be too many other biases introduced as confounders to overall outcomes. We could compare the current generation of patients treated at UTMDACC with regional ultrasound with an earlier cohort that did not use regional ultrasound, but there would not be similarities with neoadjuvant systemic therapy, radiation techniques, treatment policy, etc. We agree that a randomized study would offer the best information regarding any potential improvements in disease-free and overall survival outcomes. But when we already know that such a large portion of locally advanced breast cancer patients have their radiation treatment changed as a consequence of regional ultrasound, a randomized study may not be that straightforward to accrue or perform. Our regional ultrasound data does result in more aggressive treatment of the nodal basins from a radiation treatment perspective, which we feel offers a local control benefit and may translate into a survival benefit as well. We hope that future clinical studies will shed more light on the benefits of regional ultrasound and offer validation of the appropriateness of the additional radiation delivered as a consequence of the upstaging of the patients.
Conclusion
Our population represents the largest study investigating the effect of regional ultrasound on staging and treatment planning for patients with advanced breast cancer and reports that over one third of patients with advanced breast cancer had their clinical N stage and radiation treatments changed as a result of the regional ultrasound findings. Therefore, we suggest that all patients with newly diagnosed lymph node–positive invasive breast cancer and all patients who are to be treated with initial systemic treatment may benefit from an initial ultrasound evaluation of the regional lymph node basins.
Acknowledgments
We would like to thank members of the Breast Radiation Oncology, Medical Oncology, and Surgical Oncology Services as well as Diagnostic Imaging at UTMDACC for helpful discussions.
Supported in part by an National Institutes of Health/Medicare funded institutional training grant for residency training given to the Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center.
Presented in part as a poster at the 2008 San Antonio Breast Cancer Symposium.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Puneeth Iyengar, Eric A. Strom, Gary J. Whitman, Benjamin D. Smith, Wendy A. Woodward, Tse-Kuan Yu, Thomas A. Buchholz
Provision of study material or patients: Puneeth Iyengar, Eric A. Strom, Gary J. Whitman, Benjamin D. Smith, Wendy A. Woodward, Tse-Kuan Yu, Thomas A. Buchholz
Collection and/or assembly of data: Puneeth Iyengar, Eric A. Strom, Yu-Jing Zhang, Gary J. Whitman, Benjamin D. Smith, Wendy A. Woodward, Tse-Kuan Yu, Thomas A. Buchholz
Data analysis and interpretation: Puneeth Iyengar, Eric A. Strom, Gary J. Whitman, Benjamin D. Smith, Wendy A. Woodward, Tse-Kuan Yu, Thomas A. Buchholz
Manuscript writing: Puneeth Iyengar, Eric A. Strom, Gary J. Whitman, Benjamin D. Smith, Wendy A. Woodward, Tse-Kuan Yu, Thomas A. Buchholz
Final approval of manuscript: Puneeth Iyengar, Eric A. Strom, Yu-Jing Zhang, Gary J. Whitman, Benjamin D. Smith, Wendy A. Woodward, Tse-Kuan Yu, Thomas A. Buchholz
References
- 1.Carlson RW, Allred DC, Anderson BO, et al. Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7:122–192. doi: 10.6004/jnccn.2009.0012. [DOI] [PubMed] [Google Scholar]
- 2.Newman LA, Kuerer HM, Fornage B, et al. Adverse prognostic significance of infraclavicular lymph nodes detected by ultrasonography in patients with locally advanced breast cancer. Am J Surg. 2001;181:313–318. doi: 10.1016/s0002-9610(01)00588-8. [DOI] [PubMed] [Google Scholar]
- 3.Reed VK, Cavalcanti JL, Strom EA, et al. Risk of subclinical micrometastatic disease in the supraclavicular nodal bed according to the anatomic distribution in patients with advanced breast cancer. Int J Radiat Oncol Biol Phys. 2008;71:435–440. doi: 10.1016/j.ijrobp.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang YJ, Oh JL, Whitman GJ, et al. Clinically apparent internal mammary nodal metastasis in patients with advanced breast cancer: Incidence and local control. Int J Radiat Oncol Biol Phys. 2010;77:1113–1119. doi: 10.1016/j.ijrobp.2009.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnamurthy S. Current applications and future prospects of fine-needle aspiration biopsy of locoregional lymph nodes in the management of breast cancer. Cancer. 2009;117:451–462. doi: 10.1002/cncy.20055. [DOI] [PubMed] [Google Scholar]
- 6.Altinyollar H, Dingil G, Berberoglu U. Detection of infraclavicular lymph node metastases using ultrasonography in breast cancer. J Surg Oncol. 2005;92:299–303. doi: 10.1002/jso.20379. [DOI] [PubMed] [Google Scholar]
- 7.Meterissian S, Fornage BD, Singletary SE. Clinically occult breast carcinoma: Diagnostic approaches and role of axillary node dissection. Ann Surg Oncol. 1995;2:314–318. doi: 10.1007/BF02307063. [DOI] [PubMed] [Google Scholar]
- 8.Vlastos G, Fornage BD, Mirza NQ, et al. The correlation of axillary ultrasonography with histologic breast cancer downstaging after induction chemotherapy. Am J Surg. 2000;179:446–452. doi: 10.1016/s0002-9610(00)00382-2. [DOI] [PubMed] [Google Scholar]
- 9.Bedrosian I, Bedi D, Kuerer HM, et al. Impact of clinicopathological factors on sensitivity of axillary ultrasonography in the detection of axillary nodal metastases in patients with breast cancer. Ann Surg Oncol. 2003;10:1025–1030. doi: 10.1245/aso.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Whitman GJ, Sheppard DG, Phelps MJ, et al. Breast cancer staging. Semin Roentgenol. 2006;41:91–104. doi: 10.1053/j.ro.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Amaral BB, Meurer L, Whitman GJ, et al. Lymph node status in the breast cancer patient: Sampling techniques and prognostic significance. Semin Roentgenol. 2007;42:253–264. doi: 10.1053/j.ro.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Boughey JC, Middleton LP, Harker L, et al. Utility of ultrasound and fine-needle aspiration biopsy of the axilla in the assessment of invasive lobular carcinoma of the breast. Am J Surg. 2007;194:450–455. doi: 10.1016/j.amjsurg.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Bedi DG, Krishnamurthy R, Krishnamurthy S, et al. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: In vitro sonographic study. AJR Am J Roentgenol. 2008;191:646–652. doi: 10.2214/AJR.07.2460. [DOI] [PubMed] [Google Scholar]
- 14.Edeiken-Monroe BS, Monroe DP, Monroe BJ, et al. Metastases to intramammary lymph nodes in patients with breast cancer: Sonographic findings. J Clin Ultrasound. 2008;36:279–285. doi: 10.1002/jcu.20445. [DOI] [PubMed] [Google Scholar]
- 15.Whitman GJ, Strom EA. Workup and staging of locally advanced breast cancer. Semin Radiat Oncol. 2009;19:211–221. doi: 10.1016/j.semradonc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 16.American Joint Commission on Cancer. AJCC Staging Manual. Seventh Edition. Chicago: AJCC; 2009. pp. 1–656. [Google Scholar]