Clinical outcomes of operable breast cancer patients stratified according to a common human epidermal growth factor receptor 2 (HER-2) immunochemical testing algorithm were compared. A 2+ score combined with a negative HER-2 status on fluorescence in situ hybridization was found to be an adverse prognostic factor.
Keywords: Breast cancer, Fluorescence in situ hybridization, Immunohistochemistry, HER-2, Prognosis
Abstract
Background.
Human epidermal growth factor receptor (HER)-2 testing in patients with operable breast cancer is aimed at identifying candidates for adjuvant anti–HER-2 treatment. However, commonly defined “HER-2−” tumors express variable levels of the HER-2 protein, which can influence prognosis. We compared the clinical outcomes of operable breast cancer patients stratified according to a common HER-2 testing algorithm.
Methods.
We studied 1,150 women (median age, 58 years; range, 22–94 years) undergoing surgery for early breast cancer at our institution. HER-2 status was determined using the HercepTest™ (Dako, Glostrup, Denmark) and, when needed, by fluorescence in situ hybridization (FISH). Patients receiving adjuvant trastuzumab were excluded. The impact of HER-2 status on the disease-free survival (DFS) time was studied using multivariate Cox proportional regression analysis.
Results.
Four hundred-fifty seven (40%), 454 (39%), 116 (10%), and 123 (11%) patients were considered HER-2 0+, HER-2 1+, HER-2 2+/HER-2− by FISH, and HER-2+ (3+ or HER-2+ by FISH), respectively. Compared with a HER-2 0 or 1+ status, a HER-2 2+/HER-2− by FISH status was associated with a worse DFS outcome on multivariate analysis. Compared with a HER-2+ status, a HER-2 2+/HER-2− status showed a time-dependent effect on the DFS probability, with an initial advantage that worsened every year by a factor of 1.649.
Conclusion.
A HER-2 2+/HER-2− status is an adverse prognostic factor in patients with operable breast cancer. Because of suggestions from randomized trials that the benefits of adjuvant trastuzumab may not be limited to patients with HER-2+ tumors, patients with a HER-2 2+/HER-2− status are ideal candidates for studies testing this hypothesis.
Introduction
The human epidermal growth factor receptor 2 (HER-2) is a transmembrane tyrosine kinase receptor overexpressed in some 15%–20% of breast cancers occurring in humans [1, 2]. The highest levels of HER-2 overexpression are commonly associated with amplification of the HER-2/neu oncogene, which encodes the HER-2 protein [3]. Since its discovery, HER-2 overexpression and HER-2 amplification have been correlated with a distinct pathological breast cancer profile, consisting of a lower frequency of hormone receptor expression, a higher histopathological grade, and greater proliferative activity [4, 5]. Tumors with HER-2 overexpression and those with HER-2 amplification carry an adverse prognosis, frequently characterized by a short disease-free survival interval after surgery, visceral metastatic involvement, and resistance to hormonal therapy (when hormone receptors are coexpressed) and to anthracycline-free chemotherapy regimens [6–8]. HER-2 therapeutic targeting with the monoclonal antibody (mAb) trastuzumab, the first anti–HER-2 agent available for clinical use, has changed the natural history of patients whose tumors carry the HER-2 abnormality [9–14]. Observations in the metastatic setting have established the paradigm that trastuzumab efficacy is restricted to tumors with strong HER-2 immunohistochemical (IHC) overexpression and those with HER-2 amplification. For this reason, current HER-2 testing algorithms are aimed at identifying those patients most likely to achieve a significant benefit from HER-2 targeting. Although HER-2 expression is biologically a continuum, from no detectable expression to strong overexpression, approved IHC tests, like the HercepTest™ (Dako, Glostrup, Denmark), categorize HER-2 status on a semiquantitative scale ranging from 0 to 3+. A 3+ score corresponds to strong overexpression in >10% of tumor cells and identifies candidates for treatment. A review of 6,556 breast cancers revealed that about 92% of tumors with a HER-2 score of 3+ had HER-2/neu amplification. Conversely, HER-2 amplification was observed at lower rates in tumors with scores of 2+ (23.3%), 1+ (7.4%), and 0+ (4.1%) [15]. With HER-2 amplification as an established predictor of response to HER-2–targeting agents, the current algorithm calls for fluorescence in situ hybridization (FISH) testing of tumors with a HER-2 IHC score of 2+ [16].
Studies in cell lines revealed that the average numbers of HER-2 receptors on each cell surface were about 20,000, 100,000, 500,000, and 2,300.000 in IHC score 0, 1+, 2+, and 3+ tumors, respectively [17]. Whether or not lower degrees of HER-2 overexpression in the absence of HER-2/neu amplification are associated with breast cancer prognosis after surgery is still an open issue, which has acquired some importance as a result of observations from adjuvant randomized trials with trastuzumab. Two of those studies included subsets of patients who were enrolled based on peripheral laboratories' confirmation of HER-2 overexpression (IHC score of 3+) or HER-2 amplification that was not confirmed upon central laboratory review [18, 19]. The trastuzumab-related benefit in patients with these HER-2− tumors was about the same magnitude as in patients with HER-2+ tumors. If confirmed, these findings suggest rethinking HER-2 status with respect to prediction of trastuzumab-related benefit in patients with early breast cancer, and also, in our opinion, in prognostic terms. Although the benefit of trastuzumab in patients with HER-2− tumors will be addressed in prospective trials [20], we were interested in evaluating the prognostic value of HER-2 status defined according to the criteria used to establish eligibility for anti–HER-2 treatment in the adjuvant setting. Therefore, we compared the clinical outcome of patients with HER-2+ tumors (HercepTest™ score of 3+ or HER-2/neu amplification) with that of patients with tumors with lower degrees of HER-2 expression on IHC—score 2+ and no HER-2 amplification (hereafter, HER-2 2+/HER-2−), score 1+, and score 0+.
Materials and Methods
Patients
Data for this study were obtained from our prospectively maintained institutional database of women receiving surgery for early breast cancer in June 1996 to September 2009. To be selected for this analysis, patients had to have no prior diagnosis of another malignancy, including a prior intervention for ductal carcinoma in situ of the breast, stage I–IIIA breast cancer amenable to surgery, and no signs of metastatic disease at the time of diagnosis. For each patient, we collected the main clinical and pathological characteristics, adjuvant and neoadjuvant treatment received (neoadjuvant or adjuvant chemotherapy, adjuvant radiotherapy, adjuvant trastuzumab, adjuvant hormonal therapy), date and site of first metastasis, if this occurred, and status in vitae at the last follow-up. Patients were invited to regular follow-up visits every 6 months for the initial 5 years after surgery, and every year thereafter. The data cutoff for follow-up was November 2011.
Pathology
Tumor biopsies and surgical tumor specimens were processed according to standard routine procedures at the surgical pathology department of our institution.
HER-2 status was assessed using the HercepTest™ according to the manufacturer's instructions. HER-2 positivity was defined as a 3+ score on IHC in >10% of invasive tumor cells. Equivocal IHC cases (2+ score or 3+ score in ≤10% of invasive tumor cells) were submitted to FISH analysis. A ratio of HER-2 signals to chromosome 17 signals >2.0 was used as the cutoff to define HER-2 amplification. For patients undergoing surgery from April 2000 onward, the HercepTest™ was carried out as part of routine IHC assessments. FISH testing of cases with a 2+ HER-2 score was routinely introduced in December 2005, when trastuzumab was registered in the adjuvant setting in Italy. For all cases in which the HER-2 status was not routinely assessed (i.e., patients undergoing surgery before April 2000 and patients with a HER-2 2+ score undergoing surgery in April 2000 to December 2005), this was determined with archival material using the HercepTest™ and FISH when needed for the purpose of this study. The assessment of hormonal receptor status was carried out using IHC with the mAb to the estrogen receptor (ER) (1/100 dilution; Dako, Glostrup, Denmark) and the mAb to the progesterone receptor (PgR) (1/800 dilution; Dako). Only nuclear reactivity was taken into account for evaluation ER and PgR. Positivity was defined as immunostaining in ≥10% of invasive tumor cells. The Mib-1 monoclonal antibody (1:200 dilution; Dako) was used to assess Ki-67, which was reported as a percentage of immunoreactive cells among 2,000 tumor cells in randomly selected, high-power fields at the periphery of the tumor.
Statistical Analysis
Patients were grouped according to their HER-2 status. χ2 and Kruskal-Wallis H tests were used to compare categorical variables and continuous variables, respectively, across groups. The study endpoint was the disease-free survival (DFS) interval, which was defined as the time interval between surgery and invasive ipsilateral breast tumor recurrence, locoregional relapse, contralateral breast cancer, distant metastasis, or death, whichever occurred first. The DFS probability distribution was studied using the Kaplan–Meier product limit methods, and DFS curves were compared using the log-rank test. Patients who did not experience a DFS event were censored at the date of their last follow-up visit. Cox proportional hazards models were then fitted to determine the impact of HER-2 status on the DFS time after adjusting for patient and tumor characteristics. The proportional hazards assumption was verified using the log minus log method and by examining the smoothed plots of the rescaled Schoenfeld residuals and pointwise 95% confidence bands for each variable [21]. The following variables were studied: HER-2 status, age at initial diagnosis, tumor size, axillary lymph node involvement, histopathological type (ductal, including mixed ductal and lobular, vs. pure lobular vs. other), histopathological grade, proliferation as expressed by Ki-67 immunostaining (percentage of positive cells), hormone receptor status, adjuvant chemotherapy, and adjuvant radiotherapy. Receipt of endocrine therapy was not considered because almost all patients with a hormone receptor–positive status (any positivity) received it as part of adjuvant therapy. All the variables were initially entered into the model and then removed by backward stepwise selection. Only variables associated with the DFS time with a p-value <.05, as determined using the Wald statistic, remained in the model. Results are reported as a hazard ratio (HR) with 95% confidence interval (CI). Analyses were performed using SPSS version 17.0 software (SPSS Inc., Chicago, IL).
Results
In total, 1,295 women were initially identified for this study. Sixty-one of 196 patients with HER-2+ breast cancer undergoing breast surgery from late 2005 onward received trastuzumab as part of their adjuvant treatment and were excluded from the main analyses. Another 84 patients were excluded because of either insufficient archival material for HER-2 status assessment (25 patients) or missing follow-up information (59 patients). Table 1 summarizes the main clinical and pathological characteristics of the 1,150 patients selected for the analyses according to HER-2 status. HER-2 status was 0, 1+, 2+/HER-2−, and HER-2+ in 457 (40%), 454 (39%), 116 (10%), and 123 (11%) tumors, respectively. Compared with patients who had tumors with HER-2 scores of 0 and 1+, those with a HER-2 2+/HER-2− tumor status tended to have larger tumors at diagnosis, more frequently had high-grade tumors, had higher Ki-67 expression, and had more extensive axillary lymph node involvement. These features were similar to those of patients with HER-2+ tumors. Conversely, HER-2 2+/HER-2− tumors were more similar to tumors with HER-2 scores of 0 and 1+ with respect to hormone receptor expression and distribution of histotypes. The types of neoadjuvant and adjuvant chemotherapy are summarized in supplemental online Table S1.
Table 1.
Patient demographics according to HER-2 status
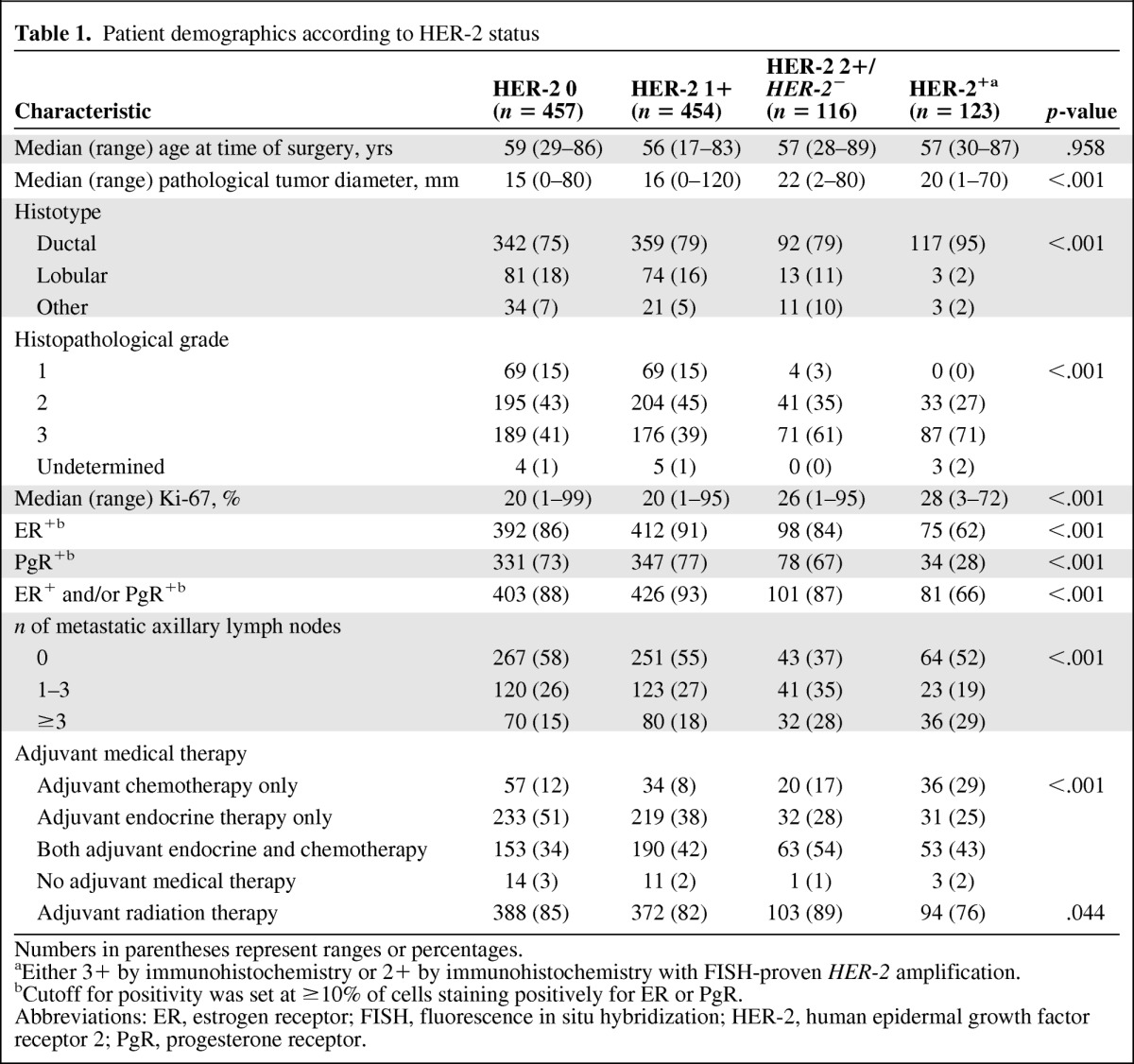
Numbers in parentheses represent ranges or percentages.
aEither 3+ by immunohistochemistry or 2+ by immunohistochemistry with FISH-proven HER-2 amplification.
bCutoff for positivity was set at ≥10% of cells staining positively for ER or PgR.
Abbreviations: ER, estrogen receptor; FISH, fluorescence in situ hybridization; HER-2, human epidermal growth factor receptor 2; PgR, progesterone receptor.
In total, 254 DFS events were registered at a median overall follow-up duration of 60 months (range, 5–175 months).
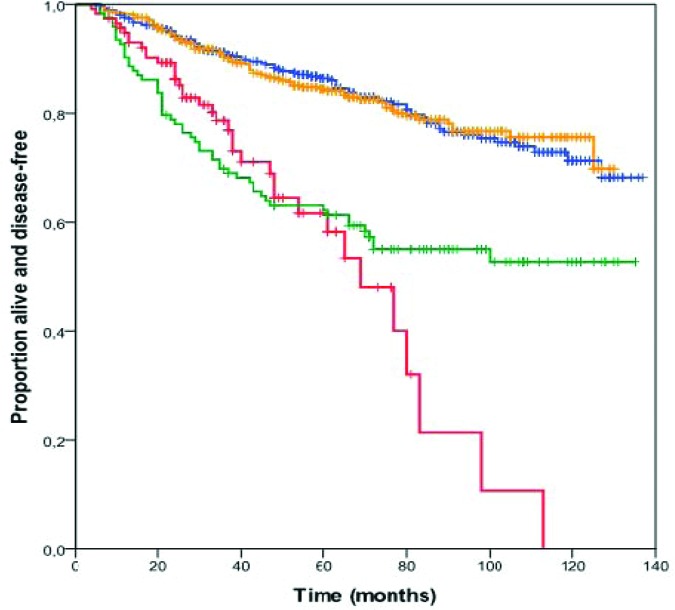
Figure 1 summarizes the DFS probability estimates according to HER-2 status. The 5-year DFS rates were 86% (95% CI, 83%–89%), 84% (95% CI, 80%–88%), 62% (95% CI, 48%–74%), and 63% (95% CI, 54%–72%) for patients with tumors categorized as HER-2 0, HER-2 1+, HER-2 2+/HER-2−, and HER-2+, respectively (p < .001). Additionally, although the DFS curves plateaued at about 60 months for patients with HER-2+ tumors, patients with HER-2 2+/HER-2− tumors displayed continued relapses beyond 60 months. HER-2 0 and HER-2 1+ were grouped together in further analyses. Although patients with HER-2+ tumors receiving adjuvant trastuzumab were excluded from this analysis, a short report that also includes this group of patients is provided in online supplemental Table S2 and Figure S1. As expected, the addition of trastuzumab resulted in a superior outcome for patients with HER-2+ tumors, which was also better than that of patients with HER-2 2+/HER-2− tumors.
Figure 1.
Kaplan–Meier estimates of disease-free survival probability according to HER-2 status. Yellow line, HER-2 0+; blue line, HER-2 1+; red line, HER-2 2+/HER-2−; green line, HER-2+.
The proportional hazards assumption was met for all the variables selected for the multivariate analysis and for the comparison of patients with HER-2 2+/HER-2− tumors versus those with HER-2 0 and 1+ tumors, but not for the comparison of patients with HER-2 2+/HER-2− tumors versus those with HER-2+ tumors (Fig. 2). We therefore decided to study two different Cox proportional hazards models for the two comparisons of HER-2 status. Table 2 shows the results for the comparison of HER-2 2+/HER-2− tumors versus HER-2 0 and 1+ tumors. Age and Ki-67 were dichotomized around the median values of 57 years and 20%, respectively. Tumor diameter was categorized as 1–10 mm, 11–20 mm, 21–50 mm, and >50 mm. A HER-2 2+/HER-2− tumor status was independently associated with a worse DFS outcome in this comparison (HR, 2.596; 95% CI, 1.782–3.781; p < .001). Table 3 shows the results for the comparison of a HER-2 2+/HER-2− tumor status with a HER-2+ tumor status. Age and Ki-67 were dichotomized around the median values of 57 years and 27%, respectively. Tumor diameter was categorized as 1–10 mm, 11–20 mm, 21–50 mm, and >50 mm. Because of the violation of the proportional hazards assumption, an extended Cox proportional model was used with the inclusion of an interaction term between HER-2 status and a newly created time-dependent covariate. In this model, HER-2 status also was independently associated with the DFS outcome, with a hazard that initially favored patients with HER-2 2+/HER-2− tumors (HR, 0.363; 95% CI, 0.169–0.779; p = .009) but worsened every year by a factor of 1.649 (95% CI, 1.327–2.049; p < .001). No significant interaction was found between HER-2 status and axillary lymph node involvement and hormone receptor status in either multivariate model (not shown).
Figure 2.
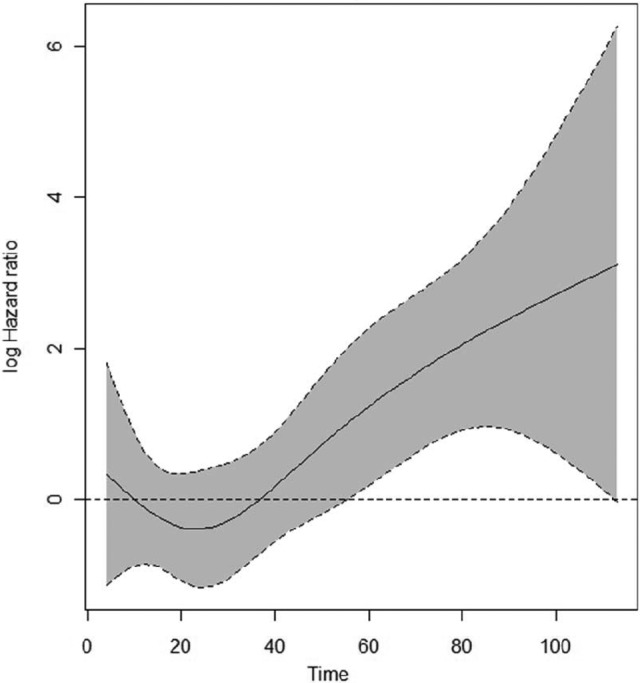
Time-dependent hazard ratio for a HER-2 2+/HER-2− versus a HER-2+ tumor status with pointwise 95% confidence interval.
Table 2.
Multivariate analysis of predictors of the disease-free survival time in patients with HER-2 0/1+ and HER-2 2+/HER-2− tumors (n = 1,018)
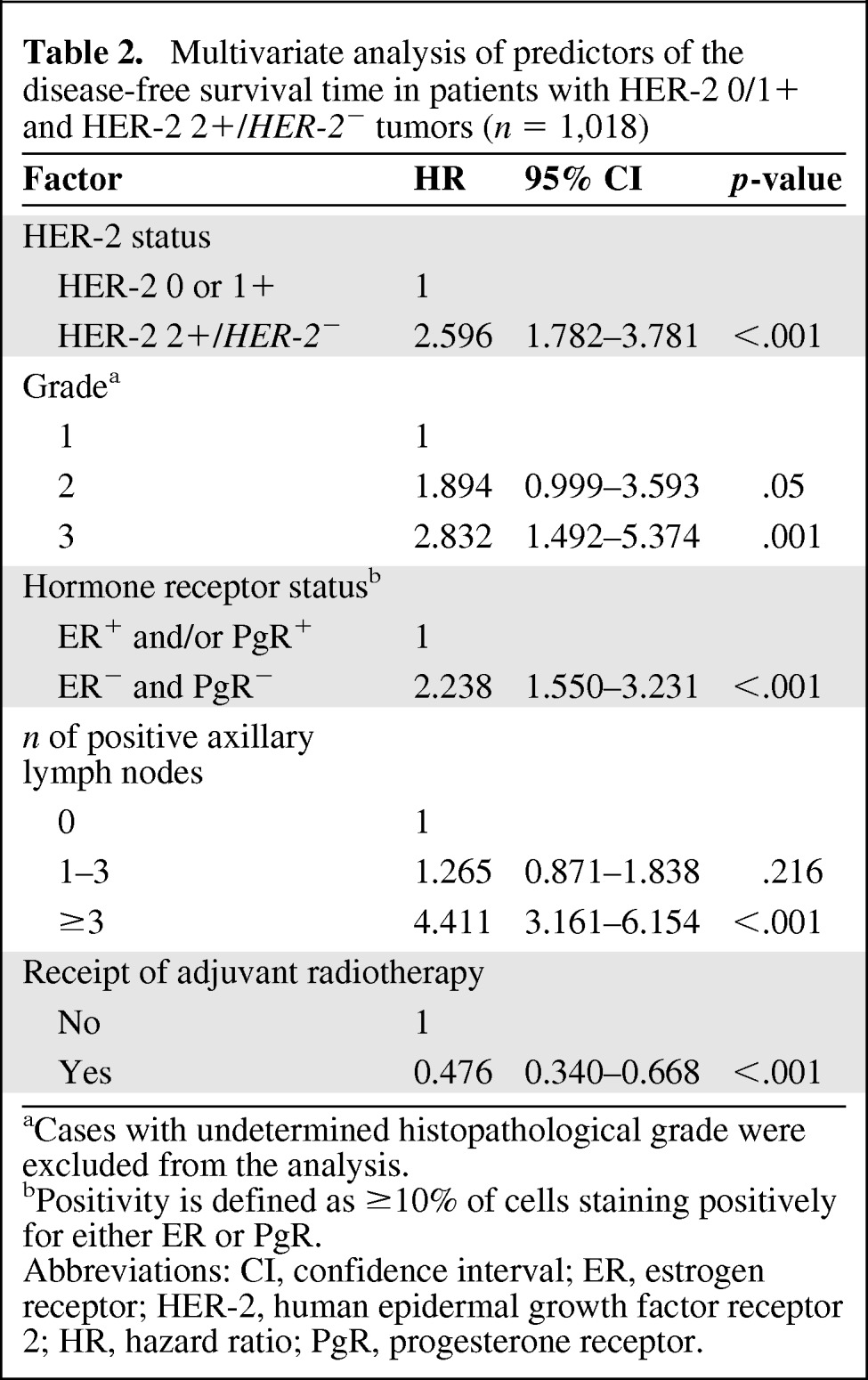
aCases with undetermined histopathological grade were excluded from the analysis.
bPositivity is defined as ≥10% of cells staining positively for either ER or PgR.
Abbreviations: CI, confidence interval; ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; HR, hazard ratio; PgR, progesterone receptor.
Table 3.
Multivariate analysis of predictors of the disease-free survival time in patients with HER-2 2+/HER-2− and HER-2+ tumors
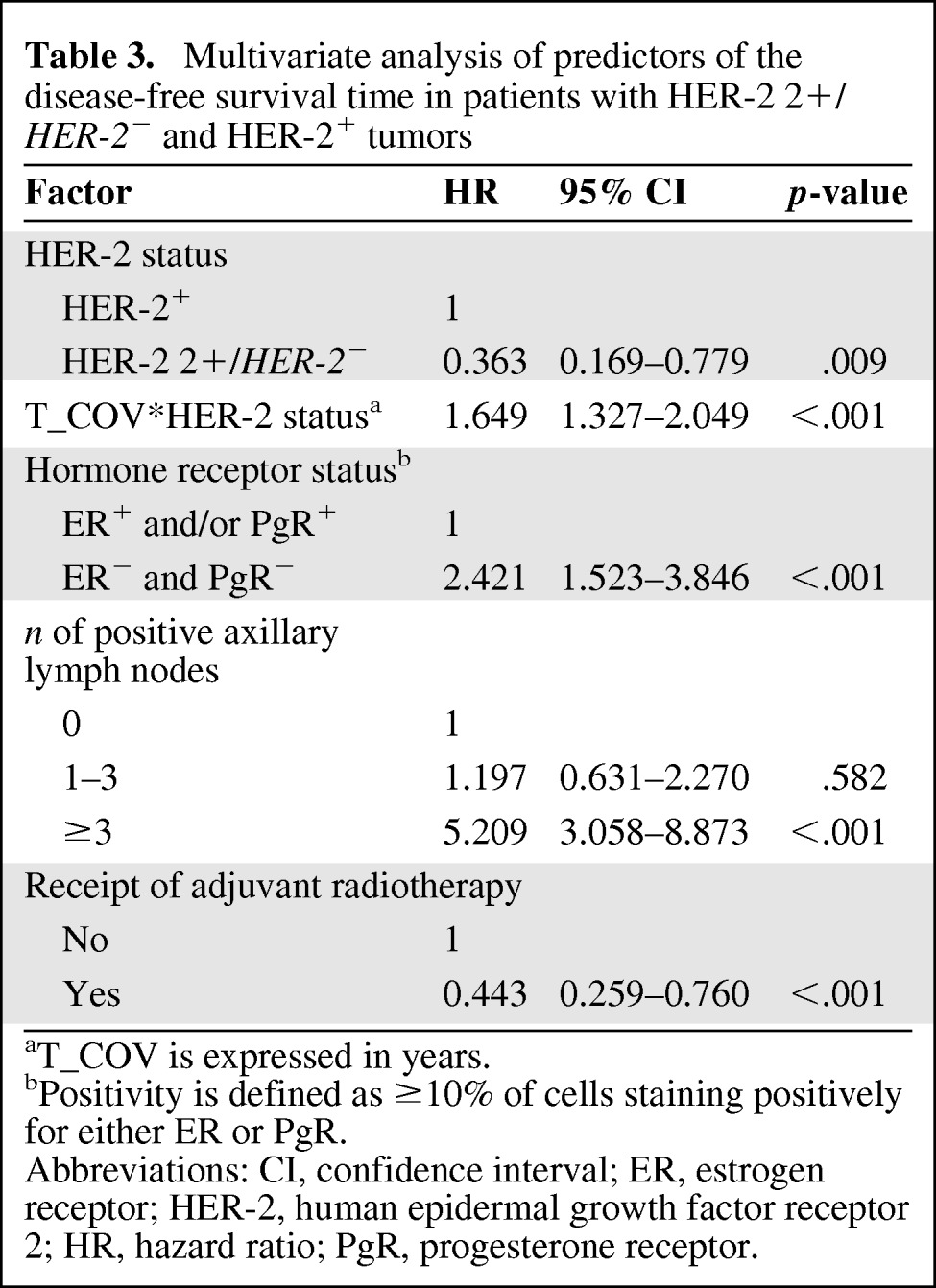
aT_COV is expressed in years.
bPositivity is defined as ≥10% of cells staining positively for either ER or PgR.
Abbreviations: CI, confidence interval; ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; HR, hazard ratio; PgR, progesterone receptor.
Because, compared with tumors with HER-2 scores of 0 and 1+, HER-2 2+/HER-2− tumors were more frequently high grade (grade 3) and showed a higher proliferation rate, we sought to elucidate whether or not the combined effect of these adverse features, rather than the HER-2 2+/HER-2− status itself, could be responsible for the observed difference in prognosis. For this purpose, tumors were categorized as luminal A (ER+, Ki-67 <14%, HER-2−), luminal B/HER-2− (ER+, Ki-67 ≥14%, HER-2−), HER-2+, and triple negative (ER−, PgR−, and HER-2−). This IHC-based tumor categorization has been shown to approximate molecularly characterized intrinsic breast cancer subtypes, each of which has been found to be associated with a distinct prognosis [22–24]. In particular, the luminal A subtype carries the best prognosis. Despite being worse than that of luminal A, the prognosis of patients with luminal B/HER-2− tumors is better than that of patients with HER-2–enriched and triple-negative tumors, which represent the most biologically aggressive subtypes of breast cancer. Totals of 297 (26%), 607 (53%), 123 (11%), and 123 (11%) tumors in our series were categorized as luminal A, luminal B/HER-2−, HER-2+, and triple negative, respectively. Kaplan–Meier and Cox proportional hazards analyses confirmed that this classification was associated with the DFS outcome (supplemental online Fig. S2). In fact, compared with HER-2 0 and 1+ tumors, a significantly higher proportion of HER-2 2+/HER-2− tumors were luminal B/HER-2− and triple negative (Table 4). However, in each of the IHC-defined tumor subtypes, a HER-2 2+ score combined with a HER-2− FISH status was still significantly associated with a worse DFS outcome (Fig. 3).
Table 4.
Prevalence of HER-2 2+/HER-2− tumors in immunohistochemically defined luminal A and luminal B/HER-2− tumors
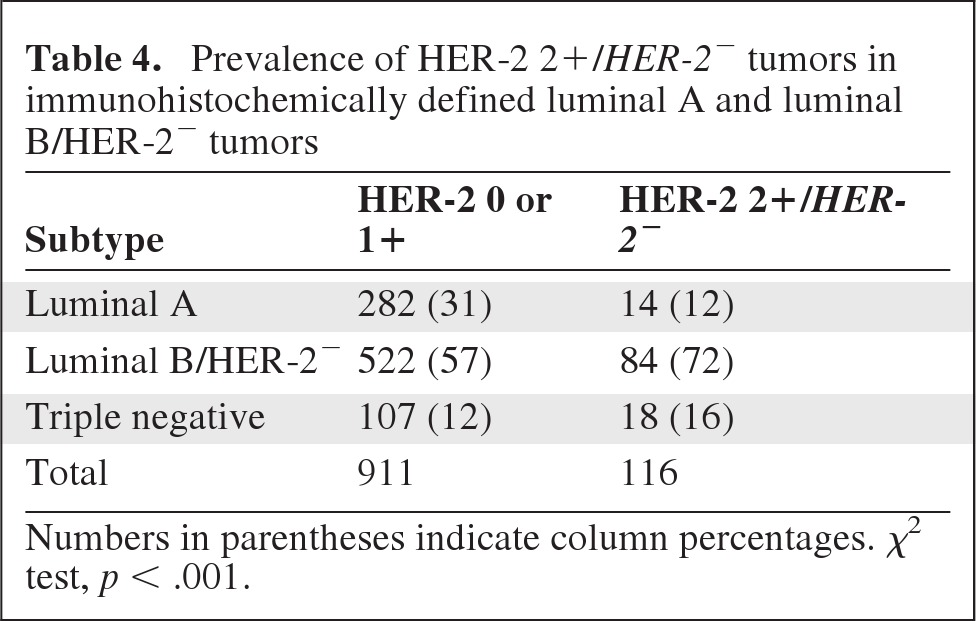
Numbers in parentheses indicate column percentages. χ2 test, p < .001.
Figure 3.
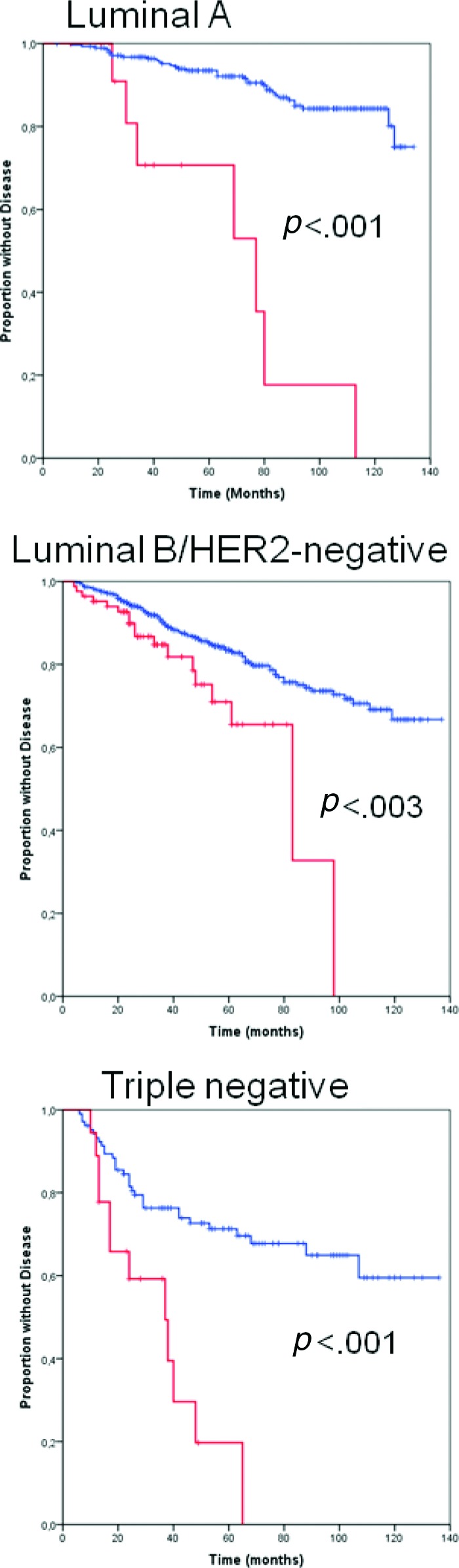
Kaplan–Meier estimates of disease-free survival probability according to HER-2 status in patients with different immunohistochemically defined tumor subtypes. Blue line, HER-2 0 and HER-2 1+; red line, HER-2 2+/HER-2−.
Discussion
In this series of patients with early breast cancer, we found that patients with tumors showing moderate immunostaining for HER-2 (2+) in the absence of HER-2 amplification had distinct pathological features and prognoses. Compared with patients with tumors with low (1+) or absent (0) HER-2 immunostaining, those with HER-2 2+/HER-2− tumors more frequently had larger diameter, grade 3 tumors, a higher Ki-67 score, and axillary lymph node involvement. All these features are similar to those of patients with HER-2+ tumors, from which HER-2 2+/HER-2− tumors differed because of more frequent hormone receptor positivity and less frequent ductal histology. In the comparison between HER-2 0 and 1+ tumors and HER-2 2+/HER-2− tumors, we confirmed that HER-2 status was an independent predictor of the DFS time. Furthermore, HER-2 2+/HER-2− tumors were more likely to be luminal B/HER-2− and triple-negative tumors than their HER-2 0 and 1+ counterparts, a factor that may have contributed to the observed differential prognosis. However, HER-2 2+/HER-2− status was significantly associated with a worse DFS outcome within each IHC-defined tumor subtype (luminal A, luminal B/HER-2−, and triple negative). Therefore, the first conclusion that we can draw from our results is that, in the clinical spectrum of commonly defined HER-2− tumors, two distinct entities do exist. The comparison between HER-2+ and HER-2 2+/HER-2− tumors revealed provoking findings. Not unexpectedly, patients with HER-2+ tumors tended to develop early DFS events. Conversely, patients with HER-2 2+/HER-2− tumors were characterized by a higher incidence of late DFS events. This translated into a time dependence of the HR for relapse when patients with these tumors were compared with those with HER-2+ tumors. Thus, the second conclusion that we can draw from our data is that, because of the better outcome in patients with HER-2+ tumors receiving anti–HER-2 adjuvant treatments, patients with HER-2 2+/HER-2− tumors might become the group with the most adverse prognosis and on which to focus research efforts.
Although the association between HER-2 status and prognosis has been known since the mid-1980s [6, 17, 25], only a few studies have analyzed the prognostic value of the definition of HER-2 status that is adopted in the clinic to establish patient eligibility for anti–HER-2 treatments. Birner et al. [26] assessed HER-2 status using the HercepTest™ and other IHC methods in 303 node-positive breast cancer patients. All tumor specimens were submitted to FISH analysis to detect HER-2 amplification. HercepTest™ 3+ cases, all of which were HER-2 amplified by FISH, had the worst prognosis in terms of DFS and overall survival outcomes. Although no significant differences were found among patients with tumors with 0, 1+, and 2+ immunostaining scores in terms of their overall survival time, 2+ cases showed a significantly longer DFS time. One of the limitations of that study was the very low number of HER-2 2+ tumors analyzed. Furthermore, and most importantly, the authors could not confirm any impact of HER-2 status on outcomes in multivariate analyses.
More recently, Ménard et al. [27] investigated the prognostic role of the HER-2 IHC score in a large multi-institutional database of 1,794 early breast cancer patients diagnosed in 2000–2001. HER-2 status was determined using different methods and reagents, including the HercepTest™ in 12% of cases, the polyclonal anti–p-185 antibody used in the HercepTest™ in 19% of cases, and the CB-11 mAb in 20% of cases. Notably, in 39% of the cases, the reagents used were not indicated. Results in the overall population confirmed that a 3+ score was associated with the worst prognosis in terms of the DFS interval. No effect was seen for the 2+ category. However, in patients with axillary lymph node–positive disease, patients whose tumors were scored 2+ had a worse DFS outcome than those with 0 or 1+ tumors, and the Kaplan–Meier curve of DFS probabilities of these patients overlapped with that of patients with 3+ tumors at about 50 months of follow-up. It would have been interesting to see whether or not longer follow-up could have revealed more late DFS events in patients with 2+ tumors, as we observed in our study.
A third, smaller study confirmed the adverse prognostic effect of both a 1+ and 2+ status over no HER-2 positivity (score of 0) in 91 patients with axillary lymph–node positive breast cancer receiving doxorubicin-based chemotherapy [28]. These findings in lymph node–positive patients are consistent with what we observed in our large, single-institution series of patients. Additionally, we could also confirm the same effect in patients with lymph node–negative tumors, regardless of the hormone receptor status. The strength of our observation is reinforced by the fact that we studied a large, single-institution series of patients with adequate postsurgical follow-up and, most importantly, centralized HER-2 testing. We therefore believe that the prognostic effect of a HER-2 2+/HER-2− status on prognosis is not a random finding and that it is worthy of further investigation. The association between a HER-2 2+/HER-2− status and adverse histopathological factors supports the hypothesis that greater signaling leading to aggressive biological behavior also can occur in the absence of HER-2 amplification. A similar phenomenon was also documented in other diseases in which HER-2 amplification is rare or absent, like prostate cancer and uterine carcinoma [29, 30]. In those studies, low to moderate HER-2 IHC positivity was found to be correlated with adverse histopathological features and prognoses.
In conclusion, we found that HER-2 2+/HER-2− tumors, which are usually defined as HER-2− by the diagnostic algorithm that is used to establish eligibility for anti–HER-2 treatments, carry a prognosis that is worse than that of tumors with no or weak HER-2 expression (IHC score of 0 or 1+). Furthermore, in the absence of adjuvant anti–HER-2 therapy, the prognosis of these patients is significantly better than that of patients with HER-2+ tumors in the initial 4–5 years of follow-up but worsens with longer follow-up.
As a result of suggestions from large, randomized, adjuvant trials that the benefits of trastuzumab may not be limited to patients with HER-2+ tumors, patients with HER-2 2+/HER-2− tumors are ideal candidates for studies testing this hypothesis.
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Valentina Rossi, Filippo Montemurro
Provision of study material or patients: Valentina Rossi, Filippo Montemurro, Ivana Sarotto, Furio Maggiorotto, Nicoletta Tomasi, Franziska Kubatzki, Rossella Martinello
Collection and/or assembly of data: Valentina Rossi, Ivana Sarotto, Giorgio Valabrega, Massimo Aglietta, Franziska Kubatzki, Rossella Martinello
Data analysis and interpretation: Valentina Rossi, Filippo Montemurro, Paola Berchialla, Massimo Aglietta, Riccardo Ponzone
Manuscript writing: Valentina Rossi, Filippo Montemurro
Final approval of manuscript: Valentina Rossi, Filippo Montemurro, Ivana Sarotto, Furio Maggiorotto, Paola Berchialla, Nicoletta Tomasi, Stefania Redana, Giorgio Valabrega, Massimo Aglietta, Riccardo Ponzone, Franziska Kubatzki, Rossella Martinello
References
- 1.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 4.Rilke F, Colnaghi MI, Cascinelli N, et al. Prognostic significance of HER-2/neu expression in breast cancer and its relationship to other prognostic factors. Int J Cancer. 1991;49:44–49. doi: 10.1002/ijc.2910490109. [DOI] [PubMed] [Google Scholar]
- 5.Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: Prognostic factor, predictive factor, and target for therapy. The Oncologist. 1998;3:237–252. [PubMed] [Google Scholar]
- 6.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 7.Berry DA, Muss HB, Thor AD, et al. HER-2/neu and p53 expression versus tamoxifen resistance in estrogen receptor-positive, node-positive breast cancer. J Clin Oncol. 2000;18:3471–3479. doi: 10.1200/JCO.2000.18.20.3471. [DOI] [PubMed] [Google Scholar]
- 8.Gennari A, Sormani MP, Pronzato P, et al. HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: A pooled analysis of randomized trials. J Natl Cancer Inst. 2008;100:14–20. doi: 10.1093/jnci/djm252. [DOI] [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 11.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 12.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianni L, Dafni U, Gelber RD, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: A 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–244. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 14.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 16.Tubbs RR, Pettay JD, Roche PC, et al. Discrepancies in clinical laboratory testing of eligibility for trastuzumab therapy: Apparent immunohistochemical false-positives do not get the message. J Clin Oncol. 2001;19:2714–2721. doi: 10.1200/JCO.2001.19.10.2714. [DOI] [PubMed] [Google Scholar]
- 17.Ross JS, Fletcher JA, Linette GP, et al. The Her-2/neu gene and protein in breast cancer 2003: Biomarker and target of therapy. The Oncologist. 2003;8:307–325. doi: 10.1634/theoncologist.8-4-307. [DOI] [PubMed] [Google Scholar]
- 18.Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358:1409–1411. doi: 10.1056/NEJMc0801440. [DOI] [PubMed] [Google Scholar]
- 19.Perez EA, Reinholz MM, Hillman DW, et al. HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J Clin Oncol. 2010;28:4307–4315. doi: 10.1200/JCO.2009.26.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Surgical Breast and Bowel Project trial. 2012. [accessed June 10, 2012]. p. 47. Available at http://www.nsabp.pitt.edu/B-47.asp.
- 21.Hilsenbeck SG, Ravdin PM, de Moor CA, et al. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res Treat. 1998;52:227–237. doi: 10.1023/a:1006133418245. [DOI] [PubMed] [Google Scholar]
- 22.Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perou CM, Sr̸lie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 24.Sr̸lie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Press MF, Bernstein L, Thomas PA, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: Poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15:2894–2904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- 26.Birner P, Oberhuber G, Stani J, et al. Evaluation of the United States Food and Drug Administration-approved scoring and test system of HER-2 protein expression in breast cancer. Clin Cancer Res. 2001;7:1669–1675. [PubMed] [Google Scholar]
- 27.Ménard S, Balsari A, Tagliabue E, et al. Biology, prognosis and response to therapy of breast carcinomas according to HER2 score. Ann Oncol. 2008;19:1706–1712. doi: 10.1093/annonc/mdn369. [DOI] [PubMed] [Google Scholar]
- 28.Gilcrease MZ, Woodward WA, Nicolas MM, et al. Even low-level HER2 expression may be associated with worse outcome in node-positive breast cancer. Am J Surg Pathol. 2009;33:759–767. doi: 10.1097/PAS.0b013e31819437f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minner S, Jessen B, Stiedenroth L, et al. Low level HER2 overexpression is associated with rapid tumor cell proliferation and poor prognosis in prostate cancer. Clin Cancer Res. 2010;16:1553–1560. doi: 10.1158/1078-0432.CCR-09-2546. [DOI] [PubMed] [Google Scholar]
- 30.Engelsen IB, Stefansson IM, Beroukhim R, et al. HER-2/neu expression is associated with high tumor cell proliferation and aggressive phenotype in a population based patient series of endometrial carcinomas. Int J Oncol. 2008;32:307–316. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.