This article explores the nondisclosure of complementary and alternative medicine use among cancer patients, including reasons for and outcomes from nondisclosure, within the context of patient-doctor communication.
Keywords: Complementary and alternative medicine, Cancer, Disclosure, Doctor-patient relations
Learning Objectives
After completing this course, the reader will be able to:
Discuss the danger inherent in nondisclosure of complementary and alternative medicine (CAM) use due to the potential for herb- or vitamin-drug interactions with conventional treatment.
Explain the need for greater patient-doctor communication about CAM use in oncology settings in order to maintain patient safety and wellbeing.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Objective.
To explore the nondisclosure of complementary and alternative medicine (CAM) use among cancer patients, including reasons for and outcomes from nondisclosure of CAM use, within the context of patient-doctor communication.
Method.
A systematic review was conducted exploring investigations surrounding the communication of CAM use for patients with cancer published until August 2011.
Results.
A total of 21 studies were located, which reported a prevalence of CAM use among patients with cancer ranging between 11% and 95%; of these patients, 20% to 77% did not disclose their CAM use. The main reasons for nondisclosure were the doctor's lack of inquiry; patient's anticipation of the doctor's disapproval, disinterest, or inability to help; and patient's perception that disclosure of CAM use is irrelevant to their conventional care. There is some evidence to suggest that patient-doctor communication about the use of CAM was associated with an enhanced patient-doctor relationship and higher patient satisfaction.
Conclusions.
Although the use of CAM by patients with cancer is high, patients frequently fail to disclose its use to their health professionals for reasons emanating from both sides of the dyadic patient-doctor relationship. Because a substantial proportion of patients with cancer may use CAM and there is potential for herb- or vitamin-drug interactions, further research in patient-doctor communication about CAM is necessary to maintain patient safety and wellbeing. The development of effective interventions to improve the disclosure of CAM use should be an integral part of this future research.
Introduction
The use and popularity of complementary and alternative medicine (CAM) to treat a multitude of conditions is increasing worldwide [1–3]. CAM has become widely used over the past decades by patients with cancer [4], with cancer survivors reporting greater use than the general population [5]. As many as 50%–83% of adult patients with cancer [6] and 84% of children with cancer in the U.S. [7], and up to 65% of adult patients with cancer [8, 9] and 46% of children [10] with cancer in Australia, report using CAM at least once after the diagnosis of cancer.
Research has shown that patients with cancer have a variety of motivations for using CAM therapies. The main reasons include to help alleviate the side effects caused by medical treatment [11]; to satisfy unmet needs from conventional medicine and doctors, including for emotional support and humanistic care [9]; and to improve quality of life and overall care [12]. Some patients with cancer use CAM because of a poor prognostic outcome [13], perceiving CAM as a last resort and as a way of finding hope [14] for cure, longer survival time, prevention of cancer recurrence, or improved immune function [15]. Other reasons given have been a desire for control; compared to nonusers, CAM users have been found to be more desirous of being involved in their health care decisions [16], are more health conscious [17], and are more likely to believe that they can improve their health by a change of lifestyle [17]. CAM is generally viewed by patients with cancer as safe, holistic [15], natural, and nontoxic in contrast to conventional medicine, which is perceived as toxic [18].
However, neither the safety nor the efficacy of many CAM therapies has been proven. CAM is defined by the National Center for Complementary and Alternative medicine (NCCAM) as “a group of diverse medical and health care systems, practices, and products that are not generally considered part of conventional medicine” [19]. For example, CAM includes nonbiological interventions (e.g., music therapy, massage) and biological interventions (e.g., vitamins, traditional Chinese medicine). The possibility of interference with conventional treatments caused by herb-drug interactions [20, 21] or vitamin-drug interactions [22] is one of the main concerns of health professionals with regard to CAM therapies [9]. Although some CAM therapies may be beneficial, it is possible that others may be directly harmful or result in reduced efficacy of chemotherapy, surgery, and radiation therapy.
Considering that a significant number of patients with cancer use or consider using CAM [18], communication about CAM use is an important part of cancer care. It may facilitate the ability of patients to weigh the safety and efficacy of CAM, ensure avoidance of harmful interactions with their conventional cancer treatments, and determine where and when they can most safely access CAM. Studies suggest that patient-doctor communication about the use of CAM may protect patients with cancer from dangerous and unproven therapies as well as maximize the potential health benefits of CAM [8, 23, 24].
Despite the recognition of the importance of patient-doctor communication about the use of CAM, few studies have explored this issue. The aim of this systematic review was to identify and summarize the existing literature on the prevalence of CAM use, patient disclosure of CAM use to doctors, communication about CAM during the consultation, and the outcomes from such practices.
Materials and Methods
Search Strategy
Electronic literature searches were performed using Medline, Pubmed, Proquest 5000, Science Direct, and Cochrane Library databases from January 1990 to August 2011. January 1990 was chosen because the last two decades essentially mark when the use of CAM during oncology treatment became a hot topic in the medical oncology community [25, 26]. The search was conducted using the following combination of terms: “complementary and alternative medicine” and “cancer” and (“communication” or “disclosure”). The identified records were initially screened for eligibility via titles and abstracts. Reference lists of identified papers and reviews in related areas were manually searched for additional studies. Articles were then selected based on analysis of the full text.
Eligibility Criteria
All studies were included that investigated the disclosure of CAM use in patients with cancer, subscribing to the definition of CAM provided by the NCCAM described above [19]. Additional eligibility criteria included provision of the CAM prevalence rate and CAM disclosure or nondisclosure rate. Studies reported in a language other than English were not included in this review. If two or more papers presented results from the same study sample, the paper providing the greatest amount of information relevant to this review was included. Papers using purposeful sampling were excluded to minimize bias, but no other restrictions on study design were made.
Quality Assessment Criteria
QualSyst [27] was used to assess the quality of the included studies. This assessment tool is regularly used to provide a systematic, reproducible, and quantitative means of assessing the quality of research across a broad range of study designs. Studies are compared against 14 criteria encompassing description of the study and results, methodology, and validity of conclusions to compute a total score. The total score ranges from 0 to 1, with a higher score indicating better quality and a score of 0.75 representing a relatively conservative cutoff point to indicate a good quality study.
Data Extraction
A review template was developed specifying the key information about each study (see Table 1). Two reviewers (E.D. and B.O.) independently applied the inclusion and quality assessment criteria. If relevant data were reported only graphically, values were estimated from the graphs. The two reviewers compared results and resolved any discrepancies by consensus.
Table 1.
Eligible studies with information about complementary and alternative medicine use and disclosure among cancer patients
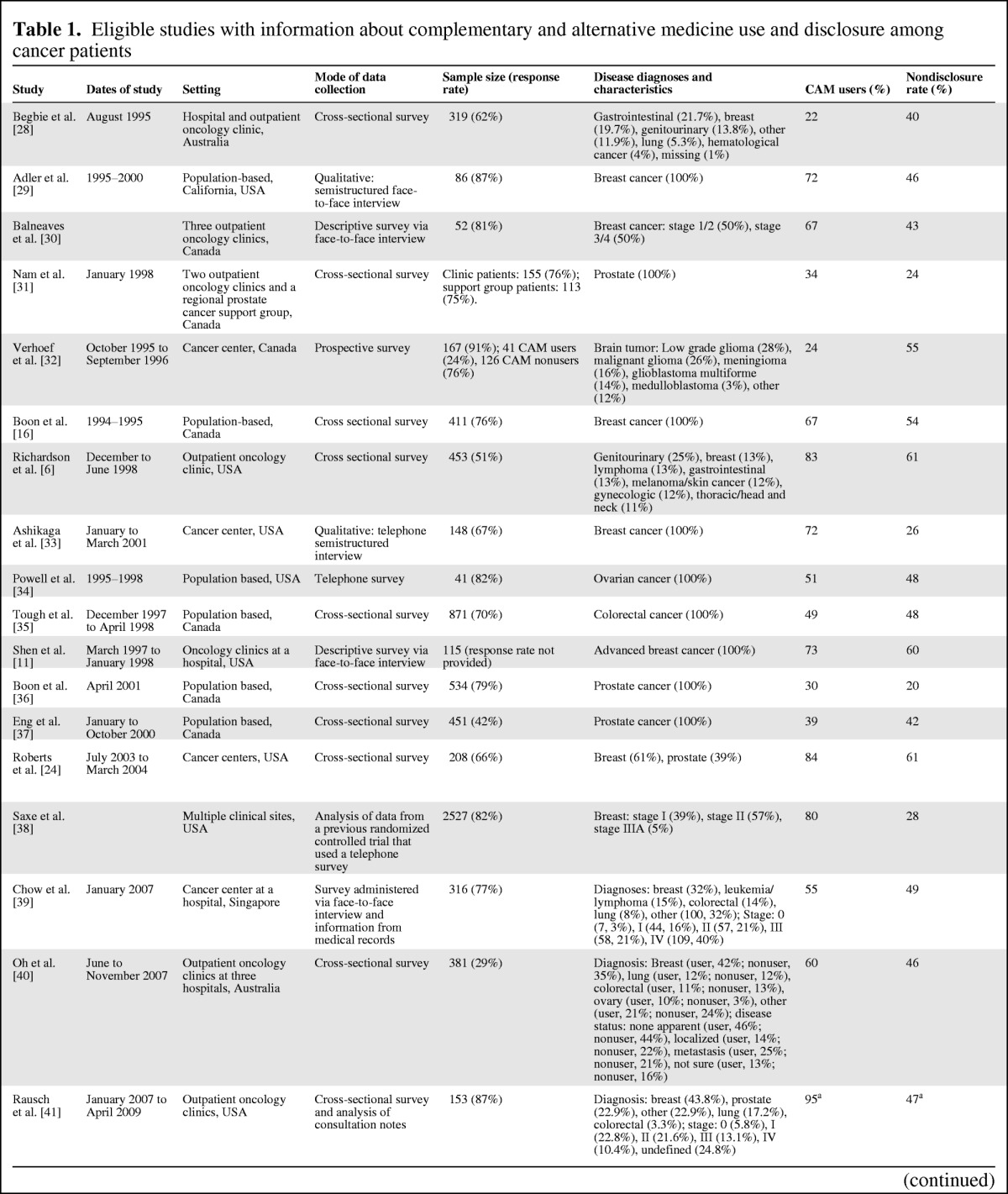
Table 1.
(Continued)
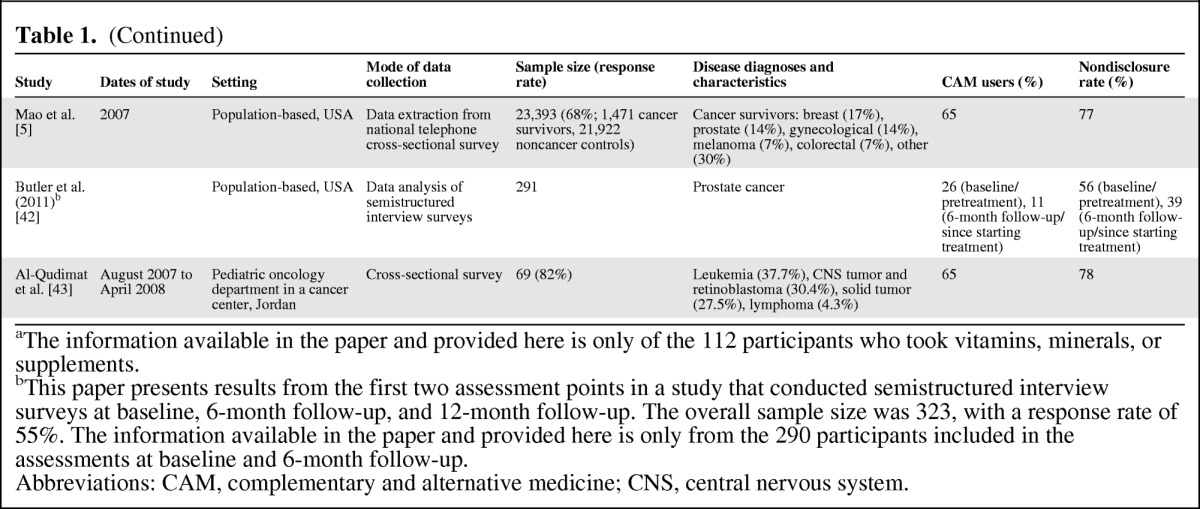
aThe information available in the paper and provided here is only of the 112 participants who took vitamins, minerals, or supplements.
bThis paper presents results from the first two assessment points in a study that conducted semistructured interview surveys at baseline, 6-month follow-up, and 12-month follow-up. The overall sample size was 323, with a response rate of 55%. The information available in the paper and provided here is only from the 290 participants included in the assessments at baseline and 6-month follow-up.
Abbreviations: CAM, complementary and alternative medicine; CNS, central nervous system.
Results
Selection of Studies
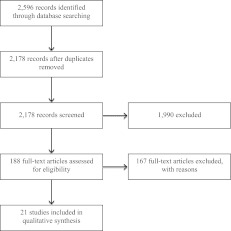
Figure 1 shows the flow of the information of the review. Excluding duplicates (n = 418), the initial search yielded 2,178 bibliographic records from the electronic databases or the reference sections of identified database publications; 87 underwent full review. On closer inspection of the full articles, only 21 studies fulfilled the eligibility criteria and were included in the review. All of the included studies were reported in published journal articles.
Figure 1.
Flow of information of the review.
Study Characteristics
An overview of the study characteristics of the selected articles are presented in Table 1. Sixteen studies were conducted using a survey, including 11 cross-sectional surveys, 3 face-to-face interviews, 1 telephone interview, and 1 prospective survey. One survey study also obtained information from consultation notes, whereas another also used information from medical records. Two studies conducted semistructured interviews, one via face-to-face interview and the other via telephone. Finally, three studies analyzed data from previous research: one from a randomized controlled trial; one from semistructured interviews conducted at baseline, 6-month follow-up, and 12-month follow-up, and the other from a national cross-sectional telephone survey. Sample sizes ranged from 41 to 23,393; however, most samples sizes were between 200 and 300. Eight studies recruited participants from the general population, whereas the remaining 13 studies recruited participants from hospitals or cancer centers. Of these, nine recruited patients from at least two sites; the remaining four studies recruited patients from one site only.
Study Quality
The overall quality of the included studies in this review was high, with an average score of 0.88 (SD = 0.10, range = 0.71–1.0). The main areas where quality was lacking were provision of a well-defined outcome measure that was robust to measurement/misclassification bias, provision of some form of variance for the main results, sufficient description of the subject group, and controlling for confounding variables. Not providing a definition of CAM was a major contributing factor for a low score in provision of a well-defined outcome measures.
Patient Characteristics
Thirteen studies were conducted in a clinical setting, of which one additionally included patients from a support group, and eight were population based. Most patients were aged 50 years or older. Of the nine studies that reported income of participants, the majority of patients were classified as mid-level according to the particular measure used. Ten studies used mixed gender samples, whereas seven used only women and four used only men. Reflective of this distribution, nine studies included patients from mixed cancer types, whereas six focused exclusively on breast cancer, three on prostate cancer, and one study for each of ovarian, brain, and colorectal cancers. All but two studies were conducted in a high-income country: 10 in the U.S., 7 in Canada, 2 in Australia/New Zealand, 1 in Singapore, and 1 in Jordan. Of the four studies that reported marital status, the most commonly reported status was married/de facto. Thirteen studies reported educational level, of which the majority reported a highly educated sample with mostly completion of education beyond high school.
Prevalence of CAM Use and Nondisclosure Rates
The 21 studies revealed an extremely wide range of prevalence of CAM use among patients with cancer, from 11% to 95%. The studies were evenly distributed over the range, and their order did not appear to be affected by patient characteristics nor the study setting or methodology.
The studies also revealed a comparatively narrower but still wide range of nondisclosure of CAM use, from 20% to 77%. The identified nondisclosure rates were predominantly clustered around the 40%–50% range, but did not appear to be affected by patient characteristics, study setting, or methodology, nor the prevalence of CAM use identified within the study.
Patients' Reasons for Disclosure/Nondisclosure
Eight studies investigated the reasons patients provided for nondisclosure of CAM use [24, 29–31, 34, 37, 40, 42]. The most common reasons for nondisclosure were doctor noninquiry [24, 31, 34, 37, 40, 42]; anticipation of doctor disapproval [24, 29, 31, 40, 42], doctor disinterest [29, 30, 34], or inability to provide information on CAM [24, 40]; and patient perception that their CAM use is irrelevant to their conventional treatment [24, 29, 31, 40, 42]. Other reasons included consultation time constraints [34] and patients seeking to maintain control over their treatment [29].
Predictors of Disclosure of CAM Use
Certain patient and doctor characteristics and the type of CAM used were also factors found to be associated with the disclosure of CAM use. One study found that younger age, higher level of education, higher family income, being married or living with a partner, and receiving chemotherapy and radiation therapy after surgery were linked to higher rates of disclosure [33]. Saxe et al. also found that the more educated participants (college graduates and postgraduates) were significantly more likely to disclose information about their use of CAM [38]. However, Oh et al. did not find patient demographics to be significantly related to disclosure [40]. Adler and Fosket found that when patients perceived their doctors to be respectful, open-minded, and willing to listen, they were more likely to reveal the use of CAM [29]. They reported that patients were also more likely to discuss their use of CAM when their doctor expected they were using some form of CAM.
Two studies reported an association between disclosure and the type of CAM used. Saxe et al. found that the highest rates of disclosure were for naturopathy (85%), followed by homeopathy (74%), acupuncture (71%), and chiropractics (47%) [38]. Shen et al. found that patients who had used biological forms of CAM (e.g., herbs, special diets, vitamins) were more likely to discuss CAM use with their health care professionals (60%–70%) than patients who had used nonbiological (chiropractic, massage, acupuncture, imagery, spiritual healing) or other noningestible CAM therapies (15%–40%) [11]. Importantly, one study found that when CAM use was discussed, such communication was the product of patient initiation in 92% of cases [34], although Roberts et al. reported that patients or their doctors were equally likely to initiate this discussion [24].
Outcomes from Disclosure
Five studies reported on the outcomes from patient disclosure of CAM use. Oh et al. found that users of biological CAM who disclosed their CAM practice reported significantly higher levels of satisfaction with their doctor [8]. This effect was not found for users of nonbiological CAM; however, overall, the majority of CAM users reported a positive response from their doctors. Most patients in another study reported a neutral (i.e., do not care) or positive response from their doctor and correspondingly believed that communication with their doctor about CAM either enhanced or did not change the doctor-patient relationship [24]. Similarly, Butler et al. reported that 56% of doctors encouraged use of CAM before beginning treatment, whereas 32% did not care and 53% of doctors encouraged continued use once treatment began (the figures comprising the remaining percentage were not reported) [42]. By contrast, patients in the Verhoef et al. study reported approximately equal proportions of positive (5/18, 28%), neutral (7/18, 39%), and negative (6/18, 33%) responses from their doctor when disclosing their CAM use [32]. Powell et al. reported that patients were disappointed by the lack of information and/or support provided by their doctor about the use of CAM [34].
Discussion
This review provides an overview of the prevalence of CAM use and the rates of nondisclosure of CAM use among patients with cancer. It is difficult to give an accurate estimate of the prevalence of CAM use, as the rates identified in the reviewed studies varied widely. Although the identified rates of patient nondisclosure also varied widely over the reviewed studies, the lowest estimate was large enough to substantiate concern over the lack of discussion regarding CAM use between doctors and their patients.
Combined, the relatively high rates of CAM use and nondisclosure of CAM use underscore the importance of understanding the barriers to disclosure and the need for interventions to reduce such barriers. Patients' reasons for nondisclosure of CAM use may result from a combination of individual and contextual factors. Patients' backgrounds, the type of CAM they use, and consultation time constraints were all identified as factors that might play a role in their decisions to disclose or not. However, it seems that perhaps the most pervasive factors are those that are the most widely influenced by patients' beliefs and attitudes regarding conventional and complementary treatments, including their expectations of their doctor's corresponding beliefs and attitudes. It is the strict demarcation between conventional medicine as scientific fact versus CAM as magical thinking and pseudoscientific belief [44] that perhaps poses the greatest barrier to achieving a synergistic relationship between these care streams. The demarcation also perpetuates the perception that the often natural, less intrusive nature of CAM therapies means that they are necessarily without risk and unrelated to traditional treatments [45].
Further impetus to improve patient-doctor communication about CAM comes from the current paradigm shift in clinical practice from traditional paternalistic approaches towards what has been termed “patient-centered” approaches in which patients become more involved in decision-making and the selection of treatment approaches [46, 47]. Communication about CAM use was viewed as highly beneficial, with patients reporting that it enhanced satisfaction with those doctors who discussed CAM [40]. Correspondingly, patients revealed disappointment in the lack of information and/or support provided by their doctor about the use of CAM [34]. The National Health and Medical Research Council, the peak body for supporting health and medical research in Australia, advocates the practice of shared decision-making and its requisite patient-doctor communication, citing such processes as integral to the provision of quality care [48]. The Salzburg statement on shared decision-making also urges it practice, with the adoption of policies that encourage shared decision-making and that support the development of skills and tools for shared decision-making [49]. Open dialogue regarding CAM may facilitate shared decision-making about treatment options for patients with cancer.
The identified barriers surrounding patients' and doctors' beliefs and attitudes of conventional and CAM treatments may be overcome by interventions targeted towards promoting open communication about CAM. In line with the Salzburg statement on shared decision-making, such interventions are recommended to encourage clinicians, patients, researchers, and policy makers to work together to be coproducers of health [49]. They might include encouraging questions about CAM in resources such as question prompt lists, developing and implementing clinical practice guidelines about communication about CAM, and inclusion of CAM within doctor communication training courses. It is possible that such education about CAM will somewhat “normalize” CAM use, including increasing doctor interest and concern about their patients' CAM use. This would increase patients' confidence to disclose their CAM use and concomitantly reduce the proportion of doctors showing a neutral or negative response in these instances. Immediate action that doctors may take to encourage disclosure of CAM use include directly asking patients and communicating to their patients that they are open-minded, as patients were shown to be more likely to disclose their use of CAM when these actions were in place [29].
Despite highlighting the significance of patient disclosure and patient-doctor communication about the use of CAM, this review has several limitations. Firstly, the reliability of the methodology used to collect information on disclosure rates is questionable, as reflected in the wide range of CAM use and disclosure rates reported. Information obtained via consecutive sampling and self-reports is subject to misrepresentation of the target population and bias, and it forms the basis of much of the data collected in the reviewed studies. Secondly, the provision of information on the length of relationship with the doctor was inconsistent. According to previous studies, patients tend to discuss other therapies once the patient-doctor relationship is established [23]. Thus, future studies are recommended to use multiple assessments of patient-doctor communication about CAM or to at least state the length of relationship with the doctor when it was assessed when reporting results.
Thirdly, although there is now a burgeoning literature on CAM, almost all of the studies discussed above were conducted with white English-speaking patients. These studies provide little information on CAM use by other races/ethnicities and non-English speakers, who are likely to show divergent use from that of English speakers and lower rates of communications about CAM. Future studies are recommended to explore CAM use and disclosure in more diverse samples.
Regarding the quality of the included studies, a major limitation was inconsistency in the therapies included as CAM and lack of clarity in the way studies defined CAM use. Although some studies did not indicate which therapies they included as CAM, others included a huge variation of therapies (e.g., from acupuncture to prayer) or focused on a particular type of therapy (e.g., herbs). In addition, some studies operationalized the use of CAM as “ever used CAM,” whereas others operationalized it as “currently using CAM.” Consequently, direct comparisons between studies were problematic and limited the accuracy of the current results. The definition of what is considered CAM in any assessment of use among patients should be explicated clearly. Standardization of both this and the operationalization of CAM use across studies would be useful.
Other key areas in which some of the included studies were inferior was the provision of some form of variance for the main results and controlling for confounding variables (e.g., education, sex, income), although this issue was less critical for this review since results were not collated. Likewise, the problem of some studies not well defining the subject group did not affect this review because being a patient with cancer was the only subject characteristic relevant to the eligibility of the included studies.
Conclusion
This review highlighted the lack of patient-doctor communication about CAM and identified factors influencing the decision on whether to disclose for patients with cancer.
Considering previous indications of a high prevalence of CAM use among patients with cancer [5–7, 10, 40] and the potential for herb-drug or vitamin-drug interactions, the implications that poor communication about CAM use has for patient safety and involvement in their treatment decisions—and thus ultimately patient well-being—underscores the importance for further research in this area. Evaluation of evidence-based interventions to improve patient-doctor communication about CAM use, such as question prompt lists, will form a critical part of this future research.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Esther L. Davis, Byeongsang O
Collection and/or assembly of data: Esther L. Davis, Byeongsang Oh
Data analysis and interpretation: Esther L. Davis, Byeongsang Oh, Phyllis N. Butow, Stephen Clarke
Manuscript writing: Esther L. Davis, Byeongsang Oh, Phyllis N. Butow, Barbara A. Mullan, Stephen Clarke
Final approval of manuscript: Esther L. Davis, Byeongsang Oh, Barbara A. Mullan, Stephen Clarke
References
- 1.U.S. Department of Health. National Health Interview Survey (NHIS) Washington, DC: U.S. Department of Health; 2002. [Google Scholar]
- 2.Hyodo I, Amano N, Eguchi K, et al. Nationwide survey on complementary and alternative medicine in cancer patients in Japan. J Clin Oncol. 2005;23:2645–2654. doi: 10.1200/JCO.2005.04.126. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson JS, Workman SB, Kronenberg F. Research on complementary/alternative medicine for patients with breast cancer: A review of the biomedical literature. J Clin Oncol. 2000;18:668–683. doi: 10.1200/JCO.2000.18.3.668. [DOI] [PubMed] [Google Scholar]
- 4.Owen DK, Lewith G, Stephens CR, et al. Can doctors respond to patients' increasing interest in complementary and alternative medicine? BMJ. 2001;322:154–158. doi: 10.1136/bmj.322.7279.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao J, Palmer C, Healy K, et al. Complementary and alternative medicine use among cancer survivors: A population-based study. J Cancer Surviv. 2011;5:8–17. doi: 10.1007/s11764-010-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson MA, Sanders T, Palmer JL, et al. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18:2505–2514. doi: 10.1200/JCO.2000.18.13.2505. [DOI] [PubMed] [Google Scholar]
- 7.Myers C, Stuber ML, Bonamer-Rheingans JI, et al. Complementary therapies and childhood cancer. Cancer Control. 2005;12:172–180. doi: 10.1177/107327480501200305. [DOI] [PubMed] [Google Scholar]
- 8.Oh B, Butow P, Mullan B, et al. Patient-doctor communication: The use of complementary and alternative medicine by adult patients with cancer. J Soc Integr Oncol. 2010;8:56–64. [PubMed] [Google Scholar]
- 9.Miller M, Boyer MJ, Butow PN, et al. The use of unproven methods of treatment by cancer patients: Frequency, expectations, and cost. Support Care Cancer. 1998;6:337–347. doi: 10.1007/s005200050175. [DOI] [PubMed] [Google Scholar]
- 10.Sawyer MG, Gannoni AF, Toogood IR, et al. The use of alternative therapies by children with cancer. Med J Aust. 1994;160:320–322. [PubMed] [Google Scholar]
- 11.Shen J, Andersen R, Albert P, et al. Use of complementary/alternative therapies by women with advanced-stage breast cancer. BMC Complement Altern Med. 2002;2:8. doi: 10.1186/1472-6882-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molassiotis A, Fernadez-Ortega P, Pud D, et al. Use of complementary and alternative medicine in cancer patients: A European survey. Ann Oncol. 2005;16:655–663. doi: 10.1093/annonc/mdi110. [DOI] [PubMed] [Google Scholar]
- 13.Ritvo P, Irvine J, Katz J, et al. The patient's motivation in seeking complementary therapies. Patient Educ Couns. 1999;38:161–165. doi: 10.1016/s0738-3991(99)00063-4. [DOI] [PubMed] [Google Scholar]
- 14.Verhoef MJ, Balneaves LG, Boon HS, et al. Reasons for and characteristics associated with complementary and alternative medicine use among adult cancer patients: A systematic review. Integr Cancer Ther. 2005;4:274–286. doi: 10.1177/1534735405282361. [DOI] [PubMed] [Google Scholar]
- 15.Helyer LK, Chin S, Chui BK, et al. The use of complementary and alternative medicines among patients with locally advanced breast cancer—A descriptive study. BMC Cancer. 2006;6:39. doi: 10.1186/1471-2407-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boon H, Stewart M, Kennard MA, et al. Use of complementary/alternative medicine by breast cancer survivors in Ontario: Prevalence and perceptions. J Clin Oncol. 2000;18:2515–2521. doi: 10.1200/JCO.2000.18.13.2515. [DOI] [PubMed] [Google Scholar]
- 17.Burstein HJ, Gelber S, Guadagnoli E, et al. Use of alternative medicine by women with early-stage breast cancer. N Engl J Med. 1999;340:1733–1739. doi: 10.1056/NEJM199906033402206. [DOI] [PubMed] [Google Scholar]
- 18.Oh B, Butow P, Mullan B, et al. The use and perceived benefits resulting from the use of complementary and alternative medicine by cancer patients in Australia. Asia Pac J Clin Oncol. 2010;6:342–349. doi: 10.1111/j.1743-7563.2010.01329.x. [DOI] [PubMed] [Google Scholar]
- 19.National Institutes of Health. What is complementary and alternative medicine? [Accessed June 20, 2008]. Available at http://nccam.nih.gov/health/whatiscam/
- 20.Richardson MA. Biopharmacologic and herbal therapies for cancer: research update from NCCAM. J Nutrition. 2001;131:3037S–30340S. doi: 10.1093/jn/131.11.3037S. [DOI] [PubMed] [Google Scholar]
- 21.Boyle FM. Adverse interaction of herbal medicine with breast cancer treatment. Med J Aust. 1997;167:286. doi: 10.5694/j.1326-5377.1997.tb125062.x. [DOI] [PubMed] [Google Scholar]
- 22.Lamson DW, Brignall MS. Antioxidants in cancer therapy; their actions and interactions with oncologic therapies. Altern Med Rev. 1999;4:304–329. [PubMed] [Google Scholar]
- 23.Tasaki K, Maskarinec G, Shumay DM, et al. Communication between physicians and cancer patients about complementary and alternative medicine: Exploring patients' perspectives. Psychooncology. 2002;11:212–220. doi: 10.1002/pon.552. [DOI] [PubMed] [Google Scholar]
- 24.Roberts CS, Baker F, Hann D, et al. Patient-physician communication regarding use of complementary therapies during cancer treatment. J Psychosoc Oncol. 2005;23:35–60. doi: 10.1300/j077v23n04_03. [DOI] [PubMed] [Google Scholar]
- 25.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997. Results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 26.Sagar SM. Integrative oncology in North America. J Soc Integr Oncol. 2006;4:27–39. [PubMed] [Google Scholar]
- 27.Kmet L, Lee RC, Cook LS. Alberta, Canada: Institute of Health Economics; 2004. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields. [Google Scholar]
- 28.Begbie SD, Kerestes ZL, Bell DR. Patterns of alternative medicine use by cancer patients. Med J Aust. 1996;165:545–548. doi: 10.5694/j.1326-5377.1996.tb138639.x. [DOI] [PubMed] [Google Scholar]
- 29.Adler SR, Fosket JR. Disclosing complementary and alternative medicine use in the medical encounter: A qualitative study in women with breast cancer. J Fam Pract. 1999;48:453–458. [PubMed] [Google Scholar]
- 30.Balneaves LG, Kristjanson LJ, Tataryn D. Beyond convention: Describing complementary therapy use by women living with breast cancer. Patient Educ Couns. 1999;38:143–153. doi: 10.1016/s0738-3991(99)00061-0. [DOI] [PubMed] [Google Scholar]
- 31.Nam RK, Fleshner N, Rakovitch E, et al. Prevalence and patterns of the use of complementary therapies among prostate cancer patients: An epidemiological analysis. J Urol. 1999;161:1521–1524. [PubMed] [Google Scholar]
- 32.Verhoef MJ, Hagen N, Pelletier G, et al. Alternative therapy use in neurologic diseases: Use in brain tumor patients. Neurology. 1999;52:617–622. doi: 10.1212/wnl.52.3.617. [DOI] [PubMed] [Google Scholar]
- 33.Ashikaga T, Bosompra K, O'Brien P, et al. Use of complimentary and alternative medicine by breast cancer patients: prevalence, patterns and communication with physicians. Support Care Cancer. 2002;10:542–548. doi: 10.1007/s00520-002-0356-1. [DOI] [PubMed] [Google Scholar]
- 34.Powell CB, Dibble SL, Dall'Era JE, et al. Use of herbs in women diagnosed with ovarian cancer. Int J Gynecol Cancer. 2002;12:214–217. doi: 10.1046/j.1525-1438.2002.01098.x. [DOI] [PubMed] [Google Scholar]
- 35.Tough SC, Johnston DW, Verhoef MJ, et al. Complementary and alternative medicine use among colorectal cancer patients in Alberta, Canada. Altern Ther Health Med, 2002;8:54–64. [PubMed] [Google Scholar]
- 36.Boon H, Westlake K, Stewart M, et al. Use of complementary/alternative medicine by men diagnosed with prostate cancer: Prevalence and characteristics. Urology. 2003;62:849–853. doi: 10.1016/s0090-4295(03)00668-x. [DOI] [PubMed] [Google Scholar]
- 37.Eng J, Ramsum D, Verhoef M, et al. A population-based survey of complementary and alternative medicine use in men recently diagnosed with prostate cancer. Integr Cancer Ther. 2003;2:212–216. doi: 10.1177/1534735403256207. [DOI] [PubMed] [Google Scholar]
- 38.Saxe GA, Madlensky L, Kealey S, et al. Disclosure to physicians of CAM use by breast cancer patients: Findings from the Women's Healthy Eating and Living Study. Integr Cancer Ther. 2008;7:122–129. doi: 10.1177/1534735408323081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chow WH, Chang P, Lee SC, et al. Complementary and alternative medicine among Singapore cancer patients. Ann Acad Med Singapore. 2010;39:129–135. [PubMed] [Google Scholar]
- 40.Oh B, Butow P, Mullan B, et al. Patient-doctor communication: The use of complementary and alternative medicine by adult patients with cancer. J Soc Integr Oncol. 2010;8:56–64. [PubMed] [Google Scholar]
- 41.Rausch SM, Winegardner F, Kruk KM, et al. Complementary and alternative medicine: Use and disclosure in radiation oncology community practice. Support Care Cancer. 2011;19:521–529. doi: 10.1007/s00520-010-0846-5. [DOI] [PubMed] [Google Scholar]
- 42.Butler S, Owen-Smith A, Diiorio C, et al. Use of complementary and alternative medicine among men with prostate cancer in a rural setting. J Community Health. 2011;36:1004–1010. doi: 10.1007/s10900-011-9402-6. [DOI] [PubMed] [Google Scholar]
- 43.Al-Qudimat MR, Rozmus CL, Farhan N. Family strategies for managing childhood cancer: Using complementary and alternative medicine in Jordan. J Adv Nurs. 2011;67:591–597. doi: 10.1111/j.1365-2648.2010.05517.x. [DOI] [PubMed] [Google Scholar]
- 44.Greasley P. Is evaluating complementary and alternative medicine equivalent to evaluating the absurd? Eval Health Prof. 2010;33:127–139. doi: 10.1177/0163278710361923. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez-Ortuno MM, Belanger L, Ivers H, et al. The use of natural products for sleep: A common practice? Sleep Med. 2009;10:982–987. doi: 10.1016/j.sleep.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Pendleton DA. Pendleton D, Hasler J. Doctor-Patient Communication. London: Academic Press; 1983. Doctor-patient communication: A review; pp. 5–53. [Google Scholar]
- 47.Charles C, Gafni A, Whelan T. Self-reported use of shared decision-making among breast cancer specialists and perceived barriers and facilitators to implementing this approach. Health Expect. 2004;7:338–348. doi: 10.1111/j.1369-7625.2004.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Health and Medical Research Council. Canberra, Australia: Australian Government Publishing Service; 2004. Communicating with Patients: Advice for Medical Practitioners. [Google Scholar]
- 49.Salzburg Global Seminar. Salzburg statement on shared decision making. BMJ. 2011;342:d1745. doi: 10.1136/bmj.d1745. [DOI] [PubMed] [Google Scholar]