Abstract
Obstructive sleep apnea (OSA) is a common disease with important neurocognitive and cardiovascular sequelae. Existing therapies are unsatisfactory, leading investigators to seek alternative forms of anatomic manipulation to influence pharyngeal mechanics. We have developed a two-dimensional computational model of the normal human upper airway based on signal averaging of MRI. Using the finite element method, we can perform various anatomic perturbations on the structure in order to assess the impact of these manipulations on pharyngeal mechanics and collapse. By design, the normal sleeping upper airway model collapses at −13 cm H2O. This closing pressure becomes more negative (ie, less collapsible) when we perform mandibular advancement (−21 cm H2O), palatal resection (−18 cm H2O), or palatal stiffening (−17 cm H2O). Where clinical data are available in the literature, the results of our model correspond reasonably well. Furthermore, our model provides information regarding the site of obstruction and provides hypotheses for clinical studies that can be undertaken in the future (eg, combination therapies). We believe that, in the future, finite element modeling will provide a useful tool to help advance our understanding of OSA and its response to various therapies.
Keywords: breathing, computational model, lung, MRI, obstructive sleep apnea, pharyngeal collapse
Obstructive sleep apnea (OSA) is an important disorder due to both its high prevalence and its well-established sequelae.1,2 Although effective therapies exist for sleep apnea,3–6 investigators continue to seek alternative methods to manipulate pharyngeal mechanics/anatomy with the ultimate goal of improving currently available therapeutic modalities.3–8
Various different methods of manipulating pharyngeal mechanics have been used to treat snoring in individuals with largely normal upper airway anatomy and to treat sleep apnea in those with a compromised pharyngeal lumen. These methods include weight loss, oral appliances, and upper airway surgery.9–11 However, these techniques have also been problematic due to variable efficacy and minimal ability to predict therapeutic response. A number of investigators have attempted to determine which patients are most likely to respond to treatment, but without great success.12–16 We believe that improvements in our understanding of normal pharyngeal anatomy and physiology may be required to gain insights into disease pathogenesis and ultimately to develop new treatment strategies.
Our group has been involved in the development of a computational model to better understand the behavior of the human upper airway.17 We have used finite element analysis, which is a mathematical technique to quantitatively analyze the behavior of a given mechanical structure. We have previously published our two-dimensional model that uses anatomically correct structure from MRI of normal human subjects.17 With this model, we have used the critical closing pressure (Pcrit) as our outcome measure to assess the collapsibility of the upper airway.18 Using finite element analysis, we sought to determine the impact of mandibular advancement, palatal resection, and palatal stiffening on the mechanics of the normal human pharyngeal airway.
Materials and Methods
For this study, we modeled the effects of various anatomic manipulations on our finite element model of the normal pharyngeal airway. We studied both mandibular advancement and palatal removal since these are techniques commonly performed clinically. In addition, we studied the role of palatal stiffening with palatal implants, as this is a technique that is currently approved by the Food and Drug Administration for the treatment of snoring in patients with otherwise normal upper airways and in mild-to-moderate OSA.
The process used in the development of the finite element model has been previously reported but will be reviewed here briefly. The human pharyngeal airway is considered a mechanical system composed of multiple materials and having complex geometric structures. A two-dimensional anatomic structure of the pharyngeal airway was constructed based on midsagittal plane MRI in normal subjects. Although the two-dimensional structure ignores the effects of the lateral walls, this model can mimic the anatomic structures in the midsagittal plane, thereby maintaining the major features of negative pressure-induced upper airway collapse in the anteroposterior dimension. A “mean structure” for a specific group of subjects was obtained by averaging the corresponding signals collected from each MRI.
Several basic approximations were used for air flow. Because the pressure drop across the upper airway is small and the flow velocity is generally much smaller than the speed of sound, we have assumed that air is essentially incompressible. We used a laminar flow model to describe the flow in the upper airway. For an already complex problem involving very irregular geometric structures and strong fluid-solid interaction, the use of a turbulent flow model would greatly increase the difficulty of the simulation, and would be computationally expensive. However, the errors introduced by this assumption are likely small for the pressure distribution along the upper airway when the pressures at the entrance and the exit to the upper airway, but not the velocity there, are given as boundary conditions. Since the posterior pharyngeal wall is thin and attached to the vertebral bodies, we modeled it as a rigid structure.
The hard palate, mandible, and bottom of the epiglottis are considered fixed boundaries with zero displacement. At the interface of two solid tissues, such as the tongue and hyoid bone, the displacement must be continuous. The anterior part of the tongue and the boundary linking the bottom of the mandible and the bottom of epiglottis are considered free boundaries. The tongue, uvula, and other soft tissue, except the parts connecting directly to fixed boundaries, can move freely under loads. Fluid-solid interaction conditions are used at the deformable front wall of the upper airway, which is composed of the air-uvula, air-tongue, and air-epiglottis interfaces. The solid model calculates the displacement and gives the kinetic condition for the fluid model, and the force distribution calculated from the fluid model gives a dynamic boundary condition for the solid part.
For the genioglossus, the major upper airway dilator muscle, we have developed a muscle contraction model that varies the stiffness of the muscle during the respiratory cycle.19 The muscle contraction model consists of a series element, a parallel element, and a contractile element. The parallel element determines the passive mechanical properties of the muscle when no muscle activity is present (ie, paralysis). When, the genioglossus is active, both a contractile element and a series element become mechanically important.20
During sleep, upper airway pharyngeal dilator muscle activity generally decreases by approximately 10 to 20% during normal breathing, which has been observed in many of our experimental studies.21–26 Most of the remaining tonic and phasic activities represent fundamentally pressure-independent inputs controlled by the respiratory central pattern generator and premotor neurons. However, muscle activation is still written, for convenience, as a function of pressure but not phase or time. During sleep, the phasic activation increases slowly when upper airway pressure becomes more negative due to a substantial reduction but not complete loss of reflex mechanisms.27
Using our sleeping upper airway model, we assessed pharyngeal collapsibility (ie, Pcrit) during normal sleep and following three anatomic manipulations: mandibular advancement, uvulopalatopharyngoplasty (UPPP), and palatal stiffening. In our model, we simulated mandibular advancement by stretching the muscle to reach a 1-cm anterior mandibular displacement. We compared the calculated collapse of the upper airway with and without mandibular advancement at the same negative pressure, and predicted the closing pressure of the upper airway with mandibular advancement. Because there have been reports of reduced upper airway dilator muscle activity following mandibular advancement, we repeated the simulation following a 50% decrease in dilator activity.28
To investigate the effects of UPPP on upper airway collapsibility, we removed the uvula/soft palate from our two-dimensional structure of the pharyngeal airway. We then simulated the air flow and tongue deformation at different upper airway negative pressures and predicted the closing pressure (Pclose) of the sleeping airway after uvula removal.
For palatal stiffening, we simulated the insertion of a palatal implant (Pillar Palatal Implant; Restore Medical; St. Paul, MN) made of polyethylentherephthalate. We have used a range of Young’s moduli (deformability) for the implant and the immediately surrounding tissue, which is likely affected by the implant (scarring, fibrosis etc). Since we have no technique of experimentally measuring implant stiffness in situ in humans, we empirically chose a range of values based on in vitro studies. We chose a position for the implant based on the recommended placement site of the manufacturer.
Results
All of the anatomic manipulations led to important decreases in the collapsibility of the normal human pharyngeal airway. By design, the normal pharyngeal airway collapsed at a pressure of −13 cm H2O during sleep based on the work of Schwartz et al,18 although a range of values have been reported in the literature.29–32 As can be seen in the figures, progressively more negative airway pressures were necessary to occlude the pharyngeal airway for all three of the experimental conditions. The effect was most pronounced for mandibular advancement (Pclose = −21 cm H2O), but less so for palatal resection (Pclose = −18 cm H2O) or palatal stiffening (Pclose = −17 cm H2O).
Mandibular Advancement
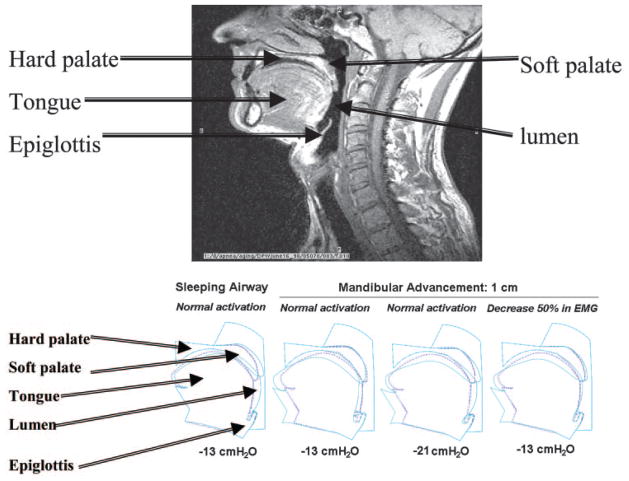
Figure 1 illustrates the effect of mandibular advancement on pharyngeal mechanics. The normal sleeping airway closes at −13 cm H2O epiglottic pressure, but remains patent at this pressure once the mandible is advanced by 1 cm. The upper airway does not actually close until −21 cm H2O is applied to the pharyngeal airway with advanced mandible. Even when muscle activity is reduced by 50%, as may occur with mandibular advancement, the pharyngeal airway remains widely patent at −13 cm H2O once the mandible has been advanced. These data illustrate that mandibular advancement leads to markedly reduced collapsibility of the pharynx, even if upper airway dilator muscle activity is reduced.
Figure 1.
MRI is provided as a reference for the labeling of our model. Below, left to right: An airway with normal sleeping muscle activation that collapses, as described above, at −13 cm H2O epiglottic pressure. The sleeping airway with 1-cm mandibular advancement and normal activation is widely patent. The sleeping airway with 1-cm mandibular advancement and normal muscle activity now has a Pclose of −21 cm H2O. If muscle activation is reduced by 50%, the upper airway remains widely patent at −13 cm H2O. EMG = electromyogram.
UPPP
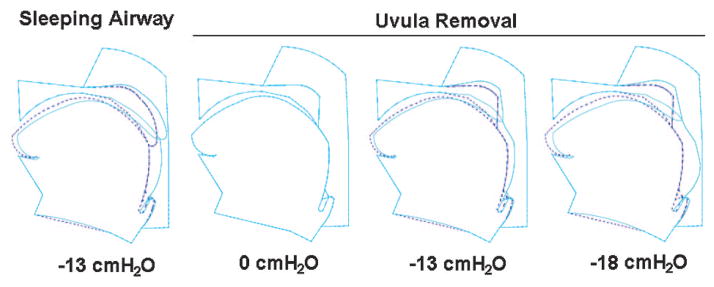
Excision of a portion of the soft palate and the uvula changes the flow pattern and pressure distribution in the pharyngeal airway substantially and, therefore, affects upper airway collapsibility. Figure 2 shows an airway during sleep with an intact palate at −13 cm H2O epiglottic pressure (Pclose), and the sleeping airway after UPPP at 0, −13, and −18 cm H2O pressure, respectively. One can see that the upper airway is still patent at −13 cm H2O pressure after UPPP. Collapse occurred at approximately −18 cm H2O pressure.
Figure 2.
Left to right: An airway during sleep with intact uvula at −13 cm H2O epiglottic pressure, and the airway during sleep without the uvula at 0, −13, and −18 cm H2O, respectively. All deformations are with normal sleeping muscle activation. The dashed line shows the initial tissue locations at zero pressure with or without the uvula. The solid line shows the structural position at a given epiglottic pressure with or without the uvula. Uvula removal makes the upper airway less collapsible and changes the site of obstruction.
Palatal Stiffening
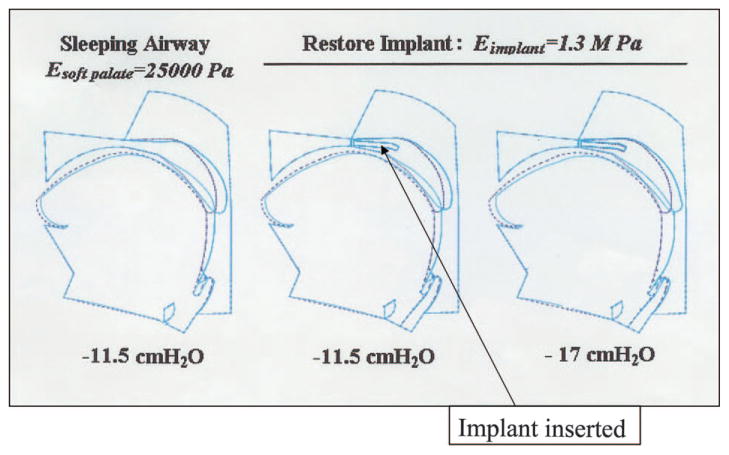
As can be seen in Table 1 and Figure 3, the palatal implant had a substantial impact on pharyngeal Pclose. Depending on the soft palate Young’s modulus and the implant stiffness, there was a consistent effect (of variable magnitude) on pharyngeal Pclose. For example, the soft palate with 25,000 Pa modulus (palate in asleep state) showed a change in Pclose from −11.5 to −17 cm H2O with an implant stiffness of 1.3 MPa. This magnitude of effect is on the order of what we have previously observed comparing normal men to normal women, suggesting the observed findings are clinically significant.17
Table 1.
Effects of Implant Stiffness on Upper Airway Pclose Values*
| E (Soft Palate), Pa | Pclose, cm H2O
|
||
|---|---|---|---|
| Without Implant | With Implant
|
||
| E (Implant) = 0.7 (MPa) | 1.3 (MPa) | ||
| 12,500 | −6.5 | −8.3 | −9 |
| 17,500 | −8.5 | −11.5 | −13 |
| 25,000 | −11.5 | −14.5 | −17 |
E is the Young’s modulus.
Figure 3.
The impact of the Restore Medical palatal implant on pharyngeal mechanics. The implant (18 mm in length, 2 mm in width including scar) was given a Young’s modulus (stiffness) of 1.3 MPa and inserted into the soft palate (stiffness or Young’s modulus [E] of 25,000 Pa). With normal genioglossal muscle contraction during sleeping conditions, the implant leads to a less collapsible pharyngeal airway (based on a more negative Pclose from −11.5 at baseline to −7 cm H2O after implant).
Discussion
The results of this study suggest that the magnitude of the change in Pcrit for the pharyngeal airway was greatest for mandibular advancement, and similar for palatal resection and palate stiffening. All procedures resulted in a considerable improvement in pharyngeal mechanics in the normal upper airway.
Previous investigators have examined the impact of anatomic manipulations of the pharyngeal airway, using a variety of different techniques. However, because of the nature of human research, it had not been possible to compare different anatomic manipulations within the same individual. There is also a paucity of data comparing the clinical effect of each of these therapeutic techniques between patients. Thus, there has been little consensus in the literature as to whether surgical therapies or dental devices have greater impact. Moreover, there is still minimal ability to predict which patients will respond to a particular therapy. Although the available clinical data are minimal in this area, they do support a greater impact of mandibular advancement as compared with uvula removal in OSA patients, as would be consistent with our model.33 We believe that finite element modeling may be an important technique in the future to predict therapeutic response in OSA patients. Clinical trials will ultimately be necessary to determine how accurately these modeling techniques predict therapeutic success.
The results of this study complement the existing literature regarding the impact of various structural manipulations on experimentally measured pharyngeal collapsibility. Schwartz et al34 reported on the Pcrit of patients undergoing UPPP measured before and after surgery. In this population with severe sleep-disordered breathing, consistent reductions were seen in Pcrit in patients undergoing surgery. Moreover, the magnitude of the change in Pcrit was predictive of the change in sleep-disordered breathing index following surgery. Because the present modeling study and the Schwartz clinical study addressed different populations, no direct comparisons can be made regarding the absolute values for Pcrit, although the magnitudes of the effects were comparable (ie, Pcrit 0.2 to −3.1 cm H2O in Schwartz et al34 vs Pcrit −13 to −18 cm H2O in present study). Of note, because the Pcrit values in normal subjects are somewhat variable in the literature, our simulations are most useful for examining the relative changes in Pclose measures, since the absolute values will depend on the initial Pclose applied to the model.
Kato et al35 measured the effect of mandibular advancement on pharyngeal mechanics in paralyzed OSA patients undergoing general anesthesia. The authors observed a dose-response effect, indicated by a more negative Pcrit with progressive mandibular advancement. For the control subjects, a Pclose of 1.3 cm H2O fell to −1.2 with 2-mm advancement, −2.4 with 4-mm advancement, and −3.5 with 6-mm advancement. Again methodologic differences preclude direct comparisons to the present study, although the changes that we observed (−13 to −21 cm H2O with 1-cm advancement) appear to be comparable.35 In addition, Ng et al36 studied the effect of mandibular advancement on upper airway Pclose on OSA patients during stage 2 sleep and during slow-wave sleep. The authors observed a less collapsible airway with mandibular advancement for both sleep stages (−1.6 cm H2O vs −3.9 cm H2O for stage 2, and −2.5 to −4.7 cm H2O for slow-wave sleep). Of note, the mean mandibular advancement was 4.6 mm in this study, perhaps explaining the more modest effects on pharyngeal mechanics as compared with our model.36
We are aware of no published data on palatal implants regarding their effects on pharyngeal mechanics. In one recent abstract, Friedman et al37 reported that apnea-hypopnea index reductions were achieved in all patients who received the implant alone or in combination with other treatments. In addition, subjective snoring (based on a 50% decrease in the visual analog scale) was reportedly improved in 80% of patients. In another abstract, Hein et al38 reported a trend toward reduction in apnea-hypopnea index from 16.3 ± 4.5 to 7.6 ± 4.6 events per hour (p < 0.001), in association with marked reductions in snoring based on visual analog scale (8.2 to 4.8, p = 0.001).
In addition to the Pclose measures observed with our model, the site of pharyngeal occlusion is also apparent after each of the therapeutic interventions. These data provide interesting speculation regarding the potential for combination therapies. For example, the combination of mandibular advancement with UPPP or palatal stiffening would be predicted to have a substantial impact on pharyngeal collapsibility. Indeed, some clinical data support this hypothesis.39 Similarly, stiffening of both the palate and the tongue may well have complementary benefit. Although some clinical data support the concept of combination therapy, we believe that further work is necessary in this area.
Despite its strengths, this study has a number of limitations. First, the existing model was based on anatomic and physiologic parameters derived from normal subjects. Therefore, one could argue that the impact of upper airway surgery on the normal airway has minimal clinical relevance. However, we believe that it was appropriate to first determine in normal subjects how anatomic manipulations affect collapsibility. We can then develop an OSA model using imaging and physiologic data from patients with disease. In addition, we would argue that individuals with simple snoring and relatively normal upper airway anatomy frequently undergo anatomic manipulations for the snoring. Thus, despite this limitation, we believe our results both provide physiologic insights and do have clinical relevance. Second, our model is two dimensional, which omits the effects in the lateral dimension and may result in some limitations when it is used for clinical predictions. However, our model is useful in predicting the behavior of the pharyngeal airway in the anteroposterior plane. Studies during both anesthesia (Shiroh Isono, MD; personal communication; December 2004) and during sleep40 have shown pharyngeal narrowing to occur primarily in an anteroposterior direction. In fact, in the study by Horner et al,40 collapse was due to posterior displacement of the tongue and soft palate “in the majority of patients,” just as we have modeled in normal subjects. Similarly Ciscar et al,41 using ultrafast MRI in normal subjects and OSA patients during sleep, observed that the changes in lateral walls were secondary to changes in airway caliber. Thus, anteroposterior collapse does appear to be physiologically and clinically important. However, upper airway collapse is fundamentally a complex three-dimensional problem. For a more accurate simulation of upper airway behaviors, we have begun to develop a more realistic computational model based on the three-dimensional structure of the pharyngeal airway. In addition, because our primary goal in this study was to examine the isolated effect of anatomic changes, any errors introduced by this and other assumptions (ie, two dimensions) are likely to affect both the before models and after models equally. We regard assumptions as essential to the modeling process itself, and would argue that they are unlikely to affect the outcomes of the present study. In fact, our early studies of the normal upper airway have demonstrated the feasibility of using such a two-dimensional approximation. For example, by matching the measured −5 cm H2O Pclose in the male passive upper airway, we predicted a 6,000 Pa Young’s modulus for the passive tongue, using our two-dimensional model. This value is almost the same as the value measured in passive skeletal muscle.42
Another assumption we have used is that flow through the upper airway is primarily laminar rather than turbulent. Although the approximation could result in some errors in the flow simulation,19 the existing literature would suggest that any errors introduced by this assumption would be small and unlikely to importantly bias our results for within-subject comparisons.43–45 Finally, we do not have any definitive data providing evidence that our predictions are accurate, which will ultimately be necessary to change clinical practice. However, our results do correspond relatively closely with those reported in clinical studies, when such data are available.
Conclusions
This article adds to the literature in this area for a number of reasons. First, it illustrates the utility of finite element analysis in the investigation of the human upper airway. Second, we have demonstrated that palatal stiffening may have an important effect on pharyngeal airway mechanics, of comparable magnitude to clinically performed anatomic manipulations. Third, these data are suggestive that palatal resection has a smaller mechanical effect than mandibular advancement on the normal human pharyngeal airway. Further investigation will be required to determine the clinical relevance of these observations in OSA patients.
Acknowledgments
This work is supported by grants from the National Institutes of Health, HL48531 (RO1) and HL60292 (specialized center of research). Dr. Malhotra is supported by a Scientific Development Grant from the American Heart Association and the Beeson Award by the National Institute of Aging (AG024837–01). Restore Medical also sponsored the work related to the Pillar palatal implant.
Abbreviations
- OSA
obstructive sleep apnea
- Pclose
closing pressure
- Pcrit
critical closing pressure
- UPPP
uvulopalatopharyngoplasty
Footnotes
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
References
- 1.Malhotra A, White D. Seminar: obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 2.Peppard P, Young T, Palta M, et al. Prospective study of the association between sleep disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.Jenkinson C, Davies RJ, Mullins R, et al. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–2105. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 4.Faccenda JF, Mackay TW, Boon NA, et al. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–348. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 5.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 6.Becker H, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 7.Engleman HM, Martin SE, Douglas NJ. Compliance with CPAP therapy in patients with the sleep apnoea/hypopnoea syndrome. Thorax. 1994;49:263–266. doi: 10.1136/thx.49.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engleman HM, Martin SE, Deary IJ, et al. Effect of CPAP therapy on daytime function in patients with mild sleep apnoea/hypopnoea syndrome. Thorax. 1997;52:114–119. doi: 10.1136/thx.52.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith PL, Gold AR, Meyers DA, et al. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med. 1985;103:850–855. doi: 10.7326/0003-4819-103-6-850. [DOI] [PubMed] [Google Scholar]
- 10.Lowe AA, Sjoholm TT, Ryan CF, et al. Treatment, airway and compliance effects of a titratable oral appliance. Sleep. 2000;15(Suppl):S172–S178. [PubMed] [Google Scholar]
- 11.Powell NB, Riley RW, Robinson A. Surgical management of obstructive sleep apnea syndrome. Clin Chest Med. 1998;19:77–86. doi: 10.1016/s0272-5231(05)70433-0. [DOI] [PubMed] [Google Scholar]
- 12.Shepard JW, Jr, Thawley SE. Localization of upper airway collapse during sleep in patients with obstructive sleep apnea. Am Rev Respir Dis. 1990;141:1350–1355. doi: 10.1164/ajrccm/141.5_Pt_1.1350. [DOI] [PubMed] [Google Scholar]
- 13.Schwab RJ. Upper airway imaging. Clin Chest Med. 1998;19:33–54. doi: 10.1016/s0272-5231(05)70430-5. [DOI] [PubMed] [Google Scholar]
- 14.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 15.Isono S, Tanaka A, Sho Y, et al. Advancement of the mandible improves velopharyngeal airway patency. J Appl Physiol. 1995;79:2132–2138. doi: 10.1152/jappl.1995.79.6.2132. [DOI] [PubMed] [Google Scholar]
- 16.Isono S, Shimada A, Tanaka A, et al. Efficacy of endoscopic static pressure/area assessment of the passive pharynx in predicting uvulopalatopharyngoplasty outcomes. Laryngoscope. 1999;109:769–774. doi: 10.1097/00005537-199905000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra A, Huang Y, Fogel R, et al. The male predisposition to pharyngeal collapse: the importance of airway length. Am J Respir Crit Care Med. 2002;166:1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz AR, Smith PL, Wise RA, et al. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64:535–542. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Malhotra A, White DP. Computational simulation of human upper airway collapse using a pressure/state-dependent model of genioglossus muscle contraction under laminar flow conditions. J Appl Physiol. 2005 doi: 10.1152/japplphysiol.00668.2004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauerland EK, Mitchell SP. Electromyographic activity of the human genioglossus muscle in response to respiration and to positional changes of the head. Bull Los Angeles Neurol Soc. 1970;35:69–73. [PubMed] [Google Scholar]
- 21.Tangel DJ, Mezzanotte WS, Sandberg EJ, et al. Influences of NREM sleep on the activity of tonic vs. inspiratory phasic muscles in normal men. J Appl Physiol. 1992;73:1058–1066. doi: 10.1152/jappl.1992.73.3.1058. [DOI] [PubMed] [Google Scholar]
- 22.Horner R, Innes J, Morrell M, et al. The effect of sleep on reflex genioglossus muscle activation by stimuli of negative airway pressure in humans. J Physiol. 1994;476:141–151. [PMC free article] [PubMed] [Google Scholar]
- 23.Wheatley J, Mezzanotte W, Tangel D, et al. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis. 1993;148:597–605. doi: 10.1164/ajrccm/148.3.597. [DOI] [PubMed] [Google Scholar]
- 24.Worsnop C, Kay A, Pierce R, et al. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85:908–920. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- 25.Worsnop C, Kay A, Kim Y, et al. Effect of age on sleep onset-related changes in respiratory pump and upper airway muscle function. J Appl Physiol. 2000;88:1831–1983. doi: 10.1152/jappl.2000.88.5.1831. [DOI] [PubMed] [Google Scholar]
- 26.Fogel R, Trinder J, Malhotra A, et al. Within-breath control of genioglossal muscle activation in humans: effect of sleep-wake state. J Physiol. 2003;550:899–910. doi: 10.1113/jphysiol.2003.038810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malhotra A, Pillar G, Fogel R, et al. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med. 2000;162:1058–1062. doi: 10.1164/ajrccm.162.3.9912067. [DOI] [PubMed] [Google Scholar]
- 28.Oshima T, Tsai W, Hajduk E, et al. Mandibular protrusion decreases genioglossal EMG during sleep in patients with obstructive sleep apnea [abstract] Am J Respir Crit Care Med. 1998;157:A655. [Google Scholar]
- 29.Issa FG, Sullivan CE. Upper airway closing pressures in snorers. J Appl Physiol. 1984;57:528–535. doi: 10.1152/jappl.1984.57.2.528. [DOI] [PubMed] [Google Scholar]
- 30.Issa FG, Sullivan CE. Upper airway closing pressures in obstructive sleep apnea. J Appl Physiol. 1984;57:520–527. doi: 10.1152/jappl.1984.57.2.520. [DOI] [PubMed] [Google Scholar]
- 31.Rowley J, Zhou X, Vergine I, et al. Influence of gender on upper airway mechanics: upper airway resistance and Pcrit. J Appl Physiol. 2001;91:2248–2254. doi: 10.1152/jappl.2001.91.5.2248. [DOI] [PubMed] [Google Scholar]
- 32.Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150:481–485. doi: 10.1164/ajrccm.150.2.8049833. [DOI] [PubMed] [Google Scholar]
- 33.Walker-Engstrom ML, Tegelberg A, Wilhelmsson B, et al. 4-year follow-up of treatment with dental appliance or uvulopalatopharyngoplasty in patients with obstructive sleep apnea: a randomized study. Chest. 2002;121:739–746. doi: 10.1378/chest.121.3.739. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz AR, Schubert N, Rothman W, et al. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1992;145:527–532. doi: 10.1164/ajrccm/145.3.527. [DOI] [PubMed] [Google Scholar]
- 35.Kato J, Isono S, Tanaka A, et al. Dose-dependent effects of mandibular advancement on pharyngeal mechanics and nocturnal oxygenation in patients with sleep-disordered breathing. Chest. 2000;117:1065–1072. doi: 10.1378/chest.117.4.1065. [DOI] [PubMed] [Google Scholar]
- 36.Ng A, Gotsopoulos H, Qian J, et al. Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;168:238–241. doi: 10.1164/rccm.200211-1275OC. [DOI] [PubMed] [Google Scholar]
- 37.Friedman M, Vidyasagar R, Bliznikas D. Patient selection and efficacy of the Pillar implant technique for snoring and OSAHS [AAO meeting abstract] New York, NY: American Academy of Otolaryngology; 2004. [DOI] [PubMed] [Google Scholar]
- 38.Hein G, Verse T, Stuck BA, et al. The Pillar palatal implant system: first results in OSA patients [ASO meeting abstract] New York, NY: Academy of Otolaryngology; 2004. [Google Scholar]
- 39.Millman RP, Rosenberg CL, Carlisle CC, et al. The efficacy of oral appliances in the treatment of persistent sleep apnea after uvulopalatopharyngoplasty. Chest. 1998;113:992–996. doi: 10.1378/chest.113.4.992. [DOI] [PubMed] [Google Scholar]
- 40.Horner RL, Shea SA, McIvor J, et al. Pharyngeal size and shape during wakefulness and sleep in patients with obstructive sleep apnoea. Q J Med. 1989;72:719–735. [PubMed] [Google Scholar]
- 41.Ciscar MA, Juan G, Martinez V, et al. Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J. 2001;17:79–86. doi: 10.1183/09031936.01.17100790. [DOI] [PubMed] [Google Scholar]
- 42.Duck F. Physical properties of tissue: a comprehensive reference book. London, UK: Academic Press; 1990. [Google Scholar]
- 43.Exar E, Collop N. Lack of efficacy of heliox in respiratory effort related arousals. Sleep. 1999;22:S59. [Google Scholar]
- 44.Collop N, Percy J, Haywood J. The effect of heliox on obstructive sleep apnea [abstract] Am Rev Respir Dis. 1993;149:A885. [Google Scholar]
- 45.Malhotra A, Pillar G, Fogel RB, et al. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med. 2002;165:71–77. doi: 10.1164/ajrccm.165.1.2011065. [DOI] [PubMed] [Google Scholar]