This meta-analysis of prospective studies examines the dose-response relationship between body mass index and primary liver cancer risk, possible confounding by hepatitis virus infection, and differences by gender or geographic location.
Keywords: Primary liver cancer, Body mass index, Meta-analysis, Relative risk
Abstract
Background.
Questions remain about the dose-response relationship between body mass index (BMI) and primary liver cancer (PLC) risk, possible confounding by hepatitis virus infection, and differences by gender or geographic location. We performed a meta-analysis of prospective studies to explore these issues.
Methods.
We searched PubMed and Embase for studies of BMI and risk of PLC through November 30, 2011. Summary relative risks with their corresponding 95% confidence intervals (CIs) were calculated using a random effects model.
Results.
A total of 21 prospective studies (including 17,624 PLC cases) were included in our analysis. The summary relative risk for a 5-unit increment in BMI (in kg/m2) was 1.39 (95% CI: 1.25–1.55), with high heterogeneity. These positive results were robust when stratified by sex, geographic location, ascertainment of exposure and outcome, the number of cases, duration of follow-up, sample source, and cofounders. There was evidence of a nonlinear association between BMI and PLC risk, with the most pronounced increase in risk among persons with a BMI >32 kg/m2. Patients with hepatitis C virus or cirrhosis (but not patients with hepatitis B virus) with excess weight had a higher risk of PLC development than general populations with excess weight.
Conclusion.
Excess weight increases PLC risk. For people with HCV infection or cirrhosis, risk increases are greater than for general population.
Introduction
Primary liver cancer (PLC) is the sixth most common cancer and the third most common cause of cancer death globally [1]. It is estimated to cause approximately 500,000 new cases worldwide annually [2], and a nearly equivalent number of persons die from this disease [3]. Hepatocellular carcinoma (HCC) is the major histological subtype, representing up to 85% of PLCs. Chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) has been recognized as the most important risk factor for HCC development [4]. In addition, excessive alcohol consumption, cigarette smoking, a history of diabetes mellitus (DM), and environmental exposure to aflatoxin B1 are also potential risk factors for HCC [5].
Interestingly, the incidence of PLC in several developed countries, including Japan, Europe, and the United States, has been increasing over the last 20 years, whereas the incidence of PLC in some developing countries has decreased [6]. It is suggested that infection with HCV may account for about half of this increase in HCC incidence in those developed countries; however, the etiology in 15%–50% of new HCC cases remains unclear [7].
Coinciding with the increased HCC incidence and mortality in developed countries, the prevalence of obesity, as measured by body mass index (BMI) ≥30 kg/m2, has also grown markedly over the past two decades. Epidemiological studies have suggested that excess weight is associated with increased several cancer risks, particularly PLC risk. A recent meta-analysis of 11 cohort studies showed increased PLC risks of 17% for overweight people (BMI 25–30 kg/m2) and 90% for obese (≥30 kg/m2) people compared to those in normal weight [8]. The authors, however, recognized the lack of allowance for potential confounders—in particular, chronic HBV and HCV infections and alcohol abuse—as potential limitations [8]. According to the World Cancer Research Fund/American Institute for Cancer Research, there is limited and inconsistent evidence suggesting that excess body weight may increase PLC risk [9]. In addition, the exact shape of the dose-risk relationship between BMI and PLC has not been clearly defined. Since this meta-analysis was published, other relevant studies on this association have been published with inconsistent results [10–21]. In the present study, we aimed to further analyze this relationship by conducting an updated meta-analysis of prospective studies following the meta-analysis of observational studies in epidemiology guidelines [22].
Methods
A comprehensive, computerized literature search was conducted in Medline and Embase from the beginning of indexing for each database to November 30, 2011, by two independent investigators (W.Y.Q. and W.B.C.). Research papers were selected using the following text words or medical subject heading terms: “body mass index,”, “BMI,” or “obesity”; “liver cancer,” “hepatocellular carcinoma,” or “HCC.” We also reviewed the reference lists of selected research papers to identify additional relevant studies. No language restrictions were imposed.
Inclusion and Exclusion Criteria
Three authors (C.H.X., W.Y.Q. and W.B.C.) independently evaluated all of the studies retrieved based on the prespecified selection criteria. To be included, the study had to meet the following criteria: (a) published as an original article; (b) used a prospective cohort or nested case-control design; (c) reported relative risk (RR) estimates with corresponding 95% confidence intervals (CIs) for more than three categories of BMI and the risk of PLC (or HCC) or provided an RR per unit (in kg/m2) increase in BMI; (d) adjusted the RR and corresponding 95% CIs at least for age. If more than one study used the same cohort and objectives, the one with the most comprehensive population or most adjusted estimate of risk associated with BMI were included. We excluded studies that reported populations with human immunodeficiency virus (HIV) infection. Studies that did not provide risk estimates, duplicate publications, or reports from non-peer-reviewed sources were also excluded. Discrepancies between the three reviewers were solved by discussion.
Data Extraction
Two investigators (C.H.X. and W.Y.Q.) independently extracted the following information from each included study using a standardized data-collection protocol: the first author's last name, publication year, country of origin, sample size, sex, age, number of cases, ascertainment of exposure and outcome, duration of follow-up, and covariates adjusted for in the analysis. For studies that reported several multivariable-adjusted RRs, we extracted the risk estimates that reflected the greatest degree of control for potential confounders. We did not assess study quality using a quality score, but investigated whether specific study characteristics—such as duration of follow-up, number of cases, and adjustment for confounders, which are indicators of study quality—influenced the results in subgroup analyses.
Statistical Analysis
Summary RR estimates with their corresponding 95% CIs for a 5-unit increment in BMI were derived using the method of DerSimonian and Laird with the assumptions of a random effects model, which incorporates between-study variability [23]. A two-tailed p value <.05 was considered statistically significant. If a study reported results specific for men and women, respectively, we combined the sex-specific RR estimates using a fixed-effects model to generate an estimate for both sexes combined. Due to its high fatality, we conducted combining analyses for the incidence and mortality of PLC.
We computed linear trends from the correlated natural logarithm of the RR across categories of BMI according to the methods described by Greenland and Longnecker [24]. We performed dose-response meta-analysis of the relationship of per 5-unit increase in the BMI and PLC risk by using generalized least-squares trend estimation analysis or by using variance-weighted least-squares regression analysis [24, 25]. Both analyses require that the medians for each category of BMI level are known. If they were not reported, we estimated the midpoint of the upper and lower boundaries in each category as the average BMI level. For open-ended categories (e.g., >30 kg/m2), we estimated the median values using data from the Calcium Polyp Prevention Study cohort in Europe for non-Asians [26, 27] or obtained the values from studies in Japanese for Asians [28]. A potential nonlinear dose-response relationship was examined by using fractional polynomial models [29]. We determined the best-fitting second-order fractional polynomial regression model, defined as the one with the lowest deviance. A likelihood ratio test was used to assess the difference between the nonlinear and linear models to test for nonlinearity [29]. We also defined body mass categories using the following BMI categories: normal (BMI 18.5–<25 kg/m2), overweight (BMI 25–<30 kg/m2), and obese (BMI ≥30 kg/m2).
Potential sources of heterogeneity were investigated in heterogeneity tests, subgrouped analyses, and meta-regression. Heterogeneity among studies was assessed using Q and I2 statistics [30]. The role of several potential sources of heterogeneity were examined by subgrouped analyses and restricted maximum likelihood (REML)-based random-effects meta-regression analysis according to geographic locations, ascertainment of exposure and outcome, the number of cases, duration of follow-up, sample source, and adjustments for confounding variables. Sensitivity analysis was also conducted to evaluate the stability of the results.
We carried out formal testing using the Begg's test [31] and Egger's to test publication bias [32]. All statistical analyses were performed using STATA version 11.0 (STATA, College Station, TX) and R-package statistical software (Version 2.11.0 beta).
Results
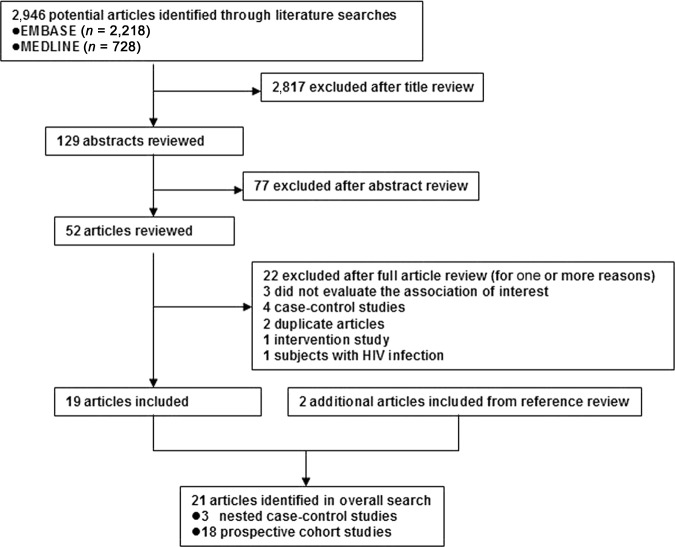
The search strategy generated 2,946 citations, of which 52 were considered of potential value and being retrieved for detailed evaluation (Fig. 1). Of these 52 articles, 33 were subsequently excluded from the meta-analysis for various reasons. An additional two articles were included from reference review. Thus, a total of 21 articles were used in this meta-analysis (Table 1). The number of participants ranged from 248 to 1,213,829, and a total of 17,624 PLC cases were identified in this meta-analysis.
Figure 1.
Flow diagram of study selection.
Abbreviation: HIV, human immunodeficiency virus.
Table 1.
Characteristics of prospective studies of body mass index per 5 kg/m2 increase and primary liver cancer risk
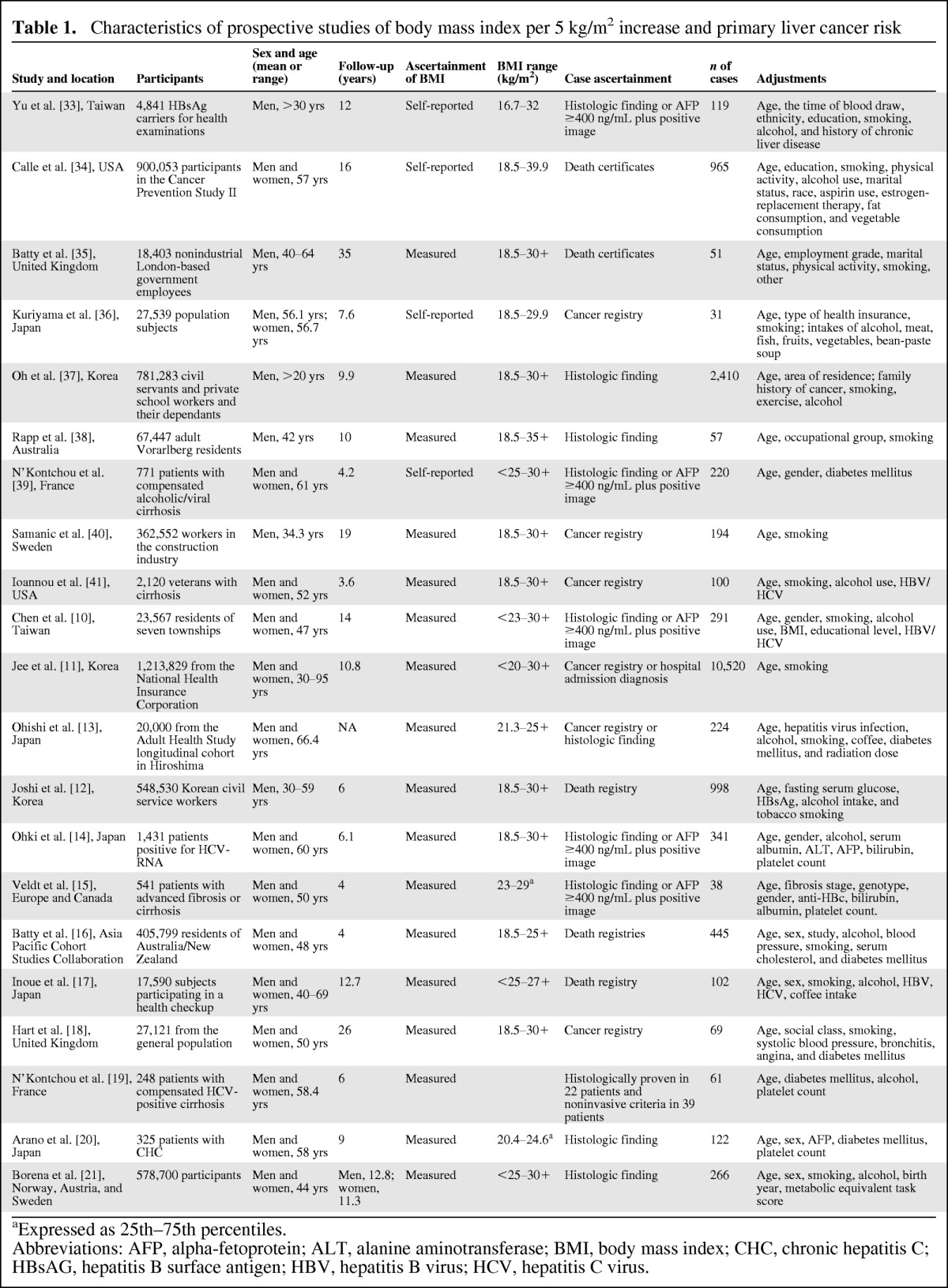
aExpressed as 25th–75th percentiles.
Abbreviations: AFP, alpha-fetoprotein; ALT, alanine aminotransferase; BMI, body mass index; CHC, chronic hepatitis C; HBsAG, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus.
Meta-analysis
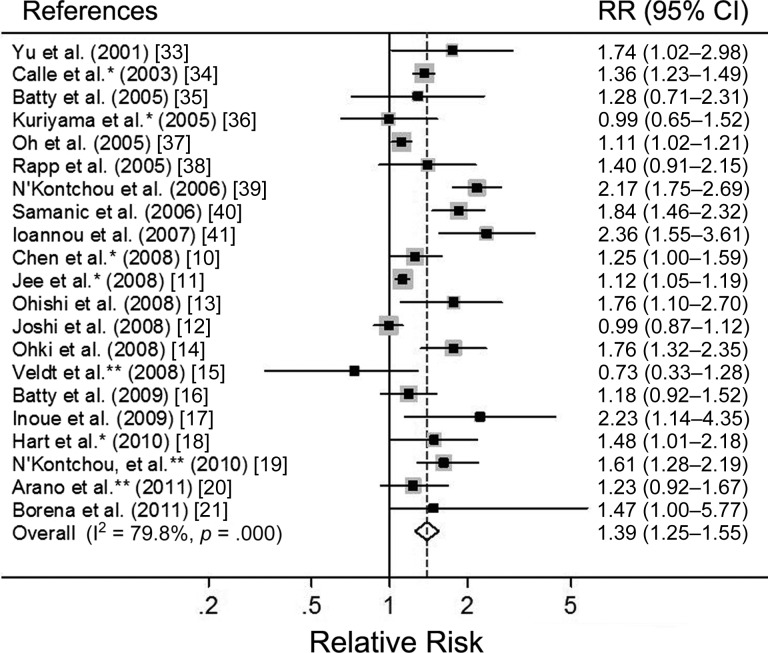
The summary relative risks (SRRs) of PLC per 5-unit increase in BMI for each study are shown in Figure 2. The meta-analysis of all 21 studies in a random-effects model found that a 5-unit increase in BMI was associated with a 39% increased risk of PLC, with significant heterogeneity among studies (SRR: 1.39, 95% CI: 1.25–1.55; p < .001, I2 = 79.8%). We conducted subgroup meta-analyses by sex, geographic locations, ascertainment of exposure and outcome, case size, duration of follow-up, sample source, and confounders (Table 2). The SRRs of the association between a 5-unit increase in BMI and risk of PLC were positive in all strata.
Figure 2.
Forest plots of risk of primary liver cancer associated with each 5-unit increase in BMI (in kg/m2). *Derived by pooling the sex-specific relative risks. **Derived from each 1-unit increment of body mass index.
Abbreviations: CI, confidence interval; RR, relative risk.
Table 2.
Stratified meta-analyses of body mass index per 5 kg/m2 and the risk of primary liver cancer
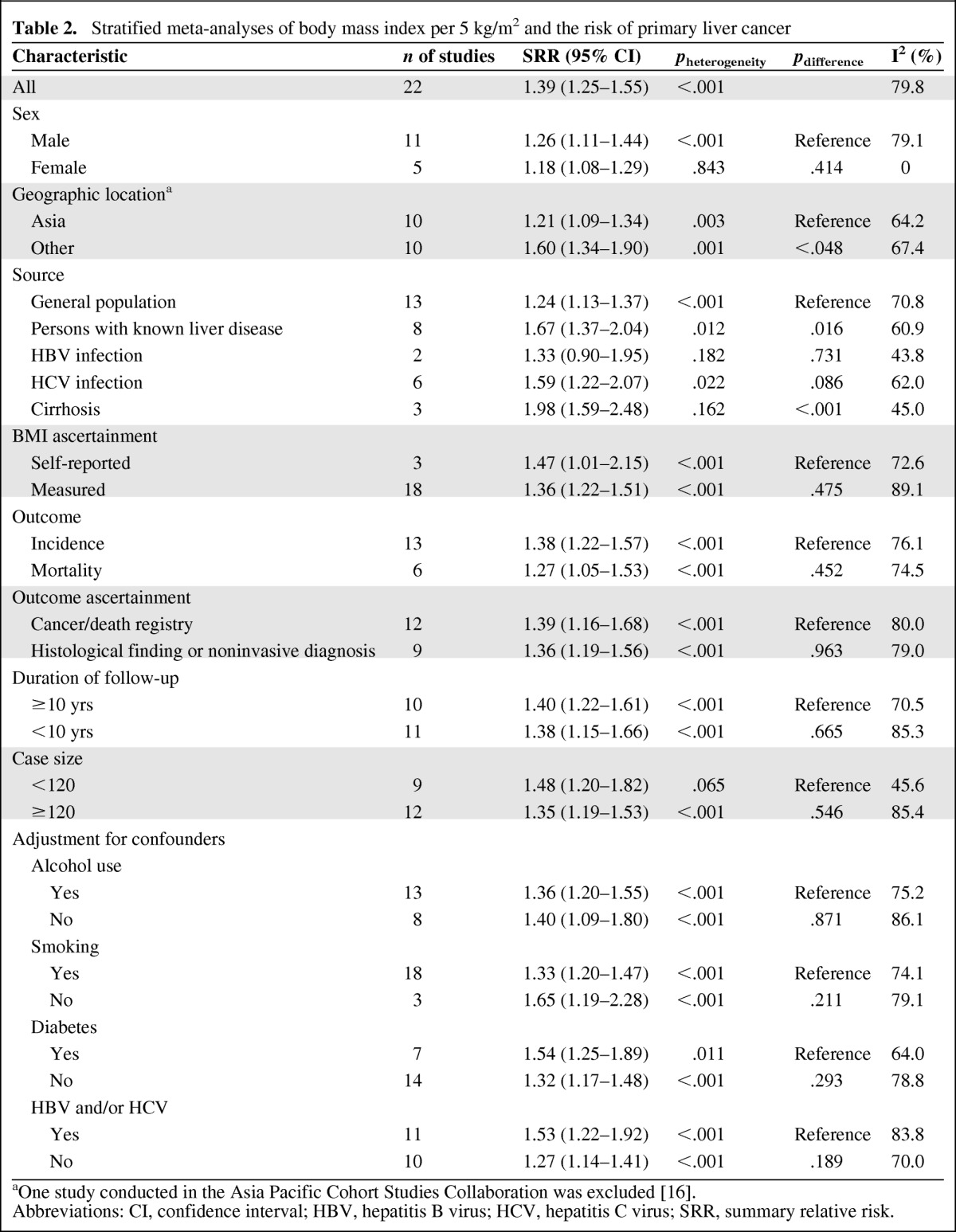
aOne study conducted in the Asia Pacific Cohort Studies Collaboration was excluded [16].
Abbreviations: CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; SRR, summary relative risk.
There was no evidence that the estimated RRs differed significantly by sex (SRR: 1.26, 95% CI: 1.11–1.44 for men vs. SRR: 1.18, 95% CI: 1.08–1.29 for women; p = .414). Similarly, there was no difference in the association between increased BMI and PLC risk between strata in ascertainment of exposure and outcome, the number of cases, duration of follow-up, and confounders (alcohol consumption, smoking, infection with HBV and/or HCV, history of DM). However, geographic location and sample source were found to significantly modify the association between increased BMI and PLC risk. The SRRs were significantly higher in non-Asian studies than in Asian studies (SRR: 1.60, 95% CI: 1.34–1.91 for non-Asians vs. SRR: 1.21, 95% CI: 1.09–1.34 for Asians; p = .048).
When combining results on studies of population with known liver disease (with HBV and/or HCV infection, cirrhosis), the SRRs were significantly stronger in people with known liver disease and excess weight than in the general population with excess weight (SRR: 1.67, 95% CI: 1.37–2.04, p = .012, I2 = 60.9% for patients with known liver disease; SRR: 1.24, 95% CI: 1.13–1.37, p < .001, I2 = 70.8%; pdifference = .016). Further analysis found that HCV-positive patients with excess weight had a higher risk of PLC development than general population (SRR: 1.59, 95% CI: 1.22–2.07; p = .086). Cirrhotic individuals with excess weight had a pooled RR of 1.98 (95% CI: 1.59–2.48), which was significant higher than general population with excess weight (p < .001). However, HBV-positive patients with excess weight had a similar risk of PLC development as the general population with excess weight (p = .731, Table 2).
There was evidence of a nonlinear association between increased BMI and PLC risk (p < .001), with the most pronounced increase in risk among persons with a BMI >32 kg/m2. Higher BMIs were associated with a further, stronger increase in PLC risk (Fig. 3).
Figure 3.
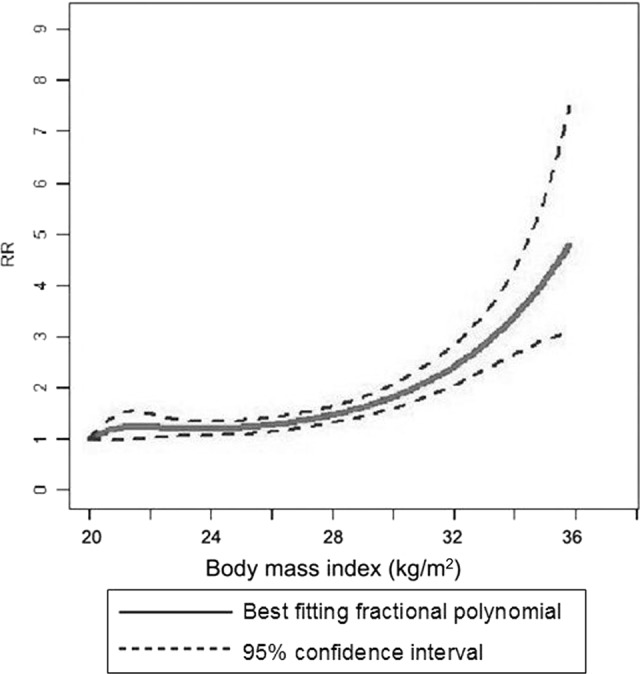
Body mass index and primary liver cancer risk, nonlinear dose-risk relationship.
Abbreviation: RR, relative risk.
We also conducted a sensitivity analysis and found that there were no changes in the direction of effect when any one study was excluded. When combining analyses of studies of PLC incidence and mortality, respectively, we found similar SRRs for PLC incidence and mortality (SRR: 1.38, 95% CI: 1.22–1.57, p < .001 for incidence; SRR: 1.27, 95% CI: 1.05–1.53, p = .001 for mortality).
We also performed a meta-analysis based on BMI categories and found that obese patients had a significantly higher risk of PLC than overweight patients did (SRR: 1.96, 95% CI: 1.66–2.32, p = .003, I2 = 56.7% for obese patients; SRR: 1.17, 95% CI: 1.07–1.27, p = .005, I2 = 53.5% for overweight patients; data not shown). We then conducted a REML-based random effects meta-regression analysis to investigate the source of the significant heterogeneity among studies. In univariate and multivariate meta-regression analyses, two variables (geographic locations and sample source) were statistically significant. The between-study variance was reduced from 0.0357 to 0.01035 based on the REML estimate, and the heterogeneity explained by these two variables was 71%. We found no statistical evidence of publication bias in this analysis (p = .928 using Begg's test; p = .143 using Egger's test).
Discussion
The comprehensive meta-analysis of observational studies on BMI and PLC risk suggests that a 5-unit increase in BMI was associated with a 39% increased risk of PLC when pooling across 21 prospective studies. This significantly positive relationship is independent of sex, geographic locations, case size, ascertainment of exposure and outcome, duration of follow-up, and confounders. Furthermore, HCV-positive or cirrhotic patients with excess weight had a higher risk of PLC development than general populations with excess weight. To our knowledge, for the first time in a meta-analysis of BMI and PLC based on linear and nonlinear analysis, we found the most pronounced increase in PLC risk was observed at a BMI >32 kg/m2.
Strengths of the present study included the quantitative analysis based on prospective studies, which might minimize the possibility that our findings were due to selection or recall bias, which might be of concern in retrospective case-control studies. All the studies included in the meta-analysis evaluated multiple potential confounders, and the relationships between BMI and PLC risk in each study were derived from regression after adjustment several potential risk factors for PLC. The large number of studies that addressed the same research question and the subsequent possibility of stratified analyses permitted us to better explore the effect of excess weight on various subgroups (especially subgroups with known liver diseases).
However, our meta-analysis has several limitations that may affect the interpretation of the results. First, great heterogeneity was observed across studies. By conducting meta-regression analysis, we found that significant heterogeneity may exist in terms of geographic locations and sample source, both of which may account for 71% of heterogeneity across studies. Second, inadequate control for confounders may bias the results toward exaggeration or underestimation of risk estimates. Although most studies adjusted for other known risk factors for PLC, residual or unknown confounding cannot be excluded. Obese persons may have unhealthy lifestyles that include smoking, heavy alcohol consumption, and a history of DM. However, adjustment for a wide range of potential confounders only marginally altered the relationship between BMI and PLC risk. Third, as in any meta-analysis, the possibility of publication bias is of concern because small studies with null results tend not to be published. However, the results obtained from formal statistical tests did not provide evidence for such bias.
The pathophysiological mechanisms underlying the association between increased BMI and the risk of PLC have been suggested. Obesity is associated with nonalcoholic fatty liver disease (NAFLD), the most common form of chronic liver disease in developed countries [42]. About 30% of individuals with NAFLD based on ultrasound were identified as having its severe form, nonalcoholic steatohepatitis (NASH), and 8% to 26% of individuals with NASH progress to cirrhosis [43]. Of patients with NASH-related cirrhosis, 40%–62% develop complications, including HCC, after 5–7 years of follow-up [44]. NASH's carcinogenic potential has been attributed to insulin resistance, which may lead to elevated levels of the proinflammatory cytokine, such as tumor necrosis factor (TNF) and interleukin (IL)-6. Both TNF and IL-6 favor the development of hepatic steatosis and inflammation and subsequent cancer of the liver [45, 46]. In addition, elevated levels of insulin may upregulate the production of insulin-like growth factor-1 (IGF-1), which stimulates cellular proliferation and inhibits apoptosis within the liver [47]. The involvement of insulin and IGF-1 in carcinogenesis of liver has been supported by in vitro studies, animal models, and epidemiologic studies [48, 49].
A previous meta-analysis showed the SRR of PLC was statistically significantly higher for obese men (RR: 2.42, 95% CI: 1.83–3.20) than that for obese women (RR: 1.67, 95% CI: 1.37–2.03; p = .03) [8]. However, the present meta-analysis showed no significant difference in the PLC risk with increased BMI between men and women, although somewhat higher risk in males than that in females (SRR: 1.26 for men vs. 1.18 for women; p = .414). We assume that use of different statistical methods and the presence of nonlinear association may partially account for this discrepancy—that is, the linear model does not fit well with the data.
In the present meta-analysis, we found evidence of a nonlinear positive association between increased BMI and PLC risk, with the greatest risk increase when increasing from high levels of BMI (>32 kg/m2). So, examining the shape of the dose-response curve seems to be important for clarifying this association. We also performed categorical meta-analysis based on included studies and found similar results with that from Larrson et al [8]—that is, obese men have a higher risk of HCC than do obese women (RR: 1.97, 95% CI: 1.50- 2.57 for men vs. RR: 1.43, 95% CI: 1.14–1.78 for women; p = .072). Thus, further studies will be necessary to dissect out the gender differences in the correlations between excess weight and the risk of PLC.
In the present meta-analysis, we found that the SRR of PLC with increased BMI in non-Asian studies was stronger than those in Asian studies. We do not know the exact mechanisms behind this phenomenon, but it may not be a chance finding because it was based on 10 prospective studies in both subgroups. It could be due to genetic factors or the prevalence of obesity. Another possibility is the difference in the prevalence of chronic liver disease between Asian and non-Asian studies. Further cohort studies of increased BMI and PLC risk in different geographic locations are needed.
Interestingly, findings from this meta-analysis indicate that the association between BMI and HCC risk is somewhat stronger for patients with HCV infection than for the general population (p = .08). We cannot completely rule out that this is a chance finding, because there were only six studies in this subgroup analysis. This phenomenon may suggest that the two risk factors, HCV and adiposity, could synergize to increase the risk of incident HCC. Adiposity is associated with hepatic steatosis and insulin resistance. Hepatic steatosis can cause hepatic inflammation and promote fibrosis through enhanced oxidative stress, increased susceptibility to apoptosis, and activation of subsinusoidal stellate cells [50]. Furthermore, HCV infection may induce insulin resistance by itself, which may also contribute to fibrosis progression [51]. More studies, including epidemiological and mechanism studies, are warranted to elucidate the exact contribution of excess weight on hepatocarcinogenesis in patients with HCV infection. In contrast to patients with HCV infection or cirrhosis, based on two studies, HBV-positive patients with excess weight were found to have a similar risk of PLC as the general population. However, because the sample size was small in these subgroups, we cannot exclude a type I error.
As obesity prevalence continues to be on an upward trajectory worldwide, the contribution of obesity to the development of PLC might constitute a significant proportion of the global burden of PLC. Obesity is an avoidable factor, as is smoking. The positive link between excessive weight and increased risk of PLC provides an excellent stimulus to intervene with individual and antiobesity treatment prior to malignant change.
In summary, this meta-analysis supports the hypothesis that excess body weight may significantly increase PLC risk. This positive association is true in both men and women; is true in North American, European, and Asian studies; and is independent of confounders. In future research, randomized trials are needed to further examine the effect of weight reduction in obese populations to decrease the risk of PLC.
Author Contributions
Conception/Design: Haixia Cao, Yuqin Wang
Provision of study materials: Haixia Cao, Yuqin Wang, Baochan Wang
Collection and/or assembly of data: Haixia Cao, Yuqin Wang, Jiangao Fan
Data analysis and interpretation: Haixia Cao, Yuqin Wang, Baochan Wang, Jiangao Fan
Manuscript writing: Haixia Cao, Yuqin Wang, Shen Feng
Final approval of manuscript: Haixia Cao, Yuqin Wang, Baochan Wang, Shen Feng, Jiangao Fan
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160:3227–3230. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 5.Yu MC, Yuan JM. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology. 2004;127:S72–78. doi: 10.1016/j.gastro.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Bosch FX, Ribes J, Diaz M, et al. Primary liver cancer: Worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Bugianesi E. Non-alcoholic steatohepatitis and cancer. Clin Liver Dis. 2007;11:191–207. doi: 10.1016/j.cld.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: A meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–1008. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polesel J, Zucchetto A, Montella M, et al. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Ann Oncol. 2009;20:353–357. doi: 10.1093/annonc/mdn565. [DOI] [PubMed] [Google Scholar]
- 10.Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: A follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 11.Jee SH, Yun JE, Park EJ, et al. Body mass index and cancer risk in Korean men and women. Int J Cancer. 2008;123:1892–1896. doi: 10.1002/ijc.23719. [DOI] [PubMed] [Google Scholar]
- 12.Joshi S, Song YM, Kim TH, et al. Socio-economic status and the risk of liver cancer mortality: A prospective study in Korean men. Public Health. 2008;122:1144–1151. doi: 10.1016/j.puhe.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Ohishi W, Fujiwara S, Cologne JB, et al. Risk factors for hepatocellular carcinoma in a Japanese population: A nested case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17:846–854. doi: 10.1158/1055-9965.EPI-07-2806. [DOI] [PubMed] [Google Scholar]
- 14.Ohki T, Tateishi R, Sato T, et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol. 2008;6:459–464. doi: 10.1016/j.cgh.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Veldt BJ, Chen W, Heathcote EJ, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47:1856–1862. doi: 10.1002/hep.22251. [DOI] [PubMed] [Google Scholar]
- 16.Batty GD, Barzi F, Huxley R, et al. Obesity and liver cancer mortality in Asia: The Asia Pacific Cohort Studies Collaboration. Cancer Epidemiol. 2009;33:469–472. doi: 10.1016/j.canep.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue M, Kurahashi N, Iwasaki M, et al. Metabolic factors and subsequent risk of hepatocellular carcinoma by hepatitis virus infection status: A large-scale population-based cohort study of Japanese men and women (JPHC Study Cohort II) Cancer Causes Control. 2009;20:741–750. doi: 10.1007/s10552-008-9287-6. [DOI] [PubMed] [Google Scholar]
- 18.Hart CL, Batty GD, Morrison DS, et al. Obesity, overweight and liver disease in the Midspan prospective cohort studies. Int J Obes (Lond) 2010;34:1051–1059. doi: 10.1038/ijo.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nkontchou G, Bastard JP, Ziol M, et al. Insulin resistance, serum leptin, and adiponectin levels and outcomes of viral hepatitis C cirrhosis. J Hepatol. 2010;53:827–833. doi: 10.1016/j.jhep.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 20.Arano T, Nakagawa H, Tateishi R, et al. Serum level of adiponectin and the risk of liver cancer development in chronic hepatitis C patients. Int J Cancer. doi: 10.1002/ijc.25861. [DOI] [PubMed] [Google Scholar]
- 21.Borena W, Strohmaier S, Lukanova A, et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer. 2012;131:193–200. doi: 10.1002/ijc.26338. [DOI] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 25.Orsini NBR, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6:40–57. [Google Scholar]
- 26.Wallace K, Baron JA, Karagas MR, et al. The association of physical activity and body mass index with the risk of large bowel polyps. Cancer Epidemiol Biomarkers Prev. 2005;14:2082–2086. doi: 10.1158/1055-9965.EPI-04-0757. [DOI] [PubMed] [Google Scholar]
- 27.Boutron-Ruault MC, Senesse P, Meance S, et al. Energy intake, body mass index, physical activity, and the colorectal adenoma-carcinoma sequence. Nutr Cancer. 2001;39:50–57. doi: 10.1207/S15327914nc391_7. [DOI] [PubMed] [Google Scholar]
- 28.Sato T, Takeda H, Sasaki Y, et al. Increased homeostasis model assessment-insulin resistance is a risk factor for colorectal adenoma in Japanese males. Tohoku J Exp Med. 2011;223:297–303. doi: 10.1620/tjem.223.297. [DOI] [PubMed] [Google Scholar]
- 29.Royston P. A strategy for modeling the effect of a continuous covariate in medicine and epidemiology. Stat Med. 2000;19:1831–1847. doi: 10.1002/1097-0258(20000730)19:14<1831::aid-sim502>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 31.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu MW, Yang YC, Yang SY, et al. Hormonal markers and hepatitis B virus-related hepatocellular carcinoma risk: A nested case-control study among men. J Natl Cancer Inst. 2001;93:1644–1651. doi: 10.1093/jnci/93.21.1644. [DOI] [PubMed] [Google Scholar]
- 34.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 35.Batty GD, Shipley MJ, Jarrett RJ, et al. Obesity and overweight in relation to organ-specific cancer mortality in London (UK): Findings from the original Whitehall study. Int J Obes (Lond) 2005;29:1267–1274. doi: 10.1038/sj.ijo.0803020. [DOI] [PubMed] [Google Scholar]
- 36.Kuriyama S, Tsubono Y, Hozawa A, et al. Obesity and risk of cancer in Japan. Int J Cancer. 2005;113:148–157. doi: 10.1002/ijc.20529. [DOI] [PubMed] [Google Scholar]
- 37.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23:4742–4754. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 38.Rapp K, Schroeder J, Klenk J, et al. Obesity and incidence of cancer: A large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93:1062–1067. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.N′Kontchou G, Paries J, Htar MT, et al. Risk factors for hepatocellular carcinoma in patients with alcoholic or viral C cirrhosis. Clin Gastroenterol Hepatol. 2006;4:1062–1068. doi: 10.1016/j.cgh.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Samanic C, Chow WH, Gridley G, et al. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17:901–909. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 41.Ioannou GN, Splan MF, Weiss NS, et al. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2007;5:938–945. doi: 10.1016/j.cgh.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 42.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 43.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 44.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 45.Papa S, Bubici C, Zazzeroni F, et al. Mechanisms of liver disease: Cross-talk between the NF-kappaB and JNK pathways. Biol Chem. 2009;390:965–976. doi: 10.1515/BC.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiencke JK. Impact of race/ethnicity on molecular pathways in human cancer. Nat Rev Cancer. 2004;4:79–84. doi: 10.1038/nrc1257. [DOI] [PubMed] [Google Scholar]
- 48.Weng CJ, Hsieh YH, Tsai CM, et al. Relationship of insulin-like growth factors system gene polymorphisms with the susceptibility and pathological development of hepatocellular carcinoma. Ann Surg Oncol. 2010;17:1808–1815. doi: 10.1245/s10434-009-0904-8. [DOI] [PubMed] [Google Scholar]
- 49.Longato L, de la Monte S, Kuzushita N, et al. Overexpression of insulin receptor substrate-1 and hepatitis Bx genes causes premalignant alterations in the liver. Hepatology. 2009;49:1935–1943. doi: 10.1002/hep.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powell EE, Jonsson JR, Clouston AD. Steatosis: Co-factor in other liver diseases. Hepatology. 2005;42:5–13. doi: 10.1002/hep.20750. [DOI] [PubMed] [Google Scholar]
- 51.Hui JM, Sud A, Farrell GC, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression. Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]