This review aims to summarize all available evidence on the association between geriatric assessments and relevant oncologic outcomes.
Keywords: Comprehensive geriatric assessment, Prognostication, Elderly, Treatment tolerance
Learning Objectives
After completing this course, the reader will be able to:
Describe the predictive value of geriatric assessments for survival in older cancer patients.
Describe the predictive value of geriatric assessments for treatment tolerance (such as toxicity of chemotherapy and perioperative complications) in older cancer patients.
Explain the concept of frailty compared to individual geriatric conditions.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
Awareness of the use of geriatric assessments for older patients with cancer is increasing. The aim of this review is to summarize all available evidence on the association between geriatric assessments and relevant oncologic outcomes.
Method.
A systematic search was conducted in Medline and Embase of studies on geriatric assessment in oncology, focusing on the association between baseline assessment and outcome.
Results.
The literature search identified 2008 reports; 51 publications from 37 studies were selected for inclusion in the review. The quality of studies was heterogeneous and generally poor. A median of five geriatric conditions were assessed per study (interquartile range: 4–8). Little consistency was found in the results of the studies. Furthermore, different tools appear to be predictive depending on the outcome measure: frailty, nutritional status, and comorbidity assessed by the Cumulative Illness Rating Scale for Geriatrics were predictive for all-cause mortality; frailty was predictive for toxicity of chemotherapy; cognitive impairment and activities of daily living impairment were predictive for chemotherapy completion; and instrumental activities of daily living impairment was predictive for perioperative complications.
Conclusion.
Although various geriatric conditions appear to be of some value in predicting outcome in elderly patients with cancer, the results are too inconsistent to guide treatment decisions. Further research is needed to elucidate the role of geriatric assessments in the oncologic decision-making process for these patients.
Introduction
Although malignant tumors occur at all ages, cancer disproportionately strikes individuals aged 65 years and older [1]. In addition, the number of elderly patients with cancer will increase substantially in the coming decades as a result of increasing life expectancy and aging of the population. Oncologists are faced with the challenge of determining the optimal treatment for these patients, with their heterogeneity in comorbidity, physical reserve, disabilities, and geriatric conditions. In this context, a myriad of editorials and review articles have been published, endorsing the use of a comprehensive geriatric assessment (CGA) in geriatric oncology [2–9]. A CGA is a systematic procedure used to objectively appraise the health status of older people, focusing on somatic, functional, and psychosocial domains [2] and aimed at constructing a multidisciplinary treatment plan. Its value in geriatric medicine has been proven extensively [10], but outside this field, the evidence is more scarce.
Oncology studies comparing treatment choices in patients that are considered fit or frail on the basis of a CGA have shown that frail patients receive less intensive treatment or receive no treatment at all [11, 12]. Although this shows that standard medical assessment overlaps in part with geriatric assessment, an additional value of the latter is its ability to identify previously unrecognized but potentially modifiable health issues, such as depressive symptoms, cognitive or functional impairment, and malnutrition [4, 5, 7]. In addition, some studies are now using CGA to assess patients for trial eligibility or to allocate them to alternative treatments regimens [13, 14]. However, the legitimacy of such decision-making protocols has been insufficiently proven thus far. It remains unclear how to translate data from the CGA to clinical practice: Should geriatric assessment only be used to classify patients as fit, vulnerable, or frail, or do individual geriatric conditions have predictive value for relevant patient outcomes?
Therefore, the aim of this systematic review is to summarize all available evidence on the association between CGA (its individual domains as well as the summarized assessment of vulnerability) and clinically relevant outcomes, such as all-cause mortality, chemotherapy toxicity, chemotherapy completion, perioperative complications, and radiotherapy tolerance.
Methods
Search Strategy and Article Selection
Our aim was to identify cohort studies that investigated the association between baseline geriatric assessment and outcome in patients with cancer, independent of age, cancer type, or stage of disease. For this purpose, a geriatric assessment was defined as an assessment using validated assessment tools composed of two or more of the following distinct domains: cognitive function, mood/depression, nutritional status, activities of daily living (ADL), instrumental activities of daily living (IADL), comorbidity, polypharmacy, mobility/falls, and frailty. Studies only using nonvalidated assessment tools or nonvalidated subscales of validated assessment tools were excluded. We also excluded studies that included other patient groups in addition to patients with cancer, as well as studies using a treatment protocol in which the outcome of the geriatric assessment determined treatment choice. For outcome, the following items were defined: all-cause mortality, toxicity of chemotherapy, chemotherapy completion, perioperative complications, and radiotherapy completion and toxicity.
We performed the following search in both Medline and Embase on February 15, 2012: ((“Geriatric Assessment”[Mesh]) OR (geriatric assessment*[tiab])) AND ((“Neoplasms”[Mesh]) OR (neoplasm*[tiab] OR cancer*[tiab] OR tumour[tiab] OR tumours[tiab] OR tumor[tiab] OR tumors[tiab] OR oncolog*[tiab] OR malignan*[tiab])) (Mesh indicates medical subheading; tiab, title/abstract). No limits in age, language, or publication date were applied to the search.
In addition, conference abstracts for the 2007–2011 scientific meetings of the American Society of Clinical Oncology, European Society of Medical Oncology, International Society of Geriatric Oncology, American Geriatric Society, and European Geriatric Medicine Society (EUGMS) were hand-searched for studies on geriatric assessments in patients with cancer to identify additional eligible studies.
The titles and abstracts of all studies retrieved by the search were assessed by one investigator (M.H.) to determine which were eligible for further investigation. All potentially relevant articles were subsequently screened as full text by two authors (M.H. and A.V.). In case only an abstract was available, we attempted to find a final report of the study by searching Embase and Medline using the names of first, second, and/or final authors as well as key words from the title. Also, in case of insufficient data in the original manuscript, the authors were contacted for additional information (e.g., about the tools used in the geriatric assessment). Finally, references of included publications were cross-referenced to retrieve any additional relevant citations.
Data Extraction
Data regarding study design and results were independently extracted by two investigators (M.H. and A.V.) for each eligible study. Items that were extracted included the type of study, study setting, study population (cancer type, cancer stage, cancer treatment), content of geriatric assessment and assessment tools used, outcome measures examined, methods of statistical analysis, and the reported results on the association between geriatric assessments and the outcome measures.
Quality Assessment
The methodological quality of each of the studies was independently assessed by two reviewers (M.H. and A.V.). Disagreements among the reviewers were discussed during a consensus meeting; in case of persisting disagreement, the assistance of a third reviewer (B.v.M.) was enlisted. We used a standardized list of 16 criteria to assess the methodological quality of the included studies. This list was a modified version of the checklist used by Kuijpers et al. [15] based on the theoretical considerations and methodological aspects described by Altman [16] (supplemental online Appendix 1a).
Data Synthesis and Analysis
As a result of heterogeneity in study designs, diversity of patient populations, and the wide variety in content of the geriatric assessment, a formal meta-analysis was not possible. Therefore, we summarized the study results to describe our main outcomes of interest. If necessary, reciprocal odds ratios or hazard ratios were calculated for optimizing comparability of data. When applicable, subgroup summaries were made based on the tools used in the assessment of the geriatric conditions.
Results
Study Characteristics
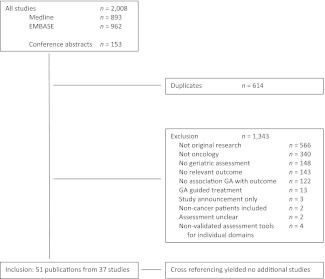
The literature search identified 1,855 citations (893 from Medline and 962 from Embase), of which 61 were duplicates. Hand-searching of conference abstracts yielded another 153 potentially relevant publications. Details on the search and reasons for exclusion can be found in Figure 1. After exclusion of 1,343 publications, 51 publications from 37 studies were included in this review [17–67]. Cross-referencing yielded no additional results.
Figure 1.
Search results and study selection.
The characteristics of these 37 studies are summarized in Table 1. The first publication is from 2002, but more than half of the studies were published in the last 2 years [17–67]. All but one study consisted of prospective cohorts [24]. The median sample size was 152 patients (range 20–1,130 patients) [17–67]. Study populations were heterogeneous, with only half focusing on a specific type of cancer [19, 20, 23, 25–27, 30–35, 45–46, 49–52, 56, 58–59, 61–63], of which eleven also focused on a specific cancer stage (30% of all studies) [19, 20, 23, 30, 32–35, 45, 52, 59, 61–63]. Furthermore, although 26 studies focused on chemotherapy (70%) [17–20, 23–36, 39–41, 45–46, 52–53, 56, 58–60, 62–66] and four studies focused on surgery (11%) [22, 47–51, 54–55], seven studies included patients receiving multiple treatment modalities (19%) [21, 37, 38, 42–44, 57, 61, 67].
Table 1.
Characteristics of included studies
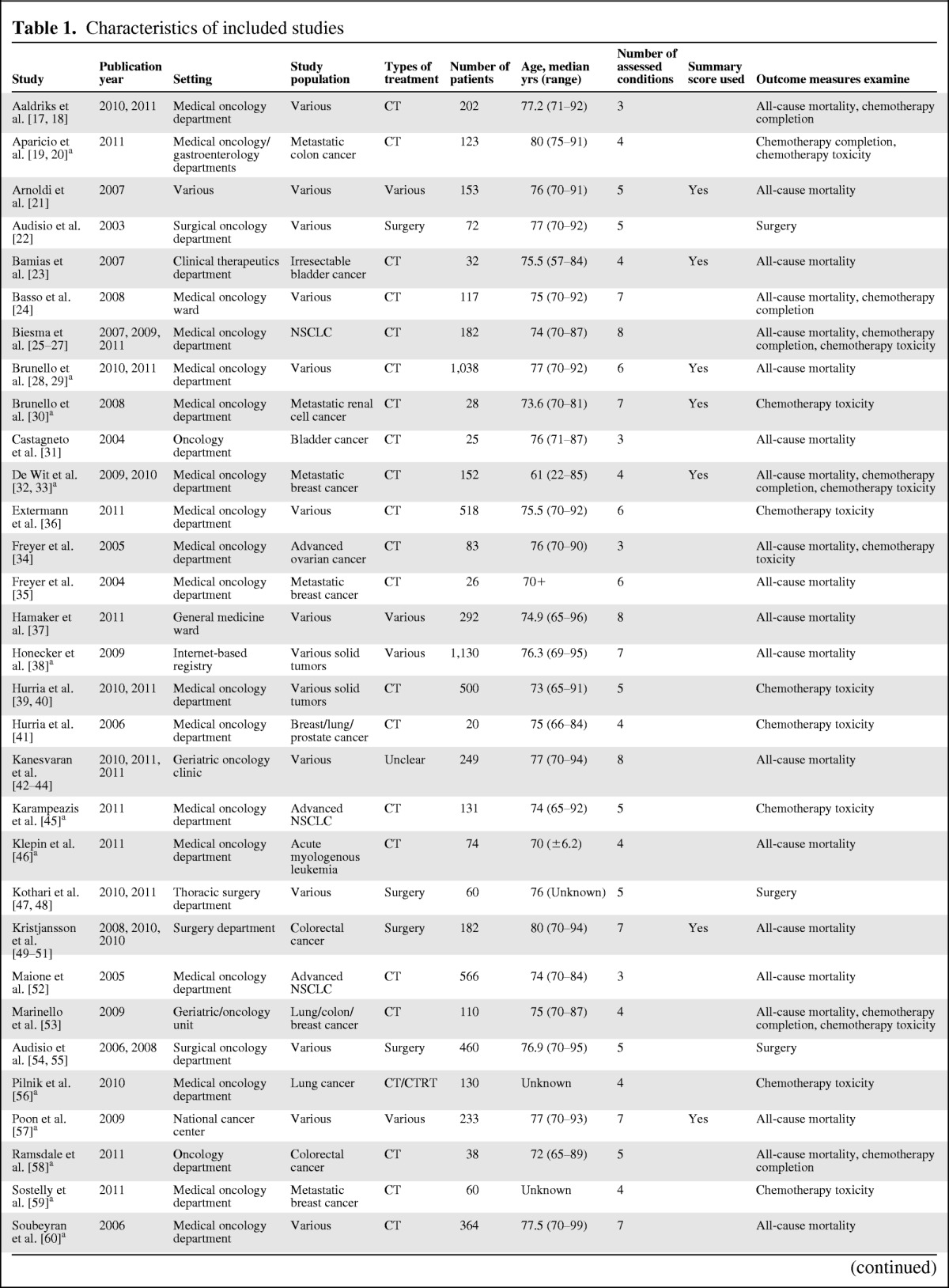
Table 1.
(Continued)
aAbstract.
Abbreviations: CT, chemotherapy; CTRT, chemoradiation; NSCLC, non-small cell lung cancer.
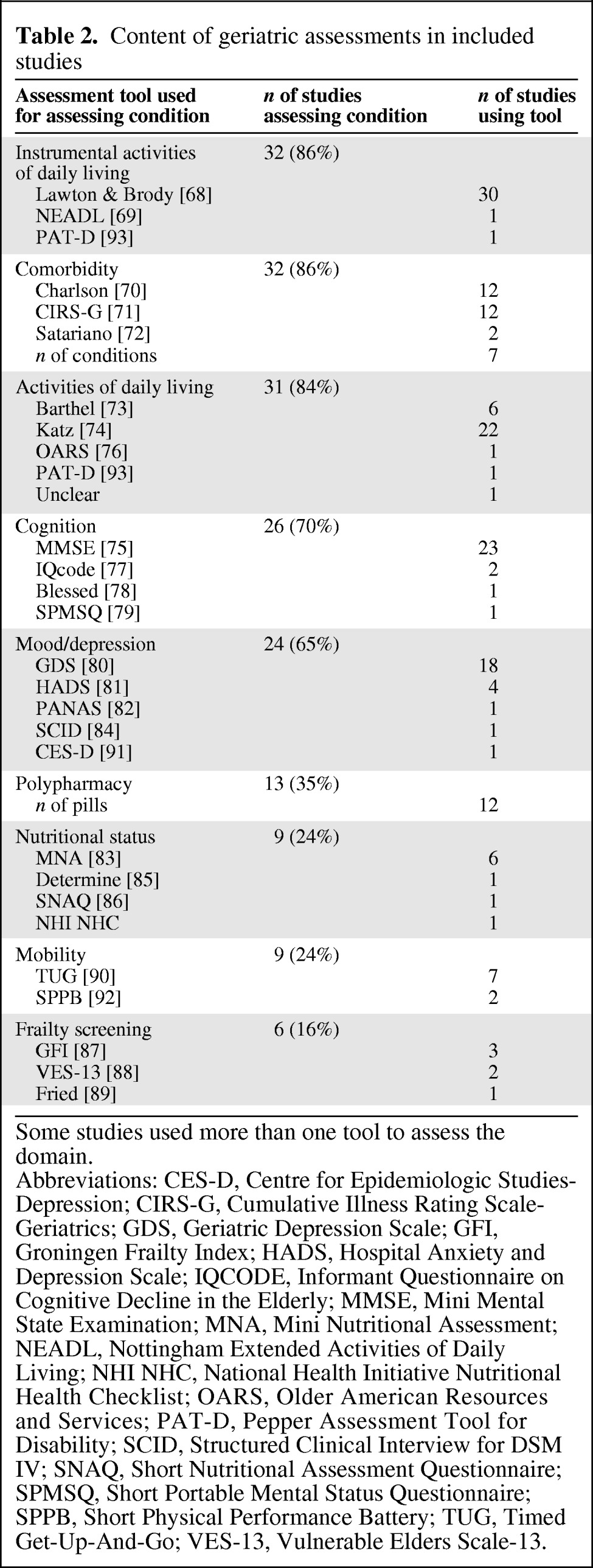
The median number of geriatric conditions that were assessed was five (interquartile range: 4–8, Table 1) [17–67]. Table 2 gives an overview of the geriatric conditions included in the studies and the method of assessment [68–93]; a more detailed overview per study can be found in supplemental online Appendix 2. Ten studies summarized results of geriatric assessment in a summary score (27%) [21, 23–24, 28–30, 32–33, 49–51, 57, 63–64]: two of these studies used the cumulative number of geriatric conditions [57, 64] as a summary and eight defined patients as frail if they were ADL-dependent, had 3 or more comorbidities (or one severe comorbid conditions), or one or more geriatric conditions. [21, 23–24, 28–30, 32–33, 49–51, 63].
Table 2.
Content of geriatric assessments in included studies
Some studies used more than one tool to assess the domain.
Abbreviations: CES-D, Centre for Epidemiologic Studies-Depression; CIRS-G, Cumulative Illness Rating Scale-Geriatrics; GDS, Geriatric Depression Scale; GFI, Groningen Frailty Index; HADS, Hospital Anxiety and Depression Scale; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; MMSE, Mini Mental State Examination; MNA, Mini Nutritional Assessment; NEADL, Nottingham Extended Activities of Daily Living; NHI NHC, National Health Initiative Nutritional Health Checklist; OARS, Older American Resources and Services; PAT-D, Pepper Assessment Tool for Disability; SCID, Structured Clinical Interview for DSM IV; SNAQ, Short Nutritional Assessment Questionnaire; SPMSQ, Short Portable Mental Status Questionnaire; SPPB, Short Physical Performance Battery; TUG, Timed Get-Up-And-Go; VES-13, Vulnerable Elders Scale-13.
The association between geriatric assessment and all-cause mortality was assessed in 25 out of 37 studies (68%, Table 1) [17, 18, 21, 23–29, 31–35, 37–38, 42–44, 46, 49–53, 57–58, 60–67], chemotherapy toxicity in 13 (35%) [19, 20, 25–27, 30–36, 39–41, 45, 53, 56, 59, 64], and chemotherapy completion in seven (19%) [17–20, 24–27, 32–33, 53, 58]. Four studies focused on the association between geriatric assessment and perioperative complications (11%) [22, 47–51, 54–55]. No studies were found on geriatric assessment in relation to radiotherapy.
Study Quality
The quality of the studies was heterogeneous, with a median score of 9 out of the 16 items on the quality checklist (interquartile range: 7–11). Reviewer agreement was >95% for all aspects. Inclusion and exclusion criteria and patient population were clearly described in 22 and 26 studies, respectively. The participation rate (i.e., the percentage of potential participants that received a geriatric assessment) was only described in nine studies. Although 21 studies listed the duration of follow-up, only eight described the number of patients lost to follow-up or compared completers with noncompleters. Only 13 studies described the completeness of data. Outcome reporting was of better quality, with 28 studies providing data from univariable analyses for the association of geriatric assessments with outcome measures and 24 presenting some form of prognostic model. However, reporting of associations differed notably between studies, with some presenting odds ratios, others hazard ratios, and others only p values to indicate a statistical significance without reporting on the actual odds/hazard ratio or confidence interval. This complicated any comparison of data and hindered combining data for a formal meta-analysis. Furthermore, three studies did not appear to have sufficient numbers for their multivariable analyses. Full results for the quality assessment can be found in supplemental online Appendix 1b.
All-Cause Mortality
The predictive value of geriatric assessments for all-cause mortality was reported in 25 studies (Table 3). Six studies addressed the association of a summary score with mortality: all six found that frail patients showed poorer overall survival (100%) [21, 23–24, 28–29, 57, 63]. In these studies, median survival was between 1.6 and 3.7 times longer for fit patients compared to frail subjects. Likewise, frailty assessed with a formal frailty screening tool was found to be associated with mortality in three out of four studies (75%) [17, 18, 23, 25, 58]. Nutritional status was found to be associated with mortality in all four studies in which it was assessed (100%) [17, 18, 42, 50, 58, 60]. For comorbidity, initial analysis revealed that only 6 out of 16 studies found an association with mortality (38%). However, when subdividing according to the assessment method used, only one out of five studies using the Charlson comorbidity index [38] and none of the four studies using the number of comorbid conditions found an association, while four out of five studies using the Cumulative Illness Rating Scale for Geriatrics (CIRS-G) found comorbidity to be associated with mortality (80%) [33, 50, 53, 60, 65]. For one study, the results for comorbidity were not clearly reported. Of the 14 studies addressing cognitive function, only two found an association between cognition and mortality [38, 61]. Only 4 out of 14 studies found an association between ADL impairment and mortality (29%) [25, 38, 46, 61, 67], and 6 out of 16 reported finding an association for IADL impairment (38%) [25, 33, 38, 46, 52, 67]. Results for mood/depression, mobility, and polypharmacy were inconclusive, with approximately equal numbers of studies that did and did not find an association. All of these results were not altered when correcting for the assessment tool that was used.
Table 3.
Association of geriatric assessment with all-cause mortality
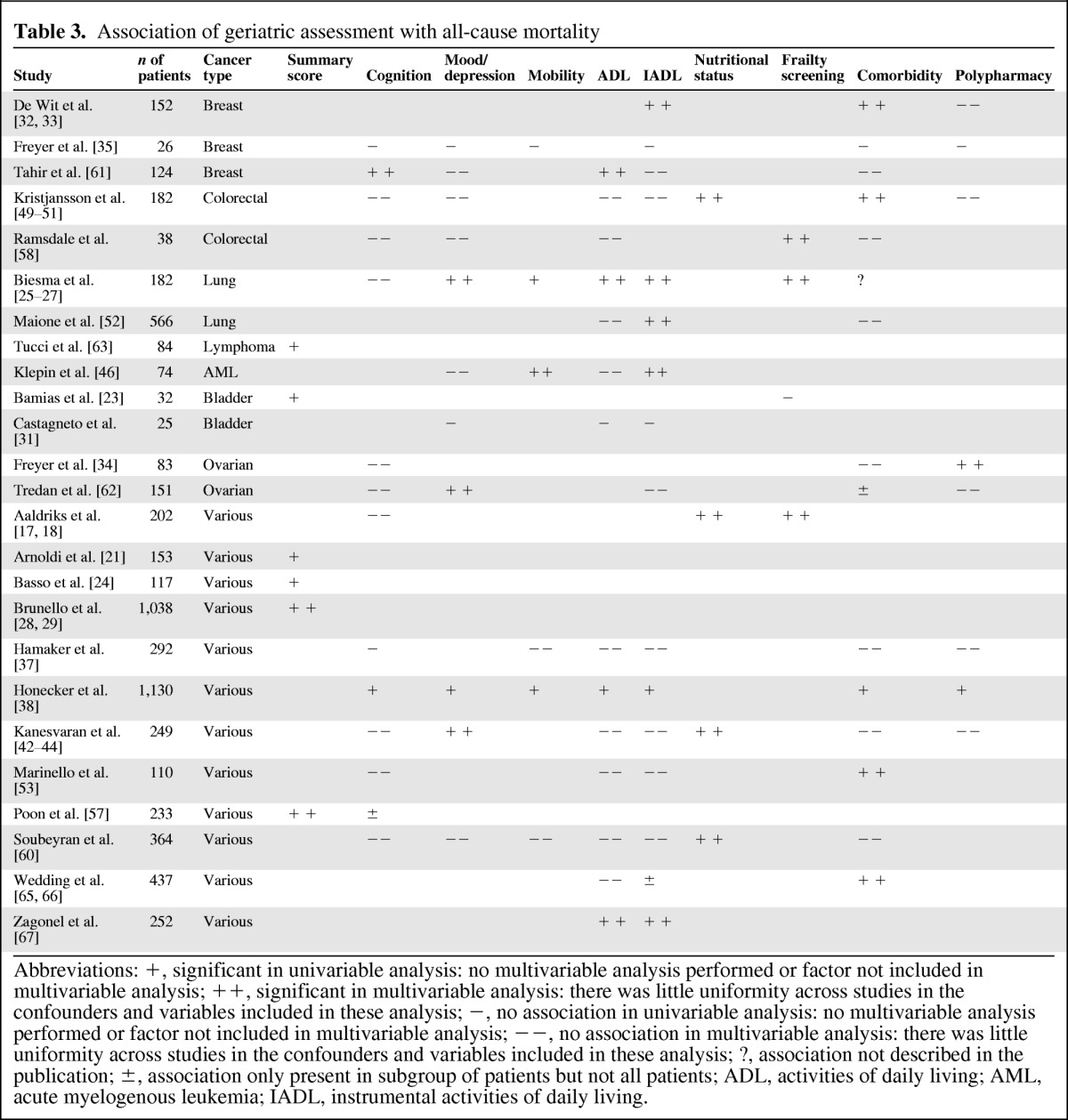
Abbreviations: +, significant in univariable analysis: no multivariable analysis performed or factor not included in multivariable analysis; ++, significant in multivariable analysis: there was little uniformity across studies in the confounders and variables included in these analysis; −, no association in univariable analysis: no multivariable analysis performed or factor not included in multivariable analysis; −−, no association in multivariable analysis: there was little uniformity across studies in the confounders and variables included in these analysis; ?, association not described in the publication; ±, association only present in subgroup of patients but not all patients; ADL, activities of daily living; AML, acute myelogenous leukemia; IADL, instrumental activities of daily living.
Toxicity of Chemotherapy
Results for toxicity of chemotherapy and chemotherapy completion are listed in Table 4. For toxicity, the score summarizing geriatric assessment was found to be associated with toxicity of chemotherapy in two out of three studies (66%), but these only reported univariable results [33, 64]. Similarly, two out of three studies found an association between toxicity and a frailty screening tool [56, 64]. For all other geriatric conditions, results were quite variable across studies. Polypharmacy was associated with toxicity in two out of four studies [33, 56]. Comorbidity was associated with toxicity of chemotherapy in only 3 out of 10 studies [53, 56, 59]. The method of assessing for comorbidity did not influence results. Toxicity of chemotherapy was associated with impaired cognition in 17% of studies, depressed mood in 13%, impaired mobility in 33%, ADL impairment in 0%, and IADL impairment in 18%, respectively (Table 4).
Table 4.
Association of geriatric assessment with treatment complications/completion
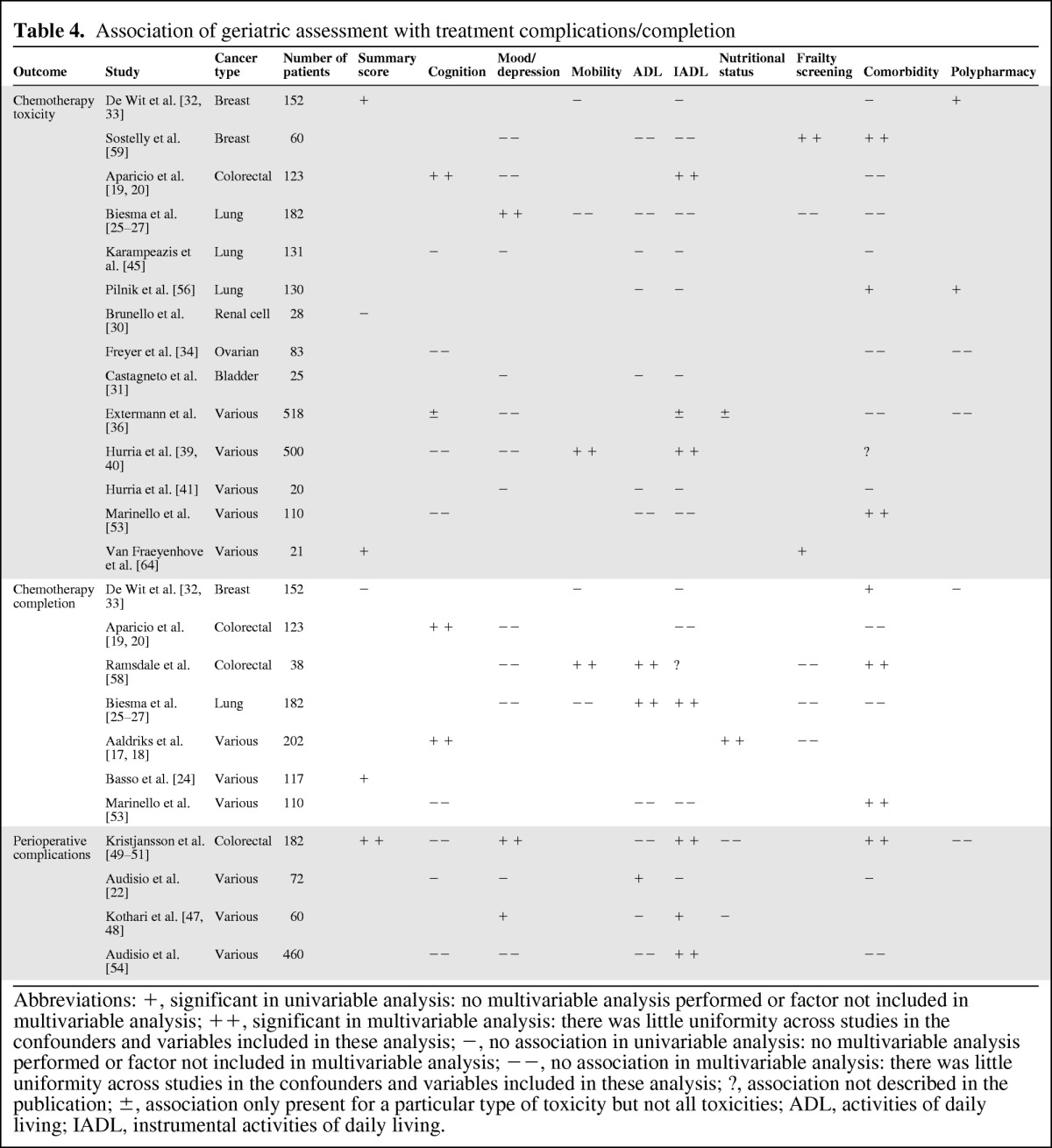
Abbreviations: +, significant in univariable analysis: no multivariable analysis performed or factor not included in multivariable analysis; ++, significant in multivariable analysis: there was little uniformity across studies in the confounders and variables included in these analysis; −, no association in univariable analysis: no multivariable analysis performed or factor not included in multivariable analysis; −−, no association in multivariable analysis: there was little uniformity across studies in the confounders and variables included in these analysis; ?, association not described in the publication; ±, association only present for a particular type of toxicity but not all toxicities; ADL, activities of daily living; IADL, instrumental activities of daily living.
Chemotherapy Completion
For completion of chemotherapy, impaired cognitive function was found to be associated with less completion or the need for dose reduction in two out of three studies (66%, Table 4) [17–20]. ADL impairment showed similar results, with association in two out of three studies [25, 58]. Furthermore, three out of five studies [33, 53, 58] found that comorbidity was predictive of lower completion rates (60%). Two of these used the CIRS-G and one the Charlson comorbidity index to assess comorbidity; the two studies that did not find an association both used the Charlson comorbidity index. One study addressed nutritional status and found an association in the multivariable analysis [17, 18]. Data was inconclusive for the summary score (association in one of two studies, 50%) and negative for depressed mood, impaired mobility, IADL impairment, and the presence of frailty (Table 4).
Perioperative Complications
Four studies addressed the association between geriatric assessment and perioperative complications (Table 4). Only one study assessed the association of a summary score and found it to be associated with perioperative complications (100%) [49]. This association was found for IADL impairment in three out of the four studies [47, 49, 54]. For depressed mood, results were inconclusive, with only two out of four studies finding an association [47, 49]. For ADL impairment, polypharmacy, nutritional status, cognitive function and comorbidity, no or little association was found. None of the studies used a frailty screening tool.
Radiotherapy Toxicity/Completion
No studies were identified that addressed the association between geriatric assessments and toxicity or completion of radiotherapy. One study assessed patients receiving chemotherapy or chemoradiation, but did not report separately on the latter group [56].
Discussion
In this review on the value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer, little consistency was found between the results of the various studies. Interestingly, different geriatric conditions appear to be predictive for the primary outcome measures: frailty, nutritional status, and comorbidity (when measured with CIRS-G) for all-cause mortality; frailty for toxicity of chemotherapy; cognitive function and ADL impairment for chemotherapy completion; and IADL impairment for perioperative complications. However, the only truly consistent finding was the association between a summary score of the geriatric assessment and mortality.
The studies included in this systematic review were heterogeneous in design, content, and reported outcomes. In addition, reporting was frequently too inadequate to assess potential sources of bias. It was often unclear whether outcome of geriatric assessment was known to the treating physician, allowing differences in overall survival to be caused by the reception of suboptimal oncologic treatment based on the outcome of geriatric assessment (and subsequently the assumption that patient would not be able to tolerate standard treatment). Another potential bias is that the patients participating in studies focusing on chemotherapy and surgery were already preselected as suitable for this treatment by their physician. Thus, although many geriatric conditions were not predictive of toxicity, one cannot conclude that patients should receive chemotherapy irrespective of their cognitive status or IADL score, for example.
These factors limited our possibilities of performing a formal meta-analysis and drawing definitive conclusions. One method to solve some of these issues would be to perform an individual patient data analysis using the original data of included studies. A second limitation of this review is that it focuses on studies assessing multiple geriatric conditions. Studies focusing on single conditions or including multiple conditions but not identified as geriatric assessment would not have been selected from Medline or Embase with our search strategy. Despite these limitations, this review does provide a thorough overview of the currently available evidence on the value of geriatric assessment for predicting relevant outcomes in older patients with cancer.
The results of this review have several clinical implications. First of all, although various geriatric conditions appear to have some predictive value for each of the four outcome measures, the lack of consistency in the findings does not support excluding patients from certain treatment options based solely on their score on a geriatric assessment tool.
A second clinical implication of this review is that although current geriatric assessments used in oncology primarily focus on cognitive function, mood/depression, and functional limitations, less frequently examined geriatric conditions, such as malnutrition and polypharmacy, appear to be of similar or even greater predictive value and are potentially modifiable; therefore, their assessment should not be omitted. Also, it appears that assessment of comorbidity without including a measure for the severity of these conditions is not useful; therefore, we recommend using the CIRS-G rather than the Charlson comorbidity index, despite that fact that the former is more time-consuming [71, 94]. Interestingly, assessment of mobility (which is one of the cornerstones of geriatric medicine) is rarely included. Given its predictive value in the general geriatric population, this element of CGA deserves further exploration [95].
Several factors may have contributed to the variation in the results of the included studies. First of all, it appears that the choice of assessment tool influences outcome, as was clearly illustrated in the assessment of comorbidity. Heterogeneity in patient populations will also have contributed to the variation in study outcomes: not only do different elements of the CGA appear to be predictive depending on the outcome measure that is examined, but it is possible that the specific characteristics and prognosis of a malignancy will also affect the predictive value of various geriatric syndromes. All of these factors mean that finding that one optimal assessment tool that will be predictive of all outcome measures in all patient populations in a broad scope of treatment settings may not be feasible.
On the other hand, the results of our systematic review suggest that in predicting outcome, it may be more important to determine whether or not a patient is frail than to determine what makes him or her frail. This fits with the definition of frailty as the final common pathway of aging [96], in which the presence of deficits in geriatric domains is the determinative factor while the particulars of each deficit are of secondary importance. If this is the case, a short frailty screening tool could potentially suffice in allocating a patient to standard treatment of tailored care and the time-consuming process of a formal geriatric assessment could be avoided [40, 47, 97]. This does require that the tool has a high sensitivity for frailty, allowing the assessor to trust that those patients deemed fit actually are fit [88]. Patients who are not fit should then receive further assessment to ascertain their ability to tolerate treatment. However, there is still much debate on the precise definition of frailty and how it should be measured; as yet, there is insufficient evidence on the quality of the various screening tools in predicting fitness in this particular setting to endorse one tool over the others [98].
Ultimately, in limiting the use of a systematic geriatric assessment in oncology practice to a decision-making tool, the potential benefit of using the CGA to optimize care for elderly patients with cancer is overlooked. For example, although a cognitive disorder does not necessarily predict chemotherapy toxicity, it potentially means that a patient may not respond adequately in case of complications or will not take oncologic or supportive medication as prescribed; these patients may require extra monitoring or home health care. Similarly, addressing previously undiagnosed depressive symptoms or malnutrition can improve a patient's resilience when undergoing treatment. A geriatric assessment could thus be seen as a starting point for further treatment and care, for improving not only the outcomes addressed in this review but also quality of life or functional capacity. However, because a formal comprehensive geriatric assessment is time consuming and a geriatric consultation is often a scarce commodity, it may be useful to develop screening tools that are particularly suitable for finding those patients at high risk for having geriatric conditions that are modifiable or require intervention [99].
In conclusion, this systematic review shows that although different geriatric conditions appear to be predictive for each of the major outcome measures, currently available evidence is too inconsistent to guide clinical decision-making. Many questions remain unanswered and will require further exploration. To elucidate the impact of the various geriatric conditions on treatment tolerance and outcome for older patients with cancer, future clinical research should use broad geriatric assessments that address all geriatric conditions and include geriatric outcome measures, such as functional capacity, in addition to standard oncologic outcomes. Furthermore, research should focus on validating screening tools that predict fitness rather than frailty and applying geriatric assessment as an intervention aimed at optimizing a patient's resilience during treatment, rather than as a decision-making tool only.
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Marije E. Hamaker, Carolien H. Smorenburg, Sophia E. de Rooij, Barbara C. van Munster
Collection and/or assembly of data: Marije E. Hamaker, Alinda G. Vos
Data analysis and interpretation: Marije E. Hamaker, Carolien H. Smorenburg, Sophia E. de Rooij, Barbara C. van Munster
Manuscript writing: Marije E. Hamaker, Carolien H. Smorenburg, Barbara C. van Munster
Final approval of manuscript: Marije E. Hamaker, Alinda G. Vos, Carolien H. Smorenburg, Sophia E. de Rooij, Barbara C. van Munster
References
- 1.Muss HB. Cancer in the elderly: A societal perspective from the United States. Clin Oncol (R Coll Radiol) 2009;21:92–98. doi: 10.1016/j.clon.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–252. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol. 2007;25:1936–1944. doi: 10.1200/JCO.2006.10.2954. [DOI] [PubMed] [Google Scholar]
- 4.Extermann M, Overcash J, Lyman GH, et al. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;6:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 5.Extermann M, Meyer J, McGinnis M, et al. A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Crit Rev Oncol Hematol. 2004;49:69–75. doi: 10.1016/s1040-8428(03)00099-4. [DOI] [PubMed] [Google Scholar]
- 6.Terret C, Zulian GB, Naiem A, Albrand G. Multidisciplinary approach to the geriatric oncology patient. J Clin Oncol. 2007;25:1876–1881. doi: 10.1200/JCO.2006.10.3291. [DOI] [PubMed] [Google Scholar]
- 7.Terret C, Albrand G, Droz JP. Multidisciplinary geriatric assessment reveals unknown medical problems in elderly cancer patients. J Clin Oncol. 2004;22(14 suppl):8167. [Google Scholar]
- 8.Gosney M. Contribution of the geriatrician to the management of cancer in older patients. Eur J Cancer. 2007;43:2153–2160. doi: 10.1016/j.ejca.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 9.Brunello A, Sandri R, Extermann M. Multidimensional geriatric evaluation for older cancer patients as a clinical and research tool. Cancer Treat Rev. 2009;35:487–492. doi: 10.1016/j.ctrv.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Ellis G, Whitehead MA, O'Neill D, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011;7:CD006211. doi: 10.1002/14651858.CD006211.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barthelemy P, Heitz D, Mathelin C, et al. Adjuvant chemotherapy in elderly patients with early breast cancer. Impact of age and comprehensive geriatric assessment on tumor board proposals. Crit Rev Oncol Hematol. 2011;79:196–204. doi: 10.1016/j.critrevonc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Klepin HD, Gajra A, Hardt N, et al. Predictors of primary dose reduction among patients aged 65 years and older receiving adjuvant chemotherapy. J Clin Oncol. 2011;29(May 20 suppl):9003. [Google Scholar]
- 13.Tirelli U, Fratino L, Balzarotti M, et al. Comprehensive geriatric assessment-adapted chemotherapy in elderly patients (>70 years) with diffuse large B-Cell non-Hodgkin's lymphoma (DLBCL): Final results and long term follow-up. Paper presented at: 51st Annual Meeting of the American Society of Hematology; December 5–8, 2009; New Orleans, LA. [Google Scholar]
- 14.Corre R, Chouaid C, Barlesi F, et al. Study ESOGIA-GFPC 08–02: Phase III, randomized multicentre trial involving subjects over age 70 with stage IV non small cell lung cancer, comparing a “classical” strategy of treatment allocation, based on performance status and age, with an “optimized” strategy allocating the same treatments according to a simplified geriatric assessmnet screening scale plus a more thorough geriatric assessment if necessary. J Clin Oncol. 2011;29(May 20 suppl):TPS219. [Google Scholar]
- 15.Kuijpers T, van der Windt DA, van der Heijden GJ, Bouter LM. Systematic review of prognostic cohort studies on shoulder disorders. Pain. 2004;109:420–431. doi: 10.1016/j.pain.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ. 2001;323:224–228. doi: 10.1136/bmj.323.7306.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aaldriks AA, Maartense E, le CS, et al. Predictive value of geriatric assessment for patients older than 70 years, treated with chemotherapy. Crit Rev Oncol Hematol. 2011;79:205–212. doi: 10.1016/j.critrevonc.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Aaldriks AA, Maartense E, Giltay EJ, et al. The impact of geriatric assessment on the outcome of older breast cancer patients treated with chemotherapy. J Geriatr Oncol. 2011;2(suppl 1):S29. [Google Scholar]
- 19.Aparicio T, Jouve JL, Teillet L, et al. Geriatric factors to predict toxicity and dose-intensity reduction in FFCD 2001–02 phase III study comparing a first-line chemotherapy of LV5FU2 or FOLFIRI in treatment of metastatic colorectal cancer in elderly patients. J Clin Oncol. 2011;29(May 20 suppl):S9111. [Google Scholar]
- 20.Aparicio T, Jouve JL, Teillet F, et al. Geriatric factors to predict toxicity and dose-intensity reduction in FFCD 2001–02 phase III study comparing a first line chemotherapy with LV5FU2 or FOLFIRI in treatment of metastatic colon cancer in elderly patients. J Geriatr Oncol. 2011;2(suppl 1):S61. [Google Scholar]
- 21.Arnoldi E, Dieli M, Mangia M, et al. Comprehensive geriatric assessment in elderly cancer patients: An experience in an outpatient population. Tumori. 2007;93:23–25. doi: 10.1177/030089160709300105. [DOI] [PubMed] [Google Scholar]
- 22.Audisio RA, Gennari R, Sunouchi K, et al. Preoperative assessment of cancer in elderly patients: A pilot study. Support Cancer Ther. 2003;1:55–60. doi: 10.3816/SCT.2003.n.005. [DOI] [PubMed] [Google Scholar]
- 23.Bamias A, Lainakis G, Kastritis E, et al. Biweekly carboplatin/gemcitabine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: Report of efficacy, quality of life and geriatric assessment. Oncology. 2007;73:290–297. doi: 10.1159/000132394. [DOI] [PubMed] [Google Scholar]
- 24.Basso U, Tonti S, Bassi C, et al. Management of frail and not-frail elderly cancer patients in a hospital-based geriatric oncology program. Crit Rev Oncol Hematol. 2008;66:163–170. doi: 10.1016/j.critrevonc.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Biesma B, Wymenga AN, Vincent A, et al. Quality of life, geriatric assessment and survival in elderly patients with non-small-cell lung cancer treated with carboplatin-gemcitabine or carboplatin-paclitaxel: NVALT-3 a phase III study. Ann Oncol. 2011;22:1520–1527. doi: 10.1093/annonc/mdq637. [DOI] [PubMed] [Google Scholar]
- 26.Wymenga M, Biesma B, Vincent A, et al. Platinum-based combination chemotherapy in the treatment of older non-small cell lung cancer (NSCLC) patients (pts): Is there a role for Complete Geriatric Assessment (CGA)? Final results from the prospective multicenter NVALT-3 study. J Clin Oncol. 2009;27(May 20 suppl):e20547. [Google Scholar]
- 27.Wymenga M, Biesma B, Vincent A, et al. Can baseline complete geriatric assessment predict toxicity in elderly non-small cell lung cancer patients? J Clin Oncol. 2007;25(18 suppl):7537. [Google Scholar]
- 28.Brunello A, Monfardini S, Falci C, et al. Prognostic role of comprehensive geriatric assessment (CGA): A prospective study of a cohort of 1038 elderly cancer patients. J Geriatr Oncol. 2011;2(suppl 1):S36. [Google Scholar]
- 29.Basso U, Falci C, Brunello A, et al. Prognostic value of multidimensional geriatric assessment on survival of a prospective cohort of 880 elderly cancer patients. J Clin Oncol. 2011;29(May 20 suppl):S9065. [Google Scholar]
- 30.Brunello A, Sacco C, Barile C, et al. Sunitinib in elderly patients (≥ 70 years) with metastatic renal cell carcinoma (MRCC): Multicenter analysis of tolerability and efficacy. Paper presented at: 34th Congress of the European Society for Medical Oncology; September 9–16, 2008; Stockholm, Sweden. [Google Scholar]
- 31.Castagneto B, Zai S, Marenco D, et al. Single-agent gemcitabine in previously untreated elderly patients with advanced bladder carcinoma: Response to treatment and correlation with the comprehensive geriatric assessment. Oncology. 2004;67:27–32. doi: 10.1159/000080282. [DOI] [PubMed] [Google Scholar]
- 32.De Wit M, Honecker F, Wedding U, et al. Incorporation of a comprehensive geriatric assessment (CGA) into a randomized phase III trial for metastatic breast cancer (MBC): The PELICAN study. J Clin Oncol. 27;15(suppl):9551. [Google Scholar]
- 33.De Wit M, Honecker F, Wedding U, et al. Incorporation of a comprehensive geriatric assessment (CGA) into a randomized phase III trial for metastatic breast cancer (MBC): The PELICAN study. J Clin Oncol. 28;15(suppl):1070. [Google Scholar]
- 34.Freyer G, Geay JF, Touzet S, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: A GINECO study. Ann Oncol. 2005;16:1795–1800. doi: 10.1093/annonc/mdi368. [DOI] [PubMed] [Google Scholar]
- 35.Freyer G, Lortholary A, Delcambre C, et al. Unexpected toxicities in elderly patients treated with oral idarubicin in metastatic breast cancer: The GINECO experience. Clin Oncol (R Coll Radiol) 2004;16:17–23. doi: 10.1016/j.clon.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 37.Hamaker ME, Buurman BM, van Munster BC, et al. The value of a comprehensive geriatric assessment for patient care in acutely hospitalized older patients with cancer. The Oncologist. 2011;16:1403–1412. doi: 10.1634/theoncologist.2010-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honecker FU, Wedding U, Rettig K, et al. Use of the Comprehensive Geriatric Assessment (CGA) in elderly patients (pts) with solid tumors to predict mortality. J Clin Oncol. 2009;27(15 suppl):9549. [Google Scholar]
- 39.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective 500 patient multicenter study. J Clin Oncol. 28(15 suppl):9001. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurria A, Fleming MT, Baker SD, et al. Pharmacokinetics and toxicity of weekly docetaxel in older patients. Clin Cancer Res. 2006;12(20 Pt 1):6100–6105. doi: 10.1158/1078-0432.CCR-06-0200. [DOI] [PubMed] [Google Scholar]
- 42.Kanesvaran R, Li H, Koo KN, Poon D. Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly Asian patients with cancer. J Clin Oncol. 2011;29:3620–3627. doi: 10.1200/JCO.2010.32.0796. [DOI] [PubMed] [Google Scholar]
- 43.Kanesvaran R, Li H, Koo K, Poon D. An analysis of prognostic factors of comprehensive geriatric assessment (CGA) and development of a clinical scoring system in elderly Asian cancer patients. J Clin Oncol. 2010;28(15 suppl):9165. doi: 10.1200/JCO.2010.32.0796. [DOI] [PubMed] [Google Scholar]
- 44.Kanesvaran R, Koo KN, Chen W, Poon D. An analysis of the prognostic value of handgrip strength and its incorporation into the comprehensive geriatric assessment in elderly Asian patients with cancer. J Clin Oncol. 2011;29(May 20 suppl):9093. doi: 10.1200/JCO.2010.32.0796. [DOI] [PubMed] [Google Scholar]
- 45.Karampeazis A, Vamvakas L, Agelaki S, et al. Baseline comprehensive geriatric assessment (CGA) and prediction of toxicity in elderly non-small cell lung cancer (NSCLC) patients receiving chemotherapy. J Clin Oncol. 2011;29(May 20 suppl):e19656. [Google Scholar]
- 46.Klepin HD, Tooze JA, Geiger AM, et al. Geriatric assessment predicts overall survival among older adults receiving induction chemotherapy for acute myologenous leukemia. J Geriatr Oncol. 2011;2(suppl 1):S32. [Google Scholar]
- 47.Kothari A, Phillips S, Bretl T, et al. Components of geriatric assessments predict thoracic surgery outcomes. J Surg Res. 2011;166:5–13. doi: 10.1016/j.jss.2010.05.050. [DOI] [PubMed] [Google Scholar]
- 48.Weigel T, Kothari A, Bretl T, Block K, LoConte NK. An abbreviated thoracic onco geriatric assessment (TOGA) and its components predict outcomes of esophagectomies. Paper presented at: 63rd Annual Cancer Symposium of the Society of Surgical Oncology; March 3–7, 2010; St. Louis, MO. [Google Scholar]
- 49.Kristjansson SR, Nesbakken A, Jordhoy MS, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: A prospective observational cohort study. Crit Rev Oncol Hematol. 2010;76:208–217. doi: 10.1016/j.critrevonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Kristjansson SR, Jordhoy MS, Nesbakken A, et al. Which elements of a comprehensive geriatric assessment (CGA) predict post-operative complications and early mortality after colorectal cancer surgery? J Geriatr Oncol. 2010;1:57–65. [Google Scholar]
- 51.Kristjansson SR, Nesbakken A, Jordhoy MS, Wyller TB. Comprehensive geriatric assessment predicts complications in elderly patients operated for colon cancer. Crit Rev Oncol Hematol. 2008;68:S24. doi: 10.1016/j.critrevonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: A prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. 2005;23:6865–6872. doi: 10.1200/JCO.2005.02.527. [DOI] [PubMed] [Google Scholar]
- 53.Marinello R, Marenco D, Roglia D, et al. Predictors of treatment failures during chemotherapy: A prospective study on 110 older cancer patients. Arch Gerontol Geriatr. 2009;48:222–226. doi: 10.1016/j.archger.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Audisio RA, Pope D, Ramesh HS, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol. 2008;65:156–163. doi: 10.1016/j.critrevonc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Ramesh HS, Pope D, Stotter A, et al. Testing CGA components to predict 30 day surgery outcome in elderly cancer patients. Crit Rev Oncol Hematol. 2006;60:S21. [Google Scholar]
- 56.Pilnik NG, Ibero MM, Carri D, Mareca O. Influence of some clinical parameters in the tolerance to treatment in elderly patients with advanced lung cancer. J Clin Oncol. 2010;28(May 20 suppl):e19600. [Google Scholar]
- 57.Poon D, Lee HH, Chan LL, et al. An exploratory analysis of comprehensive geriatric assessment results and overall survival in 233 consecutive elderly cancer patients. J Clin Oncol. 2009;27(15 suppl):9504. [Google Scholar]
- 58.Ramsdale EE, Polite N, Bylow KA, et al. Relationship between components of the comprehensive geriatric assessment, chemotherapy dose intensity, and overall survival in a colorectal cancer cohort age 65 and older. J Clin Oncol. 2011;29(May 20 suppl):9000. [Google Scholar]
- 59.Sostelly A, Falandry C, Pejuade-Lauraine E, et al. Predicting chemotherapy induced hematotoxicity in elderly patients with metastatic breast cancer treated by pegylated liposomal doxorubicin. J Clin Oncol. 2011;29(May 20 suppl):e19740. [Google Scholar]
- 60.Soubeyran P, Rainfray M, Mathoulin-Pelissier S, et al. Screening of elderly patients with cancer for early death risk. Results of a prospective multicentric study of 364 patients under chemotherapy. Crit Rev Oncol Hematol. 2006;60:S23. [Google Scholar]
- 61.Tahir M, Pretorius R, Robinson T, et al. Optimising the management of breast cancer in older patients. Paper presented at: European Journal of Surgical Oncology Conference, the Association for Cancer Surgery Scientific Conference; November 1–2, 2010; London, United Kingdom. [Google Scholar]
- 62.Tredan O, Geay JF, Touzet S, et al. Carboplatin/cyclophosphamide or carboplatin/paclitaxel in elderly patients with advanced ovarian cancer? Analysis of two consecutive trials from the Groupe d'Investigateurs Nationaux pour l'Etude des Cancers Ovariens. Ann Oncol. 2007;18:256–262. doi: 10.1093/annonc/mdl400. [DOI] [PubMed] [Google Scholar]
- 63.Tucci A, Ferrari S, Bottelli C, et al. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer. 2009;115:4547–4553. doi: 10.1002/cncr.24490. [DOI] [PubMed] [Google Scholar]
- 64.Van FF, Baitar A, De VM, et al. Prediction of chemotherapy toxicity by the groningen frailty index (GFI) and the comprehensive geriatric assessment (CGA) in elderly cancer patients (PTS): An interim analysis. Paper presented at: 35th European Society for Medical Oncology Congress; October 8–12, 2010; Milan, Italy. [Google Scholar]
- 65.Wedding U, Rohrig B, Klippstein A, et al. Age, severe comorbidity and functional impairment independently contribute to poor survival in cancer patients. J Cancer Res Clin Oncol. 2007;133:945–950. doi: 10.1007/s00432-007-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winkelmann N, Petersen I, Kiehntopf M, et al. Results of comprehensive geriatric assessment effect survival in patients with malignant lymphoma. J Cancer Res Clin Oncol. 2011;137:733–738. doi: 10.1007/s00432-010-0933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zagonel V, Fratino L, Piselli P, et al. The comprehensive geriatric assessment predicts mortality among elderly cancer patients. Proc Am Thorac Soc. 2002;21 Abstract 1458. [Google Scholar]
- 68.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 69.Nouri FM, Lincoln NB. An extended activities of daily living index for stroke patients. Clin Rehabil. 1987;1:301–305. [Google Scholar]
- 70.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 71.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 72.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120:104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 73.Barthel D. Functional evaluation: The Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 74.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychological function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 75.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 76.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 77.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 78.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 79.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 80.Sheikh JI, Yesavage JA, Brooks JO, et al. Proposed factor structure of the Geriatric Depression Scale. Int Psychogeriatr. 1991;3:23–28. doi: 10.1017/s1041610291000480. [DOI] [PubMed] [Google Scholar]
- 81.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 82.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 83.Vellas B, Guigoz Y, Garry PJ, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15:116–122. doi: 10.1016/s0899-9007(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 84.First MG, Spitzer RL, Gibbon M, Williams JB. New York: Biometrics Research Department, New York State Psychiatric Institute; 1997. Structured clinical interview for DSM-IV axis I disorders. Clinical version, administration booklet. [Google Scholar]
- 85.Posner BM, Jette AM, Smith KW, Miller DR. Nutrition and health risks in the elderly: The nutrition screening initiative. Am J Public Health. 1993;83:972–978. doi: 10.2105/ajph.83.7.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kruizenga HM, Seidell JC, de Vet HC, et al. Development and validation of a hospital screening tool for malnutrition: The short nutritional assessment questionnaire (SNAQ) Clin Nutr. 2005;24:75–82. doi: 10.1016/j.clnu.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 87.Slaets JP. Vulnerability in the elderly: Frailty. Med Clin North Am. 2006;90:593–601. doi: 10.1016/j.mcna.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 88.Saliba D, Orlando M, Wenger NS, et al. Identifying a short functional disability screen for older persons. J Gerontol A Biol Sci Med Sci. 2000;55:M750–M756. doi: 10.1093/gerona/55.12.m750. [DOI] [PubMed] [Google Scholar]
- 89.Fried LP, Tangen CM, Walston J, Newman AB, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 90.Mathias S, Nayak US, Isaacs B. Balance in elderly patients: The “get-up and go” test. Arch Phys Med Rehabil. 1986;67:387–389. [PubMed] [Google Scholar]
- 91.Radloff LS. The CES-D scale. Appl Psychol Manage. 1977;1:385–401. [Google Scholar]
- 92.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 93.Rejeski WJ, Ip EH, Marsh AP, et al. Measuring disability in older adults: The International Classification System of Functioning, Disability and Health (ICF) framework. Geriatr Gerontol Int. 2008;8:48–54. doi: 10.1111/j.1447-0594.2008.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee L, Cheung WY, Atkinson E, Krzyzanowska MK. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: A systematic review. J Clin Oncol. 2011;29:106–117. doi: 10.1200/JCO.2010.31.3049. [DOI] [PubMed] [Google Scholar]
- 95.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr., Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: A consensus report. J Am Geriatr Soc. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 97.Overcash JA, Beckstead J, Moody L, et al. The abbreviated comprehensive geriatric assessment (aCGA) for use in the older cancer patient as a prescreen: Scoring and interpretation. Crit Rev Oncol Hematol. 2006;59:205–210. doi: 10.1016/j.critrevonc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 98.Hamaker ME, Jonker JM, de Rooij SE, et al. Frailty screening tools for predicting outcome of a comprehensive geriatric assessment in older cancer patients. Lancet Oncol. 2012 doi: 10.1016/S1470-2045(12)70259-0. (in press) [DOI] [PubMed] [Google Scholar]
- 99.Maas HA, Janssen-Heijnen ML, Olde Rikkert MG, Machteld Wymenga AN. Comprehensive geriatric assessment and its clinical impact in oncology. Eur J Cancer. 2007;43:2161–2169. doi: 10.1016/j.ejca.2007.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.