The outcomes of colorectal cancer patients with lung metastases submitted to surgery were compared with those who did not receive surgery. Evidence suggesting an active role of surgery in improving survival outcomes in this patient subset is presented.
Keywords: Colorectal neoplasms, Lung metastases, Lung surgery, Overall survival
Abstract
Background.
The role of surgery for lung metastases (LM) secondary to colorectal cancer (CRC) remains controversial. The bulk of evidence is derived from single surgical series, hampering any definitive conclusions. The aim of this study was to compare the outcomes of CRC patients with LM submitted to surgery with those who were not.
Patients and Methods.
Data from 409 patients with LM as the first evidence of advanced disease were extracted from a database of 1,411 patients. Patients were divided into three groups: G1, comprised of 155 patients with pulmonary and extrapulmonary metastases; G2, comprised of 104 patients with LM only and no surgery; G3, comprised of 50 patients with LM only and submitted to surgery.
Results.
No difference in response rates emerged between G1 and G2. Median progression-free survival (PFS) times were: 10.3 months, 10.5 months, and 26.2 months for G1, G2, and G3, respectively. No difference in PFS times was observed between G1 and G2, whereas there was a statistically significant difference between G2 and G3. Median overall survival times were 24.2 months, 31.5 months, and 72.4 months, respectively. Survival times were longer in resected patients: 17 survived >5 years and three survived >10 years. In patients with LM only and no surgery, four survived for 5 years and none survived >10 years.
Conclusions.
Even though patients with resectable LM are more likely to be those with a better outcome, our study provides evidence suggesting an active role of surgery in improving survival outcomes in this patient subset.
Introduction
The clinical outcome of patients with metastatic colorectal cancer (CRC) has improved, with an increase in the median overall survival (OS) duration from 8–12 months in the 1990s to the current >20 months, along with a not negligible proportion of patients still alive at 5 years and 10 years. This improvement in treatment efficacy has been achieved mainly following the clinical use of highly active cytotoxic agents (e.g., irinotecan, oxaliplatin) and, more recently, molecular targeted therapies (e.g., cetuximab, panitumumab, and bevacizumab) [1], and through the multidisciplinary management of patients. Resection of liver metastases upfront or after neoadjuvant chemotherapy has been consistently demonstrated to result in longer survival times [2].
At the time of advanced disease presentation, pulmonary CRC metastases are revealed in ∼10%–15% of patients [3]. The best estimate of isolated lung metastases (i.e., without localization in other organs) lies in the range of 1.7%–7.4% [4]. The management of this latter subgroup of patients is a matter of debate. Surgical resection is a widespread clinical practice. Several studies describing single-institution series of resected patients reported 5-year survival rates of 21%–61.4%, exceeding those normally associated with metastatic CRC [5–7]. This notable difference in 5-year survival rates within surgical studies reflects the quality of evidence for pulmonary metastasectomy, which is insufficient to draw definitive conclusions [8]. In fact, whereas some authors demonstrated stage of the primary tumor, distribution of metastases, disease-free interval, carcinoembryonic antigen (CEA) level, gender, age, complete resection (R0), number of lung metastases, and vascular and lymphatic invasion to be variables influencing the 5-year survival rate, others reported opposite findings [9]. Moreover, inclusion criteria guidelines for lung metastasectomy published by several institutions rely on the experience of single institutions [10–12]. Despite these discrepancies, the reported outcomes are widely held to corroborate the benefit gained from lung surgery when compared with historical series. To solve the debate, a phase III, prospective, randomized clinical trial designed to compare patients with lung metastases allocated to “active monitoring” with those allocated to “active monitoring with pulmonary metastasectomy” has been advocated [13].
To our knowledge, there is no study that compared the outcomes of CRC patients with lung metastases that were surgically resected with those that were not followed and treated at the same institutions and during the same time frame. In this retrospective study, we searched the databases of three institutions and extracted data on patients consecutively followed and treated from the time of first appearance of metastatic disease. We then compared their outcomes according to whether or not they were submitted to lung metastasectomy.
Patients and Methods
Patients
Clinical databases of three institutions (University of Torino, Oncology Unit, San Luigi di Orbassano Hospital [center 1]; Institute for Cancer Research and Treatment [IRCC] Candiolo [center 2]; and University of Eastern Piedmont, Maggiore della Carità Hospital, Novara [center 3]) were retrospectively investigated. In these databases, the clinical characteristics and outcomes of all patients with metastatic CRC followed and treated from the time of first diagnosis of metastatic disease have been recorded since 1993. The data from patients with pulmonary metastases diagnosed in January 1, 1994 to June 30, 2010 were extracted and entered into a new database generated for the purpose of this study. The database included: patient demographics; primary tumor characteristics; prognostic and predictive factors (e.g., disease-free interval); CEA, lactate dehydrogenase (LDH), and alkaline phosphatase (ALP) levels at baseline (prechemotherapy or prethoracotomy); number and distribution of lung metastases; date of surgical intervention; chemotherapy history; date of first progression; and date of death or last follow-up visit. When >25, the number of lung metastases was put into the database with the absolute value of 30 and described in the results as “>25.”
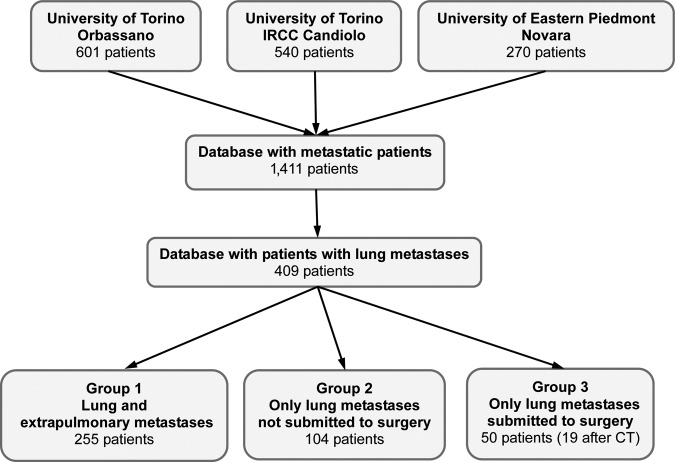
Three subgroups of patients were identified: group 1 (G1) included patients with at least one organ involved other than the lung, group 2 (G2) included patients with lung metastases as the sole site of advanced disease and no lung surgery, and group 3 (G3) included patients with lung metastases as the sole site of advanced disease who were submitted to lung surgery (Fig. 1).
Figure 1.
Consort diagram of the study.
Abbreviations: CT, chemotherapy; IRCC, Institute for Cancer Research and Treatment.
Outcome Evaluation
Response evaluation was performed under the standard assessment criteria used at each institution for the considered time frame. Up to 2001, treatment response was classified according to International Union Against Cancer criteria [14], wherein complete response was defined as the complete disappearance of all clinically detectable malignant disease, partial response was defined as a decrease ≥50% in the sum of the products of the two longest perpendicular diameters of all measurable lesions, and progressive disease was defined as an increase ≥25% in the size of measurable lesions and the development of new lesions. After 2001, centers were invited to classify responses according to the Response Evaluation Criteria In Solid Tumors (RECIST) [15], wherein response was defined as a decrease >30% in the sum of the longest diameters of target lesions and progressive disease was defined as an increase >20% of this sum. Only the best tumor response was recorded.
Progression-free survival (PFS) and OS times were estimated from first-line treatment onset until progression or death from any cause or date of last follow-up. The cutoff date for the collection of data was January 31, 2011. Patients not progressing or alive or lost to follow-up at the time of the cutoff date were censored at the time of the last follow-up examination.
Surgical Criteria
All patients with lung metastases were considered for lung resection at two thoracic surgery centers, one located at the University of Torino, San Luigi Gonzaga Hospital (also the referral center for the Oncology Unit at IRCC Candiolo) and the second one at the University of Eastern Piedmont, Maggiore della Carità Hospital, Novara. Although each institution evaluated patient eligibility for lung resection according to its own internal diagnostic workup procedures and by a multidisciplinary team that included the thoracic surgeon, the mandatory criteria requested for inclusion in this retrospective study were: resection with curative intent and with a predicted adequate residual pulmonary reserve after surgery in the absence of unresectable nonpulmonary localization. Surgery was performed upfront when resectability criteria were met. A surgical re-evaluation was planned for cases of tumor response or stabilization after chemotherapy.
Statistical Analyses
Differences between proportions were evaluated using the χ2 test with Yates correction, when necessary. Differences between groups of nonparametric unpaired variables were validated using the Mann-Whitney U test when comparing two groups and using Kruskal-Wallis analysis of variance (ANOVA) when analyzing multiple groups. Logistic regression analysis was performed to eliminate confounding parameters when examining dichotomous variables. Survival curves were plotted using the Kaplan–Meier method and validated using the log-rank test. A multivariate survival analysis was performed according to the Cox proportional hazards model. All statistical computations were performed using SPSS for Windows, version 16.0 (SPSS Inc., Chicago, IL) and STATISTICA for Windows, version 6.0 (StatSoft, Inc., Tulsa, OK) software.
Results
Patient Characteristics
Data from 1,411 CRC patients (Table 1) were retrospectively considered, 409 of whom presented with lung metastases and comprised the primary dataset for the present analyses. Patients were grouped as follows: G1 was comprised of 255 patients with lung and extrapulmonary metastases, G2 was comprised of 104 patients with the lung as the sole metastatic site and not submitted to lung resection, and G3 included the 50 patients submitted to resection (Fig. 1). Surgery was performed after neoadjuvant chemotherapy in 19 of 50 patients. Table 2 summarizes the characteristics of the 409 patients included in the study.
Table 1.
Characteristics of the entire database of patients followed and treated from the time of the first appearance of metastatic disease at three different institutions
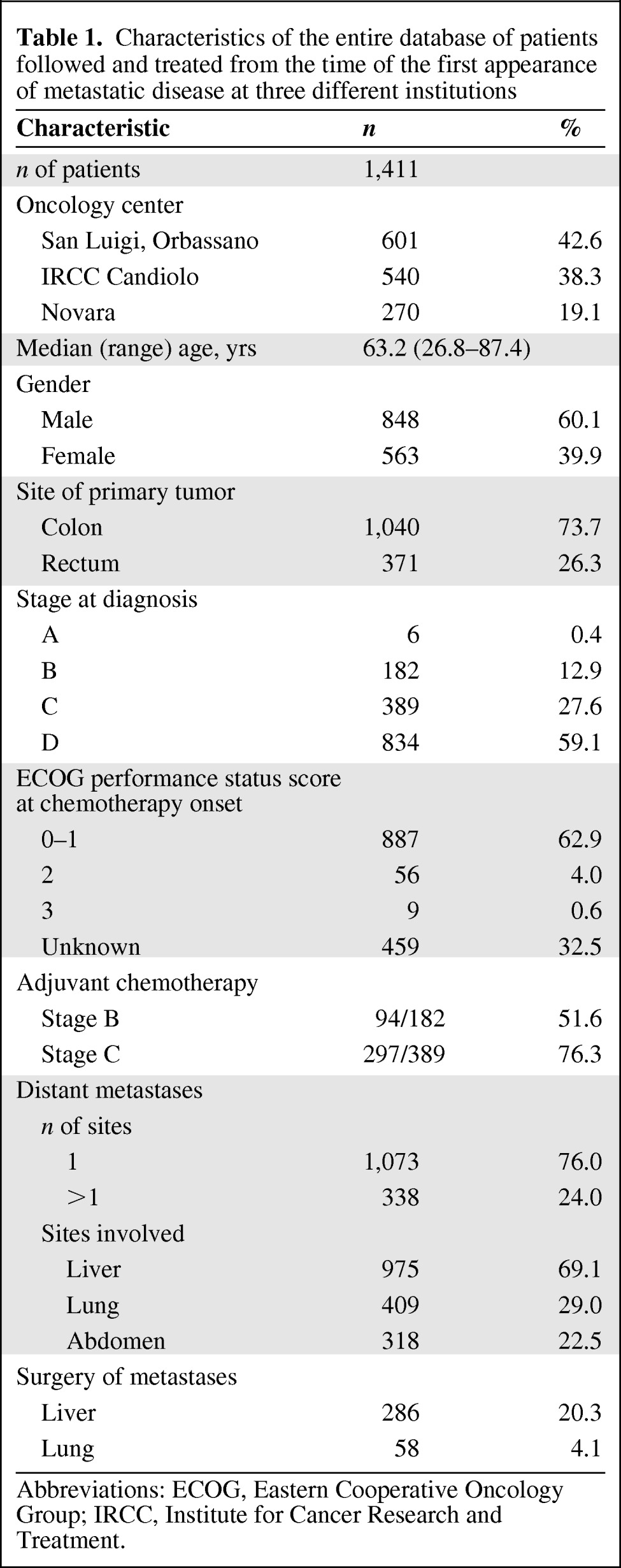
Abbreviations: ECOG, Eastern Cooperative Oncology Group; IRCC, Institute for Cancer Research and Treatment.
Table 2.
Characteristics of patients enrolled in this study
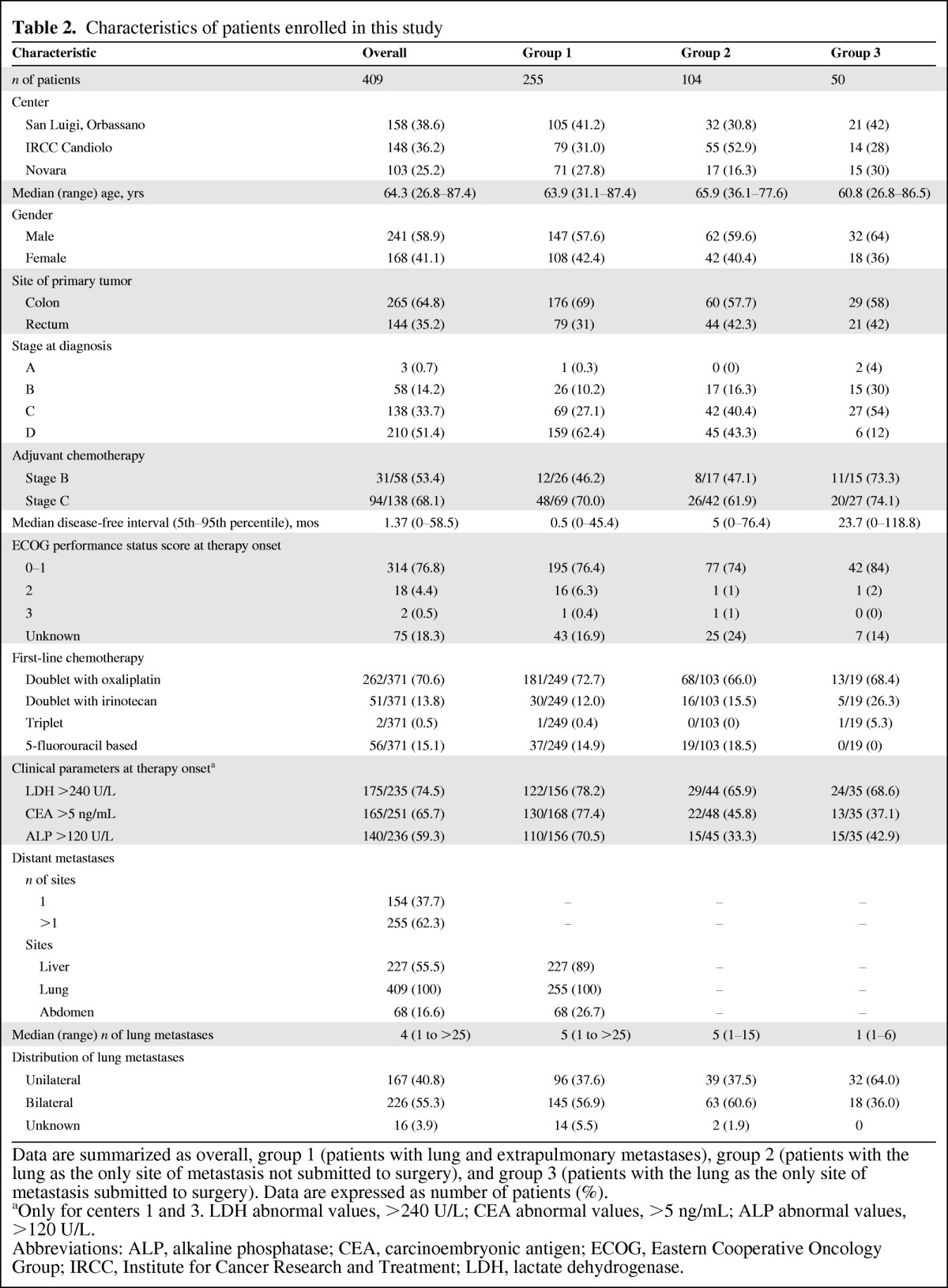
Data are summarized as overall, group 1 (patients with lung and extrapulmonary metastases), group 2 (patients with the lung as the only site of metastasis not submitted to surgery), and group 3 (patients with the lung as the only site of metastasis submitted to surgery). Data are expressed as number of patients (%).
aOnly for centers 1 and 3. LDH abnormal values, >240 U/L; CEA abnormal values, >5 ng/mL; ALP abnormal values, >120 U/L.
Abbreviations: ALP, alkaline phosphatase; CEA, carcinoembryonic antigen; ECOG, Eastern Cooperative Oncology Group; IRCC, Institute for Cancer Research and Treatment; LDH, lactate dehydrogenase.
The three groups were comparable for gender, Eastern Cooperative Oncology Group (ECOG) performance status score, and circulating serum prognostic factors such as LDH, CEA, and ALP. There was an expectedly higher proportion of colon cancers in group 1 than in the other two groups but no difference was observed between G2 and G3. Patient age and tumor stage at diagnosis were fairly similar in G1 and G2. The median age was significantly lower in G3 than in G2 (60.8 years and 65.9 years, respectively; p < .004) and there was a higher proportion of patients with metachronous tumors in G3 than in G2 (88% and 56.7%, respectively; X2 p < .0001). The median disease-free interval was longer in G3 than in G2 and G1 patients (23.7 months, 5 months, and 0.5 months, respectively; ANOVA p < .001).
Chemotherapy and Lung Surgery
In total, 371 of 409 (98.1%) patients received chemotherapy as first-line treatment and seven of 409 were not treated because of a poor performance status (n = 2), concomitant invalidating diseases (n = 2), and unknown reasons (n = 3). Thirty-one patients in G3 received lung surgery upfront and were not subsequently evaluable for chemotherapy response. The choice of systemic chemotherapy was left to each investigating center's discretion. The majority of patients (262 of 371, 70.6%) received an oxaliplatin-containing doublet, 51 of 371 (13.8%) received irinotecan-based chemotherapy, and 56 (15.1%) received fluoropyrimidine-based chemotherapy. Two patients received a triplet regimen. Nineteen patients in G2 (18.5%) received single-agent fluoropyrimidine chemotherapy because they were considered unfit (older or with other comorbidities) and one patient in G3 received a triplet regimen. No other differences in the type of chemotherapy administered between G2 and G3 patients were found (Table 2). The overall response rate was 38.0% (141 of 371 patients): 36.1% (90 of 249) in G1, 35.9% (37 of 103) in G2, and 73.7% (14 of 19) in G3. There was no difference among the three groups. All 31 patients submitted to pulmonary resection upfront received chemotherapy within 2 months of surgery. Most received 12 cycles of 5-fluorouracil, leucovorin, and oxaliplatin; only three received 12 courses of 5-fluorouracil, leucovorin, and irinotecan.
The median (range) numbers of lung metastases were five (1 to >25), five (1–15), and one (1–6) in G1, G2, and G3, respectively (ANOVA p < .001). A higher proportion of unilateral distribution was recorded in G3 (64%) than in G2 (37.5%) or in G1 (37.6%) (X2 p = .01). Resection was performed in 50 of 154 (32.5%) patients (31 upfront and 19 after neoadjuvant chemotherapy) with only metastatic lung disease. Reasons for surgery delay were: primary tumor not deemed controlled in 12 patients and complete resection not considered technically possible in seven patients. Wedge resection was performed in 34 (68%) patients, lobectomy was performed in 10 (20%) patients, segmentectomy was performed in five (10%) patients, and bilobectomy was performed in one (2%) patient. No postoperative mortality or major complications were reported. Resection was complete (R0) in 49 of 50 patients. Residual tumor was microscopically documented in the surgical margins in only one patient. One other patient underwent a second complete lung resection 1 month after the first surgical treatment.
In 21 patients in G2 who presented with resectable disease, surgery was not performed because of lung disease or other health conditions (mainly poor lung function reserve or cardiac disease). Eight patients in G1 with liver and lung metastases were submitted to liver and then to lung resection.
The proportion of patients submitted to lung resection was higher in centers with in-house thoracic surgery facilities: 21 of 53 (39.6%) in center 1, 15 of 32 (46.9%) in center 3, and 14 of 69 (20.3%) in center 2 (center 1 vs. center 2, X2 p = .01). However, there was no difference among centers in the proportion of patients with six or fewer lung metastases (the upper range of G3) not submitted to surgery (i.e., patients in G2) or in their distribution (ANOVA p > .5 for both analyses).
Clinical Outcomes
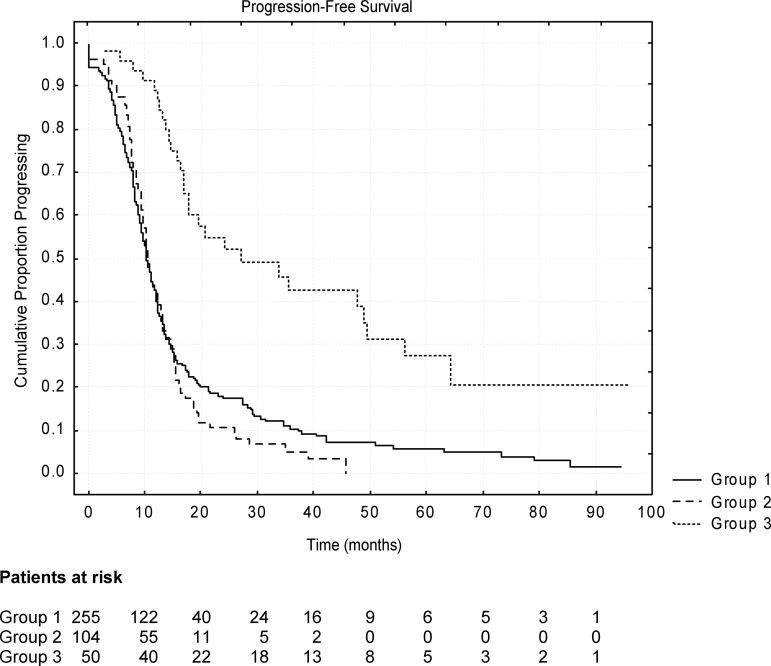
At the time of data computation (January 31, 2011), 334 of 409 (81.7%) patients had progressed. The median PFS duration of the entire population was 11.3 months. Figure 2 reports the PFS probability curves for each group. The median PFS durations were 10.3 months (95% confidence interval [CI],9.4–11.2 months) in G1, 10.5 months (95% CI, 9.6–11.4 months) in G2, and 26.2 months (95% CI, 10.4–42.0 months) in G3 (p < .001). Although the PFS duration was similar for patients with lung metastases as the sole site of disease not submitted to resection (G2) and those with lung and extrapulmonary metastases (G1) (10.5 months vs. 10.3 months; p not significant; hazard ratio [HR], 1.09; 95% CI, 0.96–1.22), it was longer in resected patients (G3) than in those with the lung as the sole metastatic site and not submitted to surgery (G2) (26.2 months vs. 10.5 months; p < .0001; HR, 0.24; 95% CI, 0.01–0.47). In the 19 patients who had received neoadjuvant chemotherapy before surgery, the median PFS duration was 26.4 months, comparable with that of patients undergoing upfront surgery (26.2 months).
Figure 2.
Kaplan–Meier curves of progression-free survival probability according to patient subgroup (see text). A statistically significant longer survival duration was evident only in patients with lung metastases submitted to surgery (group 3).
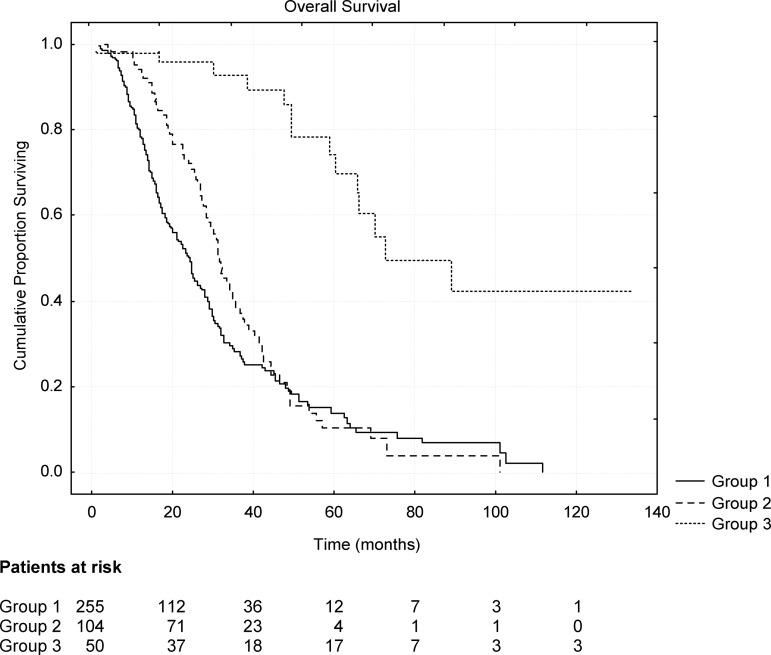
At the cutoff date for data collection (January 31, 2011), the median OS time of the entire population was 29.4 months. Figure 3 reports the Kaplan–Meier OS probability curve for each group. The median OS durations were 24.2 months (95% CI, 21.5–26.9 months) for G1, 31.5 months (95% CI, 28.8–34.2 months) for G2, and 72.4 months (95% CI, 40.7–104.1 months) for G3 (p < .001). The survival duration was longer in patients with the lung as the sole metastatic site than in those with pulmonary and extrapulmonary metastatic sites (31.5 months vs. 24.2 months; p < .03; HR, 0.76; 95% CI, 0.62–0.90). Patients who had undergone surgical resection of pulmonary metastases (G3) survived strikingly longer than those who had not (G2) (72.4 months vs. 31.5 months; p < .0001; HR, 0.17; 95% CI, 0.01–0.33). No statistically validated difference in the OS time emerged between patients submitted to neoadjuvant chemotherapy and those who were not (>70.1 months vs. >72.4 months; p = .9).
Figure 3.
Kaplan–Meier curves of overall survival probability according to patient subgroup (see text). Differences were evident and validated among groups.
At the cutoff date of data collection, 14 of 50 resected patients (G3) (28%) had died. The remaining 36 patients still alive were followed for a median of 41.3 months (range, 4.0–134.1 months). Seventeen (34%) were alive >5 years (five of them without sign of disease) and three (6%) were alive >10 years (two disease free) after their diagnosis of advanced CRC, whereas only four (4.8%) of the patients in G2 (patients with lung metastases not submitted to resection) were alive after 5 years (all with progressive disease).
Multivariate Analyses
Logistic regression analysis for surgery (value = 1) versus no surgery (value = 0) stratified for center confirmed age (HR, 0.96; 95% CI, 0.94–0.98), disease-free interval (HR, 1.03; 95% CI, 1.02–1.04), ECOG performance status score (HR, 0.54; 95% CI, 0.17–0.91), and the number of lung metastases (HR, 0.70; 95% CI, 0.62–0.70) as independent factors. Date of surgery, gender, tumor grade, CEA, LDH, ALP, distribution of lung metastases, and site of the primary did not enter the model.
In order to eliminate confounding variables, multivariate Cox analyses for predictors of PFS and OS times were performed in the entire population of 1,411 patients with metastatic disease (Tables 3 and 4).
Table 3.
Multivariate Cox proportional hazards analyses of predictive factors for progression-free survival duration in 1,411 patients with colorectal cancer followed from the time of first appearance of metastatic disease
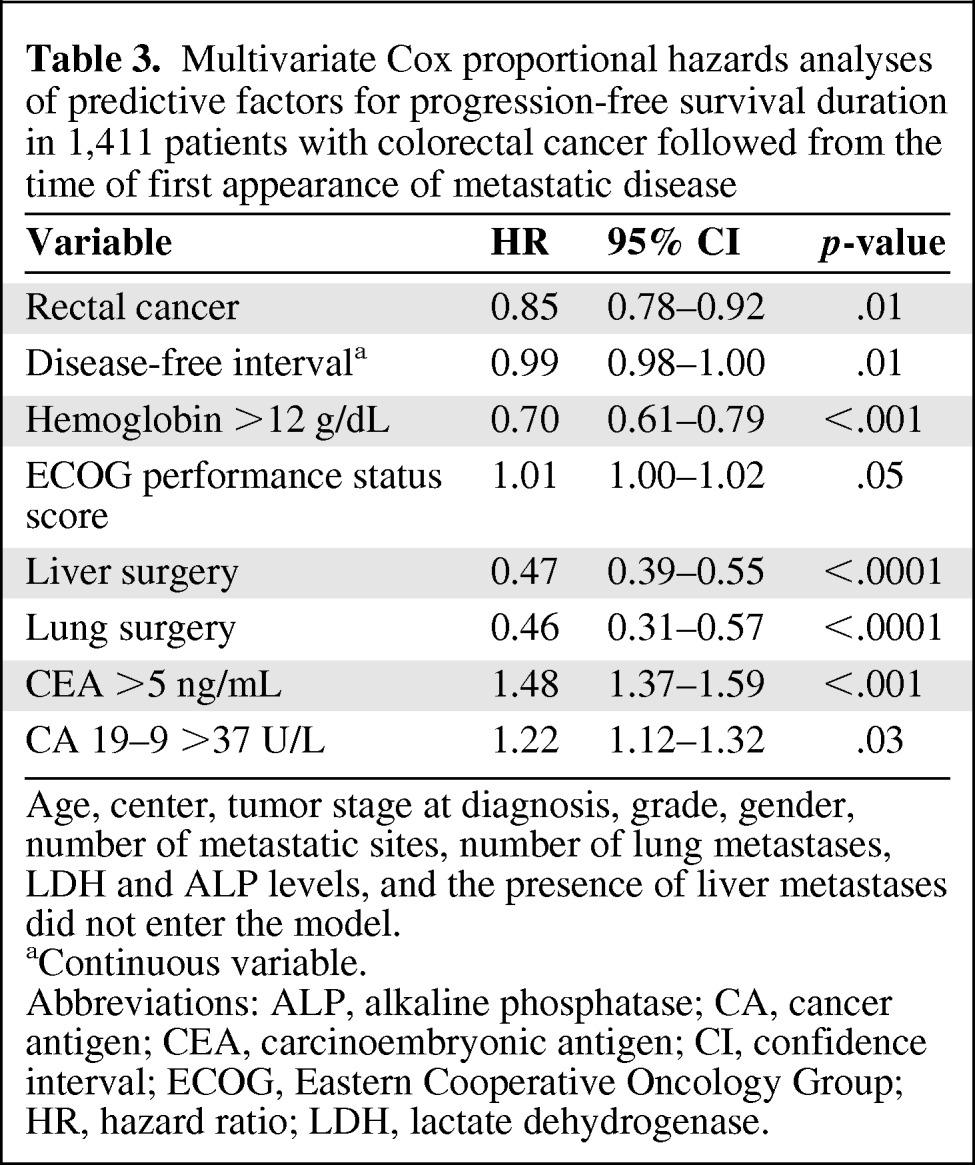
Age, center, tumor stage at diagnosis, grade, gender, number of metastatic sites, number of lung metastases, LDH and ALP levels, and the presence of liver metastases did not enter the model.
aContinuous variable.
Abbreviations: ALP, alkaline phosphatase; CA, cancer antigen; CEA, carcinoembryonic antigen; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; LDH, lactate dehydrogenase.
Table 4.
Multivariate Cox proportional hazards analyses of predictive factors for overall survival duration in 1,411 patients with colorectal cancer followed from the time of first appearance of metastatic disease
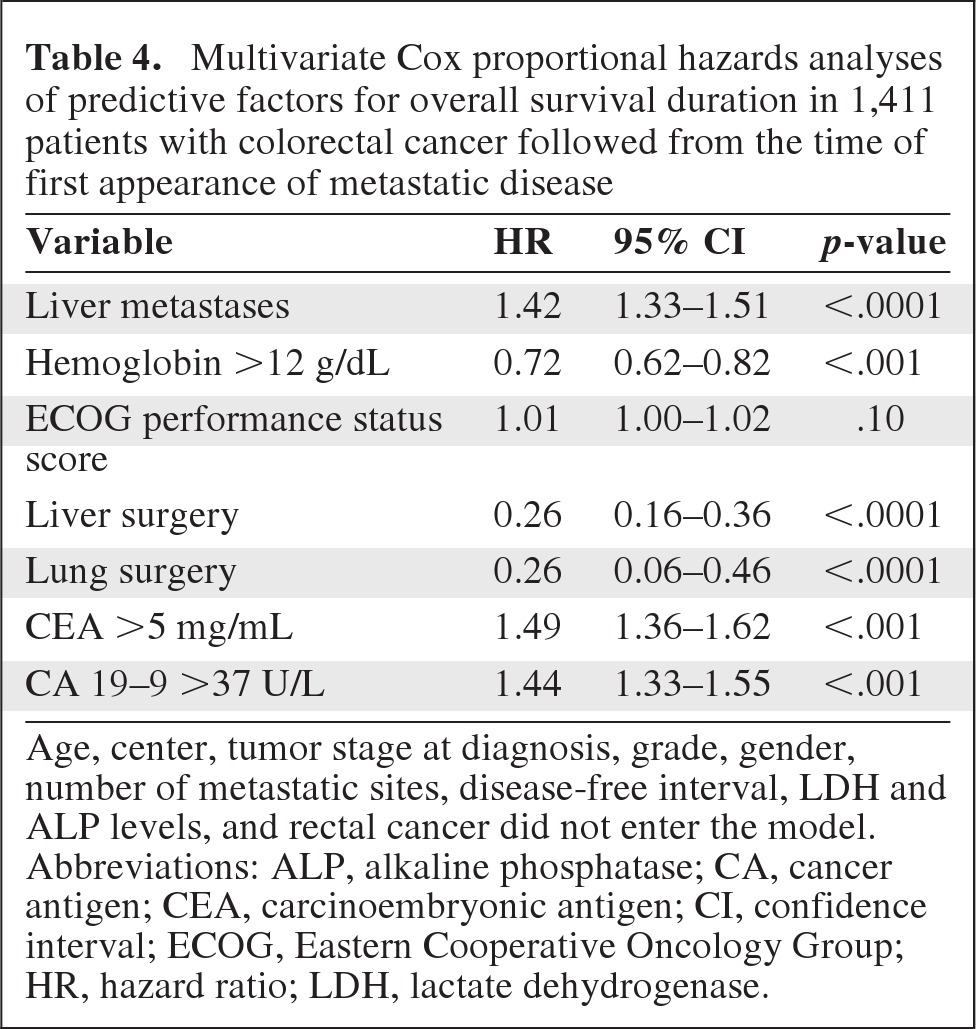
Age, center, tumor stage at diagnosis, grade, gender, number of metastatic sites, disease-free interval, LDH and ALP levels, and rectal cancer did not enter the model.
Abbreviations: ALP, alkaline phosphatase; CA, cancer antigen; CEA, carcinoembryonic antigen; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; LDH, lactate dehydrogenase.
Rectal localization, disease-free interval (as a continuous variable), hemoglobin level >12 g/dL, ECOG performance status score, and surgery of liver and lung metastases were found to be independent factors predictive of the PFS duration (Table 3). Age, treatment center, tumor stage at diagnosis, grade, gender, and number of metastatic sites did not enter the model.
The disease-free interval (as a continuous variable), the presence of liver metastases, hemoglobin level >12 g/dL, number of metastatic sites, and surgery of liver and lung metastases were found to be independent factors predictive of the OS duration (Table 4). Age, treatment center, tumor stage at diagnosis, grade, gender, ECOG performance status score, and rectal localization did not enter the model.
Discussion
In this large, retrospective study in metastatic CRC patients, we observed remarkably longer PFS and OS durations in patients submitted to resection with radical intent of their pulmonary metastases than in those who received chemotherapy alone. This is the first study to compare outcomes in the same series of patients and not against historical reports.
The incidence of synchronous lung metastases was higher in our study population than that previously reported [16]: 29% of patients presented with synchronous lung metastases and 10.9% had only lung localizations. This may be explained, in part, by the fact that the staging procedure included thoracic computed tomography, which has been demonstrated to be more sensitive than conventional x-ray [17]. Nearly one third of the patients (50 of 154) with the lung as the sole metastatic site underwent surgery. This number does not include the eight patients submitted to liver and subsequently to pulmonary resection, because they were included in G1 (patients with lung and extrapulmonary metastases) in the intention-to-treat analysis.
Not surprisingly, patients submitted to surgery were, on average, younger. The incidence of chronic lung diseases, such as chronic obstructive pulmonary disease, increases with age. As a consequence of these chronic comorbidities, older patients more frequently have poor lung function reserve and are ineligible for surgery, as was the case for 21 patients in this study. This might represent a selection bias, because younger patients are assumed to have a longer survival duration, even if other series have not shown this difference [18], or even reported an opposite pattern [19].
Quite unexpectedly, a higher number of patients with metachronous lung metastases was observed in the resected subgroup. Synchronous metastases of CRC are considered to carry a worse prognostic value than metachronous metastases, but there are few and conflicting data reported. A large, retrospective study from the Capecitabine, Irinotecan, and Oxaliplatin in Advanced Colorectal Cancer trial of the Dutch Colorectal Cancer Group concluded that, despite unfavorable clinicopathological features in patients with synchronous metastases, no difference in the median OS duration was observed [20]. A possible explanation could be the relative chemoresistance of metachronous metastases resulting from adjuvant treatments. In our study, 31 of 42 (73.8%) patients in G3 and 34 of 59 (57.6%) patients in G2 received adjuvant chemotherapy. Although not statistically validated, this difference is worth consideration.
As demonstrated by the logistic regression analysis, age, disease-free interval and the number of metastases are the variables that drove surgeons through the decision process. Even though discrepant results have been reported in the literature on the possible prognostic role of these three variables, it is reasonable to think that resected patients were those destined to have, per se, a longer survival duration because they were younger, presented with a longer disease-free interval, and had a lower number of lung metastases than those not submitted to surgery.
One open question concerns whether the strikingly longer survival duration of patients submitted to lung resection is a result of the beneficial effect of surgery itself or the selection bias described above [21, 22]. There is a large body of literature reporting the survival benefits gained from lung resection [5–7, 18–22]. However, a sort of citation cascade of the same few studies reporting these survival benefits, excluding those with negative results, might have resulted in an unfounded “authority of claim,” as recently demonstrated by Fiorentino et al. [8]. Optimally, this question could be definitively answered by a phase III trial [13]. However, if it could be indirectly demonstrated that surgery is effectively beneficial in curing patients or at least prolonging their survival duration, such a comparative trial would have lower priority from a medical oncology point of view; such was the case for surgery for the treatment of liver metastases.
Our data confirm a better outcome in patients submitted to surgery than in those treated with chemotherapy alone in the same institutions and during the same time period. In the resected subgroup, 17 patients were alive after >5 years and three were alive >10 years after the diagnosis of advanced cancer, whereas only four of those in G2 were alive after 5 years. Interestingly, of the eight patients in G1 submitted to liver and subsequently lung resection, the duration of survival was 5 years in two patients and 10 years in one patient.
Several published studies have discussed the importance of finding surrogate endpoints as outcome indicators. The response rate and PFS interval after first-line treatment have been proposed and validated in CRC patients [23–25] because their evaluation allows a reliable quantitative estimate of the efficacy of new drugs or new techniques with a lower degree of potential bias. In our retrospective study, patients were treated with chemotherapy (G1 and G2) or with surgery. Response rates to first-line treatment were similar in G1 and G2 (36.1% and 35.9%, respectively). Determining treatment response in G3 patients was difficult because 31 patients received surgery before chemotherapy: if we take into account only the 19 patients who received chemotherapy before surgery, the response rate was 73.7%; if we consider, however, surgery as an active first-line treatment, the response rate of the whole group was 98%, because residual tumor after surgery (R1) was evident in only one patient. Patients submitted to surgery had a median PFS duration of 26.2 months, more than twice that recorded in those not resected (10.5 months). Interestingly, the PFS durations were similar for G1 and G2 patients (10.3 months). On multivariate analysis of the complete patient dataset of 1,411 subjects, liver and lung surgery emerged as independent factors for both the PFS and OS outcomes. These findings further suggest that surgery is a more active treatment than chemotherapy alone.
In conclusion, the results of our retrospective study provide evidence suggesting that surgery is a more active treatment than chemotherapy alone when performed as first-line treatment. Although resectable patients are probably those destined to have a more indolent form of the disease because they have theoretically favorable prognostic factors, surgery can further improve their outcome. In our study, we recorded 10-year survivors only in G3 and G1 (one patient submitted to liver and then lung resection) and not in G2, in which some potentially resectable patients might have been included. Moreover, resected patients had a longer PFS duration, a validate surrogate endpoint for clinical outcome. From a statistical point of view, our findings are insufficient to definitively solve the question of whether or not surgery is beneficial in resectable patients because this can be demonstrated only with a prospective, randomized, phase III trial. From a medical oncology point of view, however, our results add evidence to the debate on whether or not such a study is still necessary.
Acknowledgments
This study was funded by local regional authority: “Regione Piemonte, Finanziamento di ricerca sanitaria finalizzata – anno 2009” Prot. 30258/DB2001.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Marco Tampellini, Irene Alabiso, Francesco Ardissone, Alfredo Berruti
Provision of study material or patients: Elisa Sperti, Stefania Miraglia, Oscar Alabiso, Massimo Aglietta
Collection and/or assembly of data: Azzurra Ottone, Elisa Bellini, Irene Alabiso, Chiara Baratelli, Raffaella Bitossi, Anna Ferrero, Francesco Leone, Stefania Miraglia, Laura Forti, Erica Bertona
Data analysis and interpretation: Marco Tampellini, Maria P. Brizzi, Francesco Leone, Laura Forti, Francesco Ardissone
Manuscript writing: Marco Tampellini, Francesco Ardissone
Final approval of manuscript: Marco Tampellini, Azzurra Ottone, Elisa Bellini, Irene Alabiso, Chiara Baratelli, Raffaella Bitossi, Maria P. Brizzi, Anna Ferrero, Elisa Sperti, Stefania Miraglia, Laura Forti, Erica Bertona, Francesco Ardissone, Alfredo Berruti, Oscar Alabiso, Massimo Aglietta, Giorgio V. Scagliotti
References
- 1.Catenacci DV, Kozloff M, Kindler HL, et al. Personalized colon cancer care in 2010. Semin Oncol. 2011;38:284–308. doi: 10.1053/j.seminoncol.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simmonds PC, Primrose JN, Colquitt JL, et al. Surgical resection of hepatic metastases from colorectal cancer: A systematic review of published studies. Br J Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limmer S, Unger L. Optimal management of pulmonary metastases from colorectal cancer. Expert Rev Anticancer Ther. 2011;11:1567–1575. doi: 10.1586/era.11.123. [DOI] [PubMed] [Google Scholar]
- 4.Tan KK, Lopes Gde L, Jr, Sim R. How uncommon are isolated lung metastases in colorectal cancer? A review from database of 754 patients over 4 years. J Gastrointest Surg. 2009;13:642–648. doi: 10.1007/s11605-008-0757-7. [DOI] [PubMed] [Google Scholar]
- 5.Hornbech K, Ravn J, Steinbrc̈hel DA. Outcome after pulmonary metastasectomy: Analysis of 5 years consecutive surgical resections 2002–2006. J Thorac Oncol. 2011;6:1733–1740. doi: 10.1097/JTO.0b013e3182287da2. [DOI] [PubMed] [Google Scholar]
- 6.Borasio P, Gisabella M, Billé A, et al. Role of surgical resection in colorectal lung metastases: Analysis of 137 patients. Int J Colorectal Dis. 2011;26:183–190. doi: 10.1007/s00384-010-1075-6. [DOI] [PubMed] [Google Scholar]
- 7.Girard P, Ducreux M, Baldeyrou P, et al. Surgery for lung metastases from colorectal cancer: Analysis of prognostic factors. J Clin Oncol. 1996;14:2047–2053. doi: 10.1200/JCO.1996.14.7.2047. [DOI] [PubMed] [Google Scholar]
- 8.Fiorentino F, Vasilakis C, Treasure T. Clinical reports of pulmonary metastasectomy for colorectal cancer: A citation network analysis. Br J Cancer. 2011;104:1085–1097. doi: 10.1038/sj.bjc.6606060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: A systematic review of published series. Ann Thorac Surg. 2007;84:324–338. doi: 10.1016/j.athoracsur.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence. Guidance on Cancer Services. Improving Outcomes in Colorectal Cancer: Manual Update. [accessed May 30, 2012]. Available at http://guidance.nice.org.uk/CSGCC/Guidance/pdf/English.
- 11.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Colon Cancer. [accessed May 30, 2012]. Available at http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- 12.British Thoracic Society. Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: Guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001;56:89–108. doi: 10.1136/thorax.56.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treasure T, Fallowfield L, Lees B, et al. Pulmonary metastasectomy in colorectal cancer: The PulMiCC trial. Thorax. 2012;67:185–187. doi: 10.1136/thoraxjnl-2011-200015. [DOI] [PubMed] [Google Scholar]
- 14.Hayward JL, Carbone PP, Heuson JC, et al. Assessment of response to therapy in advanced breast cancer: A project of the Programme on Clinical Oncology of the International Union Against Cancer, Geneva, Switzerland. Cancer. 1977;39:1289–1294. doi: 10.1002/1097-0142(197703)39:3<1289::aid-cncr2820390340>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Mitry E, Guiu B, Cosconea S, et al. Epidemiology, management and prognosis of colorectal cancer with lung metastases: A 30-year population-based study. Gut. 2010;59:1383–1388. doi: 10.1136/gut.2010.211557. [DOI] [PubMed] [Google Scholar]
- 17.Parnaby CN, Bailey W, Balasingam A, et al. Pulmonary staging in colorectal cancer: A review. Colorectal Dis. 2012;14:660–670. doi: 10.1111/j.1463-1318.2011.02601.x. doi: 10.1111/j.1463–1318.2011.02601.x. [DOI] [PubMed] [Google Scholar]
- 18.Stillwell AP, Ho YH, Veitch C. Systematic review of prognostic factors related to overall survival in patients with stage IV colorectal cancer and unresectable metastases. World J Surg. 2011;35:684–692. doi: 10.1007/s00268-010-0891-8. [DOI] [PubMed] [Google Scholar]
- 19.Onaitis MW, Petersen RP, Haney JC, et al. Prognostic factors for recurrence after pulmonary resection of colorectal cancer metastases. Ann Thorac Surg. 2009;87:1684–1688. doi: 10.1016/j.athoracsur.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Mekenkamp LJ, Koopman M, Teerenstra S, et al. Clinicopathological features and outcome in advanced colorectal cancer patients with synchronous vs metachronous metastases. Br J Cancer. 2010;103:159–164. doi: 10.1038/sj.bjc.6605737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfannschmidt J, Hoffmann H, Dienemann H. Reported outcome factors for pulmonary resection in metastatic colorectal cancer. J Thorac Oncol. 2010;5(suppl 2):S172–S178. doi: 10.1097/JTO.0b013e3181dca330. [DOI] [PubMed] [Google Scholar]
- 22.Mahmoud N, Bullard Dunn K. Metastasectomy for stage IV colorectal cancer. Dis Colon Rectum. 2010;53:1080–1092. doi: 10.1007/DCR.0b013e3181dcadbc. [DOI] [PubMed] [Google Scholar]
- 23.Tang PA, Bentzen SM, Chen EX, et al. Surrogate end points for median overall survival in metastatic colorectal cancer: Literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. J Clin Oncol. 2007;25:4562–4568. doi: 10.1200/JCO.2006.08.1935. [DOI] [PubMed] [Google Scholar]
- 24.Saad ED, Katz A, Hoff PM, et al. Progression-free survival as surrogate and as true end point: Insights from the breast and colorectal cancer literature. Ann Oncol. 2010;21:7–12. doi: 10.1093/annonc/mdp523. [DOI] [PubMed] [Google Scholar]
- 25.Piedbois P, Buyse M. Endpoints and surrogate endpoints in colorectal cancer: A review of recent developments. Curr Opin Oncol. 2008;20:466–471. doi: 10.1097/CCO.0b013e32830218fe. [DOI] [PubMed] [Google Scholar]